Abstract
Background.
Axillary lymph node dissection (ALND) can be avoided in node-positive patients who receive neoadjuvant chemotherapy (NAC) if ≥3 negative sentinel lymph nodes (SLNs) are retrieved. We evaluate how often node-positive patients avoid ALND with NAC, and identify predictors of identification of ≥3 SLNs and of nodal pathological complete response (pCR).
Methods.
From 11/2013–07/2019, all patients with cT1–3, biopsy-proven N1 tumors who converted to cN0 after NAC received sentinel lymph node biopsy (SLNB) with dual mapping and were identified from a prospectively maintained database.
Results.
630 consecutive N1 patients were eligible for axillary downstaging with NAC; 573 (91%) converted to cN0 and had SLNB. 531 patients (93%) had ≥3 SLNs identified. Lymphovascular invasion (OR 0.46, 95% CI 0.24–0.87,p=0.02) and increasing BMI (OR 0.77, 95% CI 0.62–0.96 per 5-unit increase,p=0.02) were significantly associated with failure to identify ≥3 SLNs. 255/573 (46%) patients achieved nodal pCR; 237 (41%) had adequate mapping. Factors associated with ALND avoidance included high grade (OR 2.51, 95% CI 1.6–3.94,p=0.001) and receptor status (HR+/HER2− [referent]: OR 1.99, 95% CI 1.15–3.46 [p=0.01] for HR−/HER2−, OR 3.93, 95% CI 2.40–6.44 [p<0.001] for HR+/HER2+, and OR 8.24, 95% CI 4.16–16.3 [p<0.001] for HR−/HER2+). LVI was associated with lower likelihood of avoiding ALND (OR 0.28, 95% CI 0.18–0.43,p<0.001).
Conclusions.
ALND was avoided in 41% of cN1 patients after NAC. Increased BMI and LVI were associated with lower retrieval rates of ≥3 SLNs. ALND avoidance rates varied with receptor status, grade, and LVI. These factors help select patients most likely to avoid ALND.
Keywords: breast cancer, neoadjuvant chemotherapy, axillary downstaging, node-positive patients
Indications for neoadjuvant chemotherapy (NAC) have evolved over time.1 Currently, in patients who are clinically node-positive at presentation, NAC is given with the aim of achieving nodal pathological complete response (pCR) and de-escalating axillary surgery.2–5 The rate of nodal response depends on tumor biology, with lower rates in hormone receptor-positive (HR+)/HER2− tumors and higher rates in triple negative (TN) and HER2 positive (HER2+) tumors.3,6,7
Patients who become clinically node-negative (cN0) after NAC are eligible for sentinel lymph node biopsy (SLNB). Four prospective multi-institutional trials have examined the accuracy of SLNB after NAC in patients who were clinically node-positive at presentation.8–11 The sentinel lymph node (SLN) identification rate in these trials ranged from 79.5% to 92.7%, and the false-negative rate (FNR) ranged from 11.9% to 14.2%, exceeding the 10% threshold considered to be clinically acceptable. All trials consistently showed that the accuracy of SLNB increased with the number of SLNs retrieved, and when 3 or more SLNs were removed and dual tracer mapping was used, the FNRs were uniformly less than 10%.8–12 Since the publication of these trials, the use of SLNB after NAC for node-positive patients at presentation has increased13–16, but little information is available on how often ALND is avoided with the use of NAC in patients presenting with nodal metastases. A previous report from our institution described our experience with 128 consecutive node-positive patients receiving NAC and found that 48% had identification of ≥ 3 SLNs and nodal pathologic complete response (pCR), and were able to avoid ALND3. In this study, we sought to confirm this finding in a larger cohort of node-positive patients receiving NAC and to identify clinicopathological factors associated with finding 3 or more SLNs and achieving nodal pCR, the two mandatory conditions required to safely avoid ALND.
METHODS
Beginning in 2013, clinically node-positive patients with biopsy-proven metastases treated at our institution received NAC with the intention of downstaging the axilla to avoid ALND. Those who presented with cT1–3 N1 disease, who converted to cN0 on physical exam, were eligible for SLNB. Sonographic evaluation of the axilla after NAC was not routine, and nodal clipping was not routinely employed. In patients presenting with clips placed in metastatic nodes elsewhere, retrieval of the clipped node was not required. Clinical T4 and cN2/3 patients were considered ineligible for SLNB irrespective of their response to NAC. SLNB was performed with dual tracer (technetium-99m sulfur colloid and isosulfan blue dye) in all patients. Sentinel nodes were defined as hot, blue, or palpably abnormal nodes. Based on the results of clinical trials demonstrating false-negative rates of < 10% with retrieval of 3 or more SLNs, ALND was omitted, if 3 or more SLNs were identified (adequate mapping) and nodal pCR in the SLNs was achieved.
Frozen section of the lymph nodes was performed intraoperatively followed by routine histological assessment; immunohistochemistry was not routinely performed. Macrometastases, micrometastases, and isolated tumor cells in the SLN post-NAC were all considered indications for ALND.
After institutional board approval, we queried our prospectively maintained database to identify consecutive stage II-III breast cancer patients with biopsy-proven nodal metastasis at presentation. Patients with a prior history of ipsilateral breast cancer were excluded. The majority of patients (85%) received dose-dense anthracycline and taxane-based chemotherapy regimens, 6% received concomitant platinum salts, and all HER2+ patients received trastuzumab and pertuzumab. Of patients who converted to cN0 and had SLNB, we compared those who had 3 or more SLNs identified (adequate mapping) with those who did not (inadequate mapping). We also compared those who had 3 or more SLNs and nodal pCR who avoided ALND versus all others. Demographic and clinical characteristics were compared between groups using Fisher’s exact test for categorical variables, and the Wilcoxon rank-sum test for continuous variables. Univariate and multivariate logistic regression was used to estimate the odds of finding 3 or more SLNs, and the odds of finding 3 or more SLNs and having nodal pCR. All tests were evaluated for statistical significance at alpha level 0.05. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Between November 2013 and July 2019, 791 biopsy-proven node-positive patients were treated with NAC; 630 patients had cN1 disease and were eligible for downstaging to SLNB. Of these, 573 (91%) converted to cN0 and had SLNB, and comprised the primary study group (Fig. 1). Table 1 shows the clinicopathological characteristics of the entire cohort and compares patients with ≥ 3 SLNs identified vs those with < 3 SLNs identified, as well as patients who were spared ALND vs those who were not. Median patient age was 49 years (range 24–82 years), and median body mass index (BMI) was 25.8 kg/m2 (range,15.9–68.3 kg/m2). The majority of patients (58%) had cT2 tumors. Clinically palpable axillary adenopathy at presentation was present in 72% of cases. Forty-two percent of patients had HR+/HER2− tumors, 38% had HER2+ tumors, and 20% had TN tumors. Ninety-nine percent of tumors were poorly or moderately differentiated, and lymphovascular invasion (LVI) was present on core biopsy (n = 101) or at final pathology (n = 112) in 213 (37%) tumors.
Fig. 1.
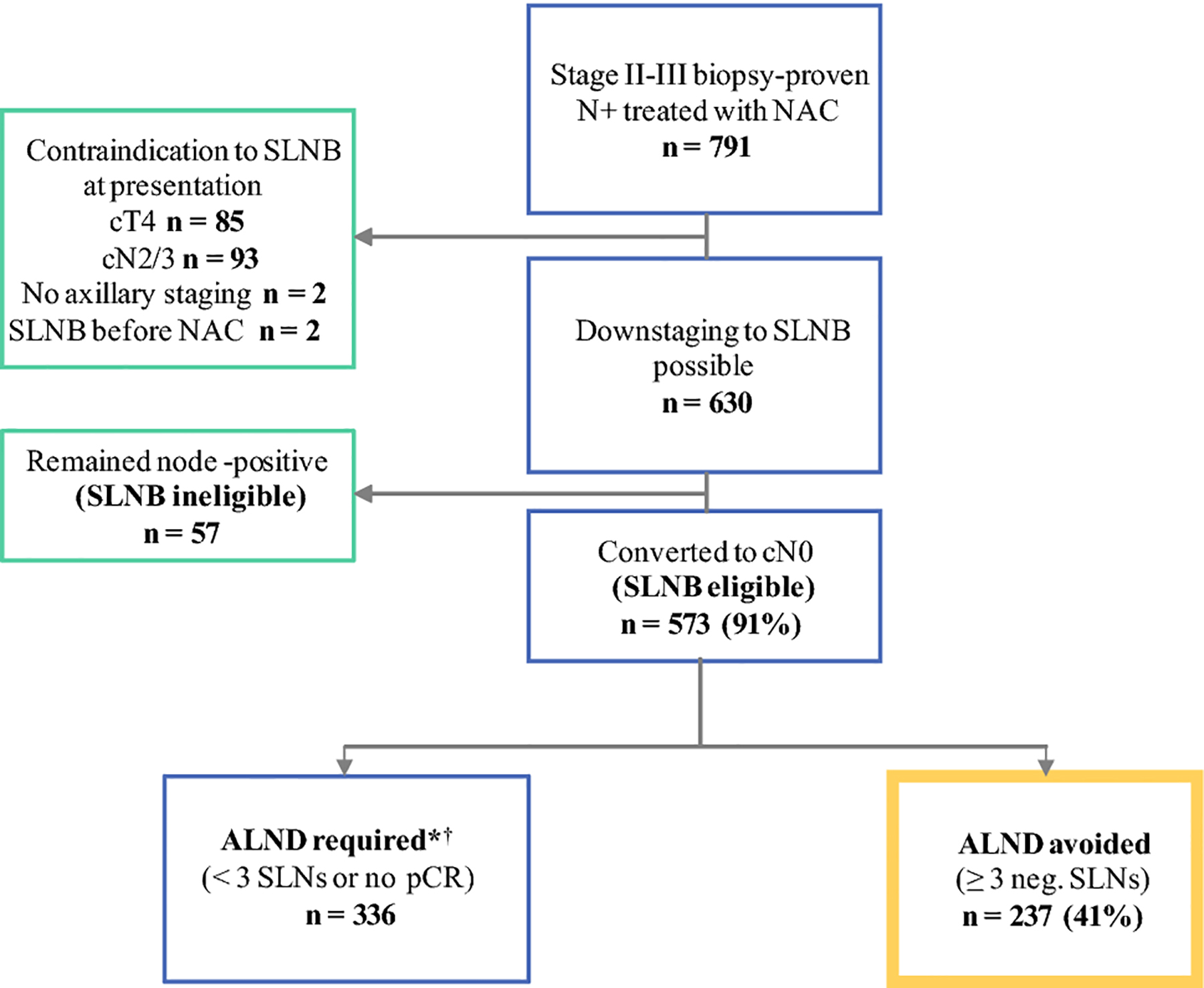
Study flowchart.
NAC neoadjuvant chemotherapy, cT clinical tumor stage, cN clinical nodal stage, SLNB sentinel lymph node biopsy, SLNs sentinel nodes, pCR pathologic complete response
*Thirty-three patients were randomized to axillary radiation therapy in the Alliance A011202 trial.
†ALND was deferred for 27 cases with residual disease by either patient preference or clinical judgment (14 patients had isolated tumor cells, 10 had micrometastases, and 3 had macrometastases).
TABLE 1.
Clinicopathological features
| Overall n = 573 | < 3 SLNs retrieved n = 42 | > 3 SLNs retrieved n = 531 | P* | ALND avoided n = 237 | ALND required n = 336 | P* | |
|---|---|---|---|---|---|---|---|
| Age, years | 49 (24, 82) | 53 (36, 78) | 49 (24, 82) | 0.07 | 49 (24, 82) | 50 (27, 78) | 0.08 |
| BMI, kg/m2 | 25.8 (15.9, 68.3) | 28.4 (17.4, 47.5) | 25.7 (15.9, 68.2) | 0.01 | 25.6 (16.9, 68.2) | 26.1 (15.9, 47.5) | 0.24 |
| Palpable node at presentation | 0.84 | 0.55 | |||||
| No | 146 | 10 (7%) | 136 (93%) | 55 (38%) | 91 (62%) | ||
| Borderline | 13 | 0 (0%) | 13 (100%) | 6 (46%) | 7 (54%) | ||
| Yes | 414 | 32 (8%) | 382 (92%) | 176 (43%) | 238 (57%) | ||
| Palpable tumor at presentation | 0.19 | 0.23 | |||||
| No | 59 | 6 (10%) | 53 (90%) | 26 (44%) | 33 (56%) | ||
| Borderline | 12 | 2 (17%) | 10 (83%) | 2 (17%) | 10 (83%) | ||
| Yes | 502 | 34 (7%) | 468 (93%) | 209 (42%) | 293 (58%) | ||
| Clinical T at presentation | 0.03 | 0.85 | |||||
| 1 | 110 | 6 (5%) | 104 (95%) | 42 (38%) | 68 (62%) | ||
| 2 | 334 | 19 (6%) | 315 (94%) | 141 (42%) | 193 (57%) | ||
| 3 | 125 | 16 (13%) | 109 (87%) | 52 (41%) | 73 (58%) | ||
| X | 4 | 1 (25%) | 3 (75%) | 2 (50%) | 2 (50%) | ||
| Subtype | 0.55 | < 0.001 | |||||
| HR+ HER2− | 241 | 14 (6%) | 227 (94%) | 49 (20%) | 192 (80%) | ||
| HR+ HER2+ | 138 | 13 (9%) | 125 (91%) | 76 (55%) | 62 (45%) | ||
| HR− HER2+ | 80 | 7 (9%) | 73 (91%) | 62 (78%) | 18 (23%) | ||
| HR− HER2− | 114 | 8 (7%) | 106 (93%) | 50 (44%) | 64 (56%) | ||
| Histology | 0.60 | 0.002 | |||||
| Ductal | 437 | 31 (7%) | 406 (93%) | 194 (44%) | 243 (56%) | ||
| Lobular and mixed | 41 | 2 (5%) | 39 (95%) | 7 (17%) | 34 (83%) | ||
| Micropapillary and mixed | 36 | 5 (14%) | 31 (86%) | 10 (28%) | 26 (72%) | ||
| Apocrine and mixed | 40 | 3 (8%) | 37 (93%) | 20 (50%) | 20 (50%) | ||
| Other | 19 | 1 (5%) | 18 (95%) | 6 (32%) | 13 (68%) | ||
| LVI† | 213 | 23 (11%) | 190 (89%) | 0.02 | 46 (22%) | 167 (78%) | < 0.001 |
| Grade | 0.72 | < 0.001 | |||||
| I | 7 | 0 (0%) | 7 (100%) | 1 (14%) | 6 (86%) | ||
| II | 228 | 19 (8%) | 209 (92%) | 55 (24%) | 173 (76%) | ||
| III | 338 | 23 (7%) | 315 (93%) | 181 (54%) | 157 (46%) |
Frequency (row percent) reported for categorical variables and median (range) reported for continuous variables
Results from Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables
LVI was present on core biopsy or final biopsy
SLN sentinel lymph node, pCR pathologic complete response, BMI body mass index, HR hormone receptor, LVI lymphovascular invasion
Predictors of Retrieval of ≥ 3 SLNs
Inadequate mapping, defined as identification of < 3 SLNs, occurred in 42 (7%) patients: failed mapping was rare and occurred in only 11 (2%) of cases. In the inadequate-mapping group, the median number of SLNs removed was 1 (range 0–2). Three or more SLNs were found in 531 (93%) cases, with a median of 4 SLNs retrieved (range 3–10). While the average number of SLNs retrieved varied by surgeon, all 16 surgeons included in the study removed, on average, more than 3 SLNs.
On univariate analysis, patients with inadequate mapping had higher BMI (median BMI 28.4 kg/m2 vs 25.7 kg/m2, p = 0.01), were more likely to present with T3 tumors (38% vs 21%, p = 0.03), and were more likely to have LVI (55% vs 36%, p = 0.02). Patients with inadequate mapping were older (median age 53 vs 49 years), but the difference did not reach statistical significance (p = 0.07). The presence of palpable nodes at presentation did not impact the accuracy of mapping (p = 0.84) (Table 1). On multivariable analysis, BMI and LVI remained independently associated with decreased odds of retrieving 3 or more SLNs (odds ratio [OR] 0.77, 95% confidence interval [CI] 0.62–0.96; and OR 0.46, 95% CI 0.24–0.87, respectively) (Table 2).
TABLE 2.
Univariate and multivariable associations between clinicopathological factors and the odds of finding 3 or more SLNs
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Age per 5-year increase | 0.88 (0.77, 1.01) | 0.07 | ||
| BMI, per 5-unit increase | 0.77 (0.62, 0.96) | 0.02 | 0.77 (0.62, 0.96) | 0.02 |
| Palpable node at presentation | 0.91 (0.43, 1.90) | 0.80 | ||
| Palpable tumor at presentation | 1.50 (0.61, 3.73) | 0.38 | ||
| cT at presentation ref: 1 | ||||
| 2 | 0.96 (0.37, 2.46) | 0.93 | ||
| 3 | 0.39 (0.15, 1.04) | 0.06 | ||
| Subtype ref: HR+ HER2− | ||||
| HR+ HER2+ | 0.59 (0.27, 1.30) | 0.19 | ||
| HR− HER2+ | 0.64 (0.25, 1.65) | 0.36 | ||
| HR− HER2− | 0.82 (0.33, 2.01) | 0.66 | ||
| Histology ref: ductal | ||||
| Lobular and mixed | 0.94 (0.27, 3.23) | 0.92 | ||
| Micropapillary and mixed | 1.49 (0.34, 6.46) | 0.60 | ||
| Apocrine and mixed | 0.47 (0.17, 1.30) | 0.15 | ||
| Other | 1.37 (0.18, 10.6) | 0.76 | ||
| LVI | 0.46 (0.24, 0.87) | 0.02 | 0.46 (0.24, 0.87) | 0.02 |
| Grade III ref I/II | 1.20 (0.64, 2.27) | 0.56 | ||
Modeling odds of finding ≥ 3 SLNs (n = 531) versus < 3 SLNs (n = 42).
CI confidence interval, BMI body mass index, HR hormone receptor, LVI lymphovascular invasion
Predictors of Avoiding ALND
Overall, 255/573 (46%) patients achieved nodal pCR. Of these, 18 had inadequate mapping and 237, or 41% of the study cohort, had adequate mapping, and were able to avoid ALND. Patients with ductal and apocrine tumors were more likely to avoid ALND compared to those with lobular and micropapillary histology (44% and 50% vs 17% and 28% respectively, p = 0.002). Omission of ALND occurred in 20% of HR+/HER2− tumors, 44% of TN tumors, 55% of HR+/HER2+ tumors, and 78% of HR−/HER2+ tumors (p < 0.001). LVI was more often present in patients requiring ALND than in those who did not require ALND (78% vs 22%, p < 0.001), and high-grade tumors were more frequently associated with avoidance of ALND than moderate- and low-grade tumors (54% vs 24% and 14%, respectively, p < 0.001) (Table 1). On multivariable analysis, factors that remained independently associated with avoidance of ALND were high grade (OR 2.51, 95% CI 1.6–3.94, p < 0.001) and receptor status (HR+/HER2− [referent]: OR 1.99, 95% CI 1.15–3.46, p = 0.01 for HR−/HER2−; OR 3.93, 95% CI 2.40–6.44, p < 0.001 for HR+/HER2+; and OR 8.24, 95% CI 4.16–16.3, p<0.001 for HR−/HER2+), while LVI (OR 0.28, 95% CI 0.18–0.43, p < 0.001) was associated with a lower likelihood of avoiding ALND (Table 3).
TABLE 3.
Univariate and multivariable associations between clinicopathological factors and avoidance of ALND (with retrieval of ≥ 3 SLNs and nodal pCR)
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Age per 5-year increase | 0.93 (0.87, 1.00) | 0.07 | ||
| BMI, per 5-unit increase | 0.95 (0.83, 1.09) | 0.46 | ||
| Palpable node at presentation | 1.23 (0.84, 1.81) | 0.30 | ||
| Palpable tumor at presentation | 0.88 (0.51, 1.52) | 0.66 | ||
| cT at presentation ref: 1 | ||||
| 2 | 1.18 (0.76, 1.84) | 0.46 | ||
| 3 | 1.15 (0.68, 1.95) | 0.59 | ||
| Subtype ref HR+ HER2− | ||||
| HR+ HER2+ | 4.80 (3.03, 7.60) | < 0.001 | 3.93 (2.40, 6.44) | < 0.001 |
| HR− HER2+ | 13.5 (7.32, 24.9) | < 0.001 | 8.24 (4.16, 16.3) | < 0.001 |
| HR− HER2− | 3.06 (1.88, 4.97) | < 0.001 | 1.99 (1.15, 3.46) | 0.01 |
| Histology ref: ductal | ||||
| Lobular and mixed | 1.25 (0.66, 2.39) | 0.50 | 0.75 (0.35, 1.62) | 0.47 |
| Micropapillary and mixed | 0.26 (0.11, 0.59) | 0.001 | 0.46 (0.19, 1.15) | 0.10 |
| Apocrine and mixed | 0.48 (0.23, 1.02) | 0.06 | 0.63 (0.25, 1.60) | 0.33 |
| Other | 0.58 (0.22, 1.55) | 0.28 | 0.55 (0.19, 1.63) | 0.28 |
| LVI | 0.24 (0.17, 0.36) | < 0.001 | 0.28 (0.18, 0.43) | < 0.001 |
| Grade III ref: I/II | 3.69 (2.55, 5.33) | < 0.001 | 2.51 (1.60, 3.94) | < 0.001 |
Modeling odds of having ≥ 3 SLNs and nodal pCR (n = 237) versus all other patients (n = 336).
ALND axillary lymph node dissection, SLN sentinel lymph node, pCR pathologic complete response, CI confidence interval, BMI body mass index, HR hormone receptor, LVI lymphovascular invasion
DISCUSSION
In this large series of biopsy-proven, stage II-III, node-positive breast cancer patients undergoing NAC, the rate of adequate SLN mapping post-NAC, defined as the identification of ≥ 3 SLNs, was 93%.
This is higher than the rate reported in previous studies. In the SENTinel NeoAdjuvant (SENTINA) and American College of Surgeons Oncology Group (ACOSOG) Z1071 trials, 3 or more SLNs were found in only 34% and 57%8,9 of cases, respectively. In a recent study from Dana-Farber Cancer Institute/Brigham and Women’s Cancer Center (DFCI/BWCC) assessing the impact of residual disease burden on SLNB outcome, Laws et al reported a rate of adequate mapping of 67% among cN1 patients after NAC.17 In a previous study from our institution evaluating the effect of clinical nodal status on the number of SLNs retrieved after NAC, Baker at al reported that the median number of SLNs retrieved after NAC in cN1 patients was 4 (range 1–14), and that the rate was stable over the 3-year study period; this is in line with the number of SLNs retrieved in the upfront surgery setting (mean 2.8, range 1–25).18,19 The low rates of identification of ≥ 3 SLNs in the SENTINA and ACOSOG Z1071 studies may be attributed to the use of dual mapping in only 28% and 79% of cases, respectively. Additionally, in the ACOSOG Z1071 trial, surgeons were only required to retrieve 2 SLNs, and in the SENTINA trial, a minimum number of SLNs was not pre-specified. In light of the protocol specifications, and the requirement for ALND in all patients, surgeons may not have been inclined to continue to search for and remove higher numbers of SLNs. Considering the high frequency of retrieval of ≥ 3 SLNs at our institution and the reproducibility of this technique among surgeons, SLNB with dual tracer is our preferred approach to avoid ALND in cN1 patients after NAC.
In the Baker et al study, the only two significant predictors of finding 3 or more SLNs after NAC were younger age (< 50 years) and cN1 status at presentation.20 In the study from the DFCI/BWCC group, the median number of SLNs was also 4, and older age and low grade were the only significant predictors of inadequate mapping.17 In our study, older age was associated with decreased odds of adequate mapping, but this did not reach statistical significance (OR 0.88, 95% CI 0.77–1.01, p = 0.07). On multivariable analysis, the only significant predictors of inadequate mapping were higher BMI and LVI. The influence of BMI on SLN mapping has been described in the upfront surgery setting21,22, with decreased lymphatic flow due to the increased fatty tissue in the breast hypothesized as the mechanism.22 The relationship between LVI and the number of SLNs identified is less well described. In the SENTINA trial, the absence of LVI was not predictive of a higher SLN detection rate9, and studies of other types of primary cancers, such as melanoma, have found the presence of LVI to be associated with an increased number of SLNs.23 In the setting of NAC, it is possible that the death of tumor cells in lymphatic vessels leads to fibrosis of these lymphatic channels with failure to take up blue dye or radioactivity, leading to identification of a fewer number of SLNs.
Although the exact mechanisms behind the association of higher BMI and LVI with failure to achieve adequate mapping remain to be elucidated, patients with these characteristics have a significantly increased risk of inadequate mapping. Of the 42 cases with inadequate mapping in our study, 18 (43%) had nodal pCR and could have been potentially spared ALND. Alternative mapping strategies independent of the number of SLNs retrieved may be useful in patients with preoperative characteristics unfavorable for SLNB mapping. In an unplanned analysis of the ACOSOG Z1071 study, the FNR for patients in whom the metastatic node was clipped at diagnosis and retrieved at the time of surgery was 6.8%.24 The combination of SLNB with retrieval of the clipped node, termed targeted axillary dissection (TAD), has also been shown to be associated with a low FNR.25 This approach is not without technical challenges, as failure to identify the clip, due to migration into the perinodal fat, has been reported in 3–30% of cases, with variation according to the localization technique used.26–28 Nevertheless, TAD may be useful in groups of patients at increased risk for inadequate mapping. Unfortunately, the concordance between pathological prognostic findings on core biopsy and final specimen is limited29, and although LVI was the only pathological factor predictive of both inadequate mapping and failure to avoid ALND, it was present on core biopsy in only 101/213 (47%) of cases, which limits the application of our findings.
Among patients with adequate mapping, 41% achieved nodal pCR and were spared ALND. Our rate of nodal pCR is consistent with the results of the ACOSOG Z1071 trial.30 We identified HER2+ and TN receptor status, high grade, ductal and apocrine histology, and absence of LVI as predictors of ALND avoidance. As reported in other studies, patients with HER2+ and TN tumors were more likely to achieve nodal pCR and be spared ALND compared to HR+/HER2− tumors.3,7,30 Dominici et al reported a nodal downstaging rate of 74% in HER2+ patients treated with trastuzumab only, with no difference based on estrogen receptor status.7 Our downstaging rate in the HER2+ group, where all patients received dual anti-HER2 therapy, was 63%, with a significant difference according to the hormone receptor status (78% in the HR−/HER2+group vs 55% in the HR+/HER2+ group; p = 0.001). This is consistent with our previous report3 and with the results of the NeoSphere trial that showed a higher rate of pCR in hormone receptor-negative, HER2+ tumors treated with dual anti-HER2 therapy.31 In univariate analysis, micropapillary histology was associated with a lower rate of nodal pCR (OR 0.26, 95% CI 0.11–0.59, p = 0.001), but this did not remain significant in multivariable analysis, likely due to the limited number of cases.32 Micropapillary tumors are known to be associated with poor prognosis33 and poor response to NAC.32 In our study, the rate of ALND avoidance in this histotype was low, at 28%, second only to the rate of ALND avoidance in the lobular histotype (17%).
In this study, we demonstrate that, with standard surgical techniques and modern neoadjuvant chemotherapy regimens, more than 40% of clinically node-positive patients avoided ALND. This rate is likely to increase with the use of new agents, alone or in combination with chemotherapy, which have been shown to increase the rate of pCR.34,35Although prospective trials have documented the accuracy of SLN biopsy after NAC, all patients underwent ALND to determine the FNR of the procedure. To the best of our knowledge, this is the first large study examining how often ALND is avoided in node-positive patients with the NAC approach, and examining an optimized SLNB procedure with the use of dual mapping and retrieval of 3 or more SLNs, as endorsed by national and international guidelines as well as expert panels.36–38
Strengths of this study include its large sample size, use of homogenous preoperative systemic therapy regimens, and standardized pathologic assessment and operative techniques. Limitations of this study include the fact that it was carried out at a single institution with dedicated surgeons and pathologists in which the SLNB procedure is highly standardized, which may limit generalizability. Data on regional recurrence after omitting ALND in patients who achieve nodal pCR are limited.39 Follow-up of this cohort will provide further evidence on the safety of this approach.
Conclusions
In this large consecutive cohort of node-positive patients treated with NAC, 3 or more SLNS were retrieved in 93% of cN0 patients post-NAC with the use of dual tracer mapping. ALND was avoided in 41% of patients who achieved nodal pCR and had 3 or more SLNs retrieved. Women with higher BMI and LVI had lower rates of adequate mapping and represent a group of patients in whom retrieval of the clipped lymph node may help to reduce the need for ALND. The correlation of LVI with axillary downstaging and SLNB identification rate should be further explored. Our results demonstrate that NAC reduces the need for ALND in clinically node-positive patients of all subtypes.
Synopsis:
Here we evaluate how often node-positive patients avoid ALND with NAC and identify predictors of retrieval of ≥3 SLNs and ALND avoidance. ALND was spared in 41% of patients; this was associated with receptor status, grade, and LVI.
ACKNOWLEDGEMENTS
The preparation of this manuscript was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center. Dr. Monica Morrow has received speaking honoraria from Genomic Health. Dr. Andrea V. Barrio has received speaking honoraria from Roche. Dr. Giacomo Montagna was supported by the Ticino Cancer League, the Hanne Liebermann Foundation, the Fondation Ancrage, and the HEMMI-Stiftung. All other authors have no conflict of interest disclosures to report. This study has been accepted for presentation in poster format at the Society of Surgical Oncology 2020 International Conference on Surgical Cancer Care, August 17–20, 2020, Boston MA.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. June 2015;12(6):335–343. [DOI] [PubMed] [Google Scholar]
- 2.Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant Chemotherapy Use in Breast Cancer is Greatest in Excellent Responders: Triple-Negative and HER2+ Subtypes. Ann Surg Oncol. August 2018;25(8):2241–2248. [DOI] [PubMed] [Google Scholar]
- 3.Mamtani A, Barrio AV, King TA, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol. October 2016;23(11):3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrow M Parsing Pathologic Complete Response in Patients Receiving Neoadjuvant Chemotherapy for Breast Cancer. JAMA Oncol. April 2016;2(4):516–517. [DOI] [PubMed] [Google Scholar]
- 5.Vila J, Mittendorf EA, Farante G, et al. Nomograms for Predicting Axillary Response to Neoadjuvant Chemotherapy in Clinically Node-Positive Patients with Breast Cancer. Ann Surg Oncol. October 2016;23(11):3501–3509. [DOI] [PubMed] [Google Scholar]
- 6.Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. December 20 2005;23(36):9304–9311. [DOI] [PubMed] [Google Scholar]
- 7.Dominici LS, Negron Gonzalez VM, Buzdar AU, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2−positive breast cancer. Cancer. June 15 2010;116(12):2884–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. October 9 2013;310(14):1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. June 2013;14(7):609–618. [DOI] [PubMed] [Google Scholar]
- 10.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. January 20 2015;33(3):258–264. [DOI] [PubMed] [Google Scholar]
- 11.Classe JM, Loaec C, Gimbergues P, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat. January 2019;173(2):343–352. [DOI] [PubMed] [Google Scholar]
- 12.Tee SR, Devane LA, Evoy D, et al. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br J Surg. November 2018;105(12):1541–1552. [DOI] [PubMed] [Google Scholar]
- 13.Wong SM, Weiss A, Mittendorf EA, King TA, Golshan M. Surgical Management of the Axilla in Clinically Node-Positive Patients Receiving Neoadjuvant Chemotherapy: A National Cancer Database Analysis. Ann Surg Oncol. October 2019;26(11):3517–3525. [DOI] [PubMed] [Google Scholar]
- 14.Caudle AS, Bedrosian I, Milton DR, et al. Use of Sentinel Lymph Node Dissection After Neoadjuvant Chemotherapy in Patients with Node-Positive Breast Cancer at Diagnosis: Practice Patterns of American Society of Breast Surgeons Members. Ann Surg Oncol. October 2017;24(10):2925–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen TT, Hoskin TL, Day CN, et al. Decreasing Use of Axillary Dissection in Node-Positive Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Ann Surg Oncol. September 2018;25(9):2596–2602. [DOI] [PubMed] [Google Scholar]
- 16.Di Micco R, Zuber V, Fiacco E, et al. Sentinel node biopsy after primary systemic therapy in node positive breast cancer patients: Time trend, imaging staging power and nodal downstaging according to molecular subtype. Eur J Surg Oncol. June 2019;45(6):969–975. [DOI] [PubMed] [Google Scholar]
- 17.Laws A, Hughes ME, Hu J, et al. Impact of Residual Nodal Disease Burden on Technical Outcomes of Sentinel Lymph Node Biopsy for Node-Positive (cN1) Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Ann Surg Oncol. November 2019;26(12):3846–3855. [DOI] [PubMed] [Google Scholar]
- 18.McCarter MD, Yeung H, Fey J, Borgen PI, Cody HS 3rd. The breast cancer patient with multiple sentinel nodes: when to stop? J Am Coll Surg. June 2001;192(6):692–697. [DOI] [PubMed] [Google Scholar]
- 19.Port ER, Patil S, Stempel M, Morrow M, Cody HS 3rd. Number of lymph nodes removed in sentinel lymph node-negative breast cancer patients is significantly related to patient age and tumor size: a new source of bias in morbidity assessment? Cancer. April 15 2010;116(8):1987–1991. [DOI] [PubMed] [Google Scholar]
- 20.Baker JL, Muhsen S, Zabor EC, Stempel M, Gemignani ML. Does Lymph Node Status Prior to Neoadjuvant Chemotherapy Influence the Number of Sentinel Nodes Removed? Ann Surg Oncol. February 2019;26(2):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow M, Rademaker AW, Bethke KP, et al. Learning sentinel node biopsy: results of a prospective randomized trial of two techniques. Surgery. October 1999;126(4):714–720; discussion 720–712. [PubMed] [Google Scholar]
- 22.Cox CE, Dupont E, Whitehead GF, et al. Age and body mass index may increase the chance of failure in sentinel lymph node biopsy for women with breast cancer. Breast J. Mar-Apr 2002;8(2):88–91. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt CR, Panageas KS, Coit DG, Patel A, Brady MS. An increased number of sentinel lymph nodes is associated with advanced Breslow depth and lymphovascular invasion in patients with primary melanoma. Ann Surg Oncol. April 2009;16(4):948–952. [DOI] [PubMed] [Google Scholar]
- 24.Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0–T4, N1–N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance). Ann Surg. April 2016;263(4):802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caudle AS, Yang WT, Krishnamurthy S, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol. April 1 2016;34(10):1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. September 2018;44(9):1307–1311. [DOI] [PubMed] [Google Scholar]
- 27.Plecha D, Bai S, Patterson H, Thompson C, Shenk R. Improving the Accuracy of Axillary Lymph Node Surgery in Breast Cancer with Ultrasound-Guided Wire Localization of Biopsy Proven Metastatic Lymph Nodes. Ann Surg Oncol. December 2015;22(13):4241–4246. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TT, Hieken TJ, Glazebrook KN, Boughey JC. Localizing the Clipped Node in Patients with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy: Early Learning Experience and Challenges. Ann Surg Oncol. October 2017;24(10):3011–3016. [DOI] [PubMed] [Google Scholar]
- 29.Sharifi S, Peterson MK, Baum JK, Raza S, Schnitt SJ. Assessment of pathologic prognostic factors in breast core needle biopsies. Mod Pathol. October 1999;12(10):941–945. [PubMed] [Google Scholar]
- 30.Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. October 2014;260(4):608–614; discussion 614–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2−positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. January 2012;13(1):25–32. [DOI] [PubMed] [Google Scholar]
- 32.Alvarado-Cabrero I, Alderete-Vazquez G, Quintal-Ramirez M, Patino M, Ruiz E. Incidence of pathologic complete response in women treated with preoperative chemotherapy for locally advanced breast cancer: correlation of histology, hormone receptor status, Her2/Neu, and gross pathologic findings. Ann Diagn Pathol. June 2009;13(3):151–157. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Fan Y, Lang RG, et al. Breast carcinoma with micropapillary features: clinicopathologic study and long-term follow-up of 100 cases. Int J Surg Pathol. April 2008;16(2):155–163. [DOI] [PubMed] [Google Scholar]
- 34.Litton JK, Scoggins ME, Hess KR, et al. Neoadjuvant Talazoparib for Patients With Operable Breast Cancer With a Germline BRCA Pathogenic Variant. J Clin Oncol. August 28 2019:JCO1901304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mamounas E, Poulos C, Goertz HP, Gonzalez JM, Pugh A, Antao V. Neoadjuvant Systemic Therapy for Breast Cancer: Factors Influencing Surgeons’ Referrals. Ann Surg Oncol. October 2016;23(11):3510–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (Breast Cancer) Version 3.2020 Version 3.2020 - March 6, 2020.
- 37.Burstein HJ, Curigliano G, Loibl S, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. October 1 2019;30(10):1541–1557. [DOI] [PubMed] [Google Scholar]
- 38.Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. August 1 2019;30(8):1194–1220. [DOI] [PubMed] [Google Scholar]
- 39.Galimberti V, Ribeiro Fontana SK, Maisonneuve P, et al. Sentinel node biopsy after neoadjuvant treatment in breast cancer: Five-year follow-up of patients with clinically node-negative or node-positive disease before treatment. Eur J Surg Oncol. March 2016;42(3):361–368. [DOI] [PubMed] [Google Scholar]


