Abstract
Purpose:
Combinations of endocrine therapy (ET) and targeted therapy (CDK4/6 or mTOR inhibitors) are standard of care for HR+/HER2- metastatic breast cancer (MBC). When ET is not effective, chemotherapy is commonly used. However, clinical outcomes of chemotherapy in the endocrine-resistant setting are limited. The purpose of this study was to identify predictive factors and the compare efficacies of chemotherapy agents in endocrine-resistant MBC.
Methods:
We conducted a retrospective study of patients with HR+/HER2- MBC who received chemotherapy after progression on ET with or without targeted therapy at MD Anderson Cancer Center from 1999–2017. We collected baseline clinicopathological and all treatment data. Primary endpoint was time to treatment failure (TTF) of first-line chemotherapy for MBC.
Results:
For the 1,258 patients analyzed, mean age was 55.3 years (range 21–91). Previous treatment with targeted therapy was recorded for 390 patients (31%): 264 with CDK4/6 inhibitor, 205 with mTOR inhibitor, and 79 treated with both. The most frequent chemotherapy agents were capecitabine (48.9%) and taxanes (28.6%). After adjustment for all factors in a multivariate model, previous treatment with a CDK4/6 inhibitor had the strongest negative effect on TTF regardless of ET duration (hazard ratio [HR] 1.84; 95%CI 1.49–2.27; p<0.001). Conversely, capecitabine had significantly longer median TTF than taxanes regardless of whether patients had prior exposure to taxanes in primary setting (6.1 vs 4.9 months; HR 0.64; 95%CI 0.55–0.75; p<0.001).
Conclusions:
Previous exposure to CDK4/6 inhibitor had a negative predictive effect for the efficacy of chemotherapy. Capecitabine had the best efficacy against endocrine-resistant breast cancer.
Keywords: breast cancer, endocrine resistant, chemotherapy, CDK4/6, mTOR, capecitabine
Introduction
Endocrine therapy (ET) is a standard treatment for metastatic breast cancer with hormone receptor-positive (HR+) and human epidermal growth factor receptor 2 negative (HER2-) status. ET as a single agent or combined with targeted therapy is preferred as initial therapy for patients without visceral crisis [1–3]. Combinations of ET and targeted therapy, such as cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors or mammalian target of rapamycin (mTOR) inhibitors, have been shown by many randomized clinical trials to confer a survival benefit with acceptable toxicities [4–7]. In contrast, chemotherapy is the standard treatment in patients with visceral crisis or concern for endocrine resistance [1–3]. National Comprehensive Cancer Network (NCCN) Guidelines [1] suggest use of chemotherapy after up to 3 sequential ET regimens because of no clinical benefit to further ET (endocrine-resistant breast cancer). Thus, chemotherapy is commonly used in patients for whom available ET has been exhausted or ET options are unavailable.
Various chemotherapy regimens have been used for metastatic breast cancer worldwide. Single-agent anthracycline- and taxane-based regimens have traditionally been considered as first-line treatment for HER2- breast cancer. However, other options are available and effective; these include capecitabine, vinorelbine, and eribulin. The decision should be individualized and considered based on toxicity profiles and patient preferences [2]. However, HR+, HER2- breast cancer is poorly sensitive to neoadjuvant chemotherapy; its rate of complete pathological response is lowest among the breast cancer subtypes [8,9]. In a preclinical model, tamoxifen-resistant breast cancer cell lines (MCF7 and T47D) expressed BARD1 and BRCA1, leading to resistance to some regimens of chemotherapy. These tamoxifen-resistant cells were resistant to doxorubicin and cisplatin but not to paclitaxel [10].
Normally, the first-line chemotherapy regimen should have the greatest benefits and fewest risks of adverse events. However, supporting clinical data on chemotherapy in endocrine-resistant breast cancer are very limited. Moreover, the optimal chemotherapy regimen for endocrine-resistant breast cancer is unclear. Therefore, we aimed to identify predictive factors and to determine the efficacy of first-line chemotherapy in patients with HR+, HER2- metastatic breast cancer who had progressed on ET with or without targeted therapy.
Methods
Study and population
This was a retrospective chart review study in patients with HR+, HER2- breast cancer who received chemotherapy after progression on ET at The University of Texas MD Anderson Cancer Center. The study was approved by the MD Anderson Institutional Review Board (PA18–1135). This study was granted a waiver for informed consent because of its retrospective nature.
We retrospectively reviewed all patients with recurrent or de novo stage IV HR+, HER2- metastatic breast cancer treated between January 1, 1999, and September 30, 2017. HR+ breast cancer was defined as estrogen receptor (ER) and/or progesterone receptor (PR) positivity ≥ 10% based on American Society of Clinical Oncology/College of American Pathologists clinical practice guidelines of 2010 [11]. Eligible patients were women aged ≥ 18 years with pathologically confirmed invasive breast cancer and ECOG performance status of 0–1 who had been treated with chemotherapy after disease progression during ET (with or without targeted therapy) for metastatic disease. Male patients and patients for whom survival outcomes were not available for analysis were excluded. Patients with discordance between their primary and recurrent breast cancer subtypes or whose subtype changed after diagnosis were excluded.
Data collection
We reviewed the Breast Medical Oncology management system database to identify patients, and then additional data were extracted from their electronic medical records. From the database, we extracted age, race, body mass index (BMI), menopausal status, clinical de novo stage IV vs recurrence status, histological subtype, inflammatory breast cancer (IBC) vs non-IBC status, hormone receptor status, nuclear grade, adjuvant therapies, number and sites of metastases, past history of ET, chemotherapy regimens, treatment stop date, and date of death.
Outcomes
The primary endpoint was time to treatment failure (TTF), defined as the time from first chemotherapy initiation in the metastatic setting until treatment discontinuation for any reason, including disease progression, treatment toxicity, patient preference, or death, whichever occurred first. The secondary endpoint was overall survival (OS), defined as the time from chemotherapy initiation until death from any cause. We also evaluated the time from starting ET until stopping chemotherapy and the time from diagnosis of metastasis until death. Date of death and treatment stop date were determined from the medical records.
Statistical analysis
Descriptive statistics were used to summarize all variables collected. For continuous variables, the mean, median, and range were presented as appropriate. For categorical variables, a frequency table was generated. Univariate and multivariate analyses of TTF and OS were performed using the Cox proportional hazard model. Kaplan-Meier methods were used to analyze the endpoints. Results were found to be significant if the p value was < 0.05.
Results
Patients and baseline characteristics
From a total of 2,096 patients with metastatic HR+, HER2- disease who received treatment at MD Anderson Cancer Center from January 1999 through September 2017, we excluded 757 patients who received only ET or received chemotherapy before ET in metastatic setting. Men diagnosed with breast cancer (23 patients) and patients with discordance in breast cancer subtype between their primary and recurrent tumors or a subtype change (58 patients) were excluded. The remaining 1,258 patients were eligible and analyzed in our study. Median follow-up time since chemotherapy initiation was 19 months. The baseline characteristics of the patients are shown in Table 1. The mean age was 55.3 years (range 21–91), and the majority of patients were white (73.8%). Forty-five patients (3.6%) had inflammatory breast cancer (IBC). Single-agent capecitabine was the most common post-ET chemotherapy regimen (48.9%); the second most common was a single taxane (paclitaxel, nab-paclitaxel, or docetaxel) (28.6%). For taxane exposure, 74.4% of adjuvant therapy and 80.1% of neoadjuvant therapy had taxane-based regimens.
Table 1.
Baseline clinical characteristics
| Clinical characteristic | No. (N = 1,258) | % |
|---|---|---|
| Age at initial chemotherapy, years | ||
| Mean (range) | 55.3 (21–91) | |
| <40 | 118 | 9.4 |
| 40–60 | 709 | 56.4 |
| >60 | 431 | 34.3 |
| Race/ethnicity | ||
| White | 929 | 73.8 |
| African-American | 123 | 9.8 |
| Hispanic | 61 | 9.9 |
| Asian | 125 | 4.8 |
| Other | 20 | 1.6 |
| BMI, kg/m2 | ||
| Underweight (<18.5) | 18 | 1.4 |
| Normal (18.5–24.99) | 413 | 32.8 |
| Over weight (25–29.99) | 375 | 29.8 |
| Obese (30 or more) | 384 | 30.5 |
| Unknown | 68 | 5.4 |
| Menopausal status | ||
| Premenopausal | 475 | 37.8 |
| Postmenopausal | 578 | 45.9 |
| Unknown | 205 | 16.3 |
| Metastasis status | ||
| De novo stage IV | 336 | 26.7 |
| Recurrence | 916 | 72.8 |
| Unknown | 6 | 0.5 |
| Histological subtype | ||
| Invasive ductal | 958 | 76.2 |
| Invasive lobular | 183 | 14.5 |
| Other | 117 | 9.3 |
| Inflammatory breast cancer | ||
| IBC | 45 | 3.6 |
| Non-IBC | 1213 | 96.4 |
| Hormone receptor status | ||
| ER+/PR+ | 981 | 78.0 |
| ER+/PR- | 249 | 19.8 |
| ER-/PR+ | 12 | 1.0 |
| Unknown ER or PR status | 16 | 1.3 |
| ER+ fraction | ||
| 0–9% | 13 | 1.0 |
| 10–55% | 104 | 8.3 |
| 56–94% | 510 | 40.5 |
| 95% or more | 367 | 29.2 |
| Nuclear grade | ||
| Grade I/II | 625 | 49.7 |
| Grade III | 482 | 38.3 |
| Neoadjuvant or adjuvant chemotherapy | ||
| Yes | 767 | 61.0 |
| No | 491 | 39.0 |
| Prior exposure to taxane in neoadjuvant or adjuvant therapy | 624 | 49.6 |
| Neoadjuvant or adjuvant hormone therapy | ||
| Yes | 792 | 63.0 |
| No | 466 | 37.0 |
| No. of organs involved | ||
| 1 or 2 | 1116 | 88.7 |
| >3 | 142 | 11.3 |
| Organ of metastasis | ||
| Lymph node | 181 | 14.4 |
| Bone only | 478 | 38.0 |
| Bone (any) | 914 | 72.7 |
| Visceral organ(s) | 466 | 37.0 |
| Brain | 55 | 4.4 |
| Prior duration of all ET for advanced disease <12 months | ||
| No targeted therapy | 503 | 40.0 |
| CDK4/6 inhibitor | 99 | 7.9 |
| mTOR inhibitor | 27 | 2.1 |
| CDK4/6 and mTOR inhibitors | 14 | 1.1 |
| Prior duration of all ET for advanced disease >12 months | ||
| No targeted therapy | 365 | 29.0 |
| CDK4/6 inhibitor | 86 | 6.8 |
| mTOR inhibitor | 99 | 7.9 |
| CDK4/6 and mTOR inhibitors | 65 | 5.2 |
| No. of ET regimens for advanced disease | ||
| 1–2 lines | 1009 | 80.2 |
| At least 3 lines | 249 | 19.8 |
| First-line chemotherapy after ET failure | ||
| Single agent | ||
| Capecitabine | 615 | 48.9 |
| Paclitaxel | 215 | 17.1 |
| Nab-paclitaxel | 88 | 7.0 |
| Docetaxel | 56 | 4.5 |
| Eribulin | 37 | 2.9 |
| Anthracycline | 14 | 1.1 |
| Other | 55 | 4.4 |
| Combination | ||
| Anthracycline-based combination (e.g., AC, FAC) | 73 | 5.8 |
| Other combination (e.g., CMF) | 105 | 8.3 |
Abbreviations: IBC, inflammatory breast cancer; ER, estrogen receptor; PR, progesterone receptor; AC, doxorubicin/cyclophosphamide; FAC, fluorouracil/doxorubicin/cyclophosphamide; CMF, cyclophosphamide/methotrexate/fluorouracil
For prior ET, 51.1% and 19.8% of patients had received second- and third-line ET regimens, respectively, before starting chemotherapy (Table 2). Combinations of ET and targeted therapy had been received in 390 patients (31.0%): 264 patients had received a CDK4/6 inhibitor, 205 patients had received an mTOR inhibitor, and 79 patients had received both targeted therapies. The most common CDK4/6 inhibitor given was palbociclib (95.1% of cases receiving CDK4/6 inhibitors); the most common mTOR inhibitor was everolimus (99.5%). The most common prior ET was a single-agent aromatase inhibitor (AI) (e.g., letrozole, anastrozole, or exemestane).
Table 2.
Prior endocrine therapy
| Type of ET | N (%) |
||
|---|---|---|---|
| First line N=1,258 | Second line N=643 | Third line N=249 | |
| Single agent | |||
| Tamoxifen | 214 (17.0) | 67 (10.4) | 27 (10.8) |
| AI (e.g.,letrozole, anastrozole, exemestane) | 674 (53.6) | 239 (37.2) | 43 (17.3) |
| Fulvestrant | 138 (11.0) | 136 (21.1) | 90 (36.2) |
| Combination | |||
| Fulvestrant plus other ET | 23 (1.8) | 10 (1.6) | 0 |
| CDK4/6 inhibitor plus ET | 167 (13.3) | 72 (11.2) | 28 (11.2) |
| mTOR inhibitor plus ET | 38 (3.0) | 112 (17.4) | 47 (18.9) |
| Other | 4 (0.3) | 7 (1.1) | 14 (5.6) |
Abbreviations: AI, aromatase inhibitor; ET, endocrine therapy
Univariate and multivariate analysis
In a univariate analysis, we identified the following factors associated with worse TTF of chemotherapy (Table 3): African-American race, IBC, nuclear grade III, prior exposure to CDK4/6 inhibitor, and treatment with a chemotherapy regimen that did not include a taxane or capecitabine. In contrast, better TTF was associated with adjuvant hormone therapy, more than two regimens of ET, and capecitabine.
Table 3.
Univariate and multivariate analysis of TTF for chemotherapy
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% Cl | P-value | Hazard ratio | 95% Cl | P-value | |
| Age, years | ||||||
| <40 | (reference) | (reference) | ||||
| 40–60 | 1.03 | (0.84–1.25) | 0.803 | 1.00 | (0.81–1.24) | 0.983 |
| >60 | 0.93 | (0.76–1.15) | 0.506 | 0.95 | (0.74–1.21) | 0.664 |
| Race/ethnicity | ||||||
| White | (reference) | (reference) | ||||
| African-American | 1.23 | (1.02–1.49) | 0.033 | 1.18 | (0.97–1.44) | 0.106 |
| Asian | 1.21 | (0.93–1.57) | 0.160 | 1.22 | (0.92–1.62) | 0.162 |
| Hispanic | 1.03 | (0.85–1.25) | 0.757 | 1.07 | (0.88–1.30) | 0.521 |
| Other | 0.99 | (0.63–1.54) | 0.950 | 1.05 | (0.67–1.66) | 0.829 |
| Obese vs non-obese | 1.00 | (0.88–1.13) | 0.943 | 0.99 | (0.87–1.12) | 0.827 |
| Postmenopausal vs premenopausal | 0.96 | (0.85–1.09) | 0.507 | 1.07 | (0.91–1.25) | 0.410 |
| De novo stage IV vs recurrence | 0.91 | (0.80–1.03) | 0.137 | 1.10 | (0.86–1.41) | 0.446 |
| Histological subtype | ||||||
| Invasive ductal | (reference) | (reference) | ||||
| Invasive lobular | 0.89 | (0.76–1.05) | 0.158 | 0.95 | (0.80–1.12) | 0.525 |
| Other | 0.85 | (0.70–1.03) | 0.095 | 0.91 | (0.74–1.11) | 0.349 |
| IBC vs non-IBC | 1.52 | (1.11–2.06) | 0.008 | 1.39 | (1.01–1.92) | 0.048 |
| Hormone receptor status | ||||||
| ER+/PR+ | (reference) | (reference) | ||||
| ER+/PR- | 0.92 | (0.80–1.06) | 0.244 | 0.91 | (0.78–1.06) | 0.222 |
| ER-/PR+ | 0.90 | (0.51–1.60) | 0.720 | 0.75 | (0.34–1.67) | 0.484 |
| ER fraction | ||||||
| 0–9% | (reference) | (reference) | ||||
| 10–55% | 0.79 | (0.44–1.40) | 0.415 | 0.79 | (0.36–1.76) | 0.564 |
| 56–94% | 0.74 | (0.43–1.29) | 0.293 | 0.73 | (0.33–1.59) | 0.428 |
| 95% or more | 0.87 | (0.50–1.51) | 0.620 | 0.82 | (0.38–1.80) | 0.622 |
| Nuclear grade (grade III vs grade I/II) | 1.17 | (1.03–1.32) | 0.012 | 1.12 | (0.99–1.28) | 0.082 |
| Neoadjuvant or adjuvant therapy | ||||||
| Chemotherapy | 0.95 | (0.84–1.06) | 0.341 | 0.91 | (0.71–1.15) | 0.418 |
| Hormone therapy | 0.85 | (0.76–0.95) | 0.006 | 0.77 | (0.62–0.94) | 0.010 |
| Prior exposure to taxane | 1.00 | (0.90–1.12) | 0.956 | 1.22 | (1.00–1.49) | 0.050 |
| No. of organs involved (>3 vs 1–2) | 0.88 | (0.74–1.06) | 0.174 | 0.92 | (0.75–1.14) | 0.443 |
| Organs of metastasis | ||||||
| Lymph node | 1.04 | (0.88–1.22) | 0.655 | 0.97 | (0.80–1.18) | 0.753 |
| Bone only | 0.97 | (0.86–1.09) | 0.569 | 0.82 | (0.68–0.99) | 0.036 |
| Bone | 0.99 | (0.87–1.12) | 0.832 | 1.07 | (0.92–1.25) | 0.362 |
| Visceral | 0.97 | (0.86–1.09) | 0.562 | 0.82 | (0.70–0.97) | 0.022 |
| Brain | 0.96 | (0.73–1.27) | 0.761 | 0.86 | (0.64–1.16) | 0.318 |
| Previous ET | ||||||
| Duration of all ET < 12 months | ||||||
| No targeted therapy | (reference) | (reference) | ||||
| CDK4/6 inhibitor | 1.59 | (1.27–2.00) | <0.001 | 1.78 | (1.35–2.33) | <0.001 |
| mTOR inhibitor | 0.80 | (0.54–1.18) | 0.252 | 0.77 | (0.51–1.16) | 0.210 |
| CDK4/6 and mTOR inhibitors | 2.20 | (1.26–3.82) | 0.005 | 2.04 | (1.15–3.62) | 0.015 |
| Duration of all ET > 12 months | ||||||
| No targeted therapy | (reference) | (reference) | ||||
| CDK4/6 inhibitor | 1.75 | (1.37–2.25) | <0.001 | 1.95 | (1.48–2.57) | <0.001 |
| mTOR inhibitor | 0.97 | (0.77–1.22) | 0.792 | 1.17 | (0.92–1.49) | 0.212 |
| CDK4/6 an mTOR inhibitors | 1.33 | (1.01–1.77) | 0.046 | 1.53 | (1.14–2.06) | 0.005 |
| Line of ET (at least 3 lines vs 1–2 lines) | 0.79 | (0.68–0.91) | 0.001 | 0.88 | (0.74–1.05) | 0.144 |
| Chemotherapy | ||||||
| Taxane | (reference) | (reference) | ||||
| Capecitabine | 0.65 | (0.57–0.75) | <0.001 | 0.64 | (0.55–0.75) | <0.001 |
| Other | 1.20 | (1.03–1.41) | 0.021 | 1.15 | (0.97–1.36) | 0.103 |
Abbreviations: IBC, inflammatory breast cancer; ER, estrogen receptor; PR, progesterone receptor
After adjustment for all factors in the multivariate analysis model (Table 3), we found that variables significantly associated with shorter TTF were IBC (adjusted hazard ratio 1.39; 95%CI 1.01–1.92; p=0.048) and prior exposure to a CDK4/6 inhibitor (adjusted hazard ratio 1.84; 95%CI 1.49–2.27; p<0.001). When we stratified patients by duration of all prior ET, we found that prior exposure to CDK4/6 inhibitor had adjusted hazard ratios of 1.78 (95%CI 1.35–2.33; p<0.001) among patients with ET duration ≤12 months and 1.95 (95%CI 1.48–2.57; p<0.001) among patients with ET duration >12 months. In contrast, a multivariate analysis model confirmed significant associations with longer TTF for capecitabine (hazard ratio 0.64; 95%CI 0.55–0.75; p<0.001) and adjuvant hormone therapy (hazard ratio 0.77; 95%CI 0.62–0.94; p=0.010), although the latter finding may have been confounded by patients who developed recurrent disease during adjuvant chemotherapy (chemotherapy resistance). Bone-only metastasis and visceral metastasis were not associated with chemotherapy TTF in univariate analysis; however, they were associated with TTF in multivariate analysis.
TTF and OS outcomes
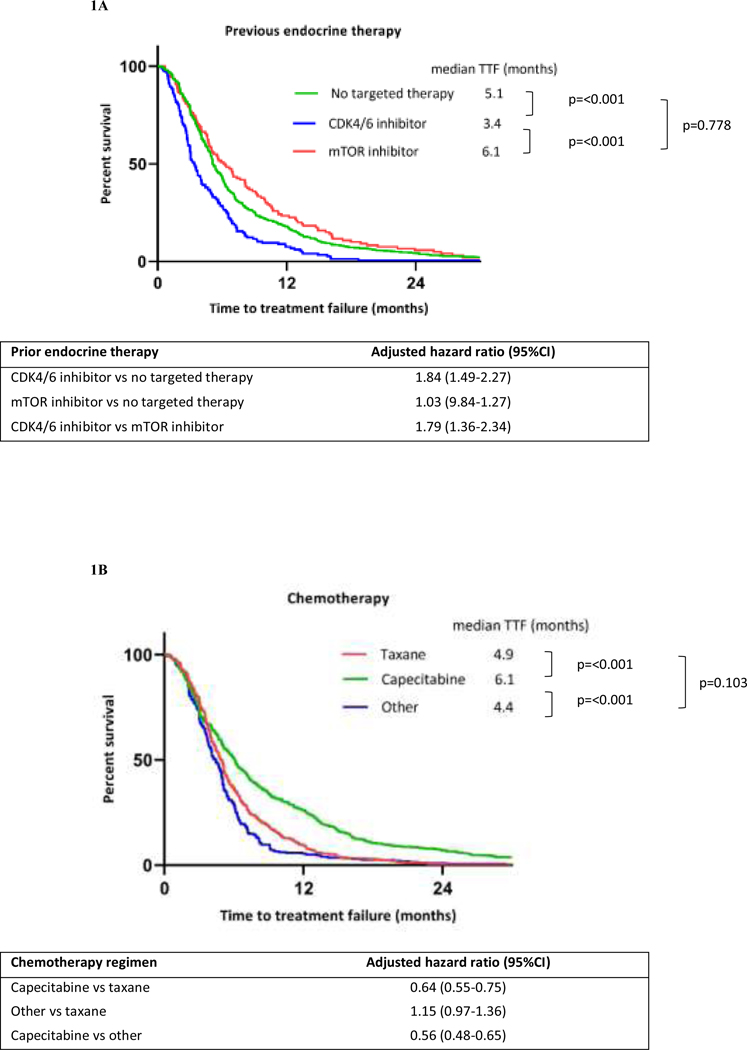
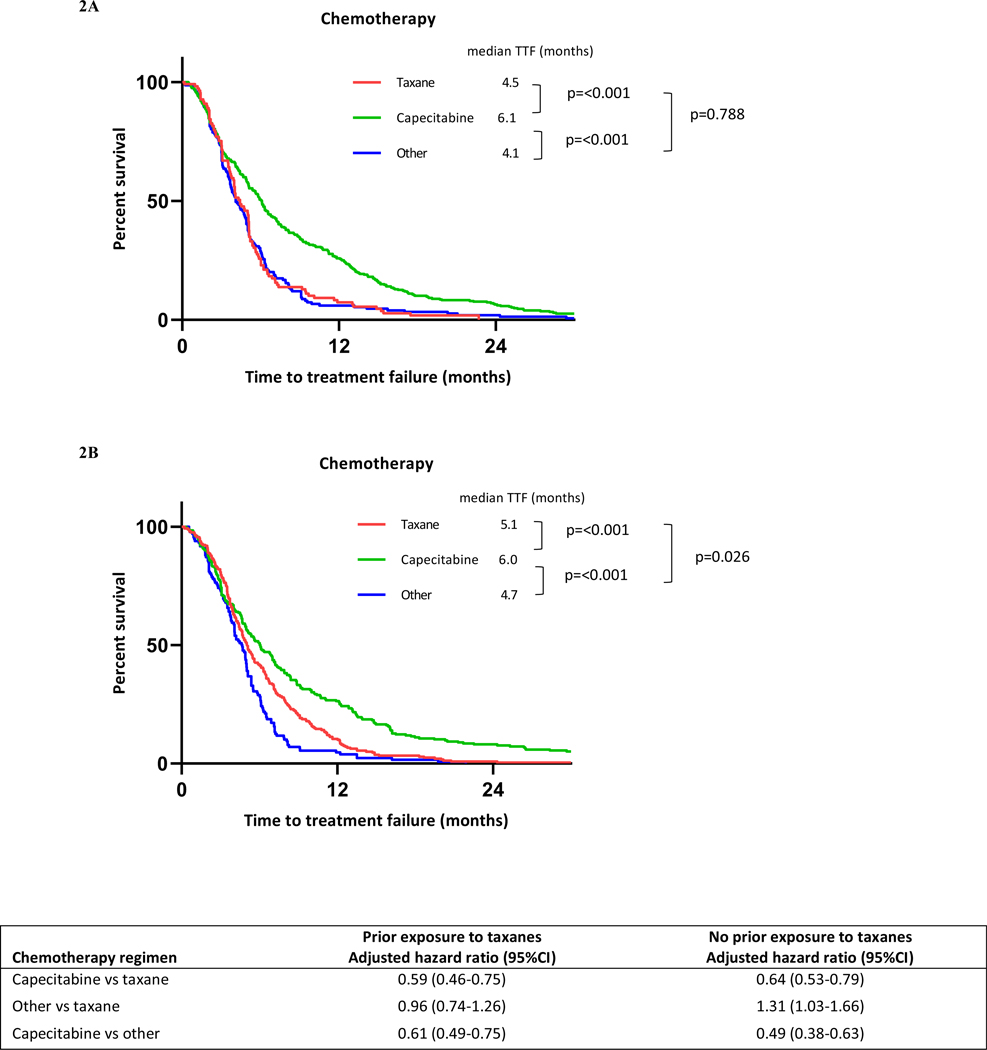
Kaplan-Meier curves for chemotherapy TTF stratified by previous ET and chemotherapy are shown in Figure 1. The median TTFs of chemotherapy in patients previously treated with ET alone, CDK4/6 inhibitor, and mTOR inhibitor were 5.1 months (95%CI 5.0–5.5), 3.4 months (95%CI 3.0–4.0), and 6.1 months (95%CI 5.03–8.13), respectively. Previous treatment with CDK4/6 inhibitor had shorter median TTF than previous treatment with ET alone (adjusted hazard ratio 1.84; 95%CI 1.49–2.27; p<0.001). Median TTFs for taxanes, capecitabine, and other chemotherapy were 4.9 months (95%CI 4.4–5.1), 6.1 months (95%CI 5.5–6.6), and 4.4 months (95%CI 4.0–4.9), respectively. The median TTF for capecitabine was significantly longer than that for taxanes (adjusted hazard ratio 0.64; 95%CI 0.55–0.75; p<0.001). The increased benefit of capecitabine over taxanes as first-line chemotherapy after ET failure was consistent regardless of whether patients had prior exposure to taxanes (Figure 2). The adjusted hazard ratios were 0.59 (95%CI 0.46–0.75; p<0.001) for patients who had received prior taxanes and 0.64 (95%CI 0.53–0.79; p<0.001) for those who had not. However, there was no significant difference in median OS stratified by these chemotherapy regimens (Figure S1). The Kaplan-Meier curve for time from starting ET until stopping first-line chemotherapy (Figure S3) showed a longer median survival time in patients previously treated with CDK4/6 inhibitors than in patients without previous CDK4/6 inhibitors (21.5 months [95%CI 19.3–24.1] vs 19.1 months [95%CI 17.9–20.3], respectively).
Figure 1.
Kaplan-Meier curves for chemotherapy TTF stratified by previous endocrine therapy (A) and chemotherapy regimens (B)
Figure 2.
Kaplan-Meier curves for chemotherapy TTF among patients who had previously received taxanes (A) or had not received taxanes (B), stratified by chemotherapy regimen
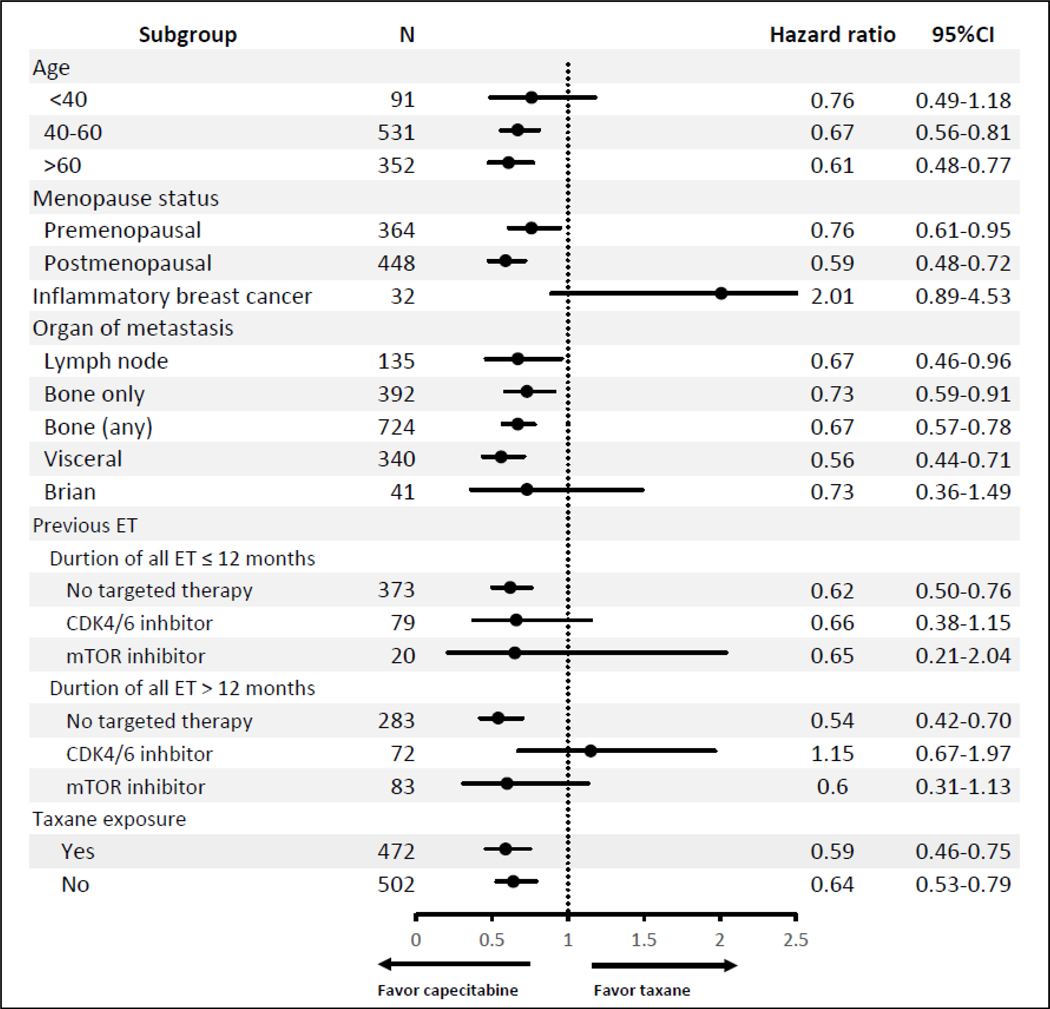
An exploratory subgroup analysis (Figure 3) evaluating the TTFs between capecitabine and taxanes showed more favorable findings for capecitabine than for taxanes, regardless of whether patients had prior exposure to taxanes. The exceptions were in patients with IBC and in those with prior duration of all ET >12 months and previous treatment with CDK4/6 inhibitor. However, neither hazard ratio was significant owing to small sample size.
Figure 3.
Subgroup analysis of TTF between capecitabine and taxane
Discussion
In this study, we evaluated the survival outcome for first-line chemotherapy after progression on ET in patients with HR+, HER2- advanced breast cancer and found that IBC, prior ET, having received adjuvant hormone therapy, and chemotherapy regimen were associated with chemotherapy TTF. Interestingly, prior exposure to ET plus CDK4/6 inhibitor had the strongest negative effect relative to TTF. In contrast, capecitabine had the strongest positive effect relative to TTF in our study.
In clinical practice, chemotherapy is often used as a subsequent treatment for endocrine-resistant breast cancer as recommended by standard guidelines [1,2]. However, supporting studies are quite limited, and the selection of chemotherapy has been based on individual physician-patient decision making [12]. Our results demonstrate factors that can apply to the chemotherapy selection. Moreover, our results may translate into future directions of clinical trial concepts.
To our knowledge, this is the first cohort study that shows the outcome of chemotherapy for patients with endocrine-resistant breast cancer who had disease progression on ET, including CDK4/6 inhibitors, which were first approved by the U.S. Food and Drug Administration in 2015. Our study found that prior types of ET received were associated with chemotherapy TTF. Patients who were previously treated with CDK4/6 inhibitors had shorter TTFs than patients treated with ET alone, regardless of prior duration of all ET. Median TTFs were 3.4 and 5.1 months in patients previously treated with CDK4/6 inhibitors plus ET and with ET alone, respectively. The adjusted hazard ratio for prior CDK4/6 inhibitor treatment was 1.84 (95%CI 1.49–2.27; p<0.001). PALOMA-3, a randomized phase 3 trial evaluating a CDK4/6 inhibitor (palbociclib) combined with ET (fulvestrant), reported a survival benefit in terms of median PFS for the palbociclib plus fulvestrant group compared with the placebo plus fulvestrant group (9.5 vs 4.6 months, respectively; p<0.001). However, no significant difference in OS (34.9 vs 28.0 months, p=0.09) was found [13,14]. When we looked at subsequent lines of therapy in that trial, more than half of patients received chemotherapy as the first line of treatment after the randomized regimen. Implication, the subsequent chemotherapy had shorter benefit after failed palbociclib plus fulvestrant than after failed placebo plus fulvestrant; therefore, the benefit of PFS did not translate into OS. An important distinction between PALOMA-3 and our study is that the inclusion criteria differed; PALOMA-3 allowed patients with no more than one line of chemotherapy in advanced disease. Thus, careful interpretation is needed.
Recently published OS data from MONARCH-2 [5], a randomized phase 3 trial comparing CDK4/6 inhibitor abemaciclib plus fulvestrant with placebo plus fulvestrant, showed an OS benefit in the abemaciclib group (median OS 46.7 vs 37.3 months, respectively; p=0.01). The exploratory analysis of subsequent treatment reported chemotherapy as the most common first line of subsequent therapy (45.3%), and the median durations of chemotherapy were 4.4 vs 4.6 months in the abemaciclib group and placebo group, respectively. However, these results are not fully parallel to our study in that almost all of our patients treated with a CDK4/6 inhibitor received palbociclib. Our results showed a small absolute difference in median TTF was 1.7 months (5.1 vs 3.4 months), but were statistically significant; however, these results need to be analyzed and confirmed in a larger cohort or prospective trial. Interestingly, if we speculate about the cumulative survival benefits of all treatments since diagnosis of metastasis, the maximum survival benefit of treatments was obtained in patients who had received a CDK4/6 inhibitor. This can translate into longer survival, even though subsequent chemotherapy after CDK4/6 inhibitor was short (Figures S2 and S3).
An ongoing question in treatment of metastatic HR+, HER2- breast cancer is what chemotherapy regimens are suitable. Even though the standard guidelines [1,2] include lists of chemotherapy regimens for this subtype, the optimal type and sequence of chemotherapy regimen have not been established. The many randomized clinical trials in HR+, HER2- breast cancer have showed a variety of chemotherapy regimens after failed ET, and there have been differences regimens in each trial [4,5,13]. However, those trials did not report the optimal regimen. In our study of association of the first line of chemotherapy with survival outcome, capecitabine had the longest TTF compared with taxanes and other regimens (median 6.1 vs 4.9 vs 4.4 months; Figure 1). The absolute benefit was 1.2 months, with a hazard ratio of 0.64 (95%CI 0.55–0.75; p<0.001) compared with taxanes, and this benefit was consistent regardless of prior exposure to taxane (Figure 2). The efficacy of first-line capecitabine has been evaluated and supported by many randomized clinical trials, with no differences in survival outcomes compared with other types of chemotherapy (paclitaxel, pegylated liposomal doxorubicin) or a combination regimen (cyclophosphamide/methotrexate/fluorouracil) regardless of whether patients were pretreated or naïve with respect to anthracyclines [15–18]. However, none of these trials enrolled only endocrine-resistant patients, as in our study. Capecitabine is a reasonable choice in endocrine-resistant patients, taking into account its favorable safety profile and lack of cumulative toxicity.
For subgroup analysis in patients previously treated with CDK4/6 inhibitor (total duration ET >12 months) and IBC, our results were more favorable for taxanes than capecitabine (Figure 3). These results were inconsistent with those for the other subgroups, which favored capecitabine. Nevertheless, the sample size was too small to interpret; a larger cohort is needed to confirm the results.
TTF was our primary endpoint and has been suggested as a clinical endpoint using real-world evidence. TTF is influenced by factors not related to efficacy alone, such as toxicity and patient preference [19]. TTF is useful in the setting in which toxicity is potentially as serious as tumor progression. Therefore, TTF is appropriate as our endpoint for evaluation in the nonaggressive subtype of HR+, HER2- breast cancer. All patients in our study had familiarity with less toxic treatment (i.e., ET with or without targeted therapy) compared to chemotherapy, so the toxicity of chemotherapy need to be considered.
Some limitations in our study need to be discussed. This was a retrospective review study conducted at a single institution, which could result in bias such as in the variety of chemotherapy regimens. Hence, we organized the regimens into three groups; furthermore, a larger sample size would reduce this bias. As noted earlier, the CDK4/6 inhibitors in our study were largely limited to palbociclib. Additional limitations are that we did not evaluate the dose intensity, patient compliance with each treatment, toxicity, or treatment delays.
Conclusions
The efficacy of chemotherapy in endocrine-resistant breast cancer could be determined based on previously treatment with a CDK4/6 inhibitor and based on chemotherapy regimen. Capecitabine was a suitable chemotherapy regimen for endocrine-resistant breast cancer patients. Our results need further validation in a larger cohort or prospective trial.
Supplementary Material
Acknowledgments
We would like to thank Sunita Patterson (Scientific Publications, Research Medical Library, MD Anderson Cancer Center) for her help in editing this article and Limin Hsu, Modesto Patangan, and Akshara Raghavendra, our data management team, for help with our database.
Funding
This research was supported by the Morgan Welch Inflammatory Breast Cancer Research Program, a State of Texas Rare and Aggressive Breast Cancer Research Program Grant, and National Institutes of Health/National Cancer Institute grant P30 CA016672 (Cancer Center Support Grant; used the Biostatistics Resource Group and the Clinical and Translational Research Center).
Abbreviations
- (ET)
Endocrine therapy
- (HR+)
Hormone receptor-positive
- (HER2-)
Human epidermal growth factor receptor 2-negative
- (CDK4/6)
Cyclin-dependent kinase 4 and 6
- (mTOR)
Mammalian target of rapamycin
- (ER)
Estrogen receptor
- (PR)
Progesterone receptor
- (IBC)
Inflammatory breast cancer
Footnotes
Compliance with ethical standards
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of University of Texas MD Anderson Cancer Center (PA18–1135) approved this study.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
The authors declare that they have no conflict of interests.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (Version 3.2019). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed January 3,2020
- 2.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, Andre F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh J, Biganzoli L, Boers-Doets CB, Cardoso MJ, Carey LA, Cortes J, Curigliano G, Dieras V, El Saghir NS, Eniu A, Fallowfield L, Francis PA, Gelmon K, Johnston SRD, Kaufman B, Koppikar S, Krop IE, Mayer M, Nakigudde G, Offersen BV, Ohno S, Pagani O, Paluch-Shimon S, Penault-Llorca F, Prat A, Rugo HS, Sledge GW, Spence D, Thomssen C, Vorobiof DA, Xu B, Norton L, Winer EP (2018) 4th ESO-ESMO International consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 29 (8):1634–1657. doi: 10.1093/annonc/mdy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, Fallowfield L, Fowble B, Ingle JN, Jahanzeb M, Johnston SR, Korde LA, Khatcheressian JL, Mehta RS, Muss HB, Burstein HJ (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J Clin Oncol 34 (25):3069–3103. doi: 10.1200/JCO.2016.67.1487 [DOI] [PubMed] [Google Scholar]
- 4.Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, Chow L, Sohn J, Lee KS, Campos-Gomez S, Villanueva-Vazquez R, Jung KH, Chakravartty A, Hughes G, Gounaris I, Rodriguez-Lorenc K, Taran T, Hurvitz S, Tripathy D (2019) Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 381 (4):307–316. doi: 10.1056/NEJMoa1903765 [DOI] [PubMed] [Google Scholar]
- 5.Sledge GW Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke E-M, Conte P, Lu Y, Barriga S, Hurt K, Frenzel M, Johnston S, Llombart-Cussac A (2019) The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, erbb2-negative breast cancer that progressed on endocrine therapy MONARCH 2: A randomized clinical trial. JAMA Oncology. doi: 10.1001/jamaoncol.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366 (6):520–529. doi: 10.1056/NEJMoa1109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sledge GW Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Frenzel M, Lin Y, Barriga S, Smith IC, Bourayou N, Llombart-Cussac A (2017) MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35 (25):2875–2884. doi: 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 8.Prat A, Fan C, Fernandez A, Hoadley KA, Martinello R, Vidal M, Viladot M, Pineda E, Arance A, Munoz M, Pare L, Cheang MC, Adamo B, Perou CM (2015) Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy. BMC Med 13:303. doi: 10.1186/s12916-015-0540-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E (2012) Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 48 (18):3342–3354. doi: 10.1016/j.ejca.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Liu Y, Zhang C, Chu J, Wu Y, Li Y, Liu J, Li Q, Li S, Shi Q, Jin L, Zhao J, Yin D, Efroni S, Su F, Yao H, Song E, Liu Q (2018) Tamoxifen-resistant breast cancer cells are resistant to DNA-damaging chemotherapy because of upregulated BARD1 and BRCA1. Nat Commun 9 (1):1595. doi: 10.1038/s41467-018-03951-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC, American Society of Clinical Oncology, Oncology College of American Pathologists (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134 (7):e48–72. doi: 10.1043/1543-2165-134.7.e48 [DOI] [PubMed] [Google Scholar]
- 12.Harbeck N, Gnant M (2017) Breast cancer. Lancet (London, England) 389 (10074):1134–1150. doi: 10.1016/s0140-6736(16)31891-8 [DOI] [PubMed] [Google Scholar]
- 13.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Andre F, Puyana Theall K, Huang X, Giorgetti C, Huang Bartlett C, Cristofanilli M (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379 (20):1926–1936. doi: 10.1056/NEJMoa1810527 [DOI] [PubMed] [Google Scholar]
- 14.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Zhang K, Theall KP, Jiang Y, Bartlett CH, Koehler M, Slamon D (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17 (4):425–439. doi: 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 15.Stockler MR, Harvey VJ, Francis PA, Byrne MJ, Ackland SP, Fitzharris B, Van Hazel G, Wilcken NR, Grimison PS, Nowak AK, Gainford MC, Fong A, Paksec L, Sourjina T, Zannino D, Gebski V, Simes RJ, Forbes JF, Coates AS (2011) Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol 29 (34):4498–4504. doi: 10.1200/JCO.2010.33.9101 [DOI] [PubMed] [Google Scholar]
- 16.Harbeck N, Saupe S, Jager E, Schmidt M, Kreienberg R, Muller L, Otremba BJ, Waldenmaier D, Dorn J, Warm M, Scholz M, Untch M, de Wit M, Barinoff J, Luck HJ, Harter P, Augustin D, Harnett P, Beckmann MW, Al-Batran SE, Pelican Investigators (2017) A randomized phase III study evaluating pegylated liposomal doxorubicin versus capecitabine as first-line therapy for metastatic breast cancer: results of the PELICAN study. Breast Cancer Res Treat 161 (1):63–72. doi: 10.1007/s10549-016-4033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talbot DC, Moiseyenko V, Van Belle S, O’Reilly SM, Alba Conejo E, Ackland S, Eisenberg P, Melnychuk D, Pienkowski T, Burger HU, Laws S, Osterwalder B (2002) Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. Br J Cancer 86 (9):1367–1372. doi: 10.1038/sj.bjc.6600261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oshaughnessy JA, Blum J, Moiseyenko V, Jones SE, Miles D, Bell D, Rosso R, Mauriac L, Osterwalder B, Burger HU, Laws S (2001) Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol 12 (9):1247–1254. doi: 10.1023/a:1012281104865 [DOI] [PubMed] [Google Scholar]
- 19.Pazdur R (2008) Endpoints for assessing drug activity in clinical trials. Oncologist 13 Suppl 2:19–21. doi: 10.1634/theoncologist.13-S2-19 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.