Abstract
Immobilizing enzymes on nanoparticles (NPs) enhances the cost-efficiency of biocatalysis; however, when the substrates are large, it becomes difficult to separate the enzyme@NP from the products while avoiding leaching or damage of enzymes in the reaction medium. Metal-Organic Framework (MOF) coated magnetic nanoparticles (MNPs) offers efficient magnetic separation and enhanced enzyme protection; however, the involved enzymes/substrates have to be smaller than MOF apertures. A potential solution to these challenges is co-precipitating metal/ligand with enzymes on MNP surface, which can partially bury (protect) the enzyme below the composite surface while exposing the rest of the enzyme to the reaction medium for catalysis of larger substrates. Here, to prove this concept, we show that using Ca2+ and terephthalic acid (BDC), large-substrate enzymes can be encapsulated in the CaBDC-MOF layers coated on MNPs via enzyme-friendly, aqueous phase one-pot synthesis. Interestingly, we found that using MNP as the nuclei of crystallization, the composite size can be tuned so that nanoscale composites were formed under low Ca2+/BDC concentrations while microscale composites under high Ca2+/BDC concentrations. While the microscale composites showed significantly enhanced reusability against a non-structured large substrate, the nanoscale composites displayed enhanced catalytic efficiency against a rigid, crystalline-like large substrate, starch, likely due to the improved diffusivity of the nanoscale composites. To our best knowledge, this is the first report on aqueous phase one-pot synthesis of size-tunable enzyme@MOF/MNP composites for large substrate biocatalysis. Our platform can be applied to immobilize other large substrate enzymes with enhanced reusability and tunable sizes.
Keywords: size-tunable composites, enzyme immobilization, magnetic nanoparticle/metal-organic framework (MNP/MOF), aqueous-phase one-pot synthesis, CaBDC MOF
Graphical Abstract

Introduction:
Degradation or conversion of large-size biological substrates are essential for research in sustainable materials, new energy sources,1, 2 and materials processing,3, 4 which find wide applications in agricultural engineering,5, 6 biomass conversion,7, 8 and antimicrobial treatment.3, 9, 10 Enzymes are optimal candidates to carry out these reactions due to their high efficiency, selectivity, and biocompatibility.11, 12 Immobilizing enzymes on the surfaces of nanoparticles (NPs) improves the cost-efficiency and reactivity of biocatalysis (due to the increased surface-to-volume ratio),13-16 but is practically challenged when the substrates are large in size (μm or larger) due to the difficulty in isolating the enzyme@NP composites from the large (unreacted) substrates or products via gravimetric separation, limiting the reusability. Hosting enzymes on magnetic NPs (MNPs) improves the separation;8, 17-19 however, enzyme@MNP composites can be challenged by leaching if the enzyme is adsorbed physically20-22 or chemical perturbation if covalently linked.23, 24 Also, the complete exposure of enzymes may lead to enzyme damage under harsher reaction conditions necessary/requested for the catalysis.13, 25
Encapsulating enzymes in “magnetic” MOFs (or MOF coated MNPs26) offers enzyme protection against harsh conditions and magnetic separation without enzyme chemical perturbation.27-29 However, the involved enzymes and substrates often have to be smaller than MOF apertures.30-33 It’s possible to create cavities or defects on the surface of MOF/MNP composites34 and host enzymes but desorption may occur.26, 35, 36 A potential solution is to co-precipitate enzyme, metal/ligand, and MNPs simultaneously,26 as inspired by our recent works on confining enzymes in MOF or MOF coated carbon nanotubes/graphene oxides.37, 38 Herein, enzymes are partially protected and partially exposed to catalyze the degradation of bacterial cell walls or starch, typical large substrates.37-39 However, coating enzyme@MOFs on MNPs face certain concerns. First, a thin layer of coating, resulting in nanoscale composites (similar size to MNPs), can offer better contact with large crystalline substrates such as starch; however, the coating may be so thin that MOFs may disassembly and leach enzymes. Larger composites likely possess enhanced composite stability and therefore, reusability, but may not contact sufficiently with structured large substrates. Depending on the substrate and application, size-tunable enzyme@MOF/MNP composites are needed but have not been explored.
In this work, we present a strategy to prepare size-tunable enzyme@MOF/MNP composites by co-precipitating Ca2+ and terephthalic acid (BDC) with model large-substrate enzymes, lysozyme or α-amylase, on MNPs in the aqueous phase. The enzyme-friendly one-pot synthesis minimizes enzyme damage during the reaction, while the composite size depends on the initial concentrations of Ca2+ and BDC (MNPs serve as the crystallization nuclei). Lower Ca2+/BDC concentrations yield nanoscale composites with a thinner layer of enzyme@CaBDC coating on MNPs, while higher concentrations result in microscale crystals with MNPs doped inside (defined as the microscale composites). Magnetic separation was then proved more effective in isolating the composites (in both sizes) from the reaction medium as compared to gravimetric separation. Furthermore, the microscale composites possess a higher reusability as compared to the nanoscale ones and allows for catalysis under weakly acidic pHs; however, the nanoscale composites show enhanced catalytic efficiency for degrading starch.
To our best knowledge, this is the first report on aqueous-phase one-pot synthesis of enzyme@MOF/MNP composites with tunable sizes targeting large-substrate catalysis. As compared to (surface or cavity) adsorption-based enzyme immobilization,40, 41 our strategy offers reduced leaching. Different from the existing enzyme@MOF/MNP works,42-45 our composites can target large-size biological substrates and offer tunable composite sizes. Tuning product size allows for designing composites depending on/according to the specific applications (nanoscale composites for structured, rigid substrates or controlled enzyme delivery with external magnets; microscale composites for expensive/precious enzymes due to the high reusability). Our approach is applicable to immobilizing enzymes with arbitrary sizes to catalyze reactions involving substrates in all sizes with enhanced reusability and product separation.
Results and Discussion
We aim to discover reactions that can form MOFs on MNP surface with the following requirements. First, the precipitation has to occur under mild conditions, so that enzyme damage is minimized during the whole process (in contrast to the high temperature/pressures for MOF synthesis and the organic phase co-precipitation of MOFs46). In addition, the size of the formed composites should be tunable via adjusting reaction conditions. Lastly, the formed composites should be stable under the typical/optimal pH conditions of the confined enzyme. After screening a few pairs of metals and ligands, we found Ca2+ and BDC satisfy these needs, as long as the carboxyl group of BDC is modified to –COONa to improve its solubility in water. Below we will show stepwise the preparation of the nanoscale and microscale composites, the advantage of magnetic separation as compared to gravimetric separation, and the activity of model enzymes on each composite.
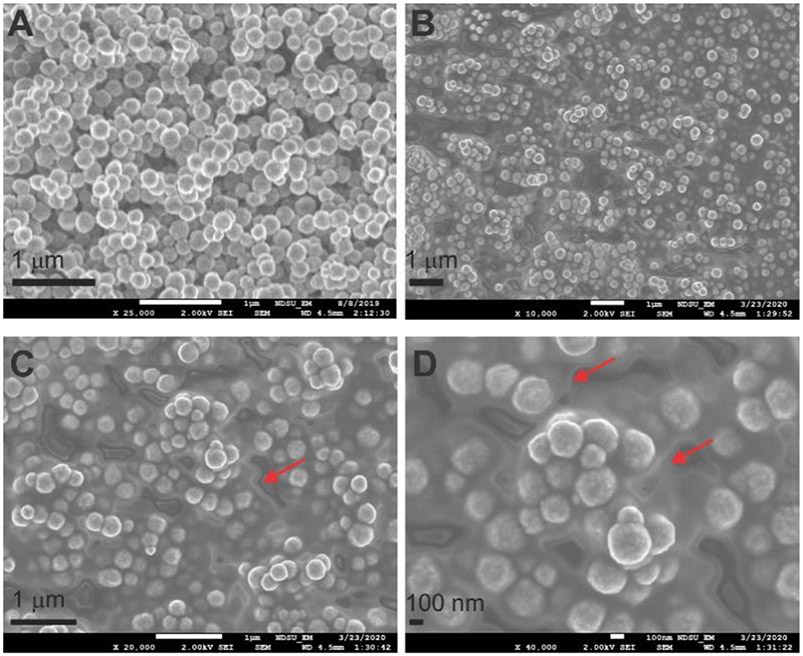
The “seed” MNPs were created by synthesizing the classic Fe3O4 MNPs following the solve-thermal method (details see the Supporting Information, SI).47 The scanning electron microscope (SEM) image of MNPs (Figure 1A) indicates uniform particle size at ~ 100-150 nm, consistent with the literature.47 To ensure the enzyme is located on MNPs before MOF layer formation (but not forming enzyme@MOF separately from MNPs), prior to co-precipitation, we coated the seed MNPs with a layer of polymer, polyacrylic acid (PAA; particle designated as MNP-PAA) and polydopamine (PDA; particle designated as MNP-PDA), one at a time, as suggested in the literature.48, 49 Because the polymer layer usually cannot be visualized from TEM, we performed a ζ-potential measurement on the formed MNPs and found the surface charge of both coated MNPs to be negative (~ −34.1 mV; MNP ~ −10.1 mV), consistent with the net charge of the polymer under neutral conditions. Next, a model large-substrate enzyme, lysozyme (5 μL, 1mM), was mixed with Ca2+ (300 μL, 10 mM final concentration) and BDC (300 μL, 10 mM final concentration) in the suspension of MNP, MNP-PAA, and MNP-PDA, one at a time, under gentle nutation at room temperature overnight. Upon removal of unreacted species via a magnet and resuspension for three rounds, three composites were generated.
Figure 1.
SEM images of the nanoscale MNPs (A). Upon incorporation of CaBDC and lysozyme, a thin grey layer as indicated is formed coating each particle (B), which can be better observed in the amplified images (C,D; see red arrows).
To determine the best polymer coating, we carried out an activity test for a model large-substrate enzyme, lysozyme. Lysozyme cleaves the 1,4-glycosidic bonds of bacterial cell walls; lysozyme activity can be assessed by monitoring the drop in optical density of 450 nm (OD450) in real time, which represents the break-down of cell walls.50 The extent or rate of OD450 decrease is often a measure of the relative scale of lysozyme activity. Our activity assay39 shows that among the three enzyme@CaBDC coated MNPs, the MNP-PAA show the best activity (Figure 2A). Therefore, for the rest of this work, we will focus on the PAA coated MNPs (and “PAA” will be neglected in the nomenclature in the rest of manuscript unless specified).
Figure 2.
(A) Activity assays of lysozyme upon encapsulation on MNP and polymer-coated MNPs via CaBDC formation. (B) Representative height profiles of particles involved in this study based on AFM images of free MNPs (C, height = 119.3 ± 4.2 nm, length = 122.9 ± 3.7 nm), PAA coated MNPs (D, height = 170.2 ± 5.6 nm, length = 175.3 ± 4.8 nm), and CaBDC and PAA coated MNPs (E, height = 197.2 ± 7.0 nm, length = 200.5 ± 13.2 nm). The increase in particle height suggests the success of each coating.
The SEM images of lys@CaBDC/MNP shown in Figures 1B-D suggest the coating of a thin layer on MNPs, wherein a grey layer of “cloud” like materials (see red arrows of Figures 1B-D) indicates the presence of the CaBDC, as compared to Figure 1A where a clear surface of each particle is visible. Similarly, the transmission electron microscopy (TEM) images also showed a grey layer on the CaBDC/MNP composites (Figures S1C&D). The successful coating of MNP with CaBDC can also be confirmed via AFM images (Figures 2B-2E) where the x-axis of Fig. 2B represents the cross-sectional distances perpendicular to the particles to extract the height profile. Since the lateral dimension of AFM images suffers from the tip-sample convolution effects, the height resolution/profile (z-axis) is commonly used to characterize any objects in AFM image analysis. In particular, the coating of PAA increases the MNP height from 119.3 ± 4.2 nm (n=10) to 170 ± 5.6 nm (n=10) while addition of the MOF layer further increased the height to 197.2 ± 7.0 nm (n=10). Unfortunately, due to the thin layer of lys@MOF on MNPs in the nanoscale composites, we could not characterize the crystallinity of the MOF scaffold on MNPs (enzyme loading capacity is ~ 0.46 %; calculation procedure is shown in the SI). To confirm that enzymes were encapsulated in the MOF layer of the formed composite, we mixed lysozyme with the MNP-PAA under the same nutation procedure without MOF, followed by the same separation and wash protocol. The activity assay on the resultant product showed almost no lysozyme activity (Figure S2 red) as compared to the enzyme@CaBDC/MNP composites (Figure 2A) and free lysozyme (Figure S2 black), indicating the presence of lysozyme in the thin layer of the enzyme@CaBDC/MNP composites.
Next, to increase the size of the composite, we increased the initial concentrations of Ca2+ and BDC to 500 mM and mixed with lysozyme and MNP under similar reaction conditions. Large crystals on the order of tens of μm with MNPs doped inside (see SEM images in Figures 3A&B). The presence of lysozyme in the crystal was confirmed via FTIC-labeled lysozyme which showed green fluorescence from confocal microscopy (Figure 3C). The loading capacity of lysozyme is ~ 2.2 % (w/w) according to protein UV absorption at 280 nm, wherein we disassembled the composites using PBS buffer (50 mM, pH 6.2), removed MNPs via a magnet, and filtered out the small molecules via an Amicon concentrator (see the SI). The microscale crystals were then characterized with powder X-ray diffraction (PXRD). The PXRD data were shown in Figure 3D, which indicate that MNPs do not display any diffraction pattern (black trace of Figure 3D), which is likely due to the fact that our MNPs have small and heterogeneous particle size, both of which limit the detection of a clean crystalline phase (see Fig. S3 of the SI), while CaBDC shows a clear one (red trace of Figure 3D). Upon doping of MNPs and encapsulation of lys, overall the PXRD pattern do not change much, although some backgrounds are visible due to MNP (cyan and blue traces of Figure 3D). Taken together, the presence of enzyme and MNP did not alter the crystallinity of CaBDC significantly in microscale crystals.
Figure 3.
SEM images of the microscale composites formed under high Ca/BDC concentrations at two different magnitudes (A, B). The white spots indicate the presence of MNPs. (C) Confocal fluorescence images of the microscale composites formed by FTIC-labeled lysozyme protein. (D) PXRD of MNPs (black), CaBDC MOF (red), CaBDC/MNPs (blue), and lysozyme@CaBDC/MNPs microscale composites (cyan).
Being able to control particle size offers an opportunity to tune the functionality of the composites: microscale crystals are expected to possess higher stability, which reduces the enzyme loss during separation/catalytic reactions and increases the reusability, while nanoscale composites are advantageous for interacting with more structured substrates or delivery of enzymes to varied systems.
To demonstrate the efficacy of magnetic separation versus centrifugation for improved reusability in large-substrate biocatalysis, we carried lysozyme activity tests for multiple cycles continuously using both the nanoscale and microscale composites. For the nanoscale composites, after monitoring the decay of OD450 for the first cycle (Figure 4A black), we separated the composites from the reaction medium via centrifugation at 14,800 rpm for 5 min. We had also tried to centrifuge at 8,000 rpm but it became difficult to completely collect all the pellets, leading to a loss in particles and reusability. At even lower g-forces such as 3,000 rpm, it became difficult to even separate the particles from the rest of the solution. After removing the supernatant, the composites were re-charged with buffer (50 mM HEPES pH 7.4), sonicated for 5 min with a 50 % duty cycle in ice-water, and centrifuged again. After three rounds of centrifugation-resuspension, the composites were mixed with the same amount of substrates. A clear reduction in OD450 was observed as compared to the first cycle (Figure 4A red vs black). In the third cycle, even more reduction in catalytic efficiency was observed (Figure 4A blue). While no clear disassembly of the composites was observed (as judged by direct visualization and SEM) after three cycles, most likely the remaining pieces of broken cell walls precipitated and coated on the surface of the nanoscale composites; sonication and resuspension were unable to remove these broken pieces which blocked the access of unreacted cell walls. It is also possible for the composite to aggregate during the centrifugation which can induce local composite aggregation and reduce the efficiency of enzyme-substrate contact.
Figure 4.
Activity assays and reusability assessment for the nanoscale (A-C) and microscale (D-F) composites formed under low and high Ca/BDC concentrations, respectively. For both composites, gravimetric separation (A, D) clearly results in more loss in activity as compared to magnetic separation (B, E). The microscale composites possess much higher reusability as compared to the nanoscale ones (F versus C). The error bars in C and F are acquired by repeating the same activity assay three times, while the relative uncertainties in A, B, D, and E are typically 3-5 % which is close to the size of the symbols and not shown for clarity of figures. The percentage of loss shown in C and F was calculated by comparing the OD450 drop in the first cycle (defined as 100 % relative activity) to that in subsequent cycles at the end point. No more than 3 cycles were conducted for the nanoscale composite as shown in C because of the obvious activity loss in the third cycle.
Magnetic separation, on the other hand, provided enhanced separation efficiency. After the first round of activity test, we placed a strong magnet on the side of the reaction container and carefully removed the rest of the solution via pipetting. After a similar resuspension and sonication, another two rounds of supernatant removal were carried out. Then, we applied the resultant composites to another batch of substrate and obtained almost identical activity curve (Figure 4B red vs black). Even the third cycle shows a reasonable reusability. These results demonstrate the advantage of magnetic separation as compared to centrifugation (Figure 4C) because the magnet can gently separate the composite from the rest of the mixture and reduce the chance for the broken cell walls to adhere to the composite surface. The reusability was found to be ~ 85 % after three cycles (Figure 4C). The reason for a substantial drop in the third cycle may be caused by the relatively poor stability of the thin layer of CaBDC MOF on the MNPs that some enzymes may be detached during magnetic separation or wash.
The microscale composites displayed a significantly improved reusability via magnetic separation. As shown in Figure 4D, the first 4 cycles of catalysis via centrifugation show a clear drop in catalytic efficiency. When switching to magnetic separation, for 7 cycles we observed almost identical catalytic curves (Figure 4E), indicating an improved reusability. The rate of OD450 drop, or catalytic efficiency, for the microscale composites is also higher than the nanoscale ones, indicating better contact efficiency of the composites with the cell walls. This is reasonable because lysozyme does not have a preferential cleavage position in the cell wall; anywhere that contains the 1,4-glycosidic bonds and contacts the composite surface can be cleaved. The large surface of the microscale composites increases the chance to contact the cell walls. The catalytic efficiency difference between the two composites can also be caused by the larger number of enzyme molecules being encapsulated in the microscale composites.
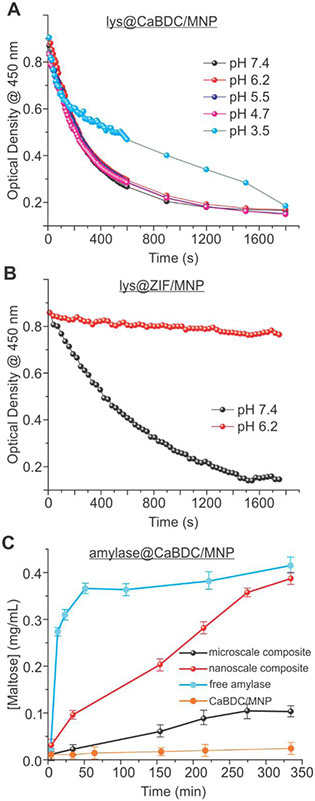
A unique advantage of the microscale composites is their pH stability as compared to the Zeolitic Imidazolate Frameworks (ZIFs). To probe the pH stability, the microscale composites were incubated for 5.5 h in a variety of buffers with pHs from 3.5 to 7.4 (buffer selection requires caution since certain buffers such as PBS disassemble the CaMOF; see the SI), washed for three times via magnetic separation, and subjected for the activity assay. As shown in Figure 5A, our composites display similar activity under most pHs except at 3.5, wherein the composites were believed to be partially disassembled. As a comparison, lysozyme@ZIF/MNP (preparation see the SI) showed almost no activity after treating with a pH 6.2 buffer (Figure 5B red) but reasonable activity under pH 7.4 (Figure 5B black).
Figure 5.
(A) Lysozyme activity assays under varied pHs on lysozyme@CaBDC/MNP microscale composites. (B) Lysozyme activity assays of lysozyme@ZIF/MNP composites treated under an acidic and basic pHs. A significant loss in activity under the acidic pH confirms the disassembly of ZIF/MNP under pH 6.2. (C) The activity assay of α-amylase against starch upon encapsulation in microscale (black) and nanoscale (red) CaBDC/MNP composites. The nanoscale composites clearly display higher catalytic efficiency. The activities of free α-amylase and microscale CaBDC/MNP composite without the enzyme were also investigated as the positive (cyan) and negative (orange) controls. Error bars were obtained by repeating the same assay three times.
The cell walls utilized above are more likely random and dynamics (“soft”) in shape, which can more efficiently contact the microscale composites. When the substrates have more stable and rigid crystal structures and only a specific site in the substrate can be catalyzed by an enzyme, the composite size may play a different role. A typical example is α-amylase, which hydrolyzes the α−1,4-glycosidic linkages bond at the end of a starch molecule and yields maltose and polysaccharides. Because starch molecules are known to possess crystalline or semi-crystalline structures,51 the microscale composites, although possess larger surface, may not be able to contact the cleavage site (the end of a starch molecule) as efficiently as compared to the nanoscale composites because a starch molecule may not necessarily contact the MOF surface with its end. For the nanoscale composites, on the other hand, because of the relatively high mobility and diffusivity, the chance for the α-amylase active site to contact the end of a starch is higher (see Figure 6A for illustration). This speculation has been proved by the α-amylase activity assay as described in our recent works,37, 38 wherein the production of maltose was quantified as a function of time upon initiation of the reaction (mixing composites with starch). As is evident from Figure 5C, the microscale composites showed a reduced activity as compared to the nanoscale ones. It is unlikely for this activity difference to be caused by different amount of α-amylase exposed on MOF surface because with comparable loading capacity for both enzymes, the lysozyme experiments showed an opposite trend of activity (higher catalytic efficiency for the microscale composites). Therefore, our rationalization to the activity data differences is that nanoscale composites have a higher chance to contact rigid substrates and thus, a higher catalytic efficiency, especially when the catalytic reaction requires the contact of enzyme with a specific position of a rigid substrate molecule.
Figure 6.
Schematic illustration of the impact of composite size on the catalytic efficiency against a rigid substrate, starch (A) and a typical soft large substrate, cell wall (B). The smaller size of the nanoscale composites allows for enhanced access to the gaps among starch branches, which was proposed as the cause of the enhanced catalytic efficiency (A). The soft nature of the cell walls can effectively contact the large crystals (B). (Inset) Representation of symbols.
Conclusion
In this work, we discovered an approach to co-precipitate enzymes with metal/ligand on MNPs under mild conditions in aqueous phase for contacting large-size biological substrates. By selecting a specific pair of metal and ligand, Ca2+ and BDC, we formed the enzyme@MOF/MNP composites via one-pot co-precipitation. The reaction condition is favorable due to the negligible damage to the target enzyme during the co-precipitation. Remarkably, by controlling the concentration of Ca2+ and BDC, we found that it is possible to control the size of the composites after co-precipitation, from sub-μm to tens of μm. In both cases, the presence of MNPs enables magnetic separation of composites from the reaction medium, which offers enhanced separation efficiency and reusability as compared to gravimetric separation. The microscale crystals possess higher stability, which improves the reusability even more (almost no loss after 7 cycles) as compared to the nanoscale crystals. The microscale crystals are also stable under acidic pHs, which opens an avenue for catalytic reactions that require lower pHs. On the other hand, when a structured substrate, such as starch, is encountered wherein the enzyme has to contact a specific position of the starch molecule, the nanoscale composites offers a higher catalytic efficiency. Our work represents the first report of large substrate biocatalysis using MOF/MNPs with tunable composite size and enhanced acidic stability. The method can be applied to immobilize other large substrate enzymes with enhanced reusability. Depending on the selection of Ca/BDC concentration, the size of the composites can be tuned, which can satisfy various needs depending on the applications such as enhanced reusability, improved mobility for delivery or contacting structured, crystalline substrates, acidic catalytic reaction conditions for optimal enzyme performance, and/or enhanced overall composite stability.
Experimental section
All chemicals and biochemical supplies were purchased from commercially resources in high purity without further purification; details are presented in the Supporting Information. All involved materials, including MNPs, polymer-coated MNPs, fluorescent labeling of enzymes, lysozyme or α-amylase@CaBDC/MNPs, and lysozyme@ZIF/MNP were prepared as detailed in the Supplementary Information. Materials characterization including control experiments, such as atomic force microscopy (AFM), powder X-ray diffraction (PXRD), transmission electron microscopy (TEM), confocal fluorescence imaging, scanning electron microscopy (SEM), and activity assays of both lysozyme and α-amylase, was carried out using local facility centers following the standard procedure of each equipment; details are presented in the Supplementary Information.
Supplementary Material
Acknowledgements
This work is supported by the New Faculty Startup Funds from the North Dakota State University and NSF ND EPSCoR seed award #FAR0032080 (Z.Y.) and NIGMS NIH R15GM122063 (Y.C.). We sincerely appreciate the help from Dr. Angel Ugrinov for the data interpretation and analysis of PXRD.
Footnotes
Supporting Information. Materials and supplies, preparation of Na2-BDC, MNPs, enzyme@MNP/MOF composites, characterization of composites with SEM, TEM, AFM, and PXRD, as well as control activity data.
References
- (1).Lynd LR; Liang X; Biddy MJ; Allee A; Cai H; Foust T; Himmel ME; Laser MS; Wang M; Wyman CE, Cellulosic Ethanol: Status and Innovation. Curr. Opin. Biotechnol 2017, 45, 202–211. [DOI] [PubMed] [Google Scholar]
- (2).Juturu V; Wu JC, Microbial Cellulases: Engineering, Production and Applications. Renew. Sustain. Energ. Rev 2014, 33, 188–203. [Google Scholar]
- (3).Kiristi M; Singh VV; Esteban-Fernández de Ávila B; Uygun M; Soto F; Aktaş Uygun D; Wang J, Lysozyme-Based Antibacterial Nanomotors. ACS Nano 2015, 9, 9252–9259. [DOI] [PubMed] [Google Scholar]
- (4).Luckarift HR; Dickerson MB; Sandhage KH; Spain JC, Rapid, Room-Temperature Synthesis of Antibacterial Bionanocomposites of Lysozyme with Amorphous Silica or Titania. Small 2006, 2, 640–643. [DOI] [PubMed] [Google Scholar]
- (5).Lee M-H; Thomas JL; Chen Y-C; Wang H-Y; Lin H-Y, Hydrolysis of Magnetic Amylase-Imprinted Poly(ethylene-co-vinyl alcohol) Composite Nanoparticles. ACS Appl. Mater. Inter 2012, 4, 916–921. [DOI] [PubMed] [Google Scholar]
- (6).Payne CM; Knott BC; Mayes HB; Hansson H; Himmel ME; Sandgren M; Ståhlberg J; Beckham GT, Fungal Cellulases. Chem. Rev 2015, 115, 1308–1448. [DOI] [PubMed] [Google Scholar]
- (7).Meng D; Wei X; Zhang Y-HPJ; Zhu Z; You C; Ma Y, Stoichiometric Conversion of Cellulosic Biomass by in Vitro Synthetic Enzymatic Biosystems for Biomanufacturing. ACS Catal. 2018, 8, 9550–9559. [Google Scholar]
- (8).Yamaguchi A; Mimura N; Shirai M; Sato O, Cascade Utilization of Biomass: Strategy for Conversion of Cellulose, Hemicellulose, and Lignin into Useful Chemicals. ACS Sustain. Chem. Eng 2019, 7, 10445–10451. [Google Scholar]
- (9).Abouhmad A; Dishisha T; Amin MA; Hatti-Kaul R, Immobilization to Positively Charged Cellulose Nanocrystals Enhances the Antibacterial Activity and Stability of Hen Egg White and T4 Lysozyme. Biomacromolecules 2017, 18, 1600–1608. [DOI] [PubMed] [Google Scholar]
- (10).Pan Y; Neupane S; Farmakes J; Oh M; Bentz K; Choi Y; Yang Z, Insights on the Structure, Molecular Weight and Activity of an Antibacterial Protein-Polymer Hybrid. ChemPhysChem 2018, 19, 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sheldon RA; Woodley JM, Role of Biocatalysis in Sustainable Chemistry. Chem. Rev 2018, 118, 801–838. [DOI] [PubMed] [Google Scholar]
- (12).Sheldon RA; Brady D, The Limits to Biocatalysis: Pushing the Envelope. Chem. Commun 2018, 54, 6088–6104. [DOI] [PubMed] [Google Scholar]
- (13).Wang M; Mohanty SK; Mahendra S, Nanomaterial-Supported Enzymes for Water Purification and Monitoring in Point-of-Use Water Supply Systems. Acc. Chem. Res 2019, 52, 876–885. [DOI] [PubMed] [Google Scholar]
- (14).Zhang Y; Ge J; Liu Z, Enhanced Activity of Immobilized or Chemically Modified Enzymes. ACS Catal. 2015, 5, 4503–4513. [Google Scholar]
- (15).Sheldon RA; van Pelt S, Enzyme Immobilisation in Biocatalysis: Why, What and How. Chem. Soc. Rev 2013, 42, 6223–6235. [DOI] [PubMed] [Google Scholar]
- (16).Laurent N; Haddoub R; Flitsch SL, Enzyme Catalysis on Solid Surfaces. Trends Biotechnol. 2008, 26, 328–337. [DOI] [PubMed] [Google Scholar]
- (17).Zakharchenko A; Guz N; Laradji AM; Katz E; Minko S, Magnetic Field Remotely Controlled Selective Biocatalysis. Nat. Catal 2018, 1, 73–81. [Google Scholar]
- (18).Huang W-C; Wang W; Xue C; Mao X, Effective Enzyme Immobilization onto A Magnetic Chitin Nanofiber Composite. ACS Sustain. Chem. Eng 2018, 6, 8118–8124. [Google Scholar]
- (19).Bilal M; Zhao Y; Rasheed T; Iqbal HMN, Magnetic Nanoparticles as Versatile Carriers for Enzymes Immobilization: A Review. Int. J. Biol. Macromol 2018, 120, 2530–2544. [DOI] [PubMed] [Google Scholar]
- (20).Yang HY; Li Y; Lee DS, Multifunctional and Stimuli-Responsive Magnetic Nanoparticle-Based Delivery Systems for Biomedical Applications. Adv. Ther 2018, 1, 1800011. [Google Scholar]
- (21).Huang J; Shu Q; Wang L; Wu H; Wang AY; Mao H, Layer-by-Layer Assembled Milk Protein Coated Magnetic Nanoparticle Enabled Oral Drug Delivery with High Stability in Stomach and Enzyme-Responsive Release in Small Intestine. Biomaterials 2015, 39, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Liu D-M; Chen J; Shi Y-P, Advances on Methods and Easy Separated Support Materials for Enzymes Immobilization. TrAC Trend. Anal. Chem 2018, 102, 332–342. [Google Scholar]
- (23).Yang Y; Zhang R; Zhou B; Song J; Su P; Yang Y, High Activity and Convenient Ratio Control: DNA-Directed Coimmobilization of Multiple Enzymes on Multifunctionalized Magnetic Nanoparticles. ACS Appl. Mater. Inter 2017, 9, 37254–37263. [DOI] [PubMed] [Google Scholar]
- (24).Lee SY; Lee S; Kho IH; Lee JH; Kim JH; Chang JH, Enzyme-Magnetic Nanoparticle Conjugates as A Rigid Biocatalyst for the Elimination of Toxic Aromatic Hydrocarbons. Chem. Commun 2011, 47, 9989–9991. [DOI] [PubMed] [Google Scholar]
- (25).Chapman R; Stenzel MH, All Wrapped up: Stabilization of Enzymes within Single Enzyme Nanoparticles. J. Am. Chem. Soc 2019, 141, 2754–2769. [DOI] [PubMed] [Google Scholar]
- (26).Hou C; Wang Y; Ding Q; Jiang L; Li M; Zhu W; Pan D; Zhu H; Liu M, Facile synthesis of Enzyme-Embedded Magnetic Metal-Organic Frameworks as A Reusable Mimic Multi-Enzyme System: Mimetic Peroxidase Properties and Colorimetric Sensor. Nanoscale 2015, 7, 18770–18779. [DOI] [PubMed] [Google Scholar]
- (27).Wu X; Hou M; Ge J, Metal-Organic Frameworks and Inorganic Nanoflowers: A Type of Emerging Inorganic Crystal Nanocarrier for Enzyme Immobilization. Catal. Sci. Technol 2015, 5, 5077–5085. [Google Scholar]
- (28).Lian X; Fang Y; Joseph E; Wang Q; Li J; Banerjee S; Lollar C; Wang X; Zhou H-C, Enzyme-MOF (Metal-Organic Framework) Composites. Chem. Soc. Rev 2017, 46, 3386–3401. [DOI] [PubMed] [Google Scholar]
- (29).Gkaniatsou E; Sicard C; Ricoux R. m.; Mahy J-P; Steunou N; Serre C, Metal-Organic Frameworks: A Novel Host Platform for Enzymatic Catalysis and Detection. Mater. Horiz 2017, 4, 55–63. [Google Scholar]
- (30).Li P; Modica JA; Howarth AJ; Vargas L E; Moghadam PZ; Snurr RQ; Mrksich M; Hupp JT; Farha OK, Toward Design Rules for Enzyme Immobilization in Hierarchical Mesoporous Metal-Organic Frameworks. Chem 2016, 1, 154–169. [Google Scholar]
- (31).Li P; Moon S-Y; Guelta MA; Harvey SP; Hupp JT; Farha OK, Encapsulation of A Nerve Agent Detoxifying Enzyme by A Mesoporous Zirconium Metal-Organic Framework Engenders Thermal and Long-Term Stability. J. Am. Chem. Soc 2016, 138, 8052–8055. [DOI] [PubMed] [Google Scholar]
- (32).Sun Q; Fu C-W; Aguila B; Perman J; Wang S; Huang H-Y; Xiao F-S; Ma S, Pore Environment Control and Enhanced Performance of Enzymes Infiltrated in Covalent Organic Frameworks. J. Am. Chem. Soc 2018, 140, 984–992. [DOI] [PubMed] [Google Scholar]
- (33).Chen Y; Lykourinou V; Vetromile C; Hoang T; Ming LJ; Larsen RW; Ma S, How Can Proteins Enter the Interior of A MOF? Investigation of Cytochrome c Translocation into A MOF Consisting of Mesoporous Cages with Microporous Windows. J. Am. Chem. Soc 2012, 134, 13188–13191. [DOI] [PubMed] [Google Scholar]
- (34).Lin C; Xu K; Zheng R; Zheng Y, Immobilization of Amidase into A Magnetic Hierarchically Porous Metal-Organic Framework for Efficient Biocatalysis. Chem. Commun 2019, 55, 5697–5700. [DOI] [PubMed] [Google Scholar]
- (35).Nadar SS; Rathod VK, Magnetic-Metal Organic Framework (magnetic-MOF): A Novel Platform for Enzyme Immobilization and Nanozyme Applications. Inter. J. Biol. Macromol 2018, 120, 2293–2302. [DOI] [PubMed] [Google Scholar]
- (36).Chen S; Wen L; Svec F; Tan T; Lv Y, Magnetic Metal-Organic Frameworks as Scaffolds for Spatial Co-Location and Positional Assembly of Multi-Enzyme Systems Enabling Enhanced Cascade Biocatalysis. RSC Adv. 2017, 7, 21205–21213. [Google Scholar]
- (37).Neupane S; Patnode K; Li H; Baryeh K; Liu G; Hu J; Chen B; Pan Y; Yang Z, Enhancing Enzyme Immobilization on Carbon Nanotubes via Metal-Organic Frameworks for Large-Substrate Biocatalysis. ACS Appl. Mater. Inter 2019, 11, 12133–12141. [DOI] [PubMed] [Google Scholar]
- (38).Farmakes J; Schuster I; Overby A; Alhalhooly L; Lenertz M; Li Q; Ugrinov A; Choi Y; Pan Y; Yang Z, Enzyme Immobilization on Graphene Oxide (GO) Surface via One-Pot Synthesis of GO/Metal-Organic Framework Composites for Large-Substrate Biocatalysis ACS Appl. Mater. Inter 2020, 12, 23119–23126. [DOI] [PubMed] [Google Scholar]
- (39).Pan Y; Li H; Farmakes J; Xiao F; Chen B; Ma S; Yang Z, How Do Enzymes Orient on Metal-Organic Framework (MOF) Surfaces? J. Am. Chem. Soc 2018, 140, 16032–16036. [DOI] [PubMed] [Google Scholar]
- (40).Santos-Martins D; Calixto AR; Fernandes PA; Ramos MJ, A Buried Water Molecule Influences Reactivity in α-Amylase on A Subnanosecond Time Scale. ACS Catal. 2018, 8, 4055–4063. [Google Scholar]
- (41).Li H; Pan Y; Farmakes J; Xiao F; Hu J; Chen B; Rao J; Yang Z, A Sulfonated Mesoporous Silica Nanoparticle for Enzyme Protection against Denaturants and Controlled Release under Reducing Conditions. J. Colloid Interface Sci 2019, 556, 292–300. [DOI] [PubMed] [Google Scholar]
- (42).He J; Sun S; Zhou Z; Yuan Q; Liu Y; Liang H, Thermostable Enzyme-Immobilized Magnetic Responsive Ni-Based Metal-Organic Framework Nanorods as Recyclable Biocatalysts for Efficient Biosynthesis of S-Adenosylmethionine. Dalton Trans. 2019, 48, 2077–2085. [DOI] [PubMed] [Google Scholar]
- (43).Xie W; Huang M, Enzymatic Production of Biodiesel Using Immobilized Lipase on Core-Shell Structured Fe3O4@MIL-100(Fe) Composites. Catalysts 2019, 9, 850. [Google Scholar]
- (44).Zhou Z; Gao Z; Shen H; Li M; He W; Su P; Song J; Yang Y, Metal-Organic Framework in situ Post-Encapsulating DNA-Enzyme Composites on A Magnetic Carrier with High Stability and Reusability. ACS Appl. Mater. Inter 2020, 12, 7510–7517. [DOI] [PubMed] [Google Scholar]
- (45).Cao S-L; Xu H; Lai L-H; Gu W-M; Xu P; Xiong J; Yin H; Li X-H; Ma Y-Z; Zhou J; Zong M-H; Lou W-Y, Magnetic ZIF-8/Cellulose/Fe3O4 Nanocomposite: Preparation, Characterization, and Enzyme Immobilization. Bioresour. Bioprocess 2017, 4, 56. [Google Scholar]
- (46).Lyu F; Zhang Y; Zare RN; Ge J; Liu Z, One-Pot Synthesis of Protein-Embedded-Supporting Information. Nano Lett. 2014, 14, 5761–5765. [DOI] [PubMed] [Google Scholar]
- (47).Zheng J; Cheng C; Fang W-J; Chen C; Yan R-W; Huai H-X; Wang C-C, Surfactant-Free Synthesis of A Fe3O4@ZIF-8 Core-Shell Heterostructure for Adsorption of Methylene Blue. CrystEngComm 2014, 16, 3960–3964. [Google Scholar]
- (48).Jin T; Yang Q; Meng C; Xu J; Liu H; Hu J; Ling H, Promoting Desulfurization Capacity and Separation Efficiency Simultaneously by the Novel Magnetic Fe3O4@PAA@MOF-199. RSC Adv. 2014, 4, 41902–41909. [Google Scholar]
- (49).Jiang J; Sun X; Li Y; Deng C; Duan G, Facile Synthesis of Fe3O4@PDA Core-Shell Microspheres Functionalized with Various Metal Ions: A Systematic Comparison of Commonly-Used Metal Ions for IMAC Enrichment. Talanta 2018, 178, 600–607. [DOI] [PubMed] [Google Scholar]
- (50).Vocadlo DJ; Davies GJ; Laine R; Withers SG, Catalysis by Hen Egg-White Lysozyme Proceeds via A Covalent Intermediate. Nature 2001, 412, 835–838. [DOI] [PubMed] [Google Scholar]
- (51).Lourdin D; Putaux J-L; Potocki-Véronèse G; Chevigny C; Rolland-Sabaté A. s.; Buléon A, Crystalline Structure in Starch In Starch: Metabolism and Structure, Nakamura Y, Ed. Springer; Japan: Tokyo, 2015; pp 61–90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.