Abstract
Purpose:
Tumor mutational burden (TMB) has emerged as a potential predictive biomarker for clinical response to ICI therapy, but whether TMB also predicts toxicity remains unknown. We investigated the relationship between TMB, objective response rate (ORR), overall survival (OS), and toxicity for ICI therapy across multiple cancer types.
Methods:
We searched MEDLINE, PubMed, and ASCO/ESMO/AACR meetings for clinical trials of anti-PD(L)1, CTLA-4, or combination in 29 cancer types. We assessed ICI administered, responses (complete or partial response), median OS, OS hazard ratio, and grade 3/4 toxicity. We conducted a systematic review, meta-analysis and meta-regression using tumor level TMB data from Foundation Medicine.
Results:
117 clinical trials, which included 12450 patients treated ICI therapy, were analyzed. Meta-regression analysis revealed that TMB was significantly associated with ORR for anti-PD(L)1, anti-CTLA-4, and combination (p<0.0001 for all), but not associated with toxicity in all treatment groups. OS data were unavailable for most studies included in our meta-analysis, and the relationship between TMB and OS in this subset was not significant (p=0.26). In high TMB tumor types (≥10 mut/megabase) the improvement of ORR and increase in grade 3/4 toxicity with combination ICI therapy as compared to PD(L)1 monotherapy were 21.13% and 25.41%, respectively, as compared to 3.73% and 18.78% in low TMB tumor types (<10 mut/megabase).
Conclusion:
There is a positive association between TMB and clinical response with anti-PD(L)1, anti-CTLA-4, and combination ICIs, but no association between TMB and toxicity. These results imply a favorable risk/benefit ratio for ICIs in tumors with a higher TMB.
Keywords: Tumor Mutational Burden, Immune Checkpoint Inhibitors, Immunotherapy Toxicity, Immunotherapy Response, Predictive Biomarker
Introduction
Targeting immune checkpoints via programmed cell death protein 1 (PD-1), its ligand (PD-L1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) has transformed treatment paradigms for numerous cancers.1–4 However, response rates have not been consistent across tumor types. Even within individual cancer types, clinical responses to immune checkpoint inhibitors (ICI) are variable, and predictive and prognostic biomarkers for ICI therapy are needed. PD-L1 expression by immunohistochemical measurement is the most commonly utilized predictive biomarker for immunotherapy, but it has major limitations.5
Tumor mutational burden (TMB), defined as the total number of nonsynonymous mutations per coding area of a tumor genome, has emerged as a novel potential predictive biomarker for response to anti-PD(L)1 immunotherapy.6–9 Rizvi and colleagues first demonstrated an association between increased TMB and clinical benefit of anti-PD-1 therapy using whole exome sequencing (WES) data from patients with advanced NSCLC.9 Since that time a relationship between TMB and clinical benefit from ICIs has been demonstrated within multiple other tumor types.10–15 In addition to predicting responses to ICIs, TMB may also predict improved survival with single and combination immunotherapy within some tumor types.16,17 TMB is a surrogate for the number of expressed tumor neoantigens; these abnormal proteins are presented on the human leukocyte antigen (HLA) complex and recognized by T cells, thereby stimulating antitumor immunity.18 Due to the high costs of WES, TMB is often estimated for clinical practice using selected targeted gene panels. TMB is independent of PD-L1 expression and may therefore provide unique information about ICI responsiveness.19
Understanding the relationship between TMB and clinical outcomes, including therapeutic response and adverse events, may have the potential to improve the clinical use and therapeutic development of ICI immunotherapy. If TMB is found to be associated with both response and toxicity, then TMB could eventually be used to identify patients who should be treated more intensively (eg, with higher doses of anti-CTLA-4 therapy) to meet the most appropriate threshold of therapeutic effect.20 By contrast, if TMB is associated with response but not toxicity, then TMB could emerge as a key biomarker for establishing which tumors benefit outweighs risks of ICI therapy. We conducted a systematic review, meta-analysis and meta-regression, to evaluate the relationship between objective response rate (ORR), overall survival (OS), toxicity and TMB for anti-PD-1, anti-PD-L1, anti-CTLA-4 monotherapy, and combination, anti-PD(L)1 plus anti-CTLA-4 therapy across multiple cancer types.
Methods
This meta-regression and meta-analysis was conducted in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.21 Three independent reviewers (A.O., A.P and M.Y) performed the literature search, assessed eligibility criteria, and performed data-extraction.
Search Strategy and Study Selection
We initially identified 29 major solid tumor types or subtypes for which TMB has been well characterized using at least 50 tumor specimens (eTable 1 in the Supplement). We conducted the literary search by screening electronic searches of MEDLINE, pubmed (from Jan 1, 2010 to Feb 20, 2019), as well as abstracts presented at ASCO, ESMO, AACR meetings 2010-2019 to identify ORR and grade 3/4 toxicity rate for all anti-PD-1, anti-PD-L1, CTLA-4 monotherapies and combination ICI therapies, anti-PD-1 or anti-PD-L1 plus anti-CTLA-4 therapies, in each of these cancer types. ORR was obtained from studies which reported either the overall ORR, or from the reported complete and partial response rate. Similarly, OS data, including median OS reported and hazard ratio (HR), was captured when available. We searched for clinical trials using the specific search terms: nivolumab, BMS-936558, pembrolizumab, MK-3475, atezolizumab, MPDL3280A, durvalumab, MEDI4736, tremelimumab, CP-675,206, Ipilimumab, BMS-734016, MDX-010, MDX-101, MEDI4736, avelumab, MSB0010718C, BMS-936559, cemiplimab, REGN2810, anti-PD-1, anti-PD-L1, anti-CTLA-4. Only English publications were considered. We also contacted experts in the field to locate additional published trials of these agents that may not have been included in our initial electronic search. We excluded trials with a total sample size or a sample size in the subgroup of interest less than 10. We also excluded studies that investigated anti-PD-1, anti-PD-L1, anti-CTLA-4 therapies in combination with other agents (not including ICI combination alone), and studies that selected patients based on PD-L1 expression or other immune-related biomarkers. Of the remaining studies, only the largest published study for each anti-PD-1, anti-PD-L1, anti-CTLA-4 monotherapy therapy or combination was included in the final assessment of ORR, OS and grade 3/4 toxicity rate for each cancer type or subtype (eTable 2 in the Supplement). For survival hazard ratio analysis, we only included studies whose control arm received standard of care treatment. For descriptive analysis of median OS data, we included all studies whose median OS for single and dual agent immunotherapy was available.
Data Extraction
For each included study or data set, we extracted the checkpoint inhibitor assessed, number of patients treated, number of responders (complete and partial response) from each treatment group, median overall survival with associated hazard ratio and total number of patients experiencing grade 3 and 4 toxicity. The number of treated, as well as number of responders and those who experienced a grade 3/4 toxicity, was used to calculate ORR and rate of grade 3/4 toxicity in each individual study and pooled estimates for each tumor type or subtype. The median TMB for each of the 29 solid tumor types was acquired from a validated targeted TMB assay performed and provided by Foundation Medicine (FoundationOne assay, Cambridge, MA, USA).22,23 Details of the assay have been previously reported, which estimates the total number of somatic, coding mutations (including synonymous and non-synonymous mutations and short indels) per megabase of tumor genome.23
Statistical Analysis:
For each monotherapy and the combination immunotherapy, to evaluate the association between TMB and tumor response, as well as association of TMB and toxicity, meta-regression was performed using a logistic-normal mixed-effects model where the median TMB of the tumor type (log-transformed) was included as a study-level fixed effect. The ability of TMB to explain the heterogeneity across tumor types was summarized as percent reduction of between-study heterogeneity in the model with and without TMB on the logit scale. To evaluate whether the association of response and TMB, as well as toxicity and TMB, differs between anti-PD(L)1 monotherapy and the combination ICI therapy, we tested the interaction term of TMB and treatment group in the logistic-normal mixed-effects model.
Meta-analysis was conducted to summarize response rate and toxicity rate for each tumor type and treatment group. The pooled estimates were obtained by a random-effects model using DerSimonian-Laird method. For tumor types with paired estimates of response rate and toxicity rate for both anti-PD(L)1 monotherapy and the combination ICI therapy, difference between treatment groups was visualized via a heat map, and spearman’s correlation coefficient was computed to correlate ORR and toxicity rate using the pooled estimates. The overall difference in response and toxicity rates between PD(L)1 monotherapy and the combination ICI therapy was obtained by a random-effects model with DerSimonian-Laird method using the pooled estimates from two treatment groups of each tumor type.
To evaluate the association between TMB and hazard ratio (log-transformed), meta-regression was performed using a mixed-effect model with DerSimonian-Laird method among studies with hazard ratio reported. Similarly, meta-analysis was conducted to summarize hazard ratios of studies with median overall survival reported using a random-effect model with inverse variance weighting method. Due to limited survival data of studies with the combination ICI therapy, these analyses were performed for anti-PD(L)1 monotherapy only.
Results
Of the 260 studies we identified, 117 ICI studies including a total of 12450 patients met the inclusion criteria and were included in our overall analysis. We identified a total of 75 studies with anti-PD(L)1 monotherapy, 14 studies with anti-CTLA-4 monotherapy, 28 studies with anti-PD(L)1 plus anti-CTLA-4 combination (Figure 1).
Figure 1: PRISMA Flowchart.
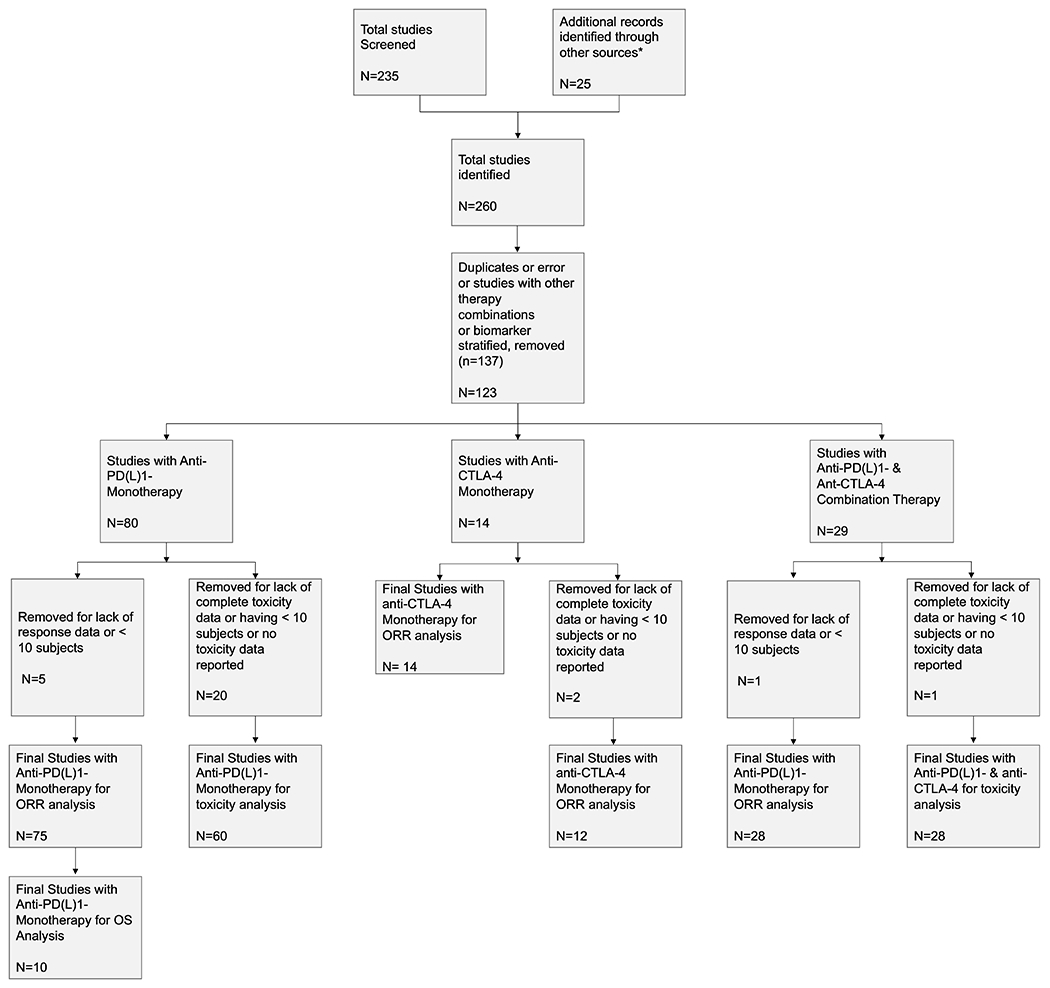
* from experts in the field to locate additional published trials of ICI agents
Tumor Objective Response and Tumor Mutational Burden
A total of 75 trials/studies were included for anti-PD(L)1 monotherapy tumor ORR analysis. In these trials, 29 tumor types were examined, where a total of 8692 patients were treated with PD(L)1 monotherapy and 1568 patients (18.04%) responded to the treatment. For anti-CTLA-4 monotherapy, a total of 14 trials were included in the final analysis of tumor response rate. In these trials, 11 tumor types were examined where a total 1377 patients were treated with anti-CTLA-4 monotherapy and 130 patients (9.44%) responded to the treatment. With regard to combination ICI therapy with anti-PD(L)1 plus anti-CTLA-4, a total of 28 trials among 19 tumor types were included, where a total of 2381 patients were treated and 791 (33.22%) patients responded to the treatment.
Meta-regression analysis revealed that TMB was positively associated with ORR, for all ICI treatment groups including anti-PD(L)1 monotherapy, anti-CTLA-4 monotherapy, and anti-PD(L)1 plus anti-CTLA-4 combination (p<0.0001, respectively; Figure 2A, Figure 2B, and Figure 2C). TMB explained 44.81%, 85.00%, and 45.93% of the heterogeneity in response across tumor types in three treatment groups, respectively. A meta-analysis of pooled response rates for each specific tumor type with each specific ICI therapy within each treatment category (anti-PD(L)1, anti-CTLA-4 and anti-PD(L)1 plus anti-CTLA-4 combination) can be found in eFigure 1, eFigure 2, and eFigure 3 in the Supplement.
Figure 2: Association Between Overall Response Rate and TMB of Single and Dual ICI Therapy.
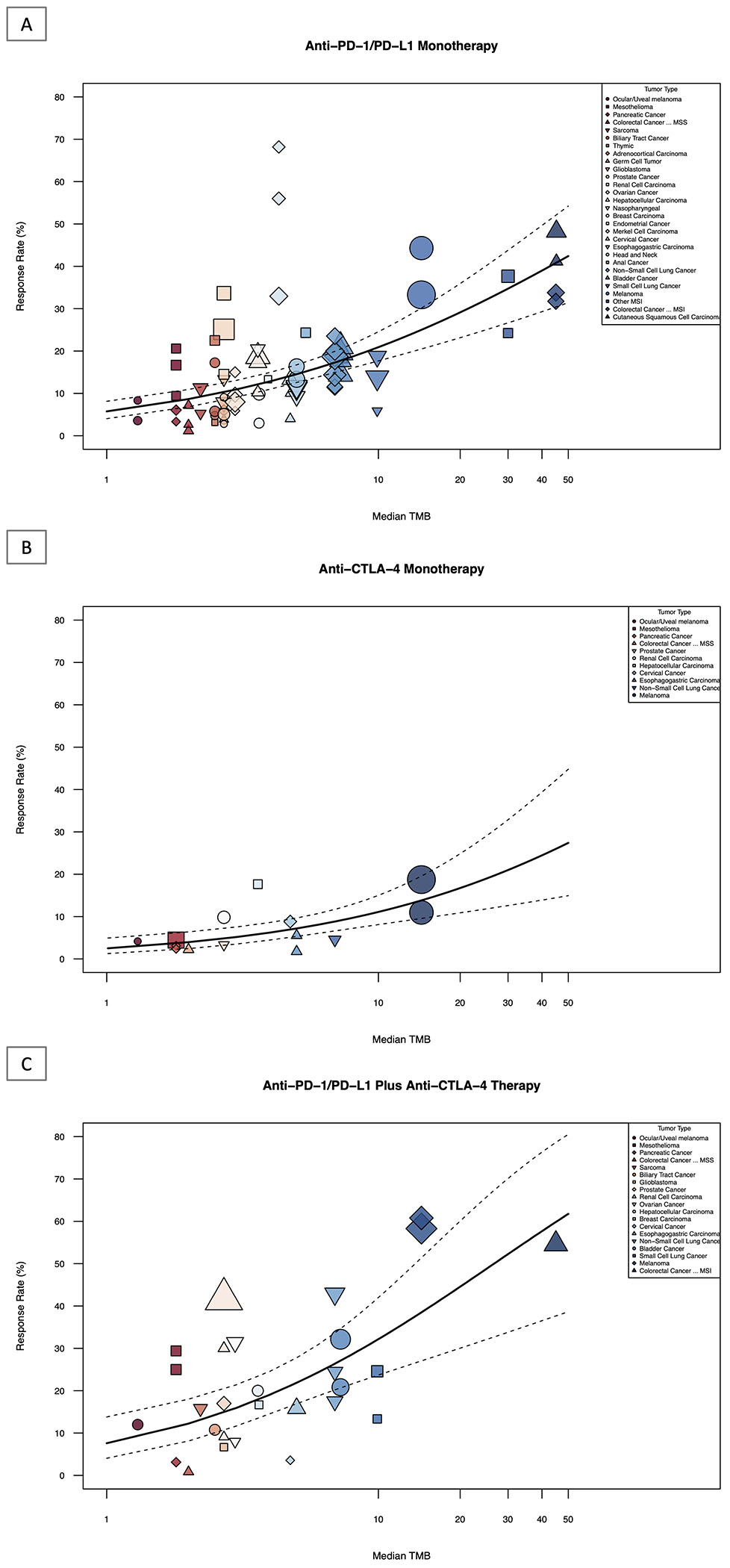
Shown above is the graphical representation of the meta-regression summary of ORR and TMB. This includes the median number of coding somatic mutations per megabase (MB) of DNA in 29 tumor types or subtypes among patients who received inhibitors of PD-1, PD-L1 and CTLA-4, as single or dual agents as described in published studies for which data regarding the objective response rate were available. The number of patients who were evaluated for the objective response rate is shown for each tumor type (size of the shape indicates weight of study as assessed by inverse-variance, color and shape indicate tumor type, see Legend Key). Data on the x axis are shown on a logarithmic scale.
2A: Association between objective response rate and TMB of anti-PD(L)1 monotherapy
2B: Association between objective response rate and TMB of anti-CTLA-4 monotherapy
2C: Association between objective response rate and TMB of anti-PD(L)1 plus anti-CTLA-4 combination therapy
Checkpoint Inhibitor Toxicity and Tumor Mutational Burden
A total of 60 trials/studies were included in analysis of ICI toxicity in patients treated with anti-PD(L)1 monotherapy. In these studies, 28 tumor types were examined where 8411 patients were treated with PD(L)1 monotherapy, of which 1262 (15.00%) patients experienced grade 3/4 toxicity. For anti-CTLA-4 monotherapy, 12 trials were analyzed among 9 tumor types that were included in the final analysis for grade 3/4 toxicity. In these studies, a total of 1317 patients were treated with anti-CTLA-4 monotherapy and among them 450 (34.17%) patients experienced grade 3 and 4 toxicity. In combination ICI therapy with anti-PD(L)1 plus anti-CTLA-4, a total of 28 trials were included in the final analysis of grade 3 and 4 toxicity. Among these studies 19 tumor types were examined, where among 2562 patients treated with ICI combination therapy, 1068 (41.69%) patients experienced a grade 3 and 4 toxicity.
Utilizing meta-regression, TMB was not significantly associated with grade 3 and 4 toxicity among all 3 ICI treatments groups (anti-PD(L)1: p=0.7819, anti-CTLA-4: p=0.6269, and anti-PD(L)1 plus anti-CTLA-4, p=0.7089; Figure 3A, Figure 3B, and Figure 3C). TMB explained only −0.12%, 8.10%, and 0.36% of the heterogeneity in toxicity across tumor types in the treatment groups, respectively. A meta-analysis of pooled grade 3 and 4 toxicity rates for each specific tumor type with each specific ICI therapy within each treatment category (anti-PD(L)1, anti-CTLA-4 and anti-PD(L)1 plus anti-CTLA-4 combination) can be found in eFigure 4, eFigure 5, and eFigure 6 in the Supplement.
Figure 3: Association Between Toxicity Rate and TMB of Single and Dual ICI Therapy.
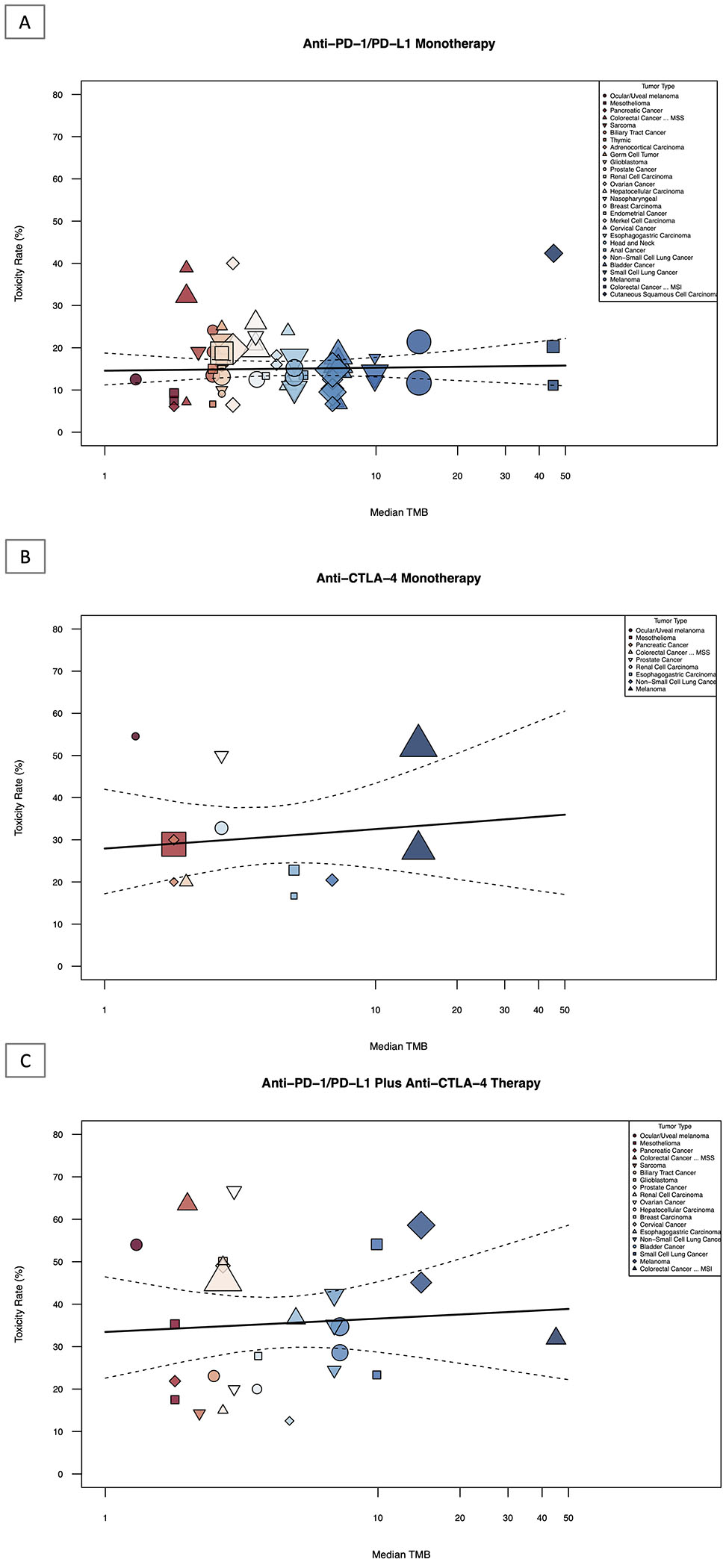
Shown above is the graphical representation of the meta-regression summary of grade 3/4 toxicity rate and TMB. This includes the median number of coding somatic mutations per megabase (MB) of DNA in 29 tumor types or subtypes among patients who received inhibitors of PD-1, PD-L1 and CTLA-4, as single or dual agents as described in published studies for which data regarding the toxicity information was available. The number of patients who were evaluated for the toxicity rate is shown for each tumor type (size of the shape indicates weight of study as assessed by inverse-variance, color and shape indicate tumor type, see Legend Key). Data on the x axis are shown on a logarithmic scale.
3A: Association between toxicity rate and TMB of anti-PD(L)1 monotherapy
3B: Association between toxicity rate and TMB of anti-CTLA-4 monotherapy
3C: Association between toxicity rate and TMB of anti-PD(L)1 plus anti-CTLA-4 combination therapy
PD(L)1 Monotherapy versus Combination Immune Checkpoint Inhibitor Therapy
Meta-regression examining the association between ORR and TMB between anti-PD(L)1 monotherapy and anti-PD(L)1 plus anti-CTLA-4 combination across tumor types, revealed that the ICI combination group had a significantly higher response rate after adjusting for TMB in comparison to anti-PD(L)1 monotherapy (p=0.0018). However, in evaluating whether the ORR difference between the combination and anti-PD(L)1 monotherapy varies with TMB, no significant differential effect was observed (test for treatment group by TMB interaction, p=0.5788; Figure 4).
Figure 4: Estimated Overall Response and Toxicity Rates from Meta-Regression of Single and Dual ICI therapy.
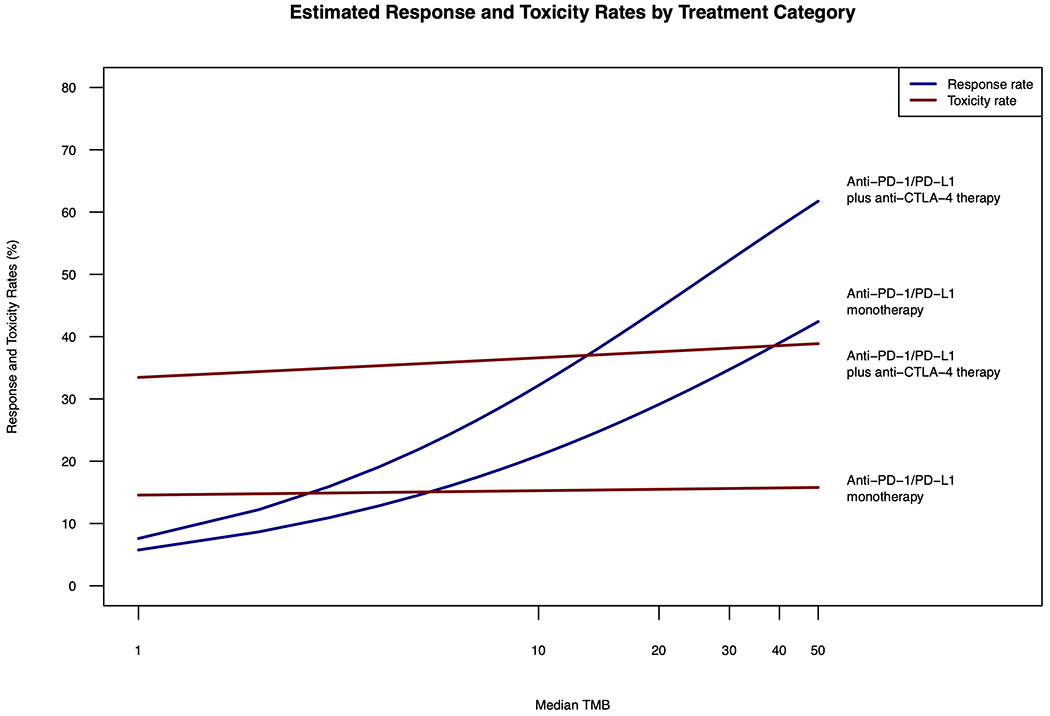
Shown above is the estimated overall response (blue) and toxicity rates (red) from meta-regression for anti-PD(L)1 monotherapy and anti-PD(L)1 plus anti-CLTA-4 combination therapy. TMB is on a logarithmic scale.
In evaluating the relationship between toxicity and TMB between anti-PD(L)1 monotherapy and anti-PD(L)1 plus anti-CTLA-4 combination, mixed-effects logistic meta-regression showed that that the ICI combination group had a significantly higher grade 3 and 4 toxicity rate compared to anti-PD(L)1monotherapy after adjusting for TMB (p<0.0001). However, change in TMB did not lead to a significant variation in toxicity difference between anti-PD(L)1 and anti-PD(L)1 plus anti-CTLA-4 combination (p=0.8847), indicating that the difference of grade 3 and 4 toxicity rates between two treatment groups does not depend on TMB (Figure 4).
ORR and grade 3/4 toxicity were simultaneously illustrated through a heatmap with both anti-PD(L)1 monotherapy and anti-PD(L)1 plus anti-CTLA-4 combination among tumor types that were studied in both treatment groups (Figure 5A). Pooled data for such individual tumors types, comparing response and toxicity rates of anti-PD(L)1 and anti-PD(L)1 plus anti-CTLA-4 for each tumor type, are presented in eTable 3 in the Supplement. ORR and toxicity rates were not significantly correlated in neither anti-PD(L)1 (Spearman correlation coefficient r=0.10, p=0.6863) nor anti-PD(L)1 plus anti-CTLA-4 combination (r=0.06, p=0.8147; Figure 5B). The estimated overall difference between anti-PD(L)1 monotherapy and combination ICI therapy among 19 major tumor types across all TMBs is 6.28% for ORR (95% interval, 0.0273, 0.0983) and 19.55% for grade 3/4 toxicity (95% interval, 0.1357, 0.2553). The estimated overall difference of ORR between anti-PD(L)1 monotherapy and combination ICI therapy in tumors with TMB <10 median mut/megabase, was 3.73% (95% interval, 0.0096, 0.0650) where tumors with TMB ≥ 10 mut/megabase was 21.13% (95% interval 0.1279, 0.2947). The estimated overall difference of grade 3/4 toxicity between anti-PD(L)1 monotherapy and combination ICI therapy in tumors with TMB <10 median mut/megabase, was 18.78% (95% interval 0.1227, 0.2529) where tumors with TMB ≥ 10 mut/megabase was 25.41% (95% interval: 0.0664, 0.4418).
Figure 5: Heatmap and Correlative Analysis of Response and Toxicity: Single and Dual ICI therapy.
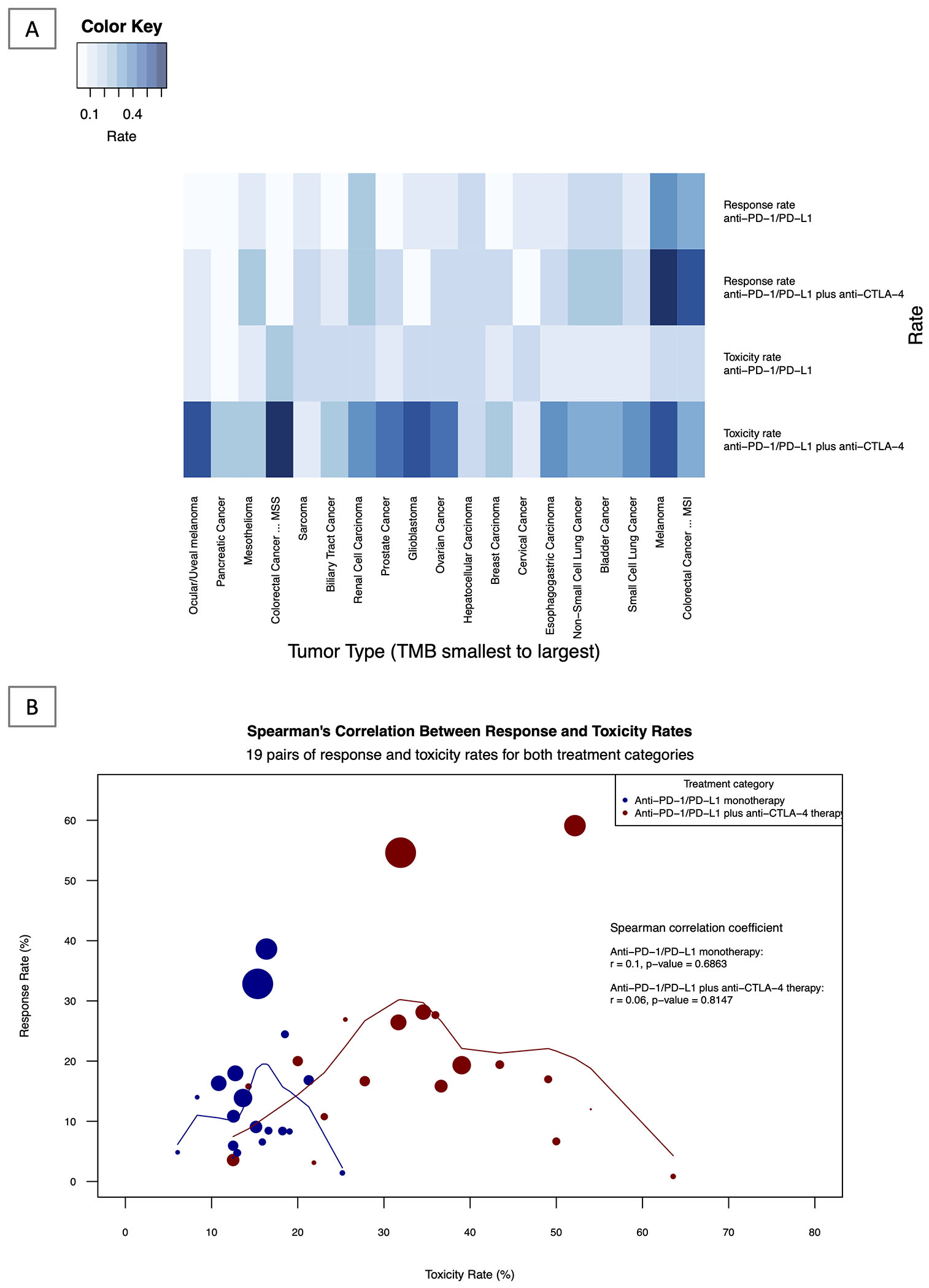
5A: Response and Toxicity Rates by Treatment Group and Tumor Type: Heatmap Analysis Shown above is the graphical heatmap representation of response and toxicity rates of 19 tumor types for which both toxicity and response data was available for both anti-PD(L)1 monotherapy and anti-PD(L)1 plus anti-CTLA-4 combination ICI therapy. Darker shading represents a higher rate of toxicity and response, whereas lighter shading indicates a lower rate of toxicity and response. eTable 3 in the Supplement shows individual data for each tumor type, including median TMB, ORR and toxicity rates.
5B: Correlative Analysis of Response and Toxicity Rates of Single vs Dual ICI therapy across 19 tumor types.
The above figure represents the Spearman’s correlation between response rate (y-axis) and toxicity rate (x-axis) of both anti-PD(L)1 monotherapy (blue line) and anti-PD(L)1 plus anti-CTLA-4 therapy (red line). The circles represent individual tumor types and varying sizes of circle represent the value of TMB, which the size of the circle is proportional to the value of TMB (higher TMB equates to larger circle). Loess smoothing curve with the default span of 0.75 is shown.
Tumor Mutational Burden and Overall Survival
Of all studies included in our meta-regression and meta-analysis, 10 studies across 8 tumor types reported overall survival hazard ratios for anti-PD(L)1 monotherapy versus a standard of care comparator. A meta-analysis of anti-PD(L)1 monotherapy using hazards ratio can be found in eFigure 7. Meta-regression examining the association between TMB and hazard ratio revealed that that there was a positive relationship between TMB and OS as defined by hazards ratio, but it did not meet statistical significance (p=0.2621; Figure 6). Descriptive statistics of median OS survival of anti-PD(L)1 monotherapy, anti-CTLA-4 and anti-PD(L)1 plus anti-CTLA-4 combination can be found in eTable 4 in the Supplement.
Figure 6: Association Between Survival Hazard Ratio and TMB of anti-PD(L)1 Monotherapy.
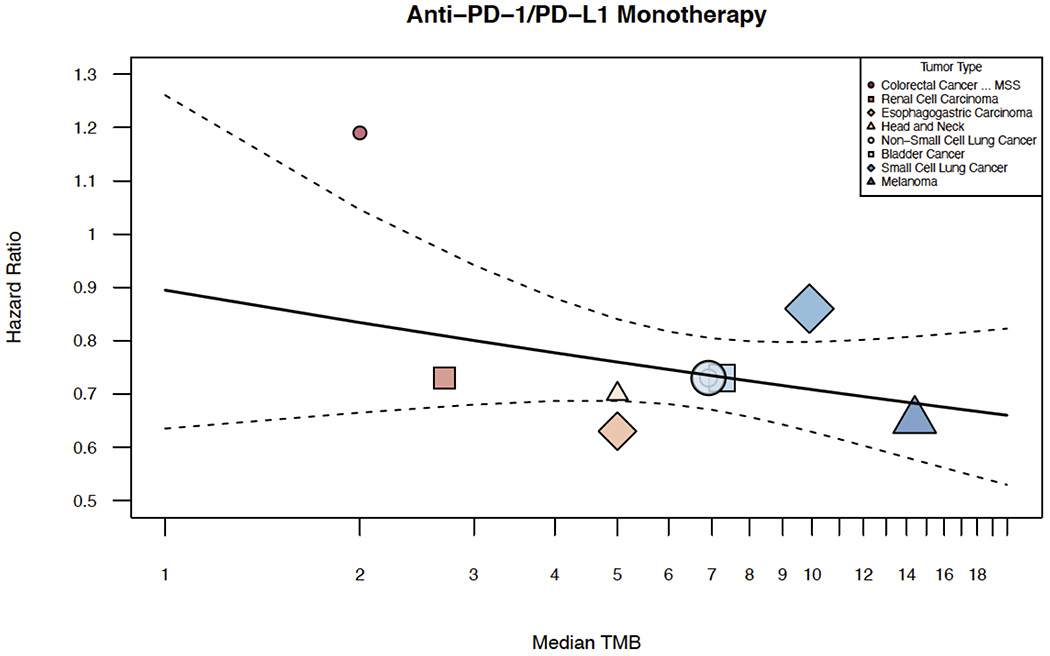
Shown above is the graphical representation of the meta-regression of survival hazards ratio and TMB. This includes the median number of coding somatic mutations per megabase (MB) of DNA in 8 tumor types or subtypes among patients who received inhibitors of PD(L)1 monotherapy for which data regarding the hazard ratio were available. A plot of hazard ratios versus median TMB was created. The meta-regression summary was graphically displayed on top of it. Inverse-variance weighting was used to vary the size of each point shape. Median TMB is shown on a log scale, but labeled on the original scale.
Discussion
We newly demonstrate using clinical trial level data that TMB is positively associated with response to single or dual checkpoint inhibitors across multiple tumor types, but is not associated with a higher likelihood of toxicity. Our results build upon the work of many other groups showing that TMB is associated with response and prolonged survival after treatment with ICI therapy.9,10,12,16,17 We were unable to confirm that an increased response rate with ICI therapy in high TMB histologies translated into an OS benefit, as OS data were immature or unavailable for the majority of studies included in our meta-analysis.
Our findings broadly suggest that the benefits of adding CTLA-4 inhibition to PD(L)1 therapy will preferentially benefit high TMB tumor types. However, the relationship between TMB and ORR is imperfect, and some tumors have a higher or lower response rate than what would otherwise be anticipated from the TMB alone. For example, both RCC and Merkel Cell Cancer (MCC) have moderate TMBs but a high response rate to ICI therapy. In RCC, ICI responses may be augmented by the presence of highly immunogenic indel mutations, whereas in the case of MCC, ICI responses may be driven by responses to Merkel Cell viral antigens.24,25 Additional work is needed to determine if the relationship between TMB and ORR can be further refined by incorporating information about mutational features in addition to mutation number. Additional work is also needed to understand if relationship between TMB and ORR is applicable to emerging checkpoint molecules, such as lymphocyte-activation gene 3 (LAG3), for which clinical data are currently limited.
Our findings have implications regarding the mechanism of immune toxicity resulting from ICI therapy. The pathogenesis and underlying mechanisms of ICI toxicity is poorly understood and it has been unclear to what extent autoimmunity in the setting of ICI therapy is driven by tumor features. 26–29 In some contexts, cancer-associated autoimmunity may result from an immune response against cancer antigens.30 For example, patients receiving ICI for melanoma often develop vitiligo, an immune mediated attack of non-malignant melanocytes, providing some initial evidence that tumors may direct the immune response against self in the setting of ICI therapy. Conversely, the observation that patients with genetic CTLA-4 deficiency often develop autoimmunity provides strong evidence that modulation of immune checkpoint pathways can result in immune related toxicities independently of tumor antigens.31 While our results are not granular enough to report an association between any specific mutations and ICI toxicity, our results broadly support the conclusion that TMB is not a significant biomarker of ICI toxicity. Our results contrast the recent findings by Bomze et al that reported a significant positive association between TMB and immune-related toxicities using postmarketing data from the US Food and Drug Administration Adverse Event Reporting System (FAERS).32 We hypothesize that while the clinical trials included in our analysis restricted enrollment to patients with a good performance status who remained on therapy for at least 12 weeks when most immune related toxicities would emerge, immune related adverse events may have been more commonly reported in the FAERS system in higher TMB tumors because such patients would have been more likely to respond and to live long enough to experience toxicities.33 FAERS reporting includes data from not only from physicians, but also other sources such as consumers, potentially resulting in heterogeneity in toxicity reporting. By contrast, our study included only clinical trial data with standardized approaches to toxicity diagnosis and grading.
Strengths of our investigation included the comprehensive nature of our analysis including trials, the use of only published toxicity and response data reported by providers on closely monitored clinical trials, and the use of TMB data from a single validated assay representing a large volume of clinical data points.22,34 Our analysis is broad and simultaneously evaluates the toxicity, survival and objective response rate of anti-PD-1, anti-PD-L1, anti-CTLA-4 monotherapy and their combination for 29 tumor types, from 117 clinical trials and 12450 patients. A limitation is TMB assessments were performed using a limited sequencing panel, and on different patients from which clinical trial responses were assessed. Inferences which arise from our findings applied to individual patients have the potential to lead to incongruent findings as a result of ecological fallacy. In addition, we cannot exclude the possibility of bias within the individual studies utilized in our clinical trial meta-analysis. Only a minority of studies included in our meta-analysis reported overall survival hazard ratios, limiting our power to determine the relationship between TMB and overall survival. The use of prospective patient level data is needed to further validate our findings; the hypothesis that TMB can identify patients for combination immunotherapy is under investigation in the CheckMate 848 study (NCT03668119).
In conclusion, a positive relationship exists between TMB and response with single or combination ICIs; however, there is no association between TMB and ICI toxicity. These findings imply that TMB may broadly define the therapeutic index for ICI therapy, with increased benefit of single and dual ICI therapy in higher TMB tumors without significant additional toxicity. Our results identify new opportunities for therapeutic development, by supporting the investigation of combination ICI therapy in higher TMB tumor types and novel combinatorial strategies that go beyond dual ICI therapy in low TMB tumors.
Supplementary Material
Translational Relevance:
TMB has emerged as a potential biomarker for clinical response to anti-PD(L)1 therapies, but whether TMB also predicts toxicity from therapy is unclear. Furthermore, the relationship between TMB, toxicity, and therapeutic response in patients treated with single or combination immunotherapies across major tumor types is unknown. Our meta-regression and meta-analysis addresses these questions and is to our knowledge, the largest comprehensive study that simultaneously assesses the relationship between TMB, ORR, OS and toxicity, of single and combination ICI therapy across multiple tumor types. We find that a higher TMB is associated with a higher response to single and dual checkpoint inhibitors across multiple tumor types, but is not associated with toxicity. Our findings also have implications for patient selection in clinical practice and for mechanisms of immune toxicity resulting from these agents.
Acknowledgments
Financial Support: This study was funded by the Linda Rubin Endowment Fellowship in Gastrointestinal & Pancreatic Cancers, Johns Hopkins Bloomberg-Kimmel Institute for Cancer Immunotherapy, the Viragh Foundation, NCI Specialized Program of Research Excellence (SPORE) in Gastrointestinal Cancers (P50 CA062924), and the NIH Center Core Grant (P30 CA006973).
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest
References:
- 1.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N Engl J Med 2018; 379: 2040–51. [DOI] [PubMed] [Google Scholar]
- 2.Hamid O, Robert C, Daud A, et al. 5-year survival outcomes in patients (pts) with advanced melanoma treated with pembrolizumab (pembro) in KEYNOTE-001. J Clin Oncol 2018; 36: 9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osipov A, Murphy A, Zheng L. From immune checkpoints to vaccines: The past, present and future of cancer immunotherapy In: Advances in Cancer Research. Academic Press, 2019: 63–144. [DOI] [PubMed] [Google Scholar]
- 5.Spencer KR, Wang J, Silk AW, Ganesan S, Kaufman HL, Mehnert JM. Biomarkers for Immunotherapy: Current Developments and Challenges. Am Soc Clin Oncol Educ B 2016; 36: e493–503. [DOI] [PubMed] [Google Scholar]
- 6.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378: 2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017; 377: 2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters S, Cho BC, Reinmuth N, et al. Abstract CT074: Tumor mutational burden (TMB) as a biomarker of survival in metastatic non-small cell lung cancer (mNSCLC): Blood and tissue TMB analysis from MYSTIC, a Phase III study of first-line durvalumab ± tremelimumab vs chemotherapy. Cancer Res 2019; 79: CT074 LP–CT074. [Google Scholar]
- 9.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (80- ) 2015; 348: 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science (80- ) 2015; 350: 207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellmann MD, Nathanson T, Rizvi H, et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018; 33: 843–852. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellmann MD, Callahan MK, Awad MM, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 2018; 33: 853–861. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder A, Makarov V, Merghoub T, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med 2014; 371: 2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015; 372: 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N Engl J Med 2019; published online Sept. DOI: 10.1056/nejmoa1910231. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Kronbichler A, Eisenhut M, et al. Tumor mutational burden and efficacy of immune checkpoint inhibitors: A systematic review and meta-analysis. Cancers (Basel) 2019. DOI: 10.3390/cancers11111798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019; 51: 202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (80-. ). 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 19.Yarchoan M, Albacker LA, Hopkins AC, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019; 4 DOI: 10.1172/jci.insight.126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y, Roy A, Masson E, Chen TT, Humphrey R, Weber JS. Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin Cancer Res 2013; 19: 3977–86. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009; 339: 332–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017; 9 DOI: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013; 31: 1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turajlic S, Litchfield K, Xu H, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol 2017; 18: 1009–21. [DOI] [PubMed] [Google Scholar]
- 25.Miller NJ, Church CD, Fling SP, et al. Merkel cell polyomavirus-specific immune responses in patients with Merkel cell carcinoma receiving anti-PD-1 therapy. J Immunother Cancer 2018; 6 DOI: 10.1186/s40425-018-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018; 378: 158–68. [DOI] [PubMed] [Google Scholar]
- 27.Daly LE, Power DG, O’Reilly Á, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer 2017; 116: 310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopkins AM, Rowland A, Kichenadasse G, et al. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer 2017; 117: 913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim SY, Lee JH, Gide TN, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1–based immunotherapy. Clin Cancer Res 2019; 25: 1557–63. [DOI] [PubMed] [Google Scholar]
- 30.Joseph CG, Darrah E, Shah AA, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science (80- ) 2014; 343: 152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma N, Burns SO, Walker LSK, Sansom DM. Immune deficiency and autoimmunity in patients with CTLA-4 (CD152) mutations. Clin. Exp. Immunol. 2017; 190: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bomze D, Hasan Ali O, Bate A, Flatz L. Association Between Immune-Related Adverse Events During Anti–PD-1 Therapy and Tumor Mutational Burden. JAMA Oncol 2019; published online Aug 22. DOI: 10.1001/jamaoncol.2019.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol 2019. DOI: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 34.Truesdell J, Miller VA, Fabrizio D. Approach to evaluating tumor mutational burden in routine clinical practice. Transl. Lung Cancer Res. 2018; 7: 678–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


