Abstract
The accessibility of adoptive T cell transfer therapies (ACT) is hindered by cost and time required for product development. Here we describe a streamlined ACT protocol using Th17 cells expanded only four days ex vivo. While shortening expansion compromised cell yield, this method licensed Th17 cells to eradicate large tumors to a greater extent than cells expanded longer term. Day 4 Th17 cells engrafted, induced release of multiple cytokines including IL-6, IL-17, MCP-1, and GM-CSF in the tumor-bearing host, and persisted as memory cells. IL-6 was a critical component for efficacy of these therapies via its promotion of long-term immunity and resistance to tumor relapse. Mechanistically, IL-6 diminished engraftment of FoxP3+ donor T cells, corresponding with robust tumor infiltration by donor effector over regulatory cells for the Day 4 Th17 cell product relative to cell products expanded longer durations ex vivo. Collectively, this work describes a method to rapidly generate therapeutic T cell products for ACT and implicates IL-6 in promoting durable immunity of Th17 cells against large, established solid tumors.
Keywords: T cell, Th17, IL-6, adoptive T cell therapy, immunooncology
Introduction
Adoptive T cell transfer therapy (ACT) is a personalized treatment effective for patients with aggressive, end stage malignancies. Administration of peripheral T cells modified with Chimeric Antigen Receptors (CAR) has resulted in remissions of up to 90% of treated patients with hematologic malignancies (1), leading to the first FDA approval for personalized gene therapy in 2017. While CAR T cells are effective in hematologic disease, T cell receptor (TCR)-based products, such as tumor-infiltrating lymphocyte (TIL) therapies, have had greater success in patients with solid tumors and are advantageous for their ability to target intracellular antigens (2). Transfer of ex vivo expanded TIL has generated up to 50% response rates in metastatic melanoma (3) in addition to complete durable responses in metastatic breast (4) and gastrointestinal (5) cancers among others.
For both CARs and TILs, however, the oppressive solid tumor microenvironment limits T cell immunity, making development of novel and safe methods to bolster antitumor potential of the transferred cell paramount. Efficacy of ACT therapies is often correlated with higher number of T cells transferred (3,6); therefore, protocols expand T cells ex vivo over long periods of time to large magnitudes (6,7). Yet, such protocols paradoxically drive T cells to a state of terminal differentiation, yielding large numbers of T cells with poor quality and persistence individually (8). Longer expansion also drives higher cost and treatment delays, which are not feasible for many patients with aggressive diseases. Thus, novel methods for generating potent T cells after shorter expansion could improve efficacy, reduce costs, and permit treatment of patients more rapidly.
We used two tumor-targeting models to determine whether shortening T cell expansion could overcome these limitations. We hypothesized that the CD4+Th17 cell subset—poised with potent immunity, inflammatory, and stemness qualities versus cytotoxic CD8+ or Th1 cells (9-12)—would be the candidate subset to address this concept. By transferring either murine TCR-specific Th17 cells into syngeneic hosts or human CAR-engineered Th17 cells against human tumors in NSG mice, we report that Th17 cells expanded only four days (termed Day 4 Th17 cells) improved antitumor responses despite obtaining fewer cells. Day 4 Th17 cells transferred into immunocompetent animals elicited profound cytokine release, including a unique induction of IL-6 relative to Th17 cells expanded longer term. IL-6 modulated the balance between Th17 and Treg cells to promote the superior antitumor responses of Day 4 Th17 cells. Herein, we describe how a shortened T cell expansion protocol with CD4+Th17 cells improves treatment outcomes and how induction of IL-6 fosters durable memory responses for Th17 cells against solid tumors.
Materials and Methods
Study design and rigor
Sample size:
Animal experiment sample sizes were selected based on power analysis while minimizing use of animals per the Medical University of South Carolina’s (MUSC) Institutional Animal Care and Use Committee (IACUC) policies. For survival experiments, power calculations for the log-rank test were based on the anticipated effect sizes (differences in survival percentages) at the end of the experiments. To achieve minimum power of 80% with 2-sided α = 0.05 for anticipated survival differences of 55% and 40%, sample sizes of n=12 and n=20 were necessary. For other continuous measures, power was based on the Student’s t-test. Our target minimum standardized effect size of 2.0 required group sizes of n=5 to achieve 80% power. Stopping data collection: experimental endpoints were determined prior to study execution. Primary antitumor efficacy was conducted over 30-40 days. Rechallenge experiments were conducted up to 100 days. Data inclusion/exclusion criteria: with experiments herein, data points were only removed for two scenarios established prospectively: 1) if injection of T cells was unsuccessful, the animal was withdrawn from study, or 2) if tumor placement resulted in an intraperitoneal tumor in contrast to a subcutaneous tumor, the animal was removed from tumor analysis as tumor burden cannot be accurately measured. Outliers: all data points are reported without removal of outliers. Randomization: for all C57BL/6 tumor experiments, female mice were randomized by tumor size and housing was mixed prior to treatment group assignment. For NSG experiments, male and female mice were randomly assigned to treatment group by tumor size, but housing of animals was not altered. Tumor sizes were measured as L x W by calipers and reported as tumor area. Assessment of T cell engraftment in peripheral blood was performed on all animals within the study when feasible. For experiments where animals were euthanized prior to endpoint for tissue distribution analysis, mice were selected using random number generation. Blinding: all tumor measurements and T cell injections were performed by a lab member blinded to treatment group.
Mice and tumor lines.
C57BL/6, TRP-1 TCR transgenic mice (Rag −/− BWTRP-1 TCR), and NOD/SCID/gamma chain knock out (NSG) mice were purchased from the Jackson laboratories and bred in house at the MUSC Hollings Cancer Center comparative medicine department. C57BL/6 CD45.1 mice were purchased from NCI Frederick laboratories for indicated studies. Tumor experiments were conducted with mice aged 6-10 weeks. NSG mice were housed in microisolator cages to maintain specific-pathogen free conditions and provided bottle access to acidified, autoclaved water and food. All housing and experiments were conducted in accordance with MUSC’s IACUC procedures and with the supervision and support of the Division of Laboratory Animal Resources (DLAR). All studies and procedures were IACUC approved prior to execution. B16F10 (H-2b) melanoma cell line was obtained from Nicholas P. Restifo and M108 mesothelioma was obtained from Carl H. June and used for in vivo tumor studies. Cell lines were validated prior to transfer and were confirmed pathogen and mycoplasma free via PCR screen most recently in March 2020. B16F10 cells were passaged less than two times and M108 were passaged less than four times after thaw prior to injection.
T cell cultures
TRP-1 cells:
TRP-1 transgenic T cells were activated in the presence of irradiated (10 Gy) feeder splenocytes pulsed with 1 μM TRP-1106-130 peptide (SGHNCGTCRPGWRGAACNQKILTVR). Feeder cells were added in a 1 cell: 5 T cell ratio. Cells were seeded at a concentration of 1.5×106/mL and polarized to Th17 phenotype using the following cocktail of cytokines: 100ng/mL recombinant human (rh) IL-6 (NIH preclinical repository), 100ng/mL rhIL-21 (Shenandoah), 30ng/mL hTGF-β1 (Biolegend), 10 ng/mL rhIL-1β (Shenandoah) 10μg/mL each of anti-mouse (αm)IFN-γ (clone XMG1.2), αmIL-4 (clone 11B11), and αmIL-2 (clone JES6-1A12). On days 2 and 3 of culture, IL-23 and IL-2 were added in concentrations of 20ng/mL and 50IU/mL, respectively. From day 4 and onward, cells were split to a density of 0.8×106/mL and fresh media added with 100IU IL-2/mL as needed. Th0 cells were expanded in IL-2 only.
Human normal donor peripheral T cells:
Peripheral blood from healthy donors (de-identified) were purchased as a buffy coat (Sylvan N. Goldman Oklahoma Blood Institute). Lymphocytes were separated from buffy coat using Lymphocyte Separation Medium (Mediatech). CD4+ T cells were isolated via untouched magnetic bead isolation kit (Dynabeads, Invitrogen) and rested overnight at 37°C in media containing 20 IU IL-2/mL. CD4+ T cells were then activated using magnetic beads decorated with antibodies targeting CD3 and ICOS at a 1 bead:5 T cell ratio, and polarized to the Th17 phenotype using the following cocktail of cytokines: 10ng/mL hIL-1β, 10ng/mL rhIL-6, 20 ng/mL IL-23, 5 μg/mL αhIL-4 and 5μg/mL αhIFN-γ. CD4+ cell cultures were maintained with 100IU IL-2/mL over duration of expansion. On day 2 after activation, cells were transduced with a CAR containing an anti-mesothelin single chain variable fragment (scFv) and a TCR CD3ζ domain with 4-1BB costimulatory domain (13). Meso-CAR was a gift of the C. H. June lab.
ACT
C57BL/6:
0.4×106 B16F10 cells were resuspended in sterile PBS and injected subcutaneously on the abdomen of mice. Tumors grew from 5-12 days to target size prior to treatment. One day prior to ACT, all mice received 5 Gy total body irradiation (TBI). For experiments with antibody blockade, 100μg antibody/mouse in sterile PBS was injected intraperitoneally every other day starting one day post ACT for a total of 5 cycles. In vivo blocking or neutralizing antibodies were purchased from BioXCell: (anti-IL-6, clone MP5-20F3; anti-IL-6R, clone 15A7; anti-IL-17A, clone 17F3; anti-IFN-γ, clone XMG1.2; anti-CD4, clone GK1.5; anti-CD8, clone 2.43; Isotype controls: IgG1 clone HRPN, IgG2b clone LTF-2, mouse IgG1, clone MOPC-21).
NSG:
M108 was injected subcutaneously in Matrigel (Corning) 47 days prior to ACT. 1.5×106 M108 cells were injected per animal in a 1:1 mix of Matrigel. Meso-CAR Th17 cells were resuspended in sterile PBS and transferred via tail vein injection.
Cytokine multiplex assay.
Murine serum samples were frozen and stored at −80°C prior to analysis. Serum was assayed with the 32-plex Mouse Cytokine Array Discovery Assay (Eve Technologies).
Tissue distribution.
Peripheral blood was collected from mandibular vein into 0.125 M EDTA, subjected to red blood cell lysis (Biolegend), and assayed via flow cytometry. Spleens, lymph nodes, and tumors were taken from animals and processed into single cell suspension by mechanical dissociation over a 70 μM filter. M108 tumors were minced and digested in 1mg/mL collagenase type IV (Worthington) at 37°C for 1 hour. Skin was minced and incubated in buffer containing 3mg/mL collagenase IV (Worthington Biochemical), and 0.2mg/mL DNase (Sigma) in Hank’s balanced salt solution (HBSS) at 37°C for 45 minutes with stirring (14). Digestion was neutralized with RPMI containing 10% FBS and 10mM EDTA. Digested and processed tissue was filtered prior to assay.
Flow cytometry:
Flow cytometry was performed using BDFACSVerse or BD Fortessa X-20 instruments and analyzed using FlowJo software (BD). For extracellular staining, samples were suspended in FACS buffer (PBS + 2% FBS) and incubated with antibodies for 20 minutes. Transcription factor staining was conducted using the FoxP3/Transcription factor kit according to manufacturer’s instructions (eBioscience). A complete list of flow antibodies can be found in Supplementary Materials Table 1.
ELISA:
TRP-1 T cells used for ELISA kinetics were washed and plated each indicated day at 0.2×106 cells/200uL in fresh media and activated with 1μM TRP-1 peptide and feeder cells at a 1:2 feeder: T cell ratio for 18 hours. Supernatant was collected and frozen prior to analysis using DuoSet ELISA kits (R&D Systems) per manufacturer instructions.
Microarray
RNA isolation was conducted using Qiagen RNeasy Mini kit for both unactivated CD4+ TRP-1 T cells and expanded/polarized TRP-1 Th17 cells. TRP-1 CD4+ T cells were combined from 6 TRP-1 transgenic animals. Naïve CD4+ T cells were isolated using mouse CD4+ negative isolation kit (Dynabeads, Invitrogen) and used as baseline comparison. Th17 polarization was conducted as described previously for cells expanded 4, 7, and 12 days. RNA was sent to the University of Chicago genomics core and sequenced with Illumina MouseRef-8. Heatmaps were constructed in GraphPad Prism (v7.0) as (+/−) log2 fold change over baseline. Microarray data are available through GEO accession number GSE149330.
Statistical Analysis
Kaplan-Meier survival curves were compared between treatment group pairs using the log-rank test. Comparisons of continuous measures between two groups were made using Mann Whitney U test or Student’s two-tailed t test as indicated, dependent on whether t-test assumptions were met. Comparisons made were primarily between Th17 treatment groups (Day 4 vs Day 7, Day 4 vs Day 14). Given the data are exploratory in nature and it was desired not to be overly restrictive, tests performed were minimized and hypothesis tests were not adjusted for multiple comparisons. P values less than 0.05 were considered significant. Plots display mean values and error bars represent standard deviations.
Results
Th17 cells expanded four days demonstrate enhanced tumor immunity
Given the robust antitumor properties of CD4+ Th17 cells compared to Th1 or Th2 cells (9,10,12,15), we hypothesized that few Th17 cells obtained using a shortened expansion protocol could effectively treat melanoma. To address this question, CD4+ T cells expressing a TCR against tyrosinase-related protein 1 (TRP-1) were used (10). TRP-1 cells were polarized to a Th17 phenotype and expanded from as little time as 12 hours up to 14 days post TCR activation, where the latter time point models clinical protocols for rapid TIL expansion (16) (Fig. 1A). The therapeutic quality of the cell product acquired at each time was assessed, accounting for yield by dividing total number of cells at the end of culture equally among mice. This resulted in doses ranging from 10,000 to 10×106 cells/mouse. Th17 cells were infused into mice bearing established B16F10 tumors preconditioned with 5 Gy total body irradiation (TBI) (Fig. 1B).
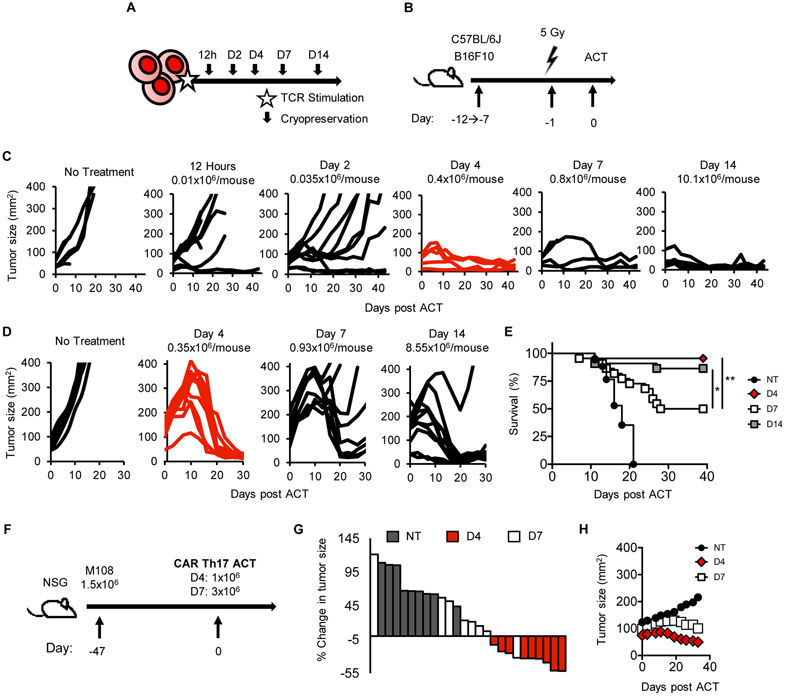
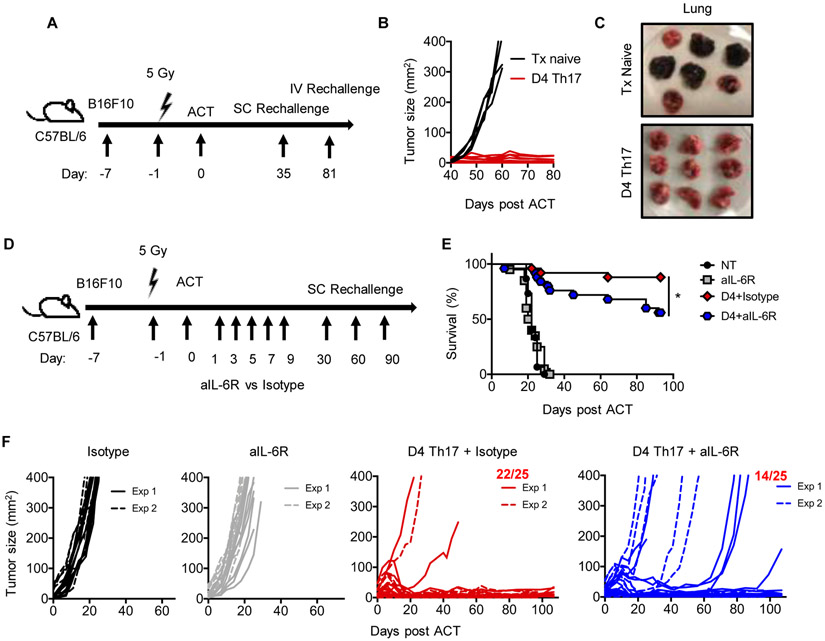
Figure 1: Four-day ex vivo expansion yields Th17 cells with potent antitumor efficacy.
A) TRP-1 Th17 cells were expanded from 12 hours to 14 days. B) ACT schematic. B16F10 tumors were established in mice from 7 days (tumor ~ 50mm2) to 12+ days (tumor ~ 140mm2). Animals were irradiated 5 Gy one day prior to ACT. C) Tumors established 7 days in mice were treated with Th17 cells. n=5-10 mice/group, representative of two experiments. D) Mice bearing B16F10 established 12 days were treated as in B) (n=10 mice/group, NT n=5). Representative of two experiments. E) Survival of mice treated in (D), combination of two experiments. n=22/group, n=17 NT. F-H) Human CD4+ T cells were polarized to Th17 phenotype and transduced with meso-CAR. F) Human M108 was inoculated 47 days pre-ACT. G) Waterfall plot relating tumor size 33 days post ACT to start. H) Serial measurements. n=8-9 mice/group, one experiment. Statistics: E) Log-rank test, *p<0.05, **p<0.01.
We discovered that four days was the minimum time after which Th17 cell products were able to eradicate tumors (Fig. 1C). A low number of Day 4 Th17 cells (0.4×106) could regress small tumors (approximately 50mm2) in mice as effectively as those treated with more Th17 cells expanded longer (Fig. 1C). Th17 cells expanded less than four days were unable to control melanoma growth.
We next questioned if mice with larger tumors could benefit from Day 4 Th17 cell therapy. We postulated that the low dose of cells yielded with four days of expansion would be insufficient to treat larger tumors (median ~140mm2). Despite administering low numbers of cells, Day 4 Th17 cells were still able to ablate large tumors (Fig. 1D). Unexpectedly, three-fold more Day 7 Th17 cells showed impaired antitumor responses and reduced survival (Fig. 1D-E). Day 14 Th17 cells were able to regress large tumors, but 25-fold more cells were infused and an extended expansion protocol was needed.
We next questioned whether our findings in murine Th17 cells would be recapitulated in human Th17 cells redirected with a CAR. To address this, human mesothelioma was established in NSG mice for a median tumor size of 100mm2 prior to treatment (Fig. 1F). Human Th17 cells were transduced with a 4-1BBζ anti-mesothelin CAR (13) and expanded for 4 or 7 days. We obtained sufficient T cells to infuse mice with 1×106 Day 4 CAR Th17 cells versus 3×106 Day 7 CAR Th17 cells (~30% transduction efficiency). Day 4 CAR Th17 cells regressed tumor in 9/10 animals (one mouse died mid-experiment). In contrast, Day 7 CAR Th17 cells regressed tumor in only one mouse (Fig. 1G-H). These findings reveal that Th17 cells expanded four days ex vivo elicit anti-tumor responses in both murine TCR and human CAR ACT models.
Four day expanded Th17 cells promote superior antitumor immunity on a per cell basis
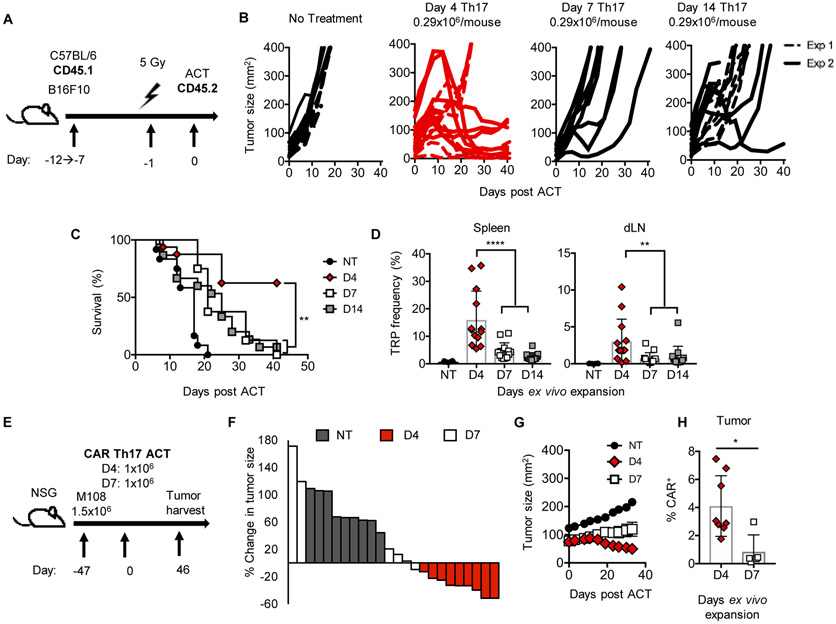
We posited that in equal number, Day 4 Th17 cells would control melanoma more effectively than Day 7 or Day 14 Th17 cells. To test this concept, we infused only ~0.3×106 Th17 cells from each group into lymphodepleted mice (Fig. 2A). At equal doses, Day 4 Th17 cells elicited greater antitumor responses and improved survival versus longer-expanded Th17 cells (Fig. 2B-C). Similar results were observed with higher doses spanning 0.8-10×106 cells/animal (Fig. S1A-B). Day 4 cells engrafted in higher frequencies (Fig. 2D) one week post transfer and persisted longer than Day 7 or Day 14 cells (Fig. S1C). Notably, we observed similar results in the human meso-CAR T cell model (Fig. 2E-H). At equal numbers, Day 4 CAR Th17 cells regressed tumors (Fig. 2F-G) and persisted more effectively at the tumor compared to Day 7 CAR Th17 cells (Fig. 2H). Collectively, our findings suggest that Day 4 Th17 cells possess greater antitumor activity with longer persistence compared to long-term expanded Th17 cells in two aggressive solid tumor models.
Figure 2: Four-day expanded Th17 cells have greater potency on a per-cell basis.
A) ACT schematic. B) Average dose of 0.29×106 TRP-1 Th17 cells were transferred. n=8 mice/group for each experiment. C) Survival of mice from experiments in B); results compiled from two experiments. D) Frequency of Th17 cells (VB14+CD45.2+) in spleen and tumor-draining lymph nodes (dLN) 7 days post treatment, n=13 mice/group, n=6 NT. Representative of two experiments. E-H) Human M108 tumors were established 47 days prior to ACT. F) Waterfall plot, percent change in tumor size day 33 versus starting tumor size. G) Serial measurements of animals treated in E). H) Frequency of CAR+ cells 46 days post transfer. n=6-9 mice/group from one experiment. NT no treatment. Statistics: C) Log-rank test, D,H) Mann-Whitney U test. ns, not significant, *p<0.05, **p<0.01, ****p<0.0001. Mean and standard deviation shown.
Day 4 Th17 cells exhibit an activated phenotype
We speculated that Th17 cells expanded for shorter duration would be less differentiated in vitro than Th17 cells expanded for one to two weeks. However, regardless of expansion time, these Th17 cells possessed an effector memory profile (CD44hiCD62Llo) in vitro (Fig. 3A). Gene array substantiated these findings, demonstrating low mRNA expression of Cd27 and Sell versus naïve CD4+ cells (Fig. 3B).
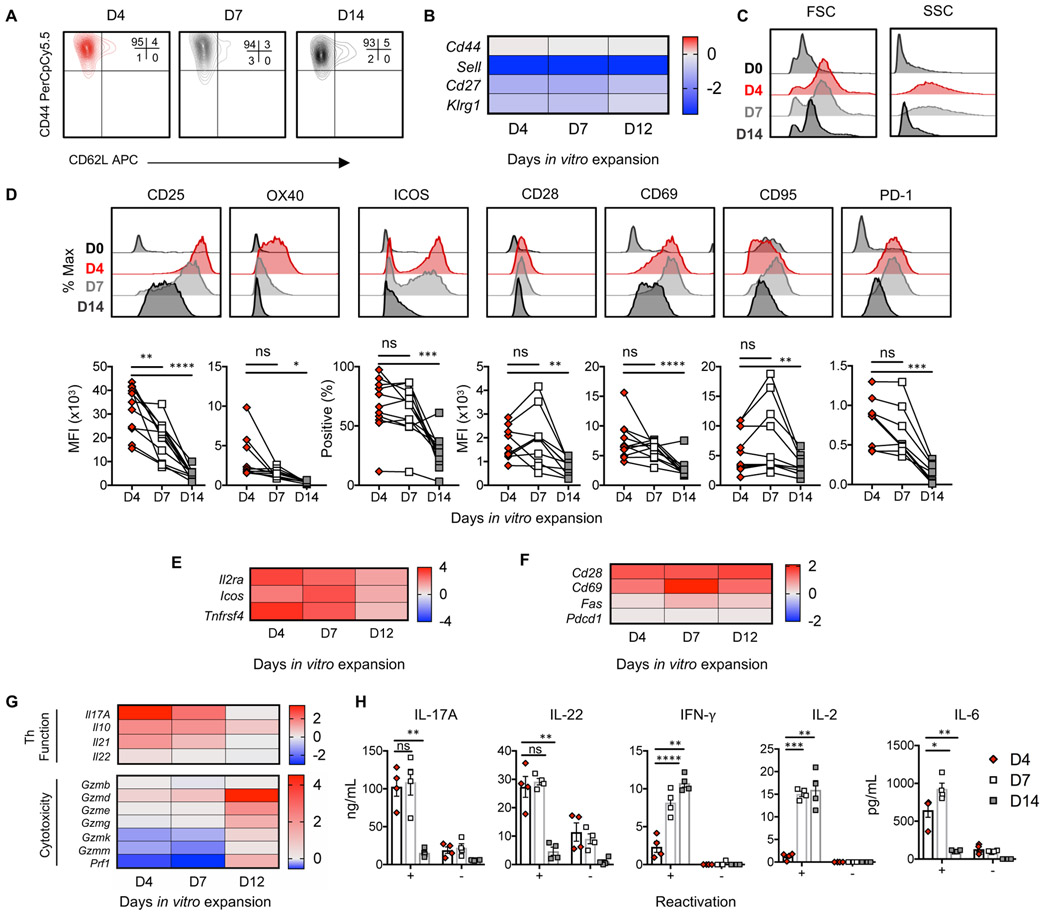
Figure 3: Day 4 Th17 cells exhibit an activated phenotype.
A) Memory phenotype of TRP-1 Th17 cells. n=7. B) Gene array heat maps display (+/−) log2 fold change of memory markers versus naïve TRP-1 CD4+ T cells compiled from 6 animals. C) FSC and SSC of TRP-1 cells prior to peptide activation/polarization (D0) versus 4, 7, and 14 days post-activation. n=7 cultures. D) Histograms and E-F) gene array depicting activation and costimulatory markers on TRP-1 Th17 cells. n=10-11 independent cultures, n=5 for PD-1. Connected lines indicate paired biological replicates. G) Heat map of (+/−) log2 fold change of mRNA expression versus naïve, unpolarized TRP-1 CD4+ T cells. Compiled from 6 animals. H) Cytokine production 18h post peptide stimulation. 4 independent cultures, representative of 2 experiments. Statistics: D,H) One-sample t test of differences. *p<0.05, **p<0.01, ***p<0.001. Mean and standard error shown in H.
Th17 cells expanded four days had a more blast-like phenotype than those expanded longer (Fig. 3C); therefore, we postulated these cells would be highly activated and functional. Four days after TCR activation, Th17 cells expressed high levels of CD25, ICOS, and OX40 which diminished over time (Fig 3D-E). CD28, CD69, CD95 and PD-1 expression similarly diminished with culture time (Fig. 3D, F). Th17 cells had a dynamic functionality during in vitro expansion; Day 4 Th17 cells produced copious IL-17A and IL-22 relative to Day 7 or Day 14 Th17 cells, while Day 7 and Day 14 cells expressed higher levels of IFN-γ and IL-2 (Fig. 3G-H). Day 4 and Day 7 Th17 cells both elicited IL-6 after peptide restimulation, which was detected at low levels after two weeks of expansion (Fig. 3H). Complementing IFN-γ production, Th17 cells expanded for more than a week had a cytotoxic profile expressing transcripts for granzymes and perforin (Fig. 3G). Our kinetic analysis underscores the heterogeneity of Th17 cells as they are expanded in vitro and supports prior literature concerning acquisition of cytotoxic properties by Th17 cells (9,11,17). Collectively, Th17 cells are highly activated and predominantly produce helper cytokines four days after stimulation, while longer in vitro expansion drives a cytotoxic cytokine repertoire.
Day 4 Th17 cells elicit systemic cytokine release
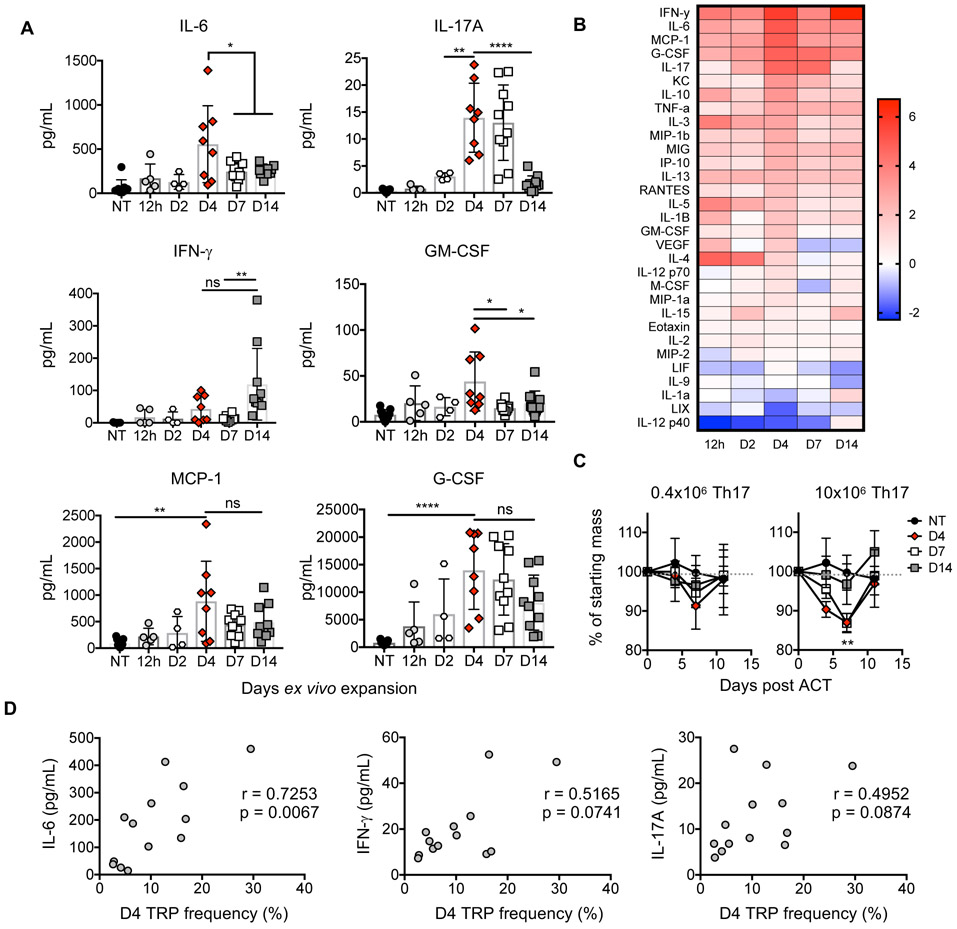
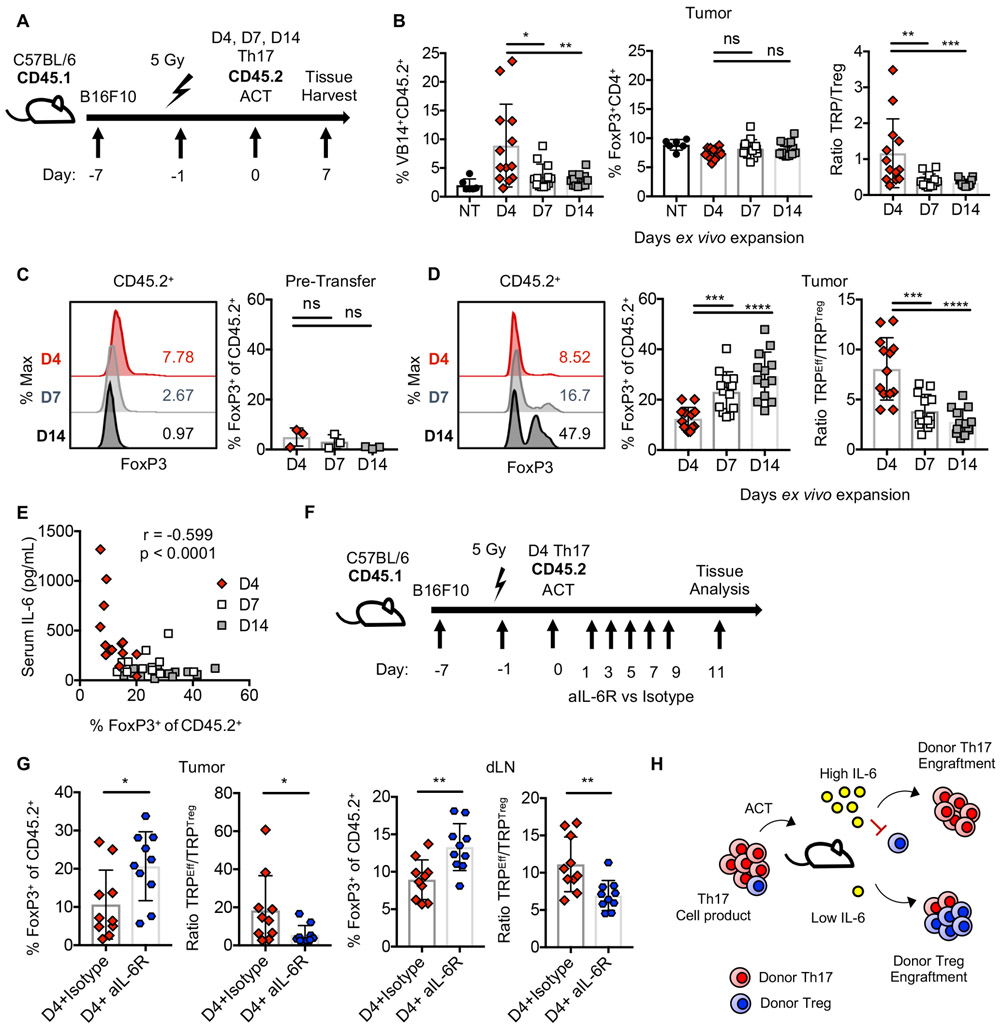
Since expanded Th17 cells have diverse ability to produce cytokines, we suspected that the cytokine profiles in the blood of treated mice would be distinct comparing Th17 cell therapies. Therefore, we transferred TRP-1 Th17 cells expanded from 12 hours to 14 days into mice with B16F10 melanoma (as in Fig. 1A) and assessed serum cytokine concentration one-week post ACT. Day 4 Th17 cells not only secreted more IL-17 but also induced heightened expression of multiple factors within the host including IL-6, MCP-1, G-CSF, and GM-CSF (Fig. 4A-B). Strikingly, this inflammatory response resulted from transfer of only 0.4×106 Day 4 Th17 cells, while in contrast, the cytokine response was diminished in animals treated with 25-fold more Day 14 Th17 cells (Fig. 4B). Consistent with an acute inflammatory response, animals treated with few Day 4 Th17 cells experienced 10% weight loss in the first week post ACT, and up to 15% weight loss with a higher dose of cells but quickly returned to baseline approximately 3 days later (Fig. 4C). Notably, IL-6 correlated with engraftment of Day 4 Th17 cells, while IL-17 and IFN-γ did not correlate with donor cell frequency (Fig. 4D).
Figure 4. Day 4 Th17 cells elicit robust cytokine release in tumor-bearing animals.
A) Tumor-bearing mice were treated as in Fig. 1A-B. Serum cytokines one-week post transfer. n=4-10 mice/group, two independent experiments. B) Heat map of (+/−) log2 fold change serum cytokine levels versus untreated animals (NT). Average from one experiment, n=4-5 mice/group. C) Percent of starting mass of animals. p<0.01 for D4 and D7 versus D14 at 10×106 cells/mouse. n=5 animals/group from one experiment, n=10 for NT. D) Correlation one-week post transfer, n=13 mice compiled from two experiments. Statistics: A,C) Student’s two-tailed t test, D) Spearman correlation. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Mean and standard deviation shown.
Th17 programming was important to cytokine induction as unpolarized CD4+ T cells (Th0) expanded four days did not induce IL-6 or other cytokines in mice (Fig. S2A-B). Yet, IL-17 was not required for antitumor responses unlike IFN-γ, as reported previously (10) (Fig. S2C-I). Overall, few Day 4 Th17 cells induce robust cytokine release in mice versus ample Th17 cells expanded longer or Th0 cells expanded four days, and concentration of IL-6 in circulation correlated with engraftment of donor Th17 cells.
CD4+ T cells are required for sustained antitumor responses
We reproducibly observed that Day 4 Th17 cells elicited durable and long-term antitumor responses in mice (Fig. 5, also see Fig. S1-5). We next addressed if mice previously cured with this therapy were protected against tumor rechallenge (Fig. 5A). While tumors grew to endpoint in control treatment-naïve animals, mice cured with Day 4 Th17 cells mounted rapid recall responses against secondary tumor (Fig. 5B). These mice were also protected against a pseudo-metastatic B16F10 injected intravascularly, which readily establishes in the lungs (Fig. 5C). Therefore, Day 4 Th17 cell therapy conferred both local and systemic memory against B16F10 tumor, permitting host protection of more than 100 days.
Figure 5. IL-6 fuels long-lived Th17 cell-mediated antitumor responses.
A) ACT sequence. 0.4×106 Day 4 TRP-1 Th17 cells were transferred into animals with B16F10 tumors; animals were rechallenged with B16F10 tumor. B) Tumor response post rechallenge of 0.5×106 B16F10 cells. Treatment (Tx) naïve, n=5; D4 Th17, n=9 from one experiment. C) Day 105 after IV injection of 0.2×106 B16F10 tumor cells on day 81. Tx naïve, n=7; D4 Th17, n=9 from one experiment. D-F) Schematic for ACT of 0.4×106 Day 4 TRP-1 Th17 cells. E) Survival and F) tumor curves of animals treated in D); two independent experiments combined. No treatment n=15, aIL-6R n=20, D4 Th17+Isotype/aIL-6R n=25. Statistics: E) Log-rank test; *p<0.05.
Since Day 4 Th17 cells induced multiple cytokines and expressed more chemokine transcripts than Day 7 or Day 14 Th17 cells (Fig. S3A), we next posited that Day 4 Th17 cells may orchestrate host lymphocytes to regress tumors. Yet, host cells, including CD8+, CD4+, and B cells, infiltrated the tumor at similar frequencies regardless of Th17 cell therapy (Fig. S3B). As Th17 cells can cooperate with CD8+ T cells (18), we depleted CD8+ T cells to determine whether Day 4 Th17 cells could influence pre-existing CD8+ T cell immunity to melanoma. CD8+ T cells were not required for tumor regression (Fig. S3C-E) or to protect against tumor rechallenge (Fig. S3F-G). Instead, depletion of CD4+ T cells resulted in relapse upon rechallenge (Fig. S3F, H-I), supporting that donor CD4+Th17 cells are more critical for durable treatment outcome.
As few Day 4 Th17 cells regress tumors unlike Day 7 or Day 14 Th17 cells, we posited that Day 4 Th17 cells may more rapidly acquire T resident memory (TRM) phenotypes in the tumor (Fig. S4A-B). Just prior to tumor regression, however, less than 1% of Th17 cells expressed CD103, CD69, or CLA (Fig. S4B). Although TRM phenotypes were not prominent early after transfer, we discovered that donor CD4+ cells persisting long term (>100 days) in animals cured with Day 4 Th17 cell therapy expressed TRM phenotypes at higher levels in the skin relative to cells persisting elsewhere (Fig. S4C-F). Therefore, Day 4 Th17 cells—able to clear tumor at low doses—persisted at the tumor site and expressed a surface signature resembling skin TRM cells (14).
IL-6 promotes long-term tumor immunity
The mechanism behind durable immunity elicited by Day 4 Th17 cells remained unknown. As Day 4 Th17 cells induced elevated IL-6 relative to long-term expanded Th17 cells, we hypothesized that in vivo, IL-6 may be important for their efficacy. After transfer of Day 4 Th17 cells, we blocked IL-6 with either an IL-6R antibody or IL-6 neutralizing antibody (Fig. 5D, S5A). Note that IL-6 induction was only transient, thus antibody was administered up to 10 days post ACT (Fig. S6). Nearly all mice cured with Day 4 Th17 cells survived relapse free (Fig. 5E-F). However, IL-6R blockade significantly impaired long-term immunity as indicated by a higher incidence of tumor relapse and reduced overall survival (Fig. 5E-F). Similar results were obtained with IL-6 neutralization (Fig. S5). IL-6 blockade did not significantly impact release of other cytokines, nor did blockade greatly mitigate acute weight loss (Fig. S6). Our results reveal, for the first time, that IL-6 promotes durable and long-lived Th17-mediated tumor immunity for ACT.
Systemic IL-6 dampens engraftment of donor T regulatory cells
IL-6 is important for the differentiation of CD4+ T cells to the Th17 phenotype (19). Additionally, IL-6 can reprogram Tregs towards a Th17 signature by downregulating FoxP3 (20). Based on this foundational work, we posited that IL-6 may preferentially maintain Th17 biology while blunting the outgrowth of Tregs in the tumor.
We first assessed the overall frequency of donor Th17 cells and Tregs in the tumor after treatment with Th17 cells expanded 4, 7, or 14 days (Fig. 6A). Day 4 Th17 cells were present in the highest frequency at the tumor; yet, the frequency of host Tregs was similar among groups (Fig. 6B). This finding corresponded with the highest ratio of Th17 to Tregs at the tumor for Day 4 Th17 cell therapies (Fig. 6B), but suggested that host Tregs may not be implicated in relapse for Day 7 or Day 14 cell therapies.
Figure 6. IL-6 drives enrichment of donor Th17 over Treg cells at the tumor.
A) Schematic for ACT of 0.4×106 Th17 cells. B) Tumor infiltration by donor (VB14+CD45.2+) cells or host Tregs one-week post ACT. NT, n=6; treated n=13/group from one experiment. C) FoxP3 expression in donor Th17 product in vitro. 3 independent cultures. D) FoxP3+ cells within donor populations 7 days post ACT. n=13/group from one experiment. E) Tumor FoxP3+ donor cells versus serum IL-6 7 days post ACT. n=39 mice, representative of two experiments. F) ACT of 0.4×106 Day 4 Th17 cells. Anti-IL-6R or isotype control were administered. G) Frequency of FoxP3+ cells within donor populations. n=10mice/group. H) Proposed model. Statistics: B, C, D, G) Mann Whitney U test, E) Spearman correlation. ns: not significant, *p<0.05, **p<0.01, ***p<0.001.
However, we discovered that a small fraction of tumor-specific Tregs remain in the Th17 cell product prior to transfer (Fig. 6C). Thus, we hypothesized that IL-6 may promote the engraftment of donor Th17 cells over donor Tregs. While Day 4 Th17 cell products may have a slightly greater frequency of FoxP3+ cells in vitro, this relationship was the opposite in vivo (Fig. 6D). Specifically, donor products expanded for one or two-weeks had a greater frequency of FoxP3+ cells infiltrating the tumor (Fig. 6D). This corresponded with a heightened ratio of TRP effector to regulatory cells in tumors for Day 4 Th17 cell therapies (Fig. 6D). Interestingly, the frequency of FoxP3+ donor cells at the tumor was inversely correlated with serum IL-6 (Fig. 6E). Therefore, we next addressed whether IL-6 was modulating the balance of Th17 and Treg donor cells (Fig. 6F). In both the tumor and tumor-draining lymph nodes, IL-6 blockade augmented the frequency of FoxP3+ donor TILs (Fig. 6G). Collectively, we propose a model where IL-6 limits the engraftment of donor Treg populations, enabling Th17 cells to thrive post transfer (Fig. 6H).
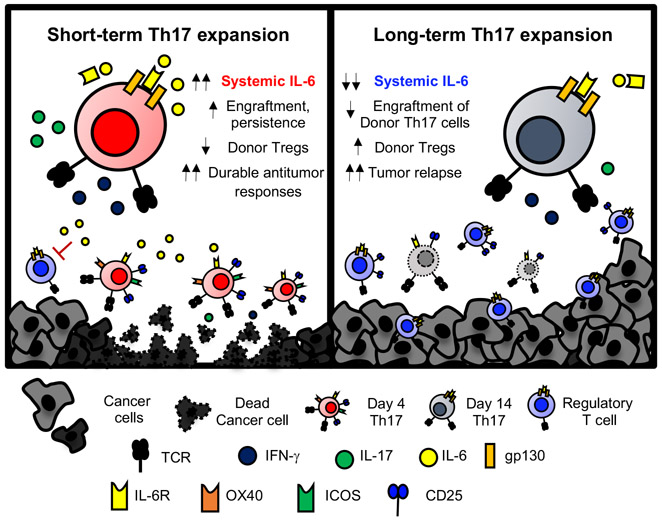
In summary, few Th17 cells—expanded only four days ex vivo—confer durable and long-term immunity, while Th17 cells expanded longer lose individual potency against solid tumors (Fig. 7). Short-term expanded Th17 cells possessed a heightened ability to engraft and persist in a transiently lymphodepleted host. Efficacy of this therapy appeared to be direct, as depletion of CD4+ but not CD8+ T cells compromised tumor control. Intriguingly, IL-6 was critical for long-term resistance to tumor relapse. IL-6 diminished engraftment of donor Tregs in the cellular product, promoting robust engraftment of cytotoxic donor Th17 cells. These findings shed a new perspective on the number of cells needed to ablate large tumors and highlight an unappreciated role for IL-6 in fostering long-lived protective immunity to tumors.
Figure 7: Th17 cells expanded only four days ex vivo confer durable antitumor responses via induction of IL-6.
Shortened ex vivo expansion of Th17 cells for ACT licenses a proinflammatory and therapeutic cell product. Th17 cells expanded only four days induce IL-6 in tumor-bearing animals, which promotes durable protection against tumor rechallenge via supporting donor Th17 over Tregs at the tumor.
Discussion
ACT therapy mediates remarkable responses in patients with aggressive cancers; yet, accessibility is limited due to its personalized nature and the advanced technology required to produce a cellular product (21). Ex vivo TIL expansion requires at least one month to obtain a product suitable for rapid expansion to large magnitudes over an additional several weeks (16). Such a treatment delay for patients with aggressive disease can limit the feasibility of this approach. CAR generation via peripheral T cell modification is naturally quicker taking up to two weeks, although generating CAR T cell products that are consistently effective against solid tumors has been challenging (2). Our data suggest that shortening ex vivo expansion licenses enhanced antitumor efficacy against solid tumors for either CAR or TIL therapies using a highly potent T cell subset: the Th17 cell. Short-term expansion of this lymphocyte population directly elicited prolonged responses against solid tumors in part by driving heightened immune activation and release of IL-6 in the host, enriching effector Th17 over Treg cells in the tumor. As briefly expanded Th17 cells could overcome some limitations of ACT, our team is designing an early-phase clinical study for safety and efficacy of this therapy.
Th17 cells play a controversial role in tumor immunity, mediating either pro-tumor or anti-tumor responses in patients, which likely depends on their ability to recognize tumor antigens or their interaction with host immune cells (22). Indeed, given the induction of chemokines and cytokines by early Th17 cells, it is possible that Th17 cells orchestrate host immune cells with either pro- or anti-tumor function. Endogenous Th17 cells have been associated with myeloid-derived suppressor cells (MDSCs), where either MDSCs can promote Th17 polarization via IL-6/IL-23/IL-1β and nitric oxide (23), or where Th17 cells can bolster the suppressive nature of MDSCs, thereby fueling cancer growth (24). IL-6 is also a key regulator of MDSC mobilization, and in the chronic setting, drives carcinogenesis (25). ACT of early Th17 cells might imply crosstalk with MDSCs particularly given IL-6 induction in the host; yet, the transient nature of peak IL-6 coupled with tumor eradication imply, at least in this context, that Th17 cells or IL-6 are not likely influencing MDSCs to foster tumor growth.
In terms of immunity, transferred CD4+ T cells can be more efficacious at tumor clearance than CD8+ T cells owing to their ability to cooperate with other immune cells (26). Tumor-specific Th17 cells elicit robust immunity compared to Th1 or Th2 cohorts, partially due to their stem-like features and self-renewal properties which permit long-term persistence (9-12). Th17 cells are plastic and can evolve into Th1-like cells, expressing IFN-γ, a property deemed critical to their antitumor efficacy (9,10). Th17 cells also promote cytotoxic T cell activity against tumors (18). Here, we showed that briefly-expanded Th17 cells could directly control tumors, while CD8+ T cells were not required for primary immunity or protection against tumor rechallenge. Yet, our work does not rule out that other host cells—including NK cells, B cells or granulocytes among others—are not contributing to the treatment outcome. A comprehensive set of studies defining how tumor-specific Th17 cells interact with host immune cells should be conducted and may clarify the debate on pro- versus anti-tumor effects of Th17 cells.
Our work is the first to show that shortening Th17 ex vivo expansion bolsters in vivo antitumor efficacy via induction of IL-6 in the host, which acts to dampen the regulatory characteristics of the transferred cells while instilling their durable antitumor properties. Preclinical models evaluating the role of IL-6 in ACT are lacking; as efficacy of human CAR T cells are explored in immunodeficient mice, these systems either fail to induce IL-6 or require additional manipulation to promote its release (27-29). Therefore, our observation of multiple cytokine release in an animal with an intact immune system is important and clinically relevant given that autologous transferred cells can interact with host immune cells.
However, stimulating a robust immune response with a cell product may come with risks via toxicities. We remain intrigued that few Day 4 Th17 cells could induce high levels of cytokines in the host, reminiscent of clinical cytokine release syndrome (CRS). CRS, seen in patients receiving cell or antibody-based cancer therapies, corresponds with heightened IL-6, IFN-γ, IL-10, GM-CSF, G-CSF, IL-2, and MCP-1 (among others) in circulation, and presents with flu-like to life-threatening symptoms (30). To circumvent toxicity, physicians commonly use tociluzumab, an FDA approved monoclonal antibody targeting the IL-6 receptor, aiming to mitigate adverse events while putatively preserving productive antitumor immunity.
CRS is a common thread relating many T cell engaging immunotherapies, yet the role of IL-6 in anti-tumor responses is debated (30-33). Blocking IL-6 can improve tumor regression in combination with anti-PD-1/PD-L1 checkpoint inhibition immunotherapies (34). Conversely, IL-6 induction has been reported in therapeutic preclinical TCR-specific ACT models (35,36) and clinically is associated with enhanced CAR T cell expansion (31,37). In fact, CAR T cells of responsive patients possessed a STAT3 signature associated with IL-6, IL-17, and IL-22 production (37). IL-6 is currently considered dispensable for cell therapy as patients who receive tocilizumab for CRS may still experience complete responses, though prospective studies directly addressing this question are lacking (38,39). Notably, IL-6 promotes CD4+ T cell memory responses to influenza virus (40,41) and suppresses development of antigen-specific Treg cells post vaccination (42), two qualities which could contribute to long-lived immunity and could be desirable in a cell therapy setting.
Our report of systemic IL-6 release is novel to Th17 ACT therapy, and importantly, we describe a role for IL-6 in regulating the balance between Th17 and Treg cells to promote durable antitumor activity. The programs for Th17 cells and Tregs are closely regulated to control immune homeostasis (19,43). IL-6 and TGF-β are critical mediators of Th17 differentiation from naïve CD4+ T cells (19,44). TGF-β alone promotes FoxP3 expression in naïve CD4+ T cells (45); yet, IL-6 can downregulate FoxP3 via activating STAT3 and promoting RORγt to drive the Th17 program (19). We discovered that IL-6, induced by Day 4 Th17 cells but not by Th17 cells expanded longer-term, was required to elicit durable resistance to tumor relapse. IL-6 was the critical mediator which diminished engraftment of donor tumor-specific Tregs from the cell product, thereby promoting a more inflammatory tumor environment. These findings harmonize with other reports showing that IL-6 promotes Th17 immunity via suppression of Treg cells (42). We further reveal that early Th17 cells persist in the skin of animals after melanoma clearance, and these cells acquire phenotypes reminiscent of TRM cells. However, it remains unknown if IL-6 plays a role in acquisition of this particular memory phenotype. Our findings are clinically meaningful as they uncover that IL-6 imprints infused antitumor Th17 cells with long-lived and protective responses against metastasis and aggressively growing solid malignancies.
Previous reports indicate that reduction of ex vivo expansion time for CAR CD8+ T cells improves their efficacy against CD19+ malignancies (46). Our work broadens applicability of this concept by demonstrating that few CD4+ T cells can ablate hard-to-treat solid tumors and highlights a previously unappreciated role for cell therapy-induced IL-6 in generating long-lasting memory responses to cancer. Yet, the efficacy of antitumor Th17 cells has yet to be explored in clinical trials. Theoretically, polarizing cytokines could be used to generate these cells or naturally-arising Th17 cells (CD4+CCR4+CCR6+ T cells) (47) could be isolated to prepare TIL, TCR, or CAR T cell products. Given our observation of high systemic IL-6 in this model of Th17 cell therapy, cytokine levels should be monitored closely in patients.
Since we describe a beneficial role for IL-6 in Th17 cell therapy, yet acknowledge that IL-6 blockade protects patients against toxicity, we advocate for methods where IL-6 could be leveraged locally to foster durable immunity without compromising the patient’s health. More broadly, understanding how IL-6 directs memory responses for other cancer immunotherapies is important to improve patient outcomes. Studies addressing timing of IL-6 blockade or levels of IL-6 which are harmful rather than immunologically beneficial are warranted. Targeting other cytokines, like IL-1 (28,29) MCP-1, or GM-CSF could differentially affect toxicity or efficacy, thus studies on the influence of specific cytokines on ACT outcomes should be conducted.
Moving forward, it is important to consider the impact of ACT expansion protocols on treatment outcomes in cancer patients. While cellular therapies have been administered for decades, clinical protocols have not adopted shorter expansion methods (3,4,6). The approach detailed herein rapidly generates potent antitumor T cells despite lower yield, which could improve feasibility for treating patients with aggressive disease. Our findings have implications for translating ACT therapies to patients worldwide.
Supplementary Material
Statement of Significance: An abbreviated, four-day ex vivo expansion method licenses Th17 cells to confer long-lived immunity against solid malignancies via induction of systemic IL-6 in tumor-bearing hosts.
Acknowledgements:
We thank our collaborators for their critical feedback; Jeff Hammerbacher, Arman Aksoy, Pinar Aksoy, Elinor Gottschalk, Dimitri Arhontoulis, Amalia Rivera-Reyes, and Reilley Chamness. We acknowledge Michael Zillox for assistance in microarray analysis.
Funding: This work was supported by NCI F30 CA243307, NIH T32 GM008716 and DE017551, and Melanoma Research Foundation (HMK); NIH T32 AI132164-01 (CJD); Hollings Cancer Center Graduate Fellowship (ASS); NIH F30 CA200272 and T32 GM008716 (JSB); NIH R50 CA233186 (MMW); NCI R01 CA222817 (MPR); NCI R01 CA175061, R01 CA208514 (CMP); and P30 CA138313 to the Cell Evaluation & Therapy Shared Resource, Hollings Cancer Center, Medical University of South Carolina.
Footnotes
Conflict of interest: HK and CP have a provisional patent regarding short term expansion of Th17 cells for adoptive transfer therapy. The remaining authors declare no competing interests.
Data and materials availability: All data are available upon reasonable request. Microarray data are available through GEO Accession number: GSE149330.
References:
- 1.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368(16):1509–18 doi 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knochelmann HM, Smith AS, Dwyer CJ, Wyatt MM, Mehrotra S, Paulos CM. CAR T Cells in Solid Tumors: Blueprints for Building Effective Therapies. Frontiers in Immunology 2018;9 doi 10.3389/fimmu.2018.01740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T Cell Transfer Immunotherapy. Clin Cancer Res 2011;17(13):4550–7 doi 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zacharakis N, Chinnasamy H, Black M, Xu H, Lu YC, Zheng Z, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med 2018. doi 10.1038/s41591-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran EH, Turcotte S, Gros A, Robbins P, Lu Y, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344:641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clinical cancer research : an official journal of the American Association for Cancer Research 2012;18(24):6758–70 doi 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res 2010;16(9):2646–55 doi 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 8.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest 2005;115(6):1616–26 doi 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 2011;35(6):972–85 doi 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muranski P, Boni A, Antony P, Cassard L, Irvine K, Kaiser AD, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 2008;112(2):362–72 doi 10.1182/blood-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowers JS, Nelson MH, Majchrzak K, Bailey SR, Rohrer B, Kaiser AD, et al. Th17 cells are refractory to senescence and retain robust antitumor activity after long-term ex vivo expansion. JCI Insight 2017;2(5):e90772 doi 10.1172/jci.insight.90772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med 2011;3(104):104ra0 doi 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A 2009;106(9):3360–5 doi 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Science Immunology 2017;2(10):eaam6346 doi 10.1126/sciimmunol.aam6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulos CM, Carpenito C, Plesa G, Suhoski MM, Varela-Rohena A, Golovina TN, et al. The Inducible Costimulator (ICOS) Is Critical for the Development of Human Th17 Cells. Science Translational Medicine 2010;2(55). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudley M, Wunderlich J, Shelton T, Even J, Rosenberg S. Generation of Tumor-Infiltrating Lymphocyte Cultures for Use in Adoptive Transfer Therapy for Melanoma Patients. J Immunother 2003;26(4):332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowers JS, Nelson MH, Kundimi S, Bailey SR, Huff LW, Schwartz KM, et al. Dendritic Cells in Irradiated Mice Trigger the Functional Plasticity and Antitumor Activity of Adoptively Transferred Tc17 Cells via IL12 Signaling. Clin Cancer Res 2015;21(11):2546–57 doi 10.1158/1078-0432.CCR-14-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009;31(5):787–98 doi 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441(7090):235–8 doi 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 20.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 2008;29(1):44–56 doi 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine B, June C. Assembly line immunotherapy. Nature 2013;498:S17. [DOI] [PubMed] [Google Scholar]
- 22.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nature reviews Immunology 2010;10(4):248–56 doi 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obermajer N, Wong JL, Edwards RP, Chen K, Scott M, Khader S, et al. Induction and stability of human Th17 cells require endogenous NOS2 and cGMP-dependent NO signaling. Journal of Experimental Medicine 2013;210(7):1433–45 doi 10.1084/jem.20121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M, et al. TGF-β Receptor II Loss Promotes Mammary Carcinoma Progression by Th17-Dependent Mechanisms. Cancer Discovery 2011;1(5):430 doi 10.1158/2159-8290.CD-11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proceedings of the National Academy of Sciences 2011;108(41):17111 doi 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Diez A, Joncker NT, Choi K, Chan WFN, Anderson CC, Lantz O, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007;109(12):5346–54 doi 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Stegen SJC, Davies DM, Wilkie S, Foster J, Sosabowski JK, Burnet J, et al. Preclinical In Vivo Modeling of Cytokine Release Syndrome Induced by ErbB-Retargeted Human T Cells: Identifying a Window of Therapeutic Opportunity? The Journal of Immunology 2013;191(9):4589 doi 10.4049/jimmunol.1301523. [DOI] [PubMed] [Google Scholar]
- 28.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nature Medicine 2018;24(6):731–8 doi 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nature Medicine 2018;24(6):739–48 doi 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 30.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASBMT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biology of Blood and Marrow Transplantation 2018. doi 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maude S, Frey N, Shaw P, Aplenc R, Barrett D, Bunin N, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. New England Journal of Medicine 2014;371(16):1507–17 doi 0.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obstfeld A, Frey N, Mansfield K, Lacey S, June C, Porter D, et al. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: clinicopathological insights Blood 2017;130(23):2569–72. [DOI] [PubMed] [Google Scholar]
- 33.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and Toxicity Management of 19–28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Science Translational Medicine 2014;6(224):224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018;67(2):320 doi 10.1136/gutjnl-2016-311585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother 2010;33(1):1–7 doi 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. Journal of Clinical Investigation 2007;117(8):2197–204 doi 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018;24(5):563–71 doi 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer journal (Sudbury, Mass) 2014;20(2):119–22 doi 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh N, Hofmann TJ, Gershenson Z, Levine BL, Grupp SA, Teachey DT, et al. Monocyte lineage-derived IL-6 does not affect chimeric antigen receptor T-cell function. Cytotherapy 2017;19(7):867–80 doi 10.1016/j.jcyt.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longhi MP, Wright K, Lauder SN, Nowell MA, Jones GW, Godkin AJ, et al. Interleukin-6 Is Crucial for Recall of Influenza-Specific Memory CD4+ T Cells. PLOS Pathogens 2008;4(2):e1000006 doi 10.1371/journal.ppat.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nish SA, Schenten D, Wunderlich FT, Pope SD, Gao Y, Hoshi N, et al. T cell-intrinsic role of IL-6 signaling in primary and memory responses. eLife 2014;3:e01949–e doi 10.7554/eLife.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proceedings of the National Academy of Sciences 2008;105(47):18460 doi 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, Mazza-McCrann JM, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cellular & Molecular Immunology 2018. doi 10.1038/s41423-018-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou L, Ivanov I, Sploski R, Min R, Shenderoc K, Egwa T, et al. IL-6 programs Th1–7 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunology 2007;8(9):967–74. [DOI] [PubMed] [Google Scholar]
- 45.Chen W, Jin W, Hardegen N, Lei K-J, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of experimental medicine 2003;198(12):1875–86 doi 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghassemi S, Nunez-Cruz S, Connor RS, Fraietta JA, Patel PR, Scholler J, et al. Reducing Ex Vivo Culture Improves the Anti-leukemic Activity of Chimeric Antigen Receptor (CAR)-T Cells. Cancer Immunology Research 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 2007;8(6):639–46 doi 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.