Abstract
Objective:
To examine the effects of a KE-supplemented diet on EE and adiposity in mice housed at 23°C versus thermoneutrality (30°C), where SNS activity is diminished.
Methods:
Thirty-two ten-week-old male C57BL/6J mice were assigned to one of four groups (n=8/group): KE23 (30% KE, 23°C), CON23 (control diet, 23°C), KE30 (30% KE, 30°C), and CON30 (control diet, 30°C). Control mice were pair-fed to the average intake of mice consuming the KE diet (ad libitum) for 8wk. Body composition, and components of energy balance were measured at completion of the study.
Results:
CON23 (mean±SD, 26.0±1.6 g) and CON30 (29.7±1.4 g) weighed more than KE groups (P<0.03 for both) and were also different from each other (CON23 vs CON30, P<0.01). However, KE23 (23.4±2.7 g) and KE30 (23.1±1.9 g) mice were not different in body weight. As expected, food intake at 30°C (2.0±0.3 g/d) was lower than at 23°C (2.6±0.3 g/d, P<0.01). Diet did not influence resting and total EE, but mice housed at 30°C had lower EE compared to mice at 23°C (P<0.01).
Conclusions:
Dietary KE attenuate body weight gain at standard (23°C) and thermoneutral (30°C) housing temperatures that is not mediated by increased EE under these conditions.
Keywords: Ketones, ketone esters, adipose tissue, energy expenditure, thermoneutrality
INTRODUCTION
Several studies have used exogenous ketone esters (KE) to induce an increase in circulating blood ketones without severe restriction of carbohydrate (CHO) (1,2,3,4,5,6), but only a few studies (7,8,9) have focused primarily on the effects of KE on components of energy balance. Mice consuming D-β-hydroxybutyrate R-1,3-butanediol (3HB-BD) as part of a mixed diet (19% CHO, 35% fat, 18% PRO, 28% KE) had increased resting energy expenditure (REE) and markers of thermogenesis and mitochondrial biogenesis in brown adipose tissue (BAT) (9). However, despite 3HB-BD increasing REE, body weight was not different from pair-fed mice receiving the control diet (47% CHO, 35% fat, 18% PRO). Obese mice consuming R,S-1,3-butanediol diacetoacetate (BD-AcAc2) in the context of a high-fat diet (HFD, 45% by kcal) displayed increased REE and total energy expenditure (TEE) and decreased body weight and adiposity compared to HFD-only pair-fed controls (10). Increased markers of thermogenesis in BAT were also observed, which was consistent with the increase in metabolic rate (10).
Studies conducted in mice to date with KE have been performed at standard housing temperatures (22-23°C), which induces activation of the sympathetic nervous system (SNS) and increases energy expenditure approximately 50% higher than at thermoneutral housing conditions (11,12). Mice singly-housed at standard temperature are under chronic cold stress, a potent stimulator of non-shivering thermogenesis in BAT, and thus, it is difficult to discriminate the previously reported effects of KE feeding on BAT thermogenesis (9,10) from those effects of cold exposure. It is important to determine if reducing sympathetic outflow to BAT, by housing mice at thermoneutrality, reduces the effect of the KE on the metabolic phenotypes produced at standard temperature. Additionally, housing mice within the thermoneutral zone may provide a better pre-clinical model of the effect of exogenous KE on components of energy balance and weight loss in humans (11). Therefore, the purpose of this study was to measure the effects of dietary BD-AcAc2 on components of energy balance at standard (23°C) and thermoneutral (30°) housing conditions.
METHODS
Overview of Study Design and Diets
Male C57BL/6J mice (n=32) were purchased from Jackson Laboratories (Bar Harbor, ME) at 10 weeks of age and acclimated for 7 days upon arrival to the UAB animal facility. At 11 weeks of age, mice were weighed and randomly assigned to one of four groups (n=8/group, diet+housing temperature): 1) control diet+23°C (CON23); 2) KE diet+23°C (KE23); 3) control diet+30°C (CON30); and 4) KE diet+30°C (KE30). Groups were not significantly different in body weight at the onset of the study. For 8 weeks, mice were pair-fed isocaloric diets, with KE mice allowed to consume food ad libitum, and CON mice pair-fed to the average of the prior day’s KE food intake. The KE diet (Dyets Inc, Bethlehem, PA, #104425) contained 30% kcals from BD-AcAc2, which replaced 30% of carbohydrate energy in the CON diet (Dyets Inc, #104419). The gross energy (GE) content of each diet was 3.7 kcal/g and details of the individual diets are provided in Table 1. The UAB Institutional Animal Care and Use Committee (IACUC) approved the investigation and all conditions conformed to the care procedures employed by the UAB Animal Resources Program.
Table 1.
AIN93-G Purified Diet Ingredients with Added Ketone Ester by % Calorie
| Ingredient | (g/kg) | (kcal/g) | ||
|---|---|---|---|---|
| Control Diet | KE | CON | KE | |
| Casein | 200 | 200 | 0.716 | 0.716 |
| L-cysteine | 3 | 3 | 0.012 | 0.012 |
| Sucrose | 100 | 100 | 0.400 | 0.400 |
| Cornstarch | 397.5 | 85.5 | 1.431 | 0.308 |
| Dyetrose | 132 | 132 | 0.502 | 0.502 |
| Soybean Oil | 70 | 70 | 0.630 | 0.630 |
| t-butylhydroquinone | 0.014 | 0.014 | 0 | 0 |
| Cellulose | 40 | 157.5 | 0 | 0 |
| Mineral Mix #210025 | 35 | 35 | 0.031 | 0.031 |
| Vitamin Mix #310025 | 10 | 10 | 0.039 | 0.039 |
| Choline Bitartrate | 2.5 | 2.5 | 0 | 0 |
| Sodium Saccharin | 10 | 10 | 0 | 0 |
| Ketone Ester | 0 | 194.5 | 0 | 1.128 |
| TOTAL (kcal/g food) | 3.761 | 3.766 | ||
| % Carbohydrate | 62.9 | 31.7 | ||
| % Fat | 16.7 | 16.7 | ||
| % Protein | 20.3 | 20.3 | ||
| % Ketone Ester | 0 | 30.0 | ||
Note: The control diet was an AIN-93G purified rodent diet. An equivalent amount of carbohydrate from the AIN-93G diet was removed and replaced with BD-AcAc2 for the KE diet.
Husbandry
Mice were single-housed in shoebox-style cages with filter tops and maintained on a standard 12:12 light-dark cycle (0400h-1600h lights on) in temperature-controlled chambers (Powers Scientific, Warminster, PA) kept at 23°C (%CV=0.20%) and 30°C (%CV=0.15%). HOBO data loggers (HOBOware, Onset Computer Corporation, Bourne, MA) were used for continuous monitoring of chamber temperatures and downloaded weekly to confirm chamber temperature stability. All animals had access to ad libitum water intake throughout the study. Food intake and body weight were recorded, and fresh food pellets were provided each day at the same time (1600h). A single measurement of total body mass, fat mass (FM) and lean body mass (LBM) was completed in the UAB Small Animal Phenotyping Core by quantitative magnetic resonance (QMR; EchoMedical MRI, Houston, TX) at the completion of the study.
Components of Energy Balance
Energy balance was determined by examining the individual components: energy intake (food intake) and energy expenditure (REE, TEE, activity counts, fecal energy, digestive efficiency [AEAE]), and all measurements were completed in the UAB Small Animal Phenotyping Core. Resting and total EE, and locomotor activity (horizontal and vertical) were measured using a TSE PhenoMaster indirect calorimetry system (TSE Instruments, Chesterfield, MO) at the end of the 8-week intervention. Mice were acclimated to the metabolic cages for 48h with measurements obtained for 24 hours the following day. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured for one minute in each of the eight measurement cages followed by the reference cage. Therefore, energy expenditure was measured every nine minutes for each animal for 24 hours. Total EE was determined by calculating the average hourly energy expenditure over the measurement period and then multiplying by 24. Resting EE was calculated by averaging the three lowest 18-minute periods of energy expenditure, with at least one hour between each period.
Fecal energy (GE feces, kcal/24h) and the energy density of the diets (GE food, kcal/g) were measured by bomb calorimetry in the UAB Small Animal Phenotyping Core. At day 45, animals were housed in new cages containing minimal bedding for a period of 72 hours, following which the mice were transferred to new clean cages. All feces produced during this time frame were carefully separated from the bedding, weighed, and frozen until the bomb calorimetry measurement. The 24h energy content (kcal/d) was calculated by multiplying the energy of the sample (kcal/sample) by the total weight (g) of the 72h sample collected for each mouse and divided by 3. Digestible energy intake was calculated by subtracting the fecal energy (kcal/d) from the gross food energy intake (kcal/d) (13). The apparent energy absorption efficiency (AEAE, a.k.a digestive efficiency) was calculated using the following equation (14):
Blood Glucose and β-Hydroxybutyrate Measurements
In order to measure the effect of the diets on blood glucose and ketone concentrations, a fasting/re-feed protocol was completed after 7 weeks on the diet. At 1200h, food was removed from all individual cages for a 4h fasting period. At normal feeding time (1600h), food was weighed and placed in the cages, and at 1800h food was weighed again to quantify the 2h food intake. At this time, a tail snip was performed to measure whole blood glucose (Precision Xtra blood glucose meter; Abbott Laboratories, Lake Bluff, IL) and D-beta-hydroxybutyrate (D-βHB; Keto-MoJo blood ketone meter; Keto-Mojo, Napa, CA) concentrations. A Pearson’s correlation analysis was also performed to determine if food intake over the 2h period was associated with D-βHB concentration.
Tissue Collection and Handling
At the completion of the study, mice were euthanized by decapitation, tissues were dissected and snap-frozen in liquid nitrogen and stored at −80°C until analysis.
Quantitative Real-Time PCR
Total RNA was isolated from brown adipose tissue using TRIreagent (Molecular Research Center Inc., Cincinnati, OH) and a RNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer’s instructions and by methods previously published (10). Genes of interest and their respective primer sequences were as follows: Ucp1: [F: GGCAAAAACAGAAGGATTGC] [R: TAAGCCGGCTGAGATCTTGT]; Pgc1α: [F: ATGTGCGCCTTCTTGCTCT] [R: CACGACCTGTGTCGAGAAAA]; Cyclophilin: [F: ATGTGGTTTTCGGCAAAGTT] [R: TGACATCCTTCAGTGGCTTG].
Western Blot Analysis
Approximately 30 mg of brown adipose tissue was homogenized in RIPA buffer with protease and phosphatase inhibitors using methods previously described (10). Briefly, protein content from homogenates was quantified by protein assay (DC Protein Assay, Bio-Rad) using fatty acid free bovine serum albumin standards. Proteins were separated by SDS-PAGE on 10% polyacrylamide gels and transferred to PVDF membranes (Bio-Rad) overnight. Membranes were then incubated with primary antibody for 2h (room temp; β-actin, Ucp1), followed by washing and subsequent incubation with secondary antibody for 1h. The Ucp1 antibody was purchased from Thermo Fisher Scientific (PIPA529575) and β-actin from Abcam (ab8227). Proteins were visualized by chemiluminescence and density of individual bands was quantified by ImageJ with normalization to β-actin.
Statistical Analysis
Normality tests were conducted on all data before analysis. Specifically, Shapiro-Wilks, Levene’s and Kolmogorov-Smirnov were used along with visualization of the data for kurtosis and skewness. No outliers were identified and the data were found to be normally distributed. Group differences were analyzed with a 2 (group) x 2 (temperature) analysis of variance (ANOVA). Differences in LBM were also assessed by ANCOVA using total body mass as a covariate, and differences in REE and TEE were assessed by ANCOVA, using fat mass (FM) and lean body mass (LBM) as covariates. Weekly body weight and food intake were analyzed using a 4 (group) x 8 (time) repeated measures ANOVA. In all analyses, if a significant interaction was detected, a simple main effect analysis was then completed. Tests requiring post hoc analysis were completed with Bonferroni adjustments. Significance was set a priori at P<0.05, and data are expressed as means±standard deviation (SD) unless otherwise noted. All statistical analyses were conducted using IBM SPSS (version 25.0; IBM Corp., Armonk, NY).
RESULTS
Dietary BD-AcAc2 Decreases Body Weight and Adiposity at Standard and Thermoneutral Housing Temperatures
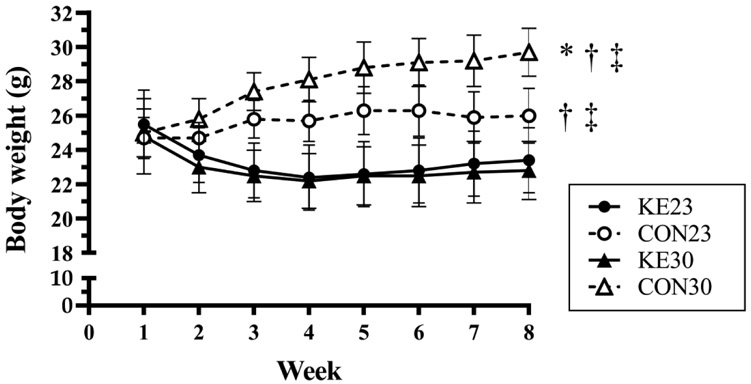
There was a significant interaction between diet and temperature for total body mass measured by QMR at the end of the study (P=0.002; Table 2A). Simple main effects identified that mice consuming the CON diet at 30°C weighed more than mice consuming the CON diet at 23° (P<0.001), and that mice consuming the KE diet had similar body weight at 8 weeks between 23°C and 30°C (P=0.765). Additionally, at both 23°C (P=0.015) and 30°C (P<0.001), mice consuming the KE diet weighed less than mice consuming the CON diet. To explore the changes in body weight over the course of the study, weekly body weight was analyzed with a 4 (group) x 8 (time) repeated measures ANOVA, and there was a significant interaction (P<0.001) between diet and temperature (Fig 1). Both KE23 and KE30 had a significant decrease in body weight from week 1 to week 2, after which body weight did not significantly change, nor differ between the two groups. Body weight in CON mice did not differ significantly from each other (CON23 vs CON30) until week 7 (P=0.014) and 8 (P=0.002). In CON23 mice, body weight was significantly increased from baseline at week 3 and appeared to “level off” around week 4, while in CON30, there was an increase in body weight until week 4.
Table 2.
Anthropometrics & Components of Energy Balance
| 23°C | 30°C | |||
|---|---|---|---|---|
| CON | KE | CON | KE | |
| A. Anthropometrics at 8 weeks | ||||
| Body Mass (g) | 26.30 ± 0.42 | 23.45 ± 0.93* | 30.47 ± 0.50*† | 23.10 ± 0.67*‡ |
| LBM (g) | 20.52 ± 0.24 | 18.44 ± 0.61* | 21.76 ± 0.33† | 17.88 ± 0.38*‡ |
| % LBM | 78.09 ± 0.70 | 78.81 ± 0.84 | 71.48 ± 1.01*† | 77.52 ± 0.84‡ |
| FM (g) | 4.78 ± 0.24 | 4.00 ± 0.33 | 7.77 ± 0.45*† | 4.40 ± 0.25‡ |
| % FM | 18.14 ± 0.67 | 16.86 ± 0.95 | 25.43 ± 1.15*† | 18.96 ± 0.62‡ |
| TBW (g) | 16.46 ± 0.20 | 14.82 ± 0.54* | 17.60 ± 0.31† | 14.29 ± 0.35*‡ |
| B. 72h Feeding & Fecal Collection | ||||
| Dry Food Intake (g/d) | 2.39 ± 0.01 | 2.41 ± 0.11 | 1.82 ± 0.01*† | 1.80 ± 0.10*† |
| Energy Intake (kcal/d) | 10.86 ± 0.04 | 11.35 ± 0.53 | 8.25 ± 0.03*† | 8.44 ± 0.48*† |
| Fecal Production (g/d) | 0.23 ± 0.01 | 0.43 ± 0.05* | 0.20 ± 0.01† | 0.30 ± 0.02† |
| Fecal Energy (kcal/g) | 3.35 ± 0.03 | 3.63 ± 0.01* | 3.54 ± 0.03* | 3.60 ± 0.01* |
| Digestible Energy Intake (kcal/d) | 10.10 ± 0.04 | 9.78 ± 0.59 | 7.56 ± 0.04*† | 7.36 ± 0.45*† |
| Digestive Efficiency (%) | 92.94 ± 0.25 | 85.91 ± 2.02* | 91.58 ± 0.53† | 87.00 ± 0.74*‡ |
| C. 24h Indirect Calorimetry | ||||
| VO2 (mL/min) | 1.39 ± 0.03 | 1.19 ± 0.04* | 0.96 ± 0.02*† | 0.74 ± 0.03*†‡ |
| VO2 (mL/g/min) | 0.053 ± 0.001 | 0.051 ± 0.001 | 0.032 ± 0.000*† | 0.032 ± 0.001*† |
| VCO2 (mL/min) | 1.27 ± 0.02 | 1.05 ± 0.04* | 0.88 ± 0.02*† | 0.65 ± 0.03*†‡ |
| RER | 0.89 ± 0.01 | 0.87 ± 0.01 | 0.90 ± 0.01† | 0.87 ± 0.00*‡ |
| REE (kcal/d) | 6.25 ± 0.32 | 5.74 ± 0.32* | 3.80 ± 0.08*† | 2.81 ± 0.09*†‡ |
| REE corrected for LBM (kcal/d) | 5.98 ± 0.21 | 6.11 ± 0.23 | 3.15 ± 0.28*† | 3.35 ± 0.26*† |
| REE corrected for LBM + FM (kcal/d) | 6.00 ± 0.24 | 6.14 ± 0.25 | 3.10 ± 0.36*† | 3.36 ± 0.26*† |
| TEE (kcal/d) | 9.91 ± 0.18 | 8.45 ± 0.29* | 6.87 ± 0.12*† | 5.27 ± 0.24*†‡ |
| TEE corrected for LBM (kcal/d) | 9.61 ± 0.18 | 8.87 ± 0.19 | 6.14 ± 0.23*† | 5.89 ± 0.21*† |
| TEE corrected for LBM + FM (kcal/d) | 9.65 ± 0.20 | 8.92 ± 0.21 | 6.04 ± 0.30*† | 5.90 ± 0.22*† |
| Activity Counts | 53250 ± 6845 | 33426 ± 3664* | 49373 ± 4147 | 37901 ± 2611 |
Note: Data presented mean ± SE. The GE calculated for each diet from bomb calorimetry was 4.54 kcal/g (CON) and 4.70 kcal/g (KE). The diets also had similar water content: 7.53% (CON) and 9.04% (KE). All groups are n=8/group, and all mice are used for each measurement.
Significantly different from CON23 (P < 0.05)
Significantly different from KE23 (P < 0.05)
Significantly different from CON30 (P < 0.05).
Abbreviations: carbon dioxide production (VCO2), fat mass (FM), lean body mass (LBM), oxygen consumption (VO2), respiratory exchange ratio (RER), resting energy expenditure (REE), total body water (TBW), total energy expenditure (TEE).
Figure 1. Weekly average body weight.
Body weight in CON30 is significantly higher compared to CON23 (*P =0.03), KE23 (†P < 0.01), and KE30 (‡P < 0.01). Body weight in CON23 is also significantly higher than KE23 (†P < 0.01) and KE30 (‡P < 0.01). There was no difference in body weight between KE23 and KE30 (P = 0.94). N=8/group.
There was a significant interaction between diet and temperature for FM (P=0.001, Table 2A). Simple main effects identified that mice consuming the CON diet at 30°C had more FM than mice consuming the CON diet at 23° (P<0.001), and that FM was similar in mice consuming the KE diet between 23°C and 30°C (P=0.352). Additionally, there was no difference in FM between CON and KE mice at 23°C (P=0.076), but FM was higher in CON compared to KE mice at 30°C (P<0.001).
There was also a significant interaction between diet and temperature for LBM (P=0.039, Table 2A). Simple main effects identified that mice consuming the CON diet at 30°C had higher absolute LBM than mice consuming the CON diet at 23° (P=0.009), and that LBM was similar in mice consuming the KE diet between 23°C and 30°C (P=0.453). Also, CON mice at both 23°C (P =0.007) and 30°C (P < 0.001) had higher LBM than KE mice at the same temperature. When LBM was adjusted for total body mass (i.e., size), there was no interaction between diet and temperature (P=0.095), and indicated a main effect for temperature on LBM (P=0.002) and no effect of diet (P=0.776). When examining the adjusted data, although statistically different, the difference in LBM between the groups (adjusted mean±SE; CON23: 20.26g±0.19g, CON30: 19.14g±0.31g, KE23: 19.78g±0.22g, KE30: 19.42g±0.23g) does not appear to be of biological relevance.
Dietary BD-AcAc2 Increases Circulating D-β-Hydroxybutyrate (βHB) Concentrations
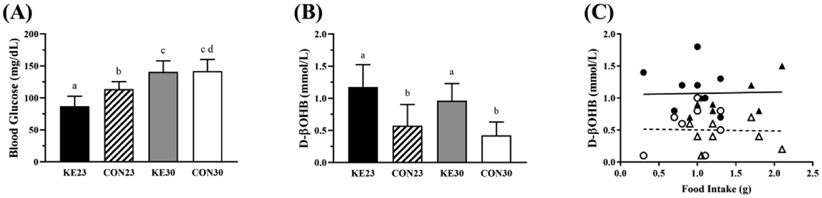
During the meal challenge, mice from each group were fasted for 4 hours and then provided food during the dark cycle (1600h) when mice are generally feeding to determine effects of the diet on blood glucose and D-βHB concentrations. There was a significant interaction between diet and temperature (P=0.032) on blood glucose during the 2h feeding period and simple main effect analysis indicated a significant effect for both diet and temperature. Blood glucose concentrations were lowest in KE23 (87.13±15.64 mg/dL), and there was no difference between KE30 (140.62±17.12 mg/dL) and CON30 (141.88±18.20 mg/dL, Fig 2A).
Figure 2. 2-h Feeding Study Results.
(A) Blood glucose was elevated at 30°C compared to 23°C, but not different between CON30 and KE30. Blood glucose was lower in KE23 vs CON23. (B) BD-AcAc2 increased R-βOHB concentrations compared to CON diet, but was not influenced by housing temperature. (C) Food intake during the 2h fast/re-feeding protocol did not affect R-βOHB concentrations, but diet did (KE diet: solid line, CON diet: hashed line, P<0.001). Open circles and triangles are CON23 and CON30, closed circles and triangles are KE23 and KE30 mice, respectively. N=8/group. Note: Lowercase letters denote significant difference between groups, groups with same letters are not significantly different from one another.
There was no interaction for D-βHB between diet and temperature (P=0.765). There was a significant main effect of diet (P<0.001) on D-βHB concentrations (CON = 0.50±0.28 mmol/L, KE = 1.07±0.32 mmol/L, Fig 2B). Correlational analysis revealed that D-βHB concentrations were not dependent on the amount of food the mouse ingested over the 2h feeding period (P=0.304), but that the diet formulation (i.e., the addition of KE) did have a significant effect on D-βHB (P<0.001), Figure 2C).
Effects of BD-AcAc2 on Components of Energy Balance
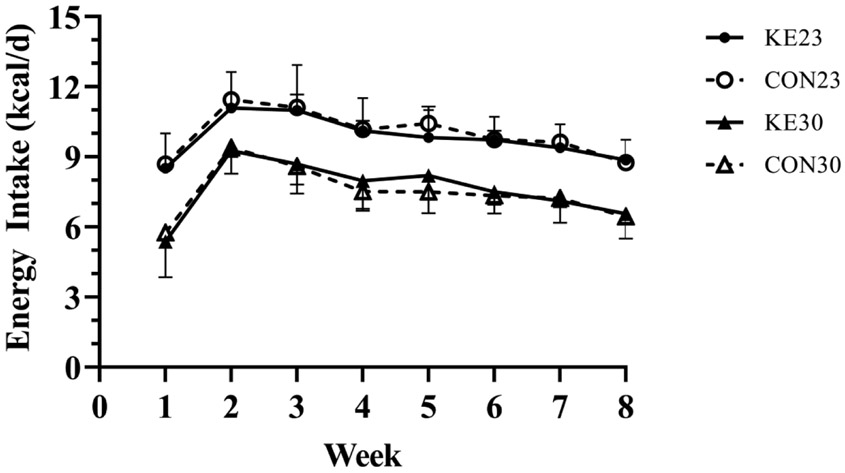
Weekly food intake was analyzed with a 4 (group) x 8 (time) repeated measures ANOVA. There was no significant interaction between diet and temperature (P=0.354). Control mice were successfully pair-fed to their respective KE group, and there was no difference in energy intake between KE and CON at their respective housing temperature. Average energy intake (Figure 3) over the 8 weeks for KE23 (9.81±1.08 kcal/d) was higher than KE30 mice (7.62±0.70 kcal/d, P<0.001), corresponding to an approximately 22% lower energy intake for KE30 compared to KE23.
Figure 3. Energy Intake.
Energy intake (kcal/day) was not different between KE and pair-fed CON mice within their respective housing temperature. However, mice housed at 23°C ate more than mice housed at 30°C. N=8/group.
There was no significant interaction between diet and temperature for fecal production (P=0.097), however, fecal production was influenced by both diet (P<0.001) and temperature (P=0.011). The KE23 mice had almost double the fecal production (g/d) compared to CON23 mice (52% greater), and the KE30 mice had ~65% greater fecal production than CON30 mice. There was also greater fecal production in mice housed at 23°C compared to 30°C regardless of diet composition (Table 2B). There was a significant interaction between diet and temperature (P<0.001) for the energy content of the feces (kcal/g) as well. Simple main effect analyses indicated that in CON mice, energy content was greater at 30°C compared to 23°C (P=0.001), but there was no difference in energy content between ketone-fed mice at 23°C and 30°C (P=0.172). At 23°C, energy content of feces was higher in KE compared to CON (P<0.001), but there was no difference between groups at 30°C (P=0.130). There was no interaction between diet and temperature for digestive efficiency (P=0.282), and there was a main effect for diet (P<0.001). Thus, digestive efficiency was significantly reduced in mice receiving KE (~86%) in their diet compared to pair-fed controls (~92%), and housing temperature did not significantly influence this response (P=0.902).
There was no significant interaction between diet and temperature for unadjusted REE (P=0.313), but there was a significant effect of both diet and housing temperature on REE. Mice receiving KE had significantly lower unadjusted REE than CON mice (P=0.003), and mice housed at 30°C had significantly lower REE than mice housed at 23°C (P<0.001). When adjusted for FM and LBM, the difference in REE between CON and KE was removed (P=0.608), but REE remained significantly lower in mice housed at 30°C (adjusted mean±SE, 3.23±0.18 kcal/d) compared to 23°C (6.07±0.18 kcal/d, P<0.001), being reduced by almost half (Table 2C). Total EE followed a similar pattern as REE. Similarly, there was no interaction between diet and housing temperature when adjusted for both FM and LBM (P=0.171) or effect of diet (P=0.156), and mice housed at 30°C had significantly lower TEE (adjusted mean±SE, 5.97±0.15) than mice housed at 23°C (9.28±0.15, P<0.001). There was no significant interaction between diet and housing temperature for RER (P=0.328) or effect of housing temperature on RER (P=0.432). However, the RER was lower in mice receiving KE compared to mice on CON diet (P<0.001). Thus, it appears that housing temperature had no effect on substrate oxidation, but RER was influenced by dietary composition (Table 2C).
Brown Adipose Tissue Markers of Thermogenesis are Unchanged by Dietary BD-AcAc2
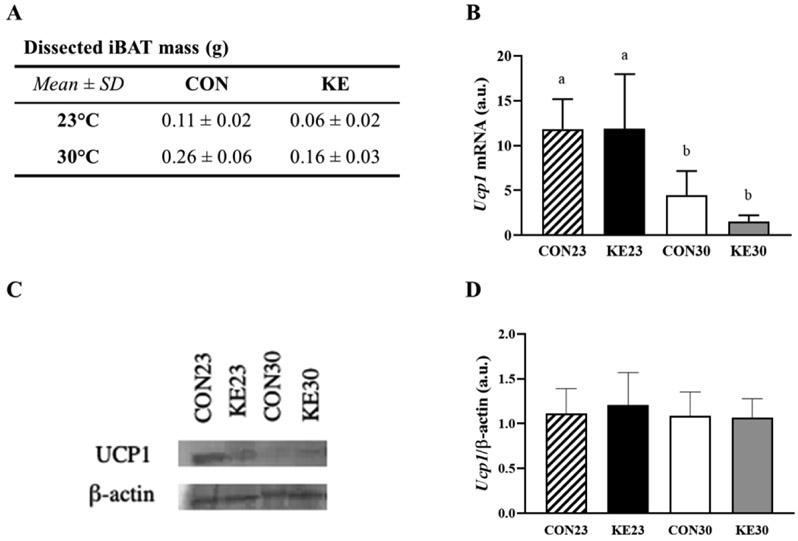
Intrascapular brown adipose tissue (iBAT) was dissected and weighed for each mouse. Mean iBAT weight for each group is displayed in Figure 4A. There was no interaction between diet and housing temperature on iBAT mass (P=0.178), but there was a significant main effect for both diet and housing temperature. Mice receiving KE had a lower iBAT mass (combined mass±SE, 0.11g±0.01g) compared to CON mice (0.18g±0.01g, P<0.001), and mice housed at 23°C had lower iBAT mass (0.09g±0.01g) than mice housed at 30°C (0.21g±0.01, P<0.001). Because the mice receiving KE were significantly smaller than CON mice, iBAT mass was adjusted for total body mass, and there remained a significant effect for housing temperature (P<0.001) only, with mice housed at 23° having about half the iBAT mass (adjusted mean±SE, 0.10g±0.01g) compared to mice housed at 30°C (0.20g±0.01g).
Figure 4. iBAT mass and Ucp1.
(A) iBAT mass (g). (B) Ucp1 expression was elevated in 23°C housed mice compared to mice housed at 30°C, but diet did not influence Ucp1 expression. N=8/group. (C) Representative Western Blot of Ucp1 protein content (N=1/group). (D) Quantified protein content from Western Blot (N=6/group). Note: Lowercase letters denote significant difference between groups, groups with same letters are not significantly different from one another.
There was no interaction between diet and housing temperature for Ucp1 mRNA expression (P=0.785). There was a main effect for housing temperature (P<0.001), but not diet (P=0.371, Figure 4B). Protein content of Ucp1 was not different between the groups (Figure 4C and 4D). There was no interaction for Pgc1α mRNA expression (P=0.895), but a significant effect for housing temperature (P=0.015), with higher mRNA expression at 23°C compared to 30°C. However, protein content by western blot was not different between groups for Pgc1α (data not shown).
DISCUSSION
This is the first study to examine the effect of the dietary ketone ester, BD-AcAc2, on components of energy balance, body weight, and adiposity in mice housed at thermoneutral conditions (30°C). Mice fed a 30% KE diet had lower body weight and FM compared to mice receiving the control diet. Although pair-fed mice housed at 30°C ate 22% fewer calories per day than mice at 23°C, body weight, FM, and LBM in mice receiving KE were not different from each other, despite CON30 mice having significantly higher body mass and fat mass than CON23. As expected, EE was lower in mice housed at 30°C compared to 23°C, but EE was not different between KE and CON mice at their respective housing temperature, after adjustment for fat and lean mass. Given that CON mice were pair-fed to KE mice; and that body weight was reduced in KE compared to CON but not different between KE mice housed at 23°C and 30°C, it seems that BD-AcAc2 has an effect on body weight independent of food intake and sympathetic activity.
It has been suggested that housing mice at 30°C (e.g., thermoneutrality) is a better alignment to human translational effects compared to standard conditions of 22-23°C (11,12), as housing mice at thermoneutrality reduces sympathetic drive, thereby lowering TEE levels similar to those observed in humans (11). Alternatively, Speakman and Keijer (2013) suggest that a housing temperature of 20-25°C actually best mimics human physiology, citing two main reasons for their argument. First, metabolic rate in humans during activities of daily living is generally 1.6-1.8 times basal metabolism, which is equivalent to a housing temperature in mice of 23-25°C; and second, the availability of bedding and nesting materials within a mouse cage provide an option of insulation, shifting the zone of thermoneutrality for singly-housed mice to 20-22°C, suggesting that these materials prevent the mouse from constant exposure to the “colder” environment (15). It is clear that rodent housing temperature cannot be ignored, and thus interventions aimed at eliciting changes in energy balance must take this into account. It was therefore a primary aim of this study to examine the role of thermoneutrality on the EE response to mice fed a diet containing 30% KE as BD-AcAc2. Previous studies (summarized in Table 3) have shown that in mice housed at standard temperatures and fed a high-fat diet (> 30% of kcal from fat), there is an increase in both REE and TEE (9, 10). However, on a low-fat diet (17% by kcal) we observed a significant decrease in both REE and TEE in mice receiving BD-AcAc2 diet compared to ad libitum fed control mice (16). Using this same low-fat diet formulation, the current study observed no difference in REE or TEE in KE mice compared to pair-fed CON, but both REE and TEE were decreased at 30°C compared to 23°C, as expected. Given that the KE mice weighed significantly less than CON mice but had similar energy intake and EE compared to CON within a housing temperature, the attenuation of weight gain in KE-supplemented mice is a puzzling result and needs further exploration.
Table 3.
Summary of Mouse Studies Examining the Effect of KE Added to Diet on Body Weight and Energy Expenditure
| Citation | KE | Housing Temp. |
CON Diet | KE Diet | Body Weight | EE | Ucp1 |
|---|---|---|---|---|---|---|---|
| High Fat Diet Studies | |||||||
| Srivastava et al. (2012) FASEB J. |
3HB-BD | 21°C | 18% PRO 35% FAT 47% CHO |
18% PRO 35% FAT 18% CHO 29% KE |
↔ compared to PF CON | ↑ REE by 14% compared to PF CON |
↑ Ucp1 protein compared to PF CON in BAT and eWAT |
| Davis et al. (2019) FASEB J |
BD-AcAc2 | 23°C | 20% PRO 45% FAT 35% CHO |
20% PRO 45% FAT 5% CHO 30% KE |
↓ compared to PF CON | ↑ REE by 30% ↑ TEE by 14% compared to PF CON |
↑ Ucp1 mRNA & protein compared to PF CON in BAT |
| Low Fat Diet Studies | |||||||
| Deemer et al. (2019) Front Nutr |
BD-AcAc2 | 23°C | 20% PRO 17% FAT 63% CHO |
20% PRO 17% FAT 33% CHO 30% KE |
↓ compared to ad lib CON |
↓ REE by 18% ↓ TEE by 8% compared to ad lib CON |
↔ Ucp1 mRNA compared to CON in BAT (unpublished data) |
| BD-AcAc2 | 23°C | 20% PRO 17% FAT 63% CHO |
20% PRO 17% FAT 33% CHO 30% KE |
↓ compared to PF CON23 | ↔ REE and TEE compared to PF CON |
↔ Ucp1 mRNA and protein compared to PF CON23 in BAT |
|
| Deemer et al. (current study) |
30°C | ↓ compared PF CON30 ↓ compared to CON23 |
↔ REE and TEE compared to PF CON ↓ compared to 23°C |
↔ Ucp1 mRNA and protein compared to PF CON30 in BAT ↓ Ucp1 mRNA and protein compared to 23 °C |
|||
Abbreviations: ketone ester (KE), D-β-hydroxybutyrate R-1,3-butanediol (3HB-BD), R,S-1,3-butanediol diacetoacetate (BD-AcAc2), protein (PRO), carbohydrate (CHO), pair-fed (PF), control (CON), resting energy expenditure (REE), total energy expenditure (TEE), uncoupling protein 1 (Ucp1), brown adipose tissue (BAT), epididymal white adipose tissue (eWAT).
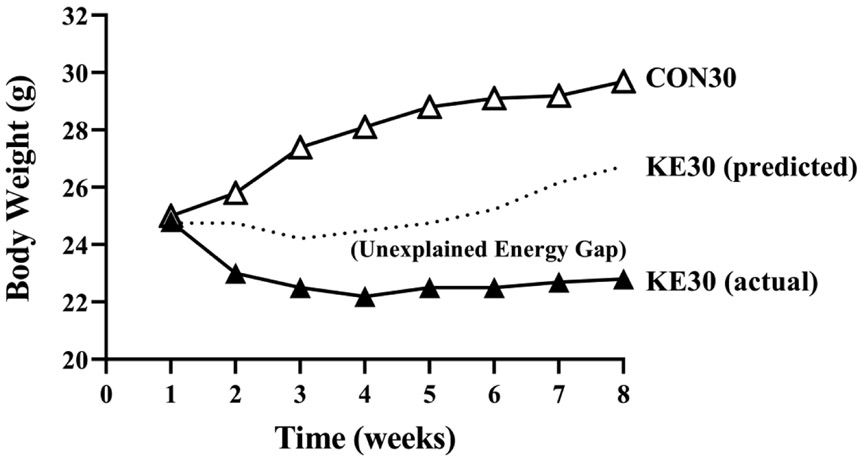
Energy balance is defined as stability between energy intake and energy expenditure such that the weight of the individual remains unchanged. An alteration to one side of the equation without compensatory alterations in the other side can result in weight gain or weight loss. Mice in this study received similar energy provisions within their respective housing temperature, but KE mice weighed less and had lower FM compared to CON. Since adjusted REE and TEE were not different between CON and KE, and energy intake was equal between CON and KE, then KE mice must be losing energy by a different mechanism, possibly though feces, urine, or breath. Indeed, fecal output and energy content was higher in the KE mice compared to CON, and these values were similar for the diet consumed independent of housing temperature. Mice housed at thermoneutrality weigh more than mice housed at 22-23°C because energy requirements needed to maintain normal body temperature are decreased, leading to low sympathetic drive and therefore an increased propensity for weight gain; and thus the fact that our KE30 mice weighed the same as our KE23 mice, is a curious finding. It is possible that because the KE30 mice consumed less food than the KE23 mice, they were able to “amplify” weight loss; however, this response would be opposite that observed in the CON30 mice, which also consumed less energy, yet weighed significantly more and had greater FM than CON23. It might be hypothesized that if KE had the same effect on body weight at thermoneutrality as they do at standard temperature, that the KE30 mice should be roughly similar in body weight to CON23 mice (Fig 5). It might then be expected that the closing of the energy gap in the KE30 mice could be explained by an increase in total fecal production or fecal energy content compared to KE23, but these actually were lower in KE30 than KE23, suggesting that this is an unlikely mechanism.
Figure 5. Hypothesized KE30 Body Weight Response.
Based on the percent change in body weight observed in the 23°C housed mice between the KE and CON diet, an estimated body weight response was calculated from observed CON30 body weights and compared to actual (observed) body weight.
Other suggested mechanisms that might contribute to the increase in weight loss seen in KE30 could be explained by alterations in thermogenic genes found in brown adipose tissue. Uncoupling protein 1 expression is increased in response to cold stress (17,18) or a high fat diet (19), and increased Ucp1 expression is related to increased metabolic inefficiency, leading to increased mitochondrial respiration, decreased ATP production, and increased heat production. However, contrary to our previous findings and those of others (9,10), Ucp1 in the current study was unchanged with consumption of BD-AcAc2 and expression was decreased in mice housed at thermoneutrality, an expected response due to reduced thermal demand for non-shivering thermogenesis. We also saw no effect of the diet on Pgc1α in iBAT, a common marker of mitochondrial biogenesis. Brown adipose tissue is also highly innervated by sympathetic fibers feeding into the hypothalamus, which includes cells that respond to circulating nutrients and appetite-regulating hormones such as ghrelin and glucagon-like peptide 1 (GLP-1), therefore making BAT activity susceptible to nutritional status as well (20). A major difference between the aforementioned studies and the current investigation is the dietary fat content, which can increase Ucp1 (19). Mice in these studies (9,10) were pair-fed, with fat content being identical between control and KE diets. It is therefore possible that a synergistic effect exists between dietary fat content and KE that has previously contributed to these observed increases in expression of Ucp1 and effects on metabolic rate (Table 3).
Additionally, it is possible that certain components of the BD-AcAc2 molecule are energetically unavailable which could explain weight loss and lower body composition of mice consuming the KE. The (R)-enantiomer of beta-hydroxybutyrate (R-βHB) is synthesized in the liver from the incomplete breakdown of fatty acids, and is also measured in circulation as d-βHB. The (S)-enantiomer of βHB (aka l-βHB), is not readily oxidized and must undergo further conversion for metabolic use. In perfused fed or starved rat liver, 80-85% of labeled S-βHB was found to be converted to ketones (R-βHB, AcAc, acetone), lipids (9-15%), and CO2 (13-15%) (21). Using a ketone salt (containing both R- and S-βHB), Stubbs et al. observed that S-βHB remained elevated in circulation longer than the R-βHB, suggesting the potential for slower metabolism of S-βHB (22). In contrast, the authors showed that the major fate of exogenous R-βHB was oxidation in peripheral tissues. Since approximately 25% of BD-AcAc2 is metabolized to S-βHB, it is possible that the difference in body weight observed between the KE and pair-fed controls is at least in part attributed to lower use of S-βHB as an energy substrate and to greater fatty acid oxidation than expected with provision of R-βHB alone. However, given the difference in energy intake between KE23 and KE30, we know that there must also be additional contributing factors to which we are as yet unaware.
Despite the rigor applied to the current study execution, a separate control group receiving ad libitum food intake was not able to be included in the study design, thus limiting the ability to determine the effect of thermoneutrality housing on food intake independent of KE supplementation. KE30 mice consumed approximately 22% less energy per day than KE23 mice. Typical differences in ad libitum feeding between thermoneutral and standard housing temperatures would suggest a 25% to as much as 50% difference in food intake (23, 24). Given the slightly lower delta between these two groups, we might be able to infer that there could be a slight food aversion to this KE-diet. Additionally, the pair-feeding design required daily provision to the control groups, while the KE groups were allowed to “free feed.” It is possible that mice in groups fed a daily provision consumed the majority of their calories within a smaller window of time than those with ad libitum access, potentially interacting with circadian rhythms in unintentional or unexpected ways. Future studies will be required to determine if timing of energy provisions impacts efficiency outcomes and/or if “missing” energy is lost in the form of urinary ketones or in breath as acetone. Another limitation of the current study design is that we were only able to measure energy expenditure once, and it was done at the end of the study when the mice were in energy balance. Future work should focus on the initial weeks of feeding of BD-AcAc2 and by what mechanisms it affects EE independent of energy intake. Finally, because a tail nick (~3μL of whole blood) was used to measure D-βHB, it was not possible to use a benchtop assay for quantification of circulating D-βHB following the fast/re-feed protocol.
CONCLUSION
In conclusion, we found that consumption of BD-AcAc2 decreased body weight resulting in lower adiposity at both 23°C and 30°C in male C57BL/6J mice compared to control-fed mice. The decrease in body weight observed in KE-fed mice occurred without an increase in either REE or TEE at either housing temperature. In addition, mice consuming KE had increased fecal output (total amount and kcal content), implying that some energy is potentially lost through excretion. The KE30 mice weighed similar to the KE23 mice throughout the intervention, despite the increased housing temperature and expected reductions in metabolic rate and sympathetic outflow when alleviating the chronic cold stress of normal housing conditions for rodents. Thus, we propose the KE BD-AcAc2 might contribute to weight loss by a mechanism that is yet to be identified. Future studies are needed to identify KE contribution to food intake regulation, and to further explore mechanisms responsible for energy loss.
Study Importance Questions:
1. What is already known about this subject?
Consumption of ketone esters (KE) are known to decrease body weight and increase energy expenditure, an effect thought to be mediated through increased expression of Ucp1. Under conditions of thermoneutrality, mice gain weight compared to mice housed at standard conditions.
2. What are the new findings of your manuscript?
With the addition of KE to the diet as BD-AcAc2, mice housed at thermoneutrality did not differ in body weight or composition from mice receiving KE housed at 23°C. This difference was not associated with changes in Ucp1 expression in BAT or an increase in energy expenditure. However, digestive efficiency was reduced in mice receiving KE. The effects of BD-AcAc2 on energy balance is not solely mediated through sympathetic mechanisms, and may be contributing to weight loss through a mechanism(s) yet to be identified.
3. How might your results change the direction of research or the focus of clinical practice?
There is rapidly growing interest in the therapeutic application of ketone esters for human obesity, and this paper adds to the body of knowledge regarding the effect of ketone esters on energy balance through translational biology by examining the additional effect of housing mice at thermoneutrality, a treatment condition considered to be more applicable to humans.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Maria S. Johnson for her effort in performing the indirect calorimetry and condensing the results for analysis, Dr. Kenda Rigdon for performing bomb calorimetry experiments and calculations, and Dr. Tim R. Nagy for his expertise with interpretation of the indirect and bomb calorimetry results.
Funding: This work was supported by a Department of Human Studies Competitive Grant to EPP from the University of Alabama at Birmingham (UAB) Department of Human Studies. SED was supported by a T32 Postdoctoral Award from the UAB Post-Doctoral Training Program in Obesity-Related Research (T32DK062710). RAHD was supported by a T32 Predoctoral Award from the UAB Pre-Doctoral Training Program in Obesity-Related Research (T32HL105349). EPP was supported by a Named New Investigator Award from the UAB NORC (P30DK056336). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
Disclosures: At the time of this publication: DD is an inventor on the following patents: Dominic P. D’Agostino; Angela M. Poff; Patrick Arnold; “Targeting Cancer with Metabolic Therapy and Hyperbaric Oxygen” (Patent Number: 9801903) & Dominic P. D’Agostino; Patrick Arnold; Shannon L. Kesl; “Compositions and Methods for Producing Elevated and Sustained Ketosis” (Patent Number: 20170266148). Patent Number: 20170266148 was developed with government support under Grant Number: N00014-13-1-0062 awarded by the Department of Defense, Office of Naval Research. DD is owner of Ketone Technologies LLC, which does consulting and public speaking events. AP is a scientific advisor to Pruvit Ventures, LLC, and is an inventor on the following patent: Dominic P. D’Agostino; Angela M. Poff; Patrick Arnold; “Targeting Cancer with Metabolic Therapy and Hyperbaric Oxygen” (Patent Number: 9801903). AP is an owner of Poff Medical Consulting and Communications, LLC and Metabolic Health Initiative, LLC. DD, AP, AK are inventors on pending patents “Compositions and Methods for Weight Loss Maintenance.” AK and DD are inventor on pending patent “Prevention of Muscle Wasting with Ketone Supplementation.” At the time of this publication, pending patents were still under review. Should patents become accepted and royalties ever accrue, AP, AK and DD will receive a share under the patent terms prescribed by the University of South Florida.
All other authors do not have financial or other relationships that may be perceived as leading to conflict of interest. All authors have approved the final version of this article.
REFERENCES
- 1.Ari C, Kovács Z, Juhasz G, Murdun C, Goldhagen CR, Koutnik AM, et al. Exogenous Ketone Supplements Reduce Anxiety-Related Behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk Rats. Front Mol Neurosci 2016;9: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciarlone SL, Grieco JC, D’Agostino DP, Weeber EJ. Ketone ester supplementation attenuates seizure activity, and improves behavior and hippocampal synaptic plasticity in an Angelman syndrome mouse model. Neurobiol Dis 2016;96: 38–46. [DOI] [PubMed] [Google Scholar]
- 3.Clarke K, Tchabanenko K, Pawlosky R, Carter E, Knight N, Murray A. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul Toxicol Pharmacol 2012;63: 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa-Veloso K. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol 2012;63: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Agostino DP, Pilla R, Held HE, Landon CS, Puchowicz M, Brunengraber H, et al. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J Physiol Regul Integr Comp Physiol 2013;304: R829–836. [DOI] [PubMed] [Google Scholar]
- 6.Desrochers S, Dubreuil P, Brunet J, Jetté M, David F, Landau BR, et al. Metabolism of (R,S)-1,3-butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am J Physiol Endocrinol Metab 1995;268: E660–E667. [DOI] [PubMed] [Google Scholar]
- 7.Kashiwaya Y, Pawlosky R, Markis W, King MT, Bergman C, Srivastava S. A ketone ester diet increases brain malonyl-CoA and uncoupling proteins 4 and 5 while decreasing food intake in the normal Wistar Rat. J Biol Chem 2010;285: 25950–25956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kesl SL, Poff AM, Ward NP, Fiorelli TN, Ari C, Van Putten AJ, et al. Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague–Dawley rats. Nutr Metab (Lond) 2016; 13: 1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava S, Kashiwaya Y, King MT, Baxa U, Tam J, Niu G, et al. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J 2012;26: 2351–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis RAH, Deemer SE, Bergeron JM, Little JT, Warren JL, Fisher G, et al. Dietary R,S-1,3-butanediol diacetoacetate reduces body weight and adiposity in obese mice fed a high-fat diet. FASEB J 2019;33: 2409–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Mol Metab 2018;7: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. J Therm Biol 2012;37: 654–685. [Google Scholar]
- 13.Mercer SW, Trayhurn P. Effect of high fat diets on energy balance and thermogenesis in brown adipose tissue of lean and genetically obese ob/ob mice. J Nutr 1987;117: 2147–2153. [DOI] [PubMed] [Google Scholar]
- 14.Hambly C, Speakman JR. Contribution of Different Mechanisms to Compensation for Energy Restriction in the Mouse. Obes Res 2005;13: 1548–1557. [DOI] [PubMed] [Google Scholar]
- 15.Speakman JR, Keijer J. Not so hot: Optimal housing temperatures for mice to mimic the thermal environment of humans. Mol Metab 2013;2: 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deemer SE, Davis RAH, Gower BA, Koutnik AP, Poff AM, Dickinson SL, et al. Concentration-Dependent Effects of a Dietary Ketone Ester on Components of Energy Balance in Mice. Front Nutr 2019;6: 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2001;1504: 82–106. [DOI] [PubMed] [Google Scholar]
- 18.Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, et al. The Biology of Mitochondrial Uncoupling Proteins. Diabetes 2004;53: S130–S135. [DOI] [PubMed] [Google Scholar]
- 19.Fromme T, Klingenspor M. Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol 2011;300: R1–R8. [DOI] [PubMed] [Google Scholar]
- 20.Hankir MK. Loading and firing the brown adipocyte. Adipocyte 2017;7: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lincoln BC, Des Rosiers C, Brunengraber H. Metabolism of S-3-hydroxybutyrate in the perfused rat liver. Arch Biochem Biophys 1987;259: 149–156. [DOI] [PubMed] [Google Scholar]
- 22.Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, et al. On the Metabolism of Exogenous Ketones in Humans. Front Physiol 2017;8: 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overton JM. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. Int J Obes (Lond) 2010;34 Suppl 2: S53–58. [DOI] [PubMed] [Google Scholar]
- 24.Overton JM, Williams TD. Behavioral and physiologic responses to caloric restriction in mice. Physiol Behav 2004;81: 749–754. [DOI] [PubMed] [Google Scholar]