Abstract
Strategies to qualitatively and quantitatively enhance the humoral response to immunizations with protein and polysaccharide antigens are of broad interest for development of new and more effective vaccines. A strategy of increasing importance is the formulation of antigens into a particulate format, mimicking the physical form of viruses. The potential benefits of enhanced B cell receptor engagement by nanoparticles have been long been appreciated, but recent studies are defining additional important factors governing how nanoparticle immunogens interact with the immune system in the context of lymphoid organs. This review will discuss findings about how nanoparticles enhance humoral immunity in vivo and factors governing the fate of nanoparticle immunogens in lymph nodes.
Keywords: Vaccines, Nanoparticles, humoral immunity
Introduction
Vaccines are a cornerstone of modern public health, and the development of vaccines against endemic pathogens has had a major impact on global mortality and morbidity from infectious diseases (1). Many vaccines have been developed through the generation of live attenuated or chemically inactivated forms of microbes, but this approach is not always successful and in some cases (e.g., HIV) is not effective and/or safe. An alternative is to design subunit vaccines based on selected protein or polysaccharide antigens from the pathogen. Here the challenge becomes promoting the right type of immune response. Because nearly all available licensed vaccines are thought to protect through induction of appropriate long-lived antibody responses (2), guiding humoral immunity is a critical element in the development of subunit vaccines.
Vaccine-elicited immunity is impacted by a number of factors, including the use of adjuvants, choice of immunization regimens, and structural design of the immunogen. One particularly effective strategy for enhancing humoral responses is via formulation of antigens in a multivalent particulate structure, mimicking the structure of a virus. Nanoparticle vaccines have a number of theoretical advantages for immune stimulation relative to soluble immunogens, including enhanced lymphatic trafficking, increased capture in lymph nodes by antigen presenting cells (APCs), and increased activation of antigen-specific B cells through receptor crosslinking (3). Formation of particulate antigens can be achieved by fusing immunogens to a protein that undergoes self-assembly with other subunits to form a nanoparticle, or through chemical linkage to synthetic lipid vesicles, polymer particles, or other inert materials. Vaccine nanoparticles are typically designed with diameters of ~100 nm or smaller, as particles in this size range exhibit effective drainage from injection sites into lymphatic vessels for effective trafficking to draining lymph nodes (3, 4). Particulate formulation has a demonstrated track record of efficacy in humans, such as the licensed HPV and hepatitis B vaccines, which are based on virus-like particle immunogens. Despite these successes, there remain many open questions about optimal design principles for nanoparticle immunogens, and how these vaccines interact with the immune system. In this review we will discuss recent advances in understanding how nanoparticle immunogens impact humoral immunity, with a particular focus on new insights into the fate of particulate antigens in vivo and how nanoparticle immunogens can be targeted to key sites for humoral immune response induction.
Factors in particulate antigen display impacting humoral immunity
Display of immunogens in a dense array on the surface of a particle is often effective to increase their immunogenicity. For example, minimal peptide immunogens that elicit poor humoral responses due to rapid clearance in vivo and lack of repetitive epitopes have been demonstrated to generate strong antigen-specific antibody responses when multimerized on a particle scaffold (5, 6). Nanoparticle display also enhances responses to T-independent immunogens, as demonstrated in the case of pneumococcal glycans: Delivery on gold nanoparticles or liposomes leads to robust class-switched antibody responses in mice (7, 8).
Nanoparticle scaffolds can also increase the stability of immunogens and maintain desired epitope conformations. Fusion of HIV Env trimer subunits with self-assembling protein scaffolds has been successfully employed to ensure the antigen maintains the correct conformation, ensuring the immunogen remains properly folded (9-12). Locking the position of an antigen can also prevent off-target responses against artificial or undesirable epitopes, helping to refocus the immune response on the antigenic site of interest (13). In the case of transmembrane antigens, an engineered lipid bilayer can be used to maintain the native conformation of an antigen that would otherwise be unstable in vivo (14, 15).
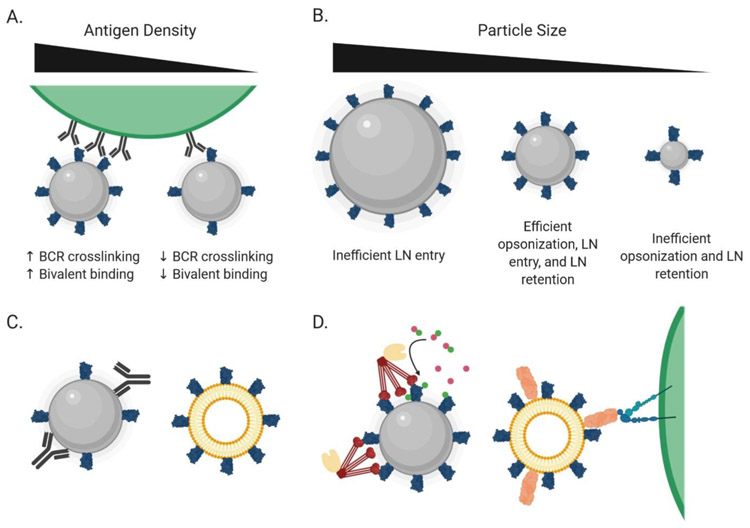
Details of how the antigen is arrayed are important in shaping the humoral response to nanoparticle vaccines. High antigen surface density appears to be important. Particles displaying antigen at a spacing allowing bivalent recognition by an individual BCR increases binding avidity (Figure 1) (10). Using a modular multicomponent protein nanoparticle system, it was shown that antigen density correlated with neutralizing responses to an RSV immunogen in mice, with optimal particles also eliciting strong neutralizing responses in non-human primates (16). Similarly, dense multimerization of engineered HIV envelope trimers on liposomes has been shown to enhance triggering of antigen-specific B cells (13, 17). Nanoparticle display has also been shown to impact humoral responses in non-human primates (NHPs) (6, 18). This is particularly highlighted by a recent NHP study of engineered HIV antigens, in which nanoparticle immunization elicited robust antibody and germinal center responses within seven days following immunization (19).
Figure 1.
Physical characteristics of nanoparticles influence their interactions with the immune system. (A) Increased antigen density on the surface of nanoparticles can improve both bivalent recognition by B cell receptors as well as B cell receptor crosslinking, inducing more robust B cell activation. (B) Small nanoparticles (<15 nm) are poorly retained within the lymph node and are poorly opsonized, while large nanoparticles (>100 nm) are often too large to freely access the lymphatic system. (C) Anti-scaffold antibody responses can distract from desirable responses against the displayed antigen and can be mitigated by utilizing a poorly immunogenic particle composition. (D) Particles can engage with innate immune defense proteins to promote trafficking to FDCs. On left, mannose-binding lectin (red) can recognize surface glycans on the particle, leading to the cleavage of C3 into C3a (magenta) and C3b (green) by MBL-associated serine proteases (tan). C3b deposition on the particle then leads to directed trafficking to lymph nodes in a complement-receptor dependent manner. On right, MFG-E8 (salmon) can recognize phosphatidylserine on the surface of liposomes. The MFG-E8 can then be bound by integrins on the surface of subcapsular sinus macrophages, leading to the direct deposition of the particles on FDCs.
Nanoparticle carriers also enable multiple antigens to be displayed simultaneously to a single B cell. For example, presentation of multiple strains of influenza hemagglutinin in close proximity on a single nanoparticle favors the expansion of B cells that recognize epitopes conserved between the distinct strains, promoting neutralizing antibody breadth (20). Sequential immunization with liposomes bearing a series of HIV Env trimers or mixtures of trimers as heterologous boosts were similarly able to elicit antibodies exhibiting broad neutralization in rabbits (21).
A challenge particularly for protein nanoparticles is the development of strong humoral responses against the “core” scaffold itself, which might immunodominate over desired epitopes on the immunogen (16, 22, 23). These responses arise in part due to the use of non-self and engineered proteins to form particle cores, and incomplete particle self-assembly in some systems (12, 23). Targeting of the particle scaffold can be minimized by using particles with nonimmunogenic compositions, such as liposomes, through engineering particle structures where the core is sterically inaccessible, or through glycan or polymer masking of the particle core. Another strategy is to design a particle composed entirely of antigen, eliminating additional material that could elicit an off-target response (24).
Trafficking of vaccine nanoparticles and engineering LN localization for vaccines
Effective design of nanoparticle vaccines must take into account the fate of these biomaterials in lymphoid tissues- how do they enter the lymph node parenchyma? Where do they localize and for how long? What cells and factors impact these processes? These are questions for which some answers are known and for which others remain unanswered, motivating further fundamental research to modulate immunogen trafficking for optimal vaccine-induced immunity.
Entry of particulate vs. soluble antigens into lymph nodes
Vaccines are typically administered parenterally. Following injection, particulate antigens with sizes greater than ~5 nm are too large to enter the blood vasculature efficiently, and are instead convected into lymphatic vessels. Soluble antigens and particles less than ~100 nm in diameter are efficiently trafficked through lymph to draining lymph nodes (4). On reaching the draining node, small soluble antigens (less than ~70 KDa in size) can be transported into the T cell areas or follicles of LNs through collagen conduits (25, 26), or via gaps in the floor of the subcapsular sinus (27). Entry into the conduits is mediated by portals in lymphatic endothelial cells capped by a size filtering complex formed by the plasmalemma vesicle-associated protein (28). Although nanoparticles have been thought to be too large to efficiently pass the PLVP filter, recent data has shown that both vaccinia and zika virus particles can access conduits and penetrate deep into LNs (29). Alternatively, particulate antigens are captured by macrophages and dendritic cells lining the subcapsular sinus (SCS) or medulla (4, 30, 31). SCS macrophages express a suite of receptors enabling antigen capture, including complement receptors, Fc receptors, and CD169, and do not rapidly internalize and degrade captured particulate antigens. Particles captured by sinus macrophages are transferred over their cell surfaces or via transcytosis to migrating follicular B cells just under the capsular floor. Dendritic cells capturing antigen in the cortical or medullary sinuses can present antigen locally or migrate into the parenchyma to initiate T and B cell priming (30, 31). In addition to direct lymphatic drainage, particles can also be captured in peripheral tissue by monocytes and dendritic cells, which can actively carry antigen into the LN parenchyma (4, 32, 33).
In addition to capture by APCs, several other mechanisms can impact antigen entry to LNs. Lymphatic endothelial cells express scavenger receptors and have been shown to capture viral particles (34). Particulate antigens are also susceptible to rapid proteolysis in the lymph and/or SCS, and protein components of particulate antigens may rapidly reach B cells deep within follicles (34, 35). Which of the above described pathways are engaged depends on the precise composition and size of the antigen/particle and is influenced by the choice of vaccine adjuvant (36). However, it is likely that multiple trafficking pathways occur simultaneously in parallel for any particular vaccine immunogen.
Transfer of particulate antigens to follicular dendritic cells
Once antigens have passed into the LN parenchyma, they may be trafficked to different sites with distinct implications for the ensuing immune response. For humoral immunity, localization of antigen in follicles and germinal centers (GCs), where antigen-triggered B cells undergo proliferation and somatic hypermutation, is critical. Follicular dendritic cells (FDCs) play an important role in GCs, by presenting immobilized antigens to B cells for extended periods (37). FDCs are primarily thought to present antigen on their dendritic surfaces via Fc and complement receptors (CR1 and CR2), and mice deficient in CR1/2 exhibit substantially impaired antibody responses to vaccination (38, 39). Thus, delivery of vaccine immunogens to FDCs is an important step for effective humoral immunity.
Nanoparticles and immune complexes can be transported to FDCs in a complement-dependent manner. Complement (C) opsonization occurs via one of 3 pathways: the classical pathway involving IgM or IgG bound to the particle, the lectin pathway involving mannose binding lectin (MBL) recognizing sugars on the particle surface, or via spontaneous deposition of complement on the particle surface through the alternative pathway (40). SCS macrophages capturing C-opsonized particles transfer the antigen to non-antigen-specific B cells migrating near the lymphatic sinuses, which subsequently deliver these antigens to FDCs in a CR1/2-dependent manner (4).
In addition to complement-mediated trafficking, other pathways for transport of particulate antigens to FDCs have been described. Medullary DCs have been shown to capture influenza viral particles via the lectin receptor SIGN-R1, and carry them toward FDCs (30), although it remains unclear if these DCs transport antigen all the way to FDCs or if transfer to B cells occurs at some intermediate point. Recently, a pathway for direct transfer of enveloped viral particles to FDCs was identified, whereby the phosphatidyl serine-binding protein MFG-E8 acts as an adaptor binding viral membranes to integrins on SCS macrophages, which can then directly transfer viral particles to abutting FDCs (41). Hence, a number of overlapping mechanisms exist to efficiently transport particulate antigens to the FDC network.
Engineering particulate immunogens to alter in vivo trafficking
As noted in the discussion above, administration of immunogens in a nanoparticle as opposed to soluble/monomeric format immediately alters LN trafficking and localization. This is clearly illustrated in studies of the LN localization of soluble vs. particulate HIV Env immunogens. Soluble HIV gp120 or gp140 trimer immunogens localize to interfollicular regions, concentrated on SIGN-R1-expressing macrophages that recognize sugars on these heavily glycosylated immunogens (12, 42). By contrast, nanoparticle forms of gp120 or Env trimers were recently shown to concentrate on FDCs within a few days of immunization, and colocalize with nascent GCs (12). This FDC localization was mediated by MBL-mediated recognition of the dense glycan coat of these NP immunogens, triggering complement deposition and subsequent CR1/2-dependent trafficking into follicles. MBL recognition of glycosylated nanoparticles increased serum antibody, GC B cell, and plasma cell responses to vaccination. A similar pattern of localization within follicles could be engineered for inert polymer nanoparticles by chemically conjugating a threshold density of minimal trimannose glycans onto particle surfaces, suggesting that nanoparticles can be engineered to improve antigen delivery to FDCs (12). In a similar manner, the recently described MFG-E8-mediated transfer of viral particles to FDCs might be engineered into liposomal vaccines (41).
Particle size is a second key parameter. Recent systematic studies using gold nanoparticles surface-conjugated with the model antigens showed that access to follicles is strongly impacted by particle size. Nanoparticles < 15 nm in diam. rapidly entered LN interfollicular regions, the SCS, and follicles following immunization, but cleared from follicles by 48 hours (43). By contrast, larger 50 or 100 nm diam. antigen-conjugated particles were largely confined to the SCS at 2 hours post immunization, but were trafficked into follicles by 12 hours and persisted there for several weeks in a C3 and complement receptor-dependent manner. Histological staining confirmed that these follicle-localized particles colocalized with FDCs and GCs at later times. TEM imaging revealed that the small nanoparticles were predominantly endocytosed by FDCs, while larger particles were immobilized on the extracellular surface of FDCs, suggesting effective localization for antigen acquisition by cognate B cells (43).
The inclusion of an adjuvant, either as a component of the nanoparticle or as an additional surface functionalization, is a third approach to humoral immunity (44-47), and adjuvant-driven inflammation alters the lymph node in diverse ways that can impact antigen trafficking. For example, it was recently shown that UV-inactivated influenza nanoparticle vaccines internalized by lymph node macrophages trigger an inflammasome-mediated necrotic-like cell death, promoting a pro-inflammatory cytokine and chemokine cascade in responding lymph nodes while simultaneously removing these macrophages as potential antigen transfer agents (48). This mechanism could potentially be active in many nanoparticle vaccines as inflammasomes are activated in response to a wide variety of nanoparticle compositions.
Conclusions
Recent studies in both small and large animal models are providing new insights into the impact of particulate formulations of vaccine antigens on humoral immunity. While much effort has focused on engineering antigen display to optimally present neutralizing epitopes, an equally important component will be consideration of engineering nanoparticle designs to exploit natural pathways for optimal entry and localization within lymph nodes. Studies to further our understanding of how the immune system processes particulate antigens should help define improved design strategies for future vaccines.
Acknowledgements
This work was supported in part by the NIAID under awards UM1AI144462, P01AI126901, and AI048240; the US Department of Defense under award W911NF-18-2-0048; and the Ragon Institute of MGH, MIT, and Harvard. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. D.J.I. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of Interest Statement
D.J.I. and B.J.R. declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Of special interest:
Of outstanding interest:
- 1.Nabel GJ, Designing tomorrow's vaccines. N Engl J Med 368, 551–560 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotkin SA, Correlates of protection induced by vaccination. Clin Vaccine Immunol 17, 1055–1065 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer TJ, Zmolek AC, Irvine DJ, Beyond antigens and adjuvants: formulating future vaccines. J Clin Invest 126, 799–808 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cyster JG, B cell follicles and antigen encounters of the third kind. Nat Immunol 11, 989–996 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Tyler M, Tumban E, Peabody DS, Chackerian B, The use of hybrid virus-like particles to enhance the immunogenicity of a broadly protective HPV vaccine. Biotechnol Bioeng 111, 2398–2406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francica JR et al. , Star nanoparticles delivering HIV-1 peptide minimal immunogens elicit near-native envelope antibody responses in nonhuman primates. PLoS Biol 17, e3000328 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai L et al. , Natural killer T (NKT)-B-cell interactions promote prolonged antibody responses and long-term memory to pneumococcal capsular polysaccharides. Proc Natl Acad Sci U S A 110, 16097–16102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetro M et al. , Preparation and immunogenicity of gold glyco-nanoparticles as antipneumococcal vaccine model. Nanomedicine (Lond) 12, 13–23 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Karch CP et al. , Design and characterization of a self-assembling protein nanoparticle displaying HIV-1 Env V1V2 loop in a native-like trimeric conformation as vaccine antigen. Nanomedicine 16, 206–216 (2019). [DOI] [PubMed] [Google Scholar]
- • 10.Brouwer PJM et al. , Enhancing and shaping the immunogenicity of native-like HIV-1 envelope trimers with a two-component protein nanoparticle. Nat Commun 10, 4272 (2019).This paper describes the application of a two-component nanoparticle to the delivery of HIV envelope trimers. The two-component nature of the system allows for the selection of only well-formed antigens for final nanoparticle incorporation. In vivo, these particles were found to be efficacious for priming humoral responses and showed enhanced antibody response quality as compared to alternative single-component nanoparticles.
- 11.Sliepen K et al. , Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology 12, 82 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 12.Tokatlian T et al. , Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 363, 649–654 (2019).This paper identifies a new mechanism for the trafficking of glycosylated nanoparticles to the FDC network. Heavily glycosylated nanoparticles were found to be opsonized by mannose-binding lectin, and a subsequent complement-mediated pathway shuttles the particles to the FDC network. This pathway has the potential to be utilized to direct the trafficking of a variety of antigen-bearing particles.
- 13.Tokatlian T et al. , Enhancing Humoral Responses Against HIV Envelope Trimers via Nanoparticle Delivery with Stabilized Synthetic Liposomes. Sci Rep 8, 16527 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frauenfeld J et al. , A saposin-lipoprotein nanoparticle system for membrane proteins. Nat Methods 13, 345–351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerritzen MJH, Martens DE, Wijffels RH, van der Pol L, Stork M, Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol Adv 35, 565–574 (2017). [DOI] [PubMed] [Google Scholar]
- • 16.Marcandalli J et al. , Induction of Potent Neutralizing Antibody Responses by a Designed Protein Nanoparticle Vaccine for Respiratory Syncytial Virus. Cell 176, 1420–1431.e1417 (2019).This paper describes the design of a two-part nanoparticle structure for immunization against RSV. The two-component nature of the system allowed the authors to ensure that only properly presented antigen was displayed on the surface of the final particles, which elicited antibody responses ten times greater than that of monomeric antigen.
- • 17.Ingale J et al. , High-Density Array of Well-Ordered HIV-1 Spikes on Synthetic Liposomal Nanoparticles Efficiently Activate B Cells. Cell Rep 15, 1986–1999 (2016).This paper describes the synthesis and characterization of HIV envelope trimer-bearing liposomes. By maintaining the trimers in close proximity and a desirable conformation the authors demonstrate enhanced activation of primary B cells and in vivo germinal center responses over the soluble trimers.
- 18.Thompson EA et al. , TLR-adjuvanted nanoparticle vaccines differentially influence the quality and longevity of responses to malaria antigen Pfs25. JCI Insight 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 19.Havenar-Daughton C et al. , Rapid Germinal Center and Antibody Responses in Non-human Primates after a Single Nanoparticle Vaccine Immunization. Cell Rep 29, 1756–1766.e1758 (2019).This paper investigates the role of the route of immunization in non-human primates using nanoparticles bearing an engineered HIV immunogen. The findings identify the subcutaneous route as being able to elicit stronger germinal center responses than the intramuscular route and show responses as early as one week following immunization. Additionally, the authors demonstrate the applicability of fine needle aspirates to track germinal center responses overtime, allowing for longitudinal studies in the same animal.
- 20.Kanekiyo M et al. , Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol 20, 362–372 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 21.Dubrovskaya V et al. , Vaccination with Glycan-Modified HIV NFL Envelope Trimer-Liposomes Elicits Broadly Neutralizing Antibodies to Multiple Sites of Vulnerability. Immunity, (2019).This paper describes the elicitation of neutralizing antibodies against HIV in rabbits using a heterologous antigen-liposome formulation, including one antibody with 87% HIV neutralization breadth. The design of the immunization highlights the benefit of nanoparticle delivery of multiple antigens to drive cross-reactive responses and represents an important step forward in efforts to developing a vaccine regimen capable of inducing broadly neutralizing responses to HIV.
- 22.Shukla GS, Sun YJ, Pero SC, Sholler GS, Krag DN, Immunization with tumor neoantigens displayed on T7 phage nanoparticles elicits plasma antibody and vaccine-draining lymph node B cell responses. J Immunol Methods 460, 51–62 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Sliepen K et al. , Structure and immunogenicity of a stabilized HIV-1 envelope trimer based on a group-M consensus sequence. Nat Commun 10, 2355 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang TZ, Stadmiller SS, Staskevicius E, Champion JA, Effects of ovalbumin protein nanoparticle vaccine size and coating on dendritic cell processing. Biomater Sci 5, 223–233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roozendaal R et al. , Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30, 264–276 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sixt M et al. , The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Pape KA, Catron DM, Itano AA, Jenkins MK, The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity 26, 491–502 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Rantakari P et al. , The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat Immunol 16, 386–396 (2015). [DOI] [PubMed] [Google Scholar]
- • 29.Reynoso GV et al. , Lymph node conduits transport virions for rapid T cell activation. Nat Immunol 20, 602–612 (2019).This paper describes the ability of virions to be transported into the lymph node interior through lymph node conduits, filling a hole in the existing model of how virions and virus-infected cells arrive within the lymph node. Additionally, the authors find that this transport of virus leads to rapid CD8+ T cell activation within the interior of the lymph node.
- 30.Gonzalez SF et al. , Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat Immunol 11, 427–434 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerner MY, Torabi-Parizi P, Germain RN, Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 42, 172–185 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Jakubzick C et al. , Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 39, 599–610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itano AA et al. , Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Junt T et al. , Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Catron DM, Pape KA, Fife BT, van Rooijen N, Jenkins MK, A protease-dependent mechanism for initiating T-dependent B cell responses to large particulate antigens. J Immunol 184, 3609–3617 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodruff MC et al. , Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. J Exp Med 211, 1611–1621 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kranich J, Krautler NJ, How Follicular Dendritic Cells Shape the B-Cell Antigenome. Front Immunol 7, 225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donius LR, Weis JJ, Weis JH, Murine complement receptor 1 is required for germinal center B cell maintenance but not initiation. Immunobiology 219, 440–449 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molina H et al. , Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci U S A 93, 3357–3361 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reis ES, Mastellos DC, Hajishengallis G, Lambris JD, New insights into the immune functions of complement. Nat Rev Immunol 19, 503–516 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 41.Park C, Kehrl JH, An Integrin/MFG-E8 shuttle loads HIV-1 viral like particles onto follicular dendritic cells in mouse lymph node. eLife 8, (2019).This paper identified a new pathway for recognition of lipid-enveloped viral particles, based on binding of the serum protein MFG-E8 to phosphatidyl serine lipids in a viral membrane. MFG-E8-opsonized particles could be captured by SCS macrophages and passed directly to FDCs. Such a pathway might be exploited in liposomal vaccines.
- 42.Park C, Arthos J, Cicala C, Kehrl JH, The HIV-1 envelope protein gp120 is captured and displayed for B cell recognition by SIGN-R1(+) lymph node macrophages. Elife 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang YN et al. , Nanoparticle Size Influences Antigen Retention and Presentation in Lymph Node Follicles for Humoral Immunity. Nano Lett 19, 7226–7235 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Fan Y et al. , Multilamellar Vaccine Particle Elicits Potent Immune Activation with Protein Antigens and Protects Mice against Ebola Virus Infection. ACS Nano 13, 11087–11096 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang S et al. , Versatile Functionalization of Ferritin Nanoparticles by Intein-Mediated Trans-Splicing for Antigen/Adjuvant Co-delivery. Nano Lett 19, 5469–5475 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Zilker C et al. , Nanoparticle-based B-cell targeting vaccines: Tailoring of humoral immune responses by functionalization with different TLR-ligands. Nanomedicine 13, 173–182 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Kasturi SP et al. , Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatziandreou N et al. , Macrophage Death following Influenza Vaccination Initiates the Inflammatory Response that Promotes Dendritic Cell Function in the Draining Lymph Node. Cell Rep 18, 2427–2440 (2017). [DOI] [PubMed] [Google Scholar]