Abstract
This review details the proceedings of a Pennington Biomedical scientific symposium entitled, “What should I eat and why? The environmental, genetic, and behavioral determinants of food choice.” The symposium was designed to review the literature about energy homeostasis, particularly related to food choice and feeding behaviors, from psychology to physiology. We discuss the intrinsic determinants of food choice, including biological mechanisms (genetics), peripheral and central signals, brain correlates, and the potential role of the microbiome. We also address the extrinsic determinants (environment) of food choice within our physical and social environments. Finally, we report the current treatment practices for the clinical management of eating-induced overweight and obesity. An improved understanding of these determinants will inform best practices for the clinical treatment and prevention of obesity. Strategies paired with systemic shifts in our public health policies and changes in our “obesogenic” environment will be most effective at attenuating the obesity epidemic.
Keywords: obesity, food intake, diets, physical activity, metabolic surgery
Introduction
Throughout history, humans and animals have evolved to exhibit complex mechanisms that regulate energy homeostasis, or the energy intake and energy expenditure required to maintain energy balance and body weight. To conclude, however, that achieving a stable weight is as simple as balancing energy intake and energy expenditure would be, at best, an oversimplification of the more complex physiology surrounding body weight regulation since so many internal and external drivers and inhibitors of food intake are at play. Moreover, a positive energy balance may reflect an underlying metabolic problem, the treatment of which may be prerequisite to successful long-term weight control.
Genetics, environmental exposures including cultural aspects, and behavioral characteristics collectively impact what, when, and how much we eat, thus all playing a role in weight regulation (1) (Figure 1). As a result, changing eating behaviors is considered to be a first-line approach to successfully control body weight, a task which is challenging in our obesogenic environment. Despite what scientists currently know about eating behavior, there is a lot that remains unexplored. Yet beyond our laboratories, individuals are not waiting for scientists to have definitive answers to questions like: which diet is best if I want to lose weight? Instead, many are conducting single-person case studies on themselves to answer their questions. Furthermore, there will always be scientific debates about what drives food choice and caloric intake, and how to make it match our energy needs in presence of frequently low physical activity levels.
Figure 1. How the Interactions between Individual Biology and the Built and Social Environments Impact What We Eat.
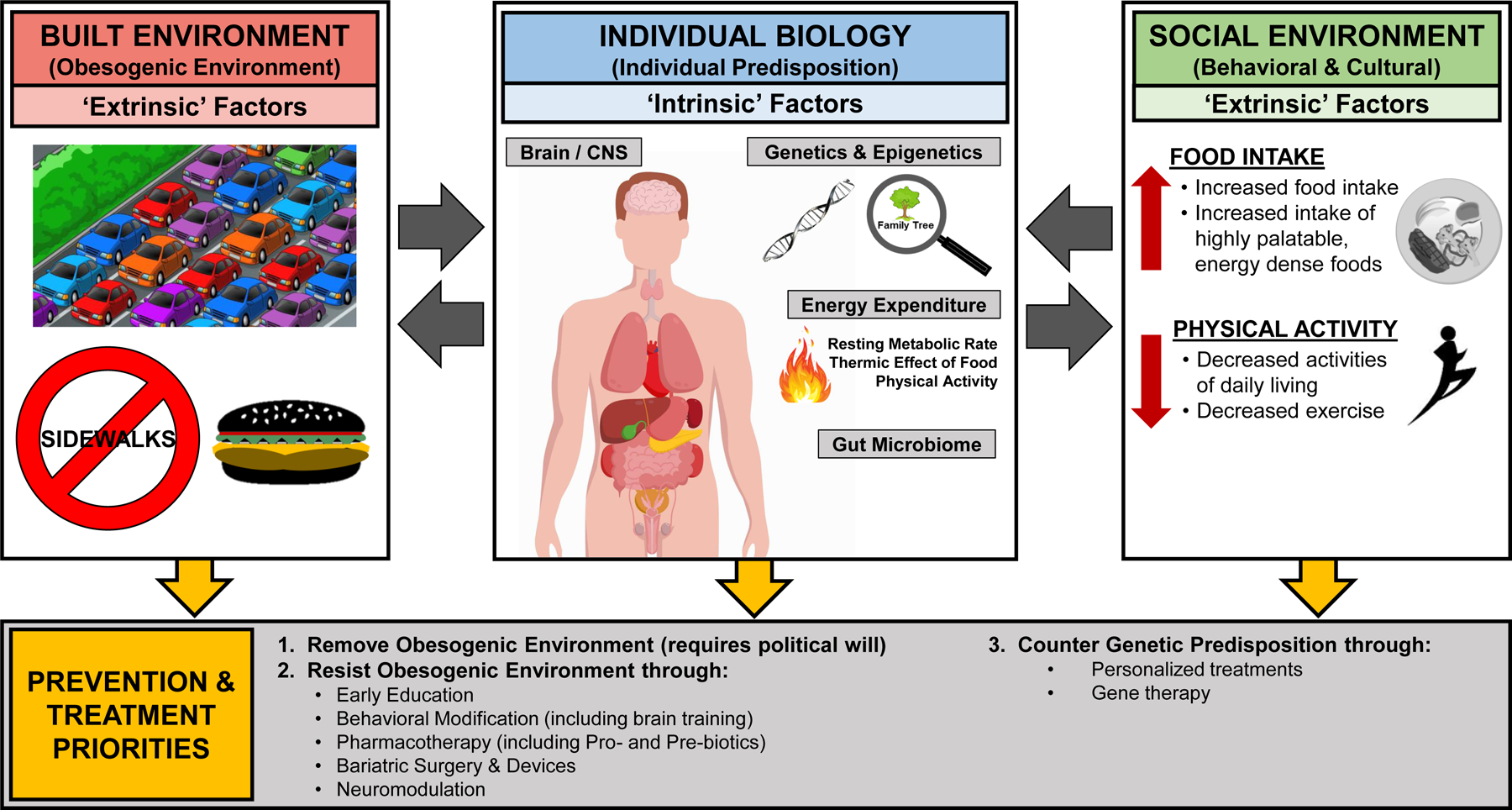
An interaction between a person’s individual biology, including genetic/epigenetic predisposition, as well as the influence of both the built and social environments is the most likely cause of the increased prevalence of obesity and metabolic diseases worldwide. Therefore, prevention and treatment should primarily focus on removing or resisting the obesogenic environment. Because removal of the obesogenic environment requires political will that seems difficult to come by, the most realistic target is to make prone individuals resist the obesogenic environment. This can be achieved through early education of children by, for example, teaching them how to disregard food advertisements/cues and choose a scientifically-proven healthy lifestyle. Through behavioral modification, either achieved by brain training with biofeedback or drugs selectively targeting the physiological systems controlling appetite and food choice and regulation of body weight/adiposity. Other approaches include bariatric surgeries and devices, as well as neuromodulation. Finally, to counter genetic predisposition, the treatment options need to be more closely personalized, taking into account the specific genetic/epigenetic defects, and selective gene therapies may be developed in the future. In all these efforts, prevention is highly preferred over treatment, as it is much more cost-effective.
Eating behavior is one of the primary contributors to the development of overweight and obesity in humans (2). There is some consensus in the field regarding the recommendations for ideal food choices that contribute to a healthy body weight and metabolic status. An overall reduction in total energy (calorie) intake, non-specific to the caloric source, is conventionally considered the primary weight management strategy when making dietary choices. However, this does not negate the importance of dietary composition, which is currently the topic of intense debate. For instance, there is abundant support for diets that favor complex carbohydrates over simple sugars, and diets that are enriched in monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) rather than saturated fats (3,4). It is highly recommended to choose “whole” or “natural” foods over highly or ultra-processed foods, which have become a dominant component of our convenient but rather unhealthy diet (5,6). Seeking variety in one’s dietary composition, such that one’s diet is composed of foods from many different food groups and sources, is encouraged and ensures the individual is consuming diverse nutrients. There is also a growing argument for choosing a diet that promotes the sustainability of our food supply and limits carbon emissions, such as reducing consumption of ultra-processed food and beverages, as well as reducing beef and dairy products. Finally, though not directly related to feeding, lifestyles that involve engagement in regular exercise and physical activity, quality sleep, stress reduction, etc. may encourage ideal food choices and reinforce a healthy metabolic status.
Many intrinsic and extrinsic factors also influence dietary intake, which may explain why eating behavior has been one of the most difficult components to address in the clinical management of overweight and obesity. Intrinsic factors influencing food choice include the human physiology that is genetically programmed resulting in a large physiological variability in biological processes. Indeed, genetic variations set the foundation for what contributes to eating behavior and predisposition to developing obesity. Large-scale genome-wide association (GWAS) studies have linked many genes to obesity, while the functional translation of the genes into physiology and/or behavior continues to be investigated (7–9). Furthermore, several monogenic mutations, including mutations to the leptin gene (LEP) and its receptor (LEPR), have been directly linked to pathological eating behaviors (7). Molecular signals such as hormones and other small signaling molecules have been well-studied with regard to eating behavior. Many of these peripheral signaling molecules are known to influence the brain, which has resulted in dedicated research to understand the neural mechanisms that influence food choice. More recently, the role of the gut microbiota in both its response to various diets and the potential of the microbiome to influence eating behavior has become increasingly investigated. Finally, the desire to eat is certainly influenced by metabolic demands, such that feeding will adapt to energy needs. Extrinsic influencers of food choice include environmental factors that strongly guide intake decisions. Indeed, our eating habits are influenced by our physical (urban versus rural living or lack of healthy foods in poverty regions), social, and cultural environment (10).
In order to develop the best strategies to combat eating-associated weight gain and dictate ideal dietary recommendations, we must understand how intrinsic and extrinsic factors determine eating behaviors. This report will review and discuss recent developments in the factors that influence eating behavior in both animals and humans. A summary of the efficacy of current strategies that are in use or under development to address issues of overeating as a means to reverse obesity and metabolic disease will also be presented.
Intrinsic Determinants of Food Choice
The biology of the human body has countless redundant mechanisms in place to ensure that individuals will seek and ingest food in order to maintain energy balance. Unfortunately, often those very mechanisms can become dysregulated and lead to excess food consumption (or positive energy balance) that ultimately leads to weight gain. Investigation of the intrinsic factors contributing to eating behavior is crucial for the development of pharmacological treatments for eating-related overweight and obesity.
Genetics and Epigenetics
There have been many studies investigating the genetic components of obesity. Initially, candidate gene-based approaches were used to identify genes related to the development of obesity. These studies led to the discovery of variants in genes such as LEP, LEPR, proopiomelanocortin (POMC), neuropeptide Y (NPY) and melanocortin 4 receptor (MC4R) all implicated in abnormal food intake behaviors (7). More recent genome-wide association studies (GWAS) have identified many more genes that are associated with obesity; however, the causal role of these genes in the development of obesity is still undefined (8,11). While these GWAS studies did not directly address eating behavior, a recent study found that a genetic risk score for obesity and BMI is partially mediated through undesirable eating behavior traits (12). Additionally, recent work has utilized twin studies to demonstrate a strong genetic influence on preferences for food and beverage selection (13,14). There is also evidence that epigenetic modifications are associated with obesity (15,16). At least one study has demonstrated a direct association between feeding and epigenetic modifications by demonstrating that POMC promoter methylation is altered by early overfeeding in a rat model (17). Yet, while many genetic studies surrounding obesity have helped us better understand the biology of the disease, these studies have uncovered only limited targets towards the treatment of obesity (18). Much work remains to comprehensively understand the implications of genetic and epigenetic modifications on eating behavior.
Metabolic Signals
Many of the genes associated with the development of obesity are responsible for the production and neural response of metabolic signals, particularly hormones and neuropeptides. Over the years, many hormones and neuropeptides have been identified that function as indicators of metabolic status and regulate feeding behavior. The most well-known include leptin, ghrelin, cholecystokinin, GLP-1, NPY and the melanocortin system (αMSH, AgRP) (19–21), but in reality a large number of other hormones and neuropeptides have been identified and demonstrated to influence feeding (22,23). The manner and sites of action through which these various signals influence feeding is both diverse and redundant, with many hormones acting on similar neural populations, some of which increase food intake and other decrease food intake. Furthermore, the triggers for the release of these hormones and neuropeptides are also variable. Our awareness of the depth and complexity of the endocrine and neural systems controlling feeding behavior illustrates the substantial progress that has been made in this area in recent years.
Despite this substantial progress in identifying hormones and neural circuits which govern food intake, it should be noted that the majority of this work has focused on feeding from a total food or energy intake standpoint, with the primary endpoints being increases or decreases in total food intake. The regulation of food choice is much more poorly understood, particularly changes in food choice in response to metabolic signals or metabolic need. Similar to the physiological defense of energy status, metabolic signals may be released in response to nutrient deficiency which drive the ingestion of the deficient nutrient. The strength of this defense appears to vary based on the nutrient, with energy and sodium balance being robustly defended, amino acids and protein moderately defended, and carbohydrates weakly defended (24,25). Recent work has highlighted a potential role for fibroblast growth factor 21 (FGF21), a liver-derived hormone which is specifically induced in the presence of macronutrient imbalance such as low protein and/or high carbohydrate diet (26,27). Deletion of FGF21, or deletion of its co-receptor bKlotho (Klb) from the brain, prevents mice from ‘sensing’ protein restriction (26,28), and FGF21 knockout mice do not alter their food intake, energy expenditure, or growth rate. Conversely, FGF21 injections have been shown to increase preference for protein while also reducing preference for sweet (29,30). Thus, FGF21 is a liver to brain signal that coordinates metabolic and behavioral responses to protein restriction and macronutrient imbalance, and particularly appears to be one of the few hormones that directly drives changes in macronutrient choice.
The list of hormones and neuropeptides affecting eating behavior in various physiological settings continues to grow. As new metabolic signaling mechanisms are discovered, it is essential to identify their physiological triggers and sites and mechanisms of action. It is also crucial to determine if and how signals such as FGF21 may interact with classic signals of energy balance such as leptin. Determining these factors will be key in developing new therapeutic targets for eating-induced metabolic diseases.
Studying Neural Mechanisms in Humans
Intrinsic metabolic signals gain access to the brain via sensory neurons or the circulation and thus have the ability to influence behavior as well as autonomic and neuroendocrine functions. Therefore, there is much to be learned by monitoring activity in the brain that is associated with behavioral responses to eating-related behaviors. Human brain imaging studies using methods such as fMRI and PET have the capacity to monitor region-specific neural activation and the activity of some major neurotransmitter systems such as dopamine. It is well-established that obesity is associated with decreased prefrontal cortex activity and decreases in goal-directed decision making (31,32), a prefrontal cortex-driven behavior known to influence food intake. Imaging studies have also demonstrated that obesity is associated with dopamine abnormalities and increased reward anticipation in the striatum (33), fitting with the hypothesis that disruptions in the dopamine reward circuit contribute to obesity via dysregulated eating behavior.
In our obesogenic environment, attention and awareness of food cues and food-related decisions are important for maintaining goal-directed decision making during episodes of food intake, which are compromised in obesity (34). Recent work has demonstrated improvements in outcome-based learning as a function of mindful eating training, which increases awareness and attention to eating behavior (35). Interestingly, patients with narcolepsy, a condition resulting from deficits in arousal and attention caused by lack of hypocretin/orexin in the lateral hypothalamus, have increased neural activity in reward regions of the brain when distracted by food cues and deficits in goal-directed decision making relative to BMI-matched controls (36,37). These data suggest an important connection between the cortical brain regions and the sub-cortical hypothalamus, which has also been demonstrated through neuroimaging studies in genetic and physiologic models of hypothalamic obesity (31,38,39).
Unfortunately, the majority of brain imaging studies have been mostly correlational. Moving forward, experimental design which studies brain function during physiologic abnormalities in connected brain regions, such as narcolepsy, will advance our understanding of the mechanisms driving obesity-induced changes in brain function and chemistry. Such advances will inform the development and validation of behavioral interventions to combat the alterations in neural functioning as a consequence of obesity.
Studying Neural Mechanisms in Animal Models
Complementary to human brain imaging studies, recent work in animal models has been able to pinpoint anatomical regions that are important for eating behavior with molecular specificity. Human models have provided significant support for the role of cortical brain regions in the response to food cues and associated behaviors, yet imaging methods are limited in resolution and, therefore, inadequate to allow analysis of smaller sub-cortical anatomical regions. Animal models have helped to bridge this gap in understanding how sub-cortical regions such as the hypothalamus regulate feeding behavior and how the hypothalamus and other sub-cortical feeding-related regions interact with cortical regions (40).
In animals, specific anatomical regions of the hypothalamus have been identified that are critical for feeding in response to hunger and exposure to palatable food. The basal hypothalamus including the arcuate nucleus is critical for hunger-mediated feeding, and the lateral hypothalamus is capable of generating robust feeding even when animals are well fed (41,42). Recent advances have included defining molecular-specific cell populations within these regions and their specific influence on feeding. For instance, the AgRP neurons in the arcuate nucleus are well documented for their role in feeding (43). Within the lateral hypothalamus, distinct neuronal populations expressing major neurotransmitters like gamma aminobutyric acids (GABA) or glutamate have been demonstrated to produce feeding and inhibit feeding, respectively, during optogenetic stimulation (44,45). Further molecular research has identified more discrete subpopulations of neurons within the larger lateral hypothalamic GABA population, marked by expression of neuropeptides such as galanin and neurotensin, which also influence feeding behavior (46,47).
Despite these advances, emerging data suggest that there are likely many subpopulations of lateral hypothalamic area (LHA) neurons that can be defined based on their gene expression and anatomical targets. These projection neurons likely encode very different aspects of feeding, with some coding for approach towards food and others coding for consummatory aspects of feeding (44). Moving forward, transcriptional profiling of single cells within an anatomical region will further characterize these regions. Nonetheless, more novel approaches are needed to study genetically-defined cell types that include the expression of more than one gene to allow more selective cell targeting strategies of specific subpopulations of neurons. Such advances in methodology will permit a shift from transcriptional profiling data to cell type specific pharmacology to manipulate feeding behavior.
Interactions between Diet and Microbiome
In the last two decades, the role of the microbiome in human health has become a growing field of research. While the microbiome affects many aspects of physiology, it is particularly relevant to metabolic health. Indeed, the gut plays a significant role in metabolism, particularly through its role in digestion and absorption of nutrients, as well as production and release of peptides and hormones that modulate eating behavior. Dietary habits are particularly critical in explaining gut microbiome variability in population studies (48,49). Thus, it is no surprise that the microbes that proliferate in the gut also influence metabolism and feeding. On the broader spectrum, data from the China Health and Nutrition Survey (CHNS) study reveal that urbanization is associated with reduced gut bacteria diversity, with strong ties between Western (versus traditional) diet with host serum metabolites and gut microbiota (50). Interestingly, reduced gut bacterial diversity and gene richness is a common feature found in overweight, obese and severely obese populations in several countries (51).
More specifically, there is growing evidence that nutrient composition and other factors (such as emulsifiers) in the diet can influence the microbiome, and reciprocally, the microbiome can influence the metabolism of all nutrients. There are strong interactions between food items, food intake patterns (including circadian aspects), as well as micronutrients and macronutrients on the profile of the gut microbiota and resulting influences on metabolic health (52,53). Depending on the starting gut microbiota of an individual, the response to a given dietary intervention targeting improvements in metabolic health can be different. For example, dietary practices such as caloric restriction alter the profile of the microbiome, and the individual response to dietary interventions is influenced by the composition of the gut microbiota (54,55). There is however more to be understood regarding the interaction between food intake patterns, nutrients, gut microbiota and individual metabolic health. Recently identified metabolites such as imidazole propionate, derived from gut microbiota histidine metabolism appear important to follow in this context (56).
It is becoming recognized that the microbiome also has a strong influence over behavior. Manipulations of the gut microbiota (via diet, probiotics, prebiotics, and/or their combination) result in changes in food intake behavior (57). Bariatric surgery, which produces various changes in the microbiota profile (51), also induces changes in food choice (58). Though these studies are largely correlational, it is hypothesized that bacterial metabolites of the microbiota are acting at targets within the gut-brain axis that are known to influence food intake. Dysbiosis of the microbiome that results from a poor diet and obesity may also influence feeding via alterations in other systems regulating behavior.
There is substantial research linking obesity to conditions of neurological impairments, such as deficits in cognitive function and increased incidence of emotional disorders including depression and anxiety (59). Recent work demonstrates that mice fed a high-fat diet developed impaired cognitive and emotional behaviors, and that the transfer of the gut microbiota from an obese mouse to a lean mouse similarly resulted in the development of disordered emotional behavior and cognitive performance, even in the absence of obesity (60).
Recently, targeting of the gut microbiome in patients is being more frequently used in clinical studies. The use of prebiotics and probiotics is common and these treatments have demonstrated some efficacy in improving multiple parameters of metabolic health in humans (61), although mechanisms of action are far from clear and results have generally small effects (62). Other strategies, such as fecal transplant to alter the gut microbiota, have great potential as evidenced by animal studies (63,64). However, the impact of fecal transplant on metabolic health in humans is still emerging (65,66). While scientific evidence surrounding the microbiome is growing, much of this research to date has been primarily correlational and more work is needed to understand the mechanisms behind how the gut microbiota influences metabolic health.
Extrinsic Determinants of Food Choice
External factors seem to have even stronger influence on eating behavior than internal factors. Throughout the lifespan, diet is dependent upon the resources available in the individual’s environment. Environmental quality is also a potential factor that may impact physiology, and indirectly, eating behavior. Of equal importance is the social environment and familial influences which contribute to early childhood exposures to different dietary compositions and eating habits. Combined, environmental conditions and social experiences extend a continuous impact on eating behavior.
Environment
Characteristics of the environment, the surroundings and conditions in which the individual lives, imposes multifaceted effects on food choice and eating. At the most basic level, food choices are first and foremost determined by the variety of foods available in the individual’s environment. Food availability is a consequence of geography (climate) and the level of industrial development, rural or urban, as well as social and economic factors. Climate, topography, and soil conditions are just a few of the geographical characteristics that determine the type and quality of food that can be produced in a region. Outside of local food resources, food availability is largely dependent on industrialization and socioeconomic factors, in that urban areas generally have a more diverse food selection due to importation of fresh and processed foods and access to prepared meals by the food service industry. Food availability also varies by socioeconomic factors, with inequitable access by income. Interestingly, characteristics of both the rural and urban food environments have been associated with obesity. Some studies have found that rural communities have less access to healthy foods, which may associate with the development of unhealthy food preferences and poor diet with increased incidence and prevalence of obesity (67). Indeed, in a study of 20 weight-stable adults, Hall et al. found that consumption of ultra-processed foods led individuals to consume more calories (~500 kcal/day) compared to consumption of unprocessed foods (6). Due to the heterogeneity of rural communities, the translatability and generalizability of research findings between these different communities remains difficult. Interestingly, the problem of access to healthy food also exists in microcosms of the urban environment. Urban environments can be broken down into neighborhoods; neighborhoods can be very heterogeneous demographically, and those demographics typically influences the type and quantity of retailers established in the neighborhood. Recent research has highlighted the possibility of “food deserts”, areas devoid of healthy food options as well as “food swamps” areas with high-density of establishments selling high-calorie fast food and junk food. Though both have had mixed findings relative to obesity. A recent review suggests a stronger association between food swamps and obesity than for the absence of full-service grocery stores (68). However, there are inconsistencies in this literature, likely attributable to the heterogeneity amongst and within such environments.
The environment can also influence physiological conditions that in turn influence eating behavior. As previously discussed, research suggests that the microbiome may relate to eating behaviors and the development of obesity. Recently, research has begun to investigate the profile of the gut microbiota and microbial metabolites in obese populations and in urban versus rural populations. Indeed, differences are found in the microbiota from urban versus rural subjects, where the composition of the urban microbiota was associated with decreased microbial diversity and increased abundance of microbes previously linked with various disease states (50). These preliminary data support the collection of more comprehensive molecular data to better understand the relationship between the environment and eating behavior. While it is clear the environment directly influences eating behavior, the environment also contributes to physiological responses that indirectly modulate food choice and metabolism.
Social Dynamics
The development of children’s eating behaviors reflects a complex interplay of intrinsic genetic and physiological predispositions around eating, such as preference for basic tastes, as well as dimensions of the social environment in which eating occurs. The family is the first and among the most fundamental sources of social influence on children’s eating behaviors. Characteristics of the family unit that are known to influence food choice in children include cultural context, socioeconomic status, educational background of the parents, and parenting style (69,70). These influences begin to shape ingestive behavior at the earliest stages of development, starting with the influence of foods selected by the mother during pregnancy and breastfeeding on flavor preferences during weaning (71). From early childhood throughout the developmental years, exposure to different types of food, eating patterns, and eating habits contributes to the formation of food preferences and habits that often perpetuate throughout adulthood (72). Early exposure to a variety of foods, especially healthy foods like vegetables and fruits, promotes acceptance, preference, and consumption of such foods in older children (73,74).
Snacking (i.e. eating between meals) behaviors among young children well illustrate the import of social nature of family influences on children’s dietary and weight outcomes. In recent decades, snacking has become nearly universal among young children and has increased in frequency as well as contributions to daily energy, currently providing more than 1 out of every 4 calories consumed daily by US preschoolers. Recent data have associated the number, size, and energy-density of snacking episodes with the diet quality and the weight status of young children (75). Caregivers including parents make important decision about the quality and amounts of foods offered to as snacks and also shape children’s behaviors through feeding practices surrounding snacks. These data illustrate how social influences shape norms for eating and point to the need for evidence-based guidance to develop early interventions to support the development of eating habits of children.
Strategies to Target Eating-Induced Overweight and Obesity
Despite our current understanding of the intrinsic and extrinsic determinants of food choice, there remain several tried-and-true strategies that physicians recommend or that individuals try themselves to help manage weight. Targeted strategies such as behavioral modifications (diet and exercise), neuromodulation, environmental and social change, use of pharmacotherapy, bariatric surgery, seem to have a positive yet highly variable effect on weight management. Targeted behavioral therapies and psychological-based approaches to get individuals to adhere to specific dietary and exercise regimens are useful when undertaking any lifestyle modification program. Moreover, a combination of these approaches often offer superior and likely synergistic effects on weight management. There are also environmental and social factors that can impact weight loss success.
Behavioral Modifications
Overweight and obesity are thought to be the result of the chronic imbalance between energy intake and energy expenditure, where energy intake exceeds energy expenditure over an extended period. Although standard weight loss tactics often involve adopting healthier lifestyle habits that reduce energy intake (calories consumed) and/or increase energy expenditure (physical activity, exercise), alternative approaches focusing on macronutrient ratio and quality have been proposed.
Energy (Macronutrient) Intake.
We have known for many years that maintaining energy balance (weight) requires the balance of intake and oxidation of the three main macronutrients—protein, carbohydrate, and fat (76). During weight gain, the amount of excess calories consumed matters more if they come from fat. Protein and carbohydrate (and alcohol) stores are tightly controlled and regulated by the human body (76–79), whereas fat is the only macronutrient that contributes to a chronic imbalance between intake and oxidation and thus leads to the accumulation of adipose mass (77,80). During spontaneous overfeeding, all excess fat intake that is not oxidized is stored as body fat (78). Additionally, ingestion of a mixed meal results in increased carbohydrate oxidation and decreased fat oxidation, and the addition of extra fat does not appear to alter this macronutrient oxidation relationship (76,79). There is growing evidence to suggest that the quality of calories consumed also matters. Indeed, consuming different proportions of protein, carbohydrate, and fat (and often alcohol) all cause different physiological responses, including the rise and fall of circulating hormones and substrates, that can ultimately impact eating behavior and energy expenditure (81).
According to the “carbohydrate-insulin model” of obesity, an increased proportion of carbohydrates (especially those with a high glycemic load) in the diet results in elevated postprandial insulin that shifts substrate partitioning away from oxidation and toward calorie deposition in adipose tissue (82,83). The decreased availability of metabolic fuels, including free fatty acids and glucose, in the late postprandial period elicits a starvation-like response, driving an adaptive increase in energy (food) intake and a decrease in energy expenditure. Such hypothesis seems to be somewhat supported by data showing that low-carbohydrate (high-fat) diets may have a modest advantage for weight loss compared to low-fat diets (84,85). After initial weight loss, a low-carbohydrate (high-fat) isocaloric diet was associated with a smaller reduction in energy expenditure compared to low-fat (high-carbohydrate) (86). While short-term studies often reveal variability in the effects of diet composition on metabolism, a recent 5-month study found a higher energy expenditure via doubly labeled water [209 kcal/day (91 to 326)] with a low-carbohydrate (high-fat) diet during weight loss maintenance (87). This higher energy expenditure with a low-carbohydrate (high-fat) diet, however, was not observed to the same extent in a well-controlled inpatient 4-week trial (though not randomized) with only 57±13 kcal/day higher 24-hour energy expenditure measured in a metabolic chamber and no fat mass loss (88).
Indeed, not all feeding studies agree on the relative effects of dietary carbohydrate and fat. Several randomized, controlled trials have performed important head-to-head comparisons of different diets of differing macronutrient compositions on weight loss (85,89–92). These data reveal that low-carbohydrate (high-fat) and Mediterranean-style diets may be superior for weight loss while a low-fat (high-carbohydrate) diet may provide a better approach for maintaining weight loss (93). In contrast to the aforementioned benefits of a low-fat (high-carbohydrate) diet, a recent meta-analysis of 32 controlled “isocaloric” feeding studies revealed very small effect size differences in energy expenditure and fat loss between the low-fat and low-carbohydrate diets with equal protein (3). The median length of the 32 studies was less than 1-week, which may be insufficient to account for transient adaptive responses following carbohydrate restriction. Overall, this report concludes that “a calorie is a calorie” when it comes to body fat and energy expenditure differences between controlled isocaloric diets varying in ratio of carbohydrate and fat (3). Another well-controlled, isocaloric diet further revealed that a diet low in dietary fat (high-carbohydrate) results in more fat loss (94). Despite this ongoing debate, many macronutrient-focused behavioral studies show little difference in long-term weight loss among different diets (95–97). Such finding may indicate the need to select diets according to personal behavioral and biological factors predicting long-term compliance as well as a need for intensive, high-quality dietary trials (98). Furthermore, introducing functional foods that promote weight loss and satiety may have an important practical application in increasing compliance to a negative energy balance (99).
Future weight management studies should: (1) extend the duration of the dietary intervention so that the long-term benefits of different diets can be adequately examined; (2) assess differences in ad libitum food intake in response to prescribed, tightly controlled intake of different dietary compositions; (3) assess why humans do not automatically adjust energy intake to energy expenditure, as well as why certain individuals respond differently to dietary intake (and the interaction of differences in body composition); and examine calorie-independent effects of diet composition on various components of energy expenditure.
Energy (Exercise) Expenditure.
Exercise is commonly recommended for weight management, yet actual weight loss is only ~30–40% of expected based on measured caloric expenditure in more long-term studies (more than 6 months) (100–102). This discrepancy is defined as a metabolic compensation (or adaptation), and is likely the result of reduced metabolic rate, decreased spontaneous activity, and/or increased energy intake that results from weight loss. Important findings from the Examination of Mechanisms of Exercise-induced Weight Compensation (E-MECHANIC) study (103) revealed no difference in weight loss following two different doses of controlled aerobic exercise training [900 vs. 2,250 kcal/week] after 6 months. The findings suggest that higher doses of exercise resulted in greater ad libitum compensation, which was primarily due to increased energy intake and appetite (104). Furthermore, it has been suggested that clinically meaningful weight loss—unless the overall volume of exercise is very high (105–107)—is unlikely to occur in response to aerobic exercise training (108). Specifically, exercise-related energy expenditure may need to exceed 500–600 kcals per exercise session to induce weight loss and visceral fat loss, which is well above the recommended levels of physical activity (105). Poor adherence to exercise prescriptions further supports that targeting energy expenditure for weight loss may not be efficacious, and that exercise (both aerobic or resistance) may be a better strategy for weight maintenance after initial weight loss due to increased ad libitum feeding rather than an immediate first-line approach (104).
Future studies should: (1) explore the long-term effect of exercise on macronutrient intake, snacking, and ingestion of calorie-containing beverages; (2) assess the impact of exercise prescription on the remaining energy expenditure budget, including spontaneous physical activity and sleep quality and duration; and (3) assess the individual variability in exercise responses and how this affects the ability of exercise to promote weight loss and/or facilitate long-term weight loss maintenance.
Neuromodulation
The brain is the origin of where decisions are made with respect to what we eat and how much we eat. Exciting new developments in the area of non-invasive neuromodulation (stimulation) and neurofeedback research has provided promising information on our current knowledge of appetite control and disordered eating behaviour (109,110). Non-invasive techniques include transcranial magnetic stimulation (rTMS), transcranial direct-current stimulation (tDCS), and fMRI neurofeedback. These non-invasive neuromodulation techniques can cause acute or even permanent changes in brain circuits and brain physiology in a safe manner thereby opening an entire new avenue of ways to potentially regulate appetite (111). Reducing food cravings by altering motivational and cognitive brain processes is one such benefit, and proof-of-principle studies are currently underway to better understand the exact mechanisms that regulate appetite (112,113). If we are to treat eating disorders and obesity with the use of these techniques, standardized protocols and treatment paradigms are needed and are of important relevance (114). The prospect of combining non-invasive techniques with other treatments should also be explored in addition to understanding the long-term effects of such combined therapies on eating behaviour.
Pharmacotherapy
All the medications currently approved by the United States FDA (Food and Drug Administration) for weight management, with one exception (Orlistat), produce weight loss by targeting appetite centrally to reduce energy (food) intake. Orlistat, a pancreatic lipase inhibitor, is prescribed with instructions to avoid fat in meals or snacks. Specifically, Orlistat blocks the absorption of 30% of ingested fat, and if taken as directed three times daily, will rapidly produce steatorrhea if fatty foods are ingested. Orlistat is the only medication that is FDA approved for weight management in adolescents with obesity. The other FDA-approved medications that target appetite and are approved for long-term (chronic) treatment of obesity include lorcaserin, liraglutide, phentermine, and the combinations of phentermine/topiramate extended-release (ER), as well as naltrexone/bupropion sustained-release (SR) (115). The exact mechanisms by which these medications target appetite, however, are not entirely understood. In practice, when patients with obesity are treated with medications (or surgery of lifestyle alone) there is enormous variation in weight loss response, with 20–40% of patients (depending on medication and dose) not achieving weight loss of even 5% at 6 months. Despite this observation, the phenotypic and genotypic characteristics of non-responders or responders have not been identified. In addition, there are no reported head-to-head comparisons of these medications to evaluate their relative weight loss efficacy. An important meta-analysis of weight-loss medications, however, reports the proportion of patients who achieve >5% weight loss at one year in those patients treated with placebo (23%), orlistat (44%), lorcaserin (49%), naltrexone/bupropion SR (55%), liraglutide (63%), and phentermine/topiramate ER (75%) (116).
Advances in understanding the biology of food intake regulation and identification of targets from human single gene defects, animal studies, and studies of bariatric surgery, have yielded new directions for anti-obesity medications. Older medications relied on targeting monoamines (including phentermine, lorcaserin, bupropion) for drug development or simply the observation of weight loss with a drug (topiramate). Greater knowledge of food intake biology has resulted in identifying an emerging group of medications (e.g., GLP-1 receptor agonists liraglutide and semaglutide; setmelanotide, and agonist of the melanocortin 4 receptor; single molecules with dual or triple targets around GLP-1, Glucagon and GIP; GDF15 identified from fed and fasted rodent proteomics). Specifically, semaglutide is a very exciting, new therapy that is currently being tested in phase III trials and has been shown to reduce cravings, glycemia, and lower the rate of cardiovascular death and cardiovascular disease in patients with type 2 diabetes (117). Semaglutide 2.4 mg weekly is expected to produce average weight loss >10% in persons with obesity. The same semaglutide is also being studied in a cardiovascular outcome trial in patients with pre-existing cardiovascular disease, but with diabetes. If this trial demonstrates a reduction in cardiovascular events, it will be a game-changer for the obesity field. Other emerging pathways forward in drug development have been a precision medicine approach, using knowledge of single gene defects (e.g., leptin and setmelanotide) (118,119). However, these approaches are only going to be approved for rare genetic obesities, including individuals with rare variants of the leptin-melanocortin pathway.
The development of combination drugs (i.e., phentermine/topiramate ER, naltrexone/bupropion SR) are harbingers of newer approaches to pharmacologic weight management, where we will use medications for obesity like we do for treating hypertension; using multiple agents together to maximize efficacy and safety. An innovative combination approach is to develop single molecule, dual action drugs by combining members of the glucagon family (peptides from both GLP1 with GIP or GLP1 with glucagon) or even single molecule triple action (GLP, GIP, and glucagon combinations). These drugs called coagonists and triagonists (120) are also rising to phase II studies in potential new treatment avenues. Newer medications produce more weight loss on average, and combination approaches also produce greater mean weight losses. Within 10 years, such novel approaches may produce twice the mean weight loss that we have been able to achieve today.
Metabolic (Bariatric) Surgery Treatment
The growing epidemic of obesity, along with unsatisfactory results of conventional weight reduction programs, has led to a remarkable rise in the number of bariatric surgeries performed in the last decade. Compared to 2015, the total number of metabolic and bariatric procedures performed in the United States in 2016 increased from approximately 196,000 to 216,000 (or +10%). According to the American Society for Metabolic and Bariatric Surgery (ASMBS), the most common bariatric operations in 2017 include sleeve gastrectomy (59%), Roux-en-Y gastric bypass (RYGB; 18%), gastric banding (3%), biliopancreatic diversion (1%), and others (19%). To date, bariatric surgery is the only known treatment for obesity that has been shown to yield durable weight loss (>20% body weight) beyond 10 years. Indeed, the most well-studied effect of bariatric surgery is the immediate and sustained improvement (and in some cases complete remission) of type 2 diabetes (121–123). A reduction in overall mortality of 30 to 80% has also been observed (124–127) in observational studies but not yet in prospective randomized trials.
The mechanisms by which bariatric surgery—particularly RYGB—causes long-term weight loss have not been fully elucidated. We do know that the sustained reductions in appetite and enhanced satiety that occur post-surgery are likely associated with observed weight loss. A host of gut-derived hormones (e.g., ghrelin, GLP1, PYY, and CCK) are also altered post-surgery and can play important roles in energy homeostasis (128). Of all the surgery techniques, RYGB is currently the most effective treatment in common use for severe obesity. There are clinical and preclinical studies suggesting that RYGB not only decreases food intake but changes preference in favor of foods and fluids with lower fat and sugar content (129,130). However, the decrease in relative fat and sugar intake does not appear to be driven by RYGB-induced decreases in the palatability of the taste of these nutrients (129).
Moving forward, more comprehensive studies are needed in both human and animal models to assess whether the sensory, motivational, and physiological domains of taste function are impacted by RYGB and other bariatric surgery models. Conversely, human research on the effects of RYGB on eating, food preference, and taste-driven motivation should focus less on verbal reporting of these traits (such as scaling, surveys, and dietary recall) and more on direct measures of the targeted behaviors. Improved understanding of mechanisms that drive appetite reduction and altered taste perception after surgery may ultimately translate to more effective and scalable dietary and pharmacologic interventions. Despite the benefits of bariatric surgery only 1% of eligible patients undergo surgery, thus, greater access to surgery could enable many more to benefit. On the other hand, not all individuals are ideal candidates for surgery, consequently, public health tactics to prevent or revert the increasing obesity trends is of upmost importance.
Public Health Approaches
The prevalence of obesity is increasing in every region of the world. While many evidence-based policy recommendations aimed at halting and reversing obesity rates have been endorsed by the public and many governing bodies at the global, country, and state/province level, very little progress has been made (10). According to the Lancet Commission on Obesity, much of the lack of progress is due to policy inertia, or the inadequate political leadership and governance to enact policies in the face of powerful commercial interests and lobbying forces with opposing interests. While public opinion supports the need for health improvement policies that combat obesity, the lack of demand for policy action by the public is also to blame (10). Unfortunately, external factors like climate change, and the similar denial observed among commercial interest and lobbying groups, will further compound the rise in obesity and malnutrition if no action is taken. It is important that we understand the integration of biological, social (behavioral and cultural), and environmental factors and its relationship to the heterogeneity of obesity.
Indeed, a focus on modifying and uniting “complex adaptive systems,” which include health systems, school, and families, has been suggested to combat existing policy resistance and create a more homeostatic health environment. Mandates, restrictions, reimbursements, economic incentives or excise taxes are just some of the things our health systems could utilize to change the way that schools and families interact and potentially alter the course of obesity (131). A recent systematic review of natural- or quasi-experiments found that local and school policies to improve food environments (e.g., bans/restrictions on unhealthy foods, mandates offering healthier foods, and economic purchasing regulations) were more successful than other interventions (e.g., menu labelling, new supermarkets) (132). In order to implement such policy changes, however, it is critical that research scientists work together with policy makers and influencers to draft such policies (133). Indeed, policy changes are often accompanied by negative perceptions for how they will affect both business’ bottom-line and consumers’ wallets. Using the Philadelphia Beverage Tax as a model for how policy change can affect purchasing behavior, a 1.5 cents per ounce tax on sugar- and artificially-sweetened beverages was implemented in January 2017. This tax was associated with a 38% reduction in taxed beverage sales, after accounting for some individuals shopping outside the City to avoid the tax (134).
Parental influence of young children’s eating habits also serve as important models of social influence and subsequent eating behavior (74). For instance, snacking is nearly universal among young children in the United States and represents close to one-third of daily energy intake—more than any other single meal. These snacks are often high in solid fats and added sugars for non-nutritive reasons, which further contributes to weight gain. While a few studies have shown that repeated exposure to healthier foods earlier in life actually help children learn to prefer healthier foods over unhealthier options (135), data indicating the types of parenting practices that support healthful snacking patterns in young children is limited and should be explored more in depth.
Conclusion
In order to reduce the prevalence of obesity and its economic burden, there is a growing need for widespread dietary change. Furthermore, and on a larger scale, there is a valid argument for choosing dietary patterns that promote the sustainability of our food supply and limit carbon emissions (greenhouse gases), such as limiting consumption of ultra-processed food, as well as meat and dairy products. Yet, while changing one’s dietary intake may appear straight forward, there are many determinants that affect food choices that are important to understand. Indeed, each person’s eating habits are likely influenced by a unique combination of both intrinsic and extrinsic factors that regulate feeding behavior. As such, the strategies used to improve food choice must be tailored to fit individual needs rather than a one-size fits all model. And while adherence to specific diets (low-fat versus high-fat) is often a topic of intense debate, many macronutrient-focused studies have reported little difference in long-term weight loss outcomes. However, better designed trials are needed to answer the efficacy and safety of specific diet on weight management outcomes. While there are indeed pros and cons of many of these diets, choosing a diet you are best able to adhere to over the long-term is arguably important. At the same time, dietary composition may influence an individual’s ability to adherence to a diet through biological mechanisms, including increased hunger and lower energy expenditure. As pharmacotherapy and metabolic surgery approaches to treat obesity are becoming increasingly prevalent, the long-term consequences of such treatment approaches are not entirely understood and are important for studies to disentangle in the years to come. Despite the progress that scientists have made in reducing the prevalence and economic burden of obesity, a stalemate persists between what is known and what is actually implemented at the local, state, national, and global levels. Society must consider what changes can be instated, either by cultural movement or policy-driven mandates/public health practices, to shift the food environment to favor healthier and more sustainable food choices. Bridging the gap between what we know as scientists and what policy makers promote is critical in order to instill long-term change.
Study Important Questions.
What major reviews have already been published on this subject?
Leaders in the scientific community surrounding the determinants of food choice convened at the Pennington Biomedical for a scientific symposium entitled “What should I eat and why?” Our findings are summarized in the present review.
What are the new findings in your manuscript?
This review is unique to other reviews as it discussed the environmental, genetic, and behavioral determinants of food choice and its impact on weight gain and obesity.
How might your results change the direction of research or the focus of clinical practice?
We hope that our review will provide a concise summary of where the scientific field is heading to curb the obesity epidemic.
Acknowledgements
Brief Statement of Assistance: The authors thank David S. Ludwig, MD, PhD, of the Harvard School of Public Health and Harvard Medical School, as well as Christina A. Roberto, PhD, of the Perelman School of Medicine at the University of Pennsylvania, for their participation in the 2018 Pennington Biomedical Scientific Symposium Series and their contributions to this manuscript.
Funding: Supported by an “Educational Grant from Novo Nordisk Inc.”, Amway, Medtronic, Blue Cross and Blue Shield of Louisiana, Louisiana NORC, and Pennington Biomedical Research Center. KLM was also supported by the NIDDK sponsored Ruth L. Kirschstein National Research Service T32 Research Training Grant (T32-DK064584).
Disclosure Statement: T.S.C. is an employee of Naturally Slim, and is also the founder, shareholder, and board member of Genetic Direction, Catapult Health, and Fitness Interactive Experience. D.H.R. has served as a consultant or advisor to Alyvent, Amgen, Bausch Health, Boeringer Ingelheim, Epitomee, Gila Therapeutics, IFA Celtic, Janssen, Novo Nordisk, Phenomix, real appeal (United Health), ReDesign Health, Sanofi, and Scientific Intake. D.H.R. has also been a speaker for Novo Nordisk, and has equity in Alyvent, Gila Therapeutics, Phenomix, Xeno Bioscience, Epitomee, ReDesign Health, and Scientific Intake. P.R.S. is supported by Ethicon, Medtronic, and Pacira; serves on the advisory panel of GI Dynamics and Keyron; is a shareholder of SE Healthcare LLC; and is a consultant for WL Gore, BD Surgical, Global Academy for Medical Education, Medtronic, and Ethicon.
Footnotes
ClinicalTrials.gov Registration: N/A
All other authors have no conflicts of interest to report.
References
- 1.Speakman JR. Obesity: The Integrated Roles of Environment and Genetics. J Nutr. 2004;134(8):2090S–2105S. doi: 10.1093/jn/134.8.2090s [DOI] [PubMed] [Google Scholar]
- 2.Guyenet SJ, Schwartz MW. Regulation of food intake, energy balance, and body fat mass: Implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab. 2012;97(3):745–755. doi: 10.1210/jc.2011-2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall KD, Guo J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology. 2017;152(7):1718–1727.e3. doi: 10.1053/j.gastro.2017.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paniagua JA, Pérez-Martinez P, Gjelstad IMF, et al. A low-fat high-carbohydrate diet supplemented with long-chain n-3 PUFA reduces the risk of the metabolic syndrome. Atherosclerosis. 2011;218(2):443–450. doi: 10.1016/j.atherosclerosis.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 5.Martínez Steele E, Popkin BM, Swinburn B, Monteiro CA. The share of ultra-processed foods and the overall nutritional quality of diets in the US: Evidence from a nationally representative cross-sectional study. Popul Health Metr. 2017;15(1):6. doi: 10.1186/s12963-017-0119-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall KD, Ayuketah A, Brychta R, et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019;30(1):67–77.e3. doi: 10.1016/j.cmet.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh S, Bouchard C. Convergence between biological, behavioural and genetic determinants of obesity. Nat Rev Genet. 2017;18(12):731–748. doi: 10.1038/nrg.2017.72 [DOI] [PubMed] [Google Scholar]
- 8.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y, Day FR, Gustafsson S, et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat Commun. 2016;7:10495. doi: 10.1038/ncomms10495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swinburn BA, Kraak VI, Allender S, et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet. 2019. doi: 10.1016/S0140-6736(18)32822-8 [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Day FR, Gustafsson S, et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat Commun. 2016. doi: 10.1038/ncomms10495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacob R, Drapeau V, Tremblay A, Provencher V, Bouchard C, Pérusse L. The role of eating behavior traits in mediating genetic susceptibility to obesity. Am J Clin Nutr. 2018;108(3):445–452. doi: 10.1093/ajcn/nqy130 [DOI] [PubMed] [Google Scholar]
- 13.Smith AD, Fildes A, Cooke L, et al. Genetic and environmental influences on food preferences in adolescence. Am J Clin Nutr. 2016;104(2):446–453. doi: 10.3945/ajcn.116.133983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AD, Fildes A, Forwood S, Cooke L, Llewellyn C. The individual environment, not the family is the most important influence on preferences for common non-Alcoholic beverages in adolescence. Sci Rep. 2017;7(1). doi: 10.1038/s41598-017-17020-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: An evolutionary-developmental perspective. Int J Obes. 2008. doi: 10.1038/ijo.2008.240 [DOI] [PubMed] [Google Scholar]
- 16.Lillycrop KA, Burdge GC. Epigenetic changes in early life and future risk of obesity. Int J Obes. 2011;35(1):72–83. doi: 10.1038/ijo.2010.122 [DOI] [PubMed] [Google Scholar]
- 17.Plagemann A, Harder T, Brunn M, et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: An epigenetic model of obesity and the metabolic syndrome. J Physiol. 2009;587(20):4963–4976. doi: 10.1113/jphysiol.2009.176156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loos RJF, Janssens ACJW. Predicting Polygenic Obesity Using Genetic Information. Cell Metab. 2017. doi: 10.1016/j.cmet.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 19.Baile CA, Della-Fera MA. Central nervous system cholecystokinin and the control of feeding. Ann N Y Acad Sci. 1985;448:424–430. [DOI] [PubMed] [Google Scholar]
- 20.Bates SH, Myers MG. The role of leptin receptor signaling in feeding and neuroendocrine function. Trends Endocrinol Metab. 2003;14(10):447–452. doi: 10.1016/j.tem.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 21.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. doi: 10.1038/35051587 [DOI] [PubMed] [Google Scholar]
- 22.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534 [DOI] [PubMed] [Google Scholar]
- 23.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18(2):158–168. doi: 10.1016/j.numecd.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 24.Solon-Biet SM, McMahon AC, Ballard JWO, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19(3):418–430. doi: 10.1016/j.cmet.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berthoud HR, Münzberg H, Richards BK, Morrison CD. Neural and metabolic regulation of macronutrient intake and selection. In: Proceedings of the Nutrition Society.; 2012. doi: 10.1017/S0029665112000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laeger T, Henagan TM, Albarado DC, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–3922. doi: 10.1172/JCI74915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iroz A, Montagner A, Benhamed F, et al. A Specific ChREBP and PPARα Cross-Talk Is Required for the Glucose-Mediated FGF21 Response. Cell Rep. 2017. doi: 10.1016/j.celrep.2017.09.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill CM, Laeger T, Dehner M, et al. FGF21 Signals Protein Status to the Brain and Adaptively Regulates Food Choice and Metabolism. Cell Rep. 2019. doi: 10.1016/j.celrep.2019.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talukdar S, Owen BM, Song P, et al. FGF21 regulates sweet and alcohol preference. Cell Metab. 2016;23(2):344–349. doi: 10.1016/j.cmet.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Von Holstein-Rathlou S, Bondurant LD, Peltekian L, et al. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 2016;23(2):335–343. doi: 10.1016/j.cmet.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: Current knowledge and future directions. Obes Rev. 2012;13(1):43–56. doi: 10.1111/j.1467-789X.2011.00927.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gluck ME, Viswanath P, Stinson EJ. Obesity, appetite, and the prefrontal cortex. Curr Obes Rep. 2017;6(4):380–388. doi: 10.1007/s13679-017-0289-0 [DOI] [PubMed] [Google Scholar]
- 33.Val-Laillet D, Aarts E, Weber B, et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage Clin. 2015;8:1–31. doi: 10.1016/j.nicl.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen LK, Duif I, van Loon I, et al. Loss of lateral prefrontal cortex control in food-directed attention and goal-directed food choice in obesity. Neuroimage. 2017;146:148–156. doi: 10.1016/j.neuroimage.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 35.Janssen LK, Duif I, Van Loon I, et al. Greater mindful eating practice is associated with better reversal learning. Sci Rep. 2018;8(1):5702. doi: 10.1038/s41598-018-24001-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Holst RJ, van der Cruijsen L, van Mierlo P, et al. Aberrant food choices after satiation in human orexin-deficient narcolepsy Type 1. Sleep. 2016;39(11):1951–1959. doi: 10.5665/sleep.6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Holst RJ, Janssen LK, van Mierlo P, et al. Enhanced food-related responses in the ventral medial prefrontal cortex in narcolepsy type 1. Sci Rep. 2018. doi: 10.1038/s41598-018-34647-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth CL, Aylward E, Liang O, Kleinhans NM, Pauley G, Schur EA. Functional neuroimaging in craniopharyngioma: A useful tool to better understand hypothalamic obesity? Obes Facts. 2012;5(2):243–253. doi: 10.1159/000338695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Der Klaauw AA, Von Dem Hagen EAH, Keogh JM, et al. Obesity-associated melanocortin-4 receptor mutations are associated with changes in the brain response to food cues. J Clin Endocrinol Metab. 2014;99(10):E2101–E2106. doi: 10.1210/jc.2014-1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berthoud HR, Münzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: From electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104(1):29–39. doi: 10.1016/j.physbeh.2011.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qualls-Creekmore E, Münzberg H. Modulation of feeding and associated behaviors by lateral hypothalamic circuits. Endocrinology. 2018;159(11):3631–3642. doi: 10.1210/en.2018-00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sohn JW, Elmquist JK, Williams KW. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013. doi: 10.1016/j.tins.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas MA, Xue B. Mechanisms for AgRP neuron-mediated regulation of appetitive behaviors in rodents. Physiol Behav. 2018;190:34–42. doi: 10.1016/j.physbeh.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jennings JH, Ung RL, Resendez SL, et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160(3):516–527. doi: 10.1016/j.cell.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stamatakis AM, Van Swieten M, Basiri ML, Blair GA, Kantak P, Stuber GD. Lateral Hypothalamic Area Glutamatergic Neurons and Their Projections to the Lateral Habenula Regulate Feeding and Reward. J Neurosci. 2016. doi: 10.1523/jneurosci.1202-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qualls-Creekmore E, Yu S, Francois M, et al. Galanin-Expressing GABA Neurons in the Lateral Hypothalamus Modulate Food Reward and Noncompulsive Locomotion. J Neurosci. 2017;37(25):6053–6065. doi: 10.1523/jneurosci.0155-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodworth HL, Beekly BG, Batchelor HM, et al. Lateral Hypothalamic Neurotensin Neurons Orchestrate Dual Weight Loss Behaviors via Distinct Mechanisms. Cell Rep. 2017;21(11):3116–3128. doi: 10.1016/j.celrep.2017.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018. doi: 10.1038/nature25973 [DOI] [PubMed] [Google Scholar]
- 49.Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science (80- ). 2016. doi: 10.1126/science.aad3503 [DOI] [PubMed] [Google Scholar]
- 50.Winglee K, Howard AG, Sha W, et al. Recent urbanization in China is correlated with a Westernized microbiome encoding increased virulence and antibiotic resistance genes. Microbiome. 2017;5(1):121. doi: 10.1186/s40168-017-0338-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aron-Wisnewsky J, Prifti E, Belda E, et al. Major microbiota dysbiosis in severe obesity: Fate after bariatric surgery. Gut. 2019;68(1):70–82. doi: 10.1136/gutjnl-2018-316103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-Induced Obesity Is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome. Cell Host Microbe. 2008;3(4):213–223. doi: 10.1016/j.chom.2008.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maruvada P, Leone V, Kaplan LM, Chang EB. The Human Microbiome and Obesity: Moving beyond Associations. Cell Host Microbe. 2017;22(5):589–599. doi: 10.1016/j.chom.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 54.Griffin NW, Ahern PP, Cheng J, et al. Prior Dietary Practices and Connections to a Human Gut Microbial Metacommunity Alter Responses to Diet Interventions. Cell Host Microbe. 2017;21(1):84–96. doi: 10.1016/j.chom.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013. doi: 10.1038/nature12480 [DOI] [PubMed] [Google Scholar]
- 56.Koh A, Molinaro A, Ståhlman M, et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell. 2018. doi: 10.1016/j.cell.2018.09.055 [DOI] [PubMed] [Google Scholar]
- 57.Fetissov SO. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat Rev Endocrinol. 2017;13(1):11–25. doi: 10.1038/nrendo.2016.150 [DOI] [PubMed] [Google Scholar]
- 58.Al-Najim W, Docherty NG, le Roux CW. Food Intake and Eating Behavior After Bariatric Surgery. Physiol Rev. 2018;98(3):1113–1141. doi: 10.1152/physrev.00021.2017 [DOI] [PubMed] [Google Scholar]
- 59.Atlantis E, Baker M. Obesity effects on depression: Systematic review of epidemiological studies. Int J Obes. 2008;32(6):881–891. doi: 10.1038/ijo.2008.54 [DOI] [PubMed] [Google Scholar]
- 60.Bruce-Keller AJ, Salbaum JM, Luo M, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77(7):607–615. doi: 10.1016/j.biopsych.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Markowiak P, Ślizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(9):1021. doi: 10.3390/nu9091021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koutnikova H, Genser B, Monteiro-Sepulveda M, et al. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2019. doi: 10.1136/bmjopen-2017-017995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soto M, Herzog C, Pacheco JA, et al. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol Psychiatry. 2018;23(12):2287–2301. doi: 10.1038/s41380-018-0086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lai ZL, Tseng CH, Ho HJ, et al. Fecal microbiota transplantation confers beneficial metabolic effects of diet and exercise on diet-induced obese mice. Sci Rep. 2018. doi: 10.1038/s41598-018-33893-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9(2):88–96. doi: 10.1038/nrgastro.2011.244 [DOI] [PubMed] [Google Scholar]
- 66.Chang CS, Ruan JW, Kao CY. An overview of microbiome based strategies on anti obesity. Kaohsiung J Med Sci. 2019;35(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lenardson JD, Hansen AY, Hartley D. Rural and remote food environments and obesity. Curr Obes Rep. 2015;4(1):46–53. doi: 10.1007/s13679-014-0136-5 [DOI] [PubMed] [Google Scholar]
- 68.Cooksey-Stowers K, Schwartz MB, Brownell KD. Food swamps predict obesity rates better than food deserts in the United States. Int J Environ Res Public Health. 2017. doi: 10.3390/ijerph14111366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savage JS, Fisher JO, Birch LL. Parental influence on eating behavior: Conception to adolescence. In: Journal of Law, Medicine and Ethics. ; 2007. doi: 10.1111/j.1748-720X.2007.00111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scaglioni S, De Cosmi V, Ciappolino V, Parazzini F, Brambilla P, Agostoni C. Factors influencing children’s eating behaviours. Nutrients. 2018;10(6). doi: 10.3390/nu10060706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mennella JA, Daniels LM, Reiter AR. Learning to like vegetables during breastfeeding: A randomized clinical trial of lactating mothers and infants. Am J Clin Nutr. 2017. doi: 10.3945/ajcn.116.143982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101(Supplement 2):539–549. [PubMed] [Google Scholar]
- 73.Wadhera D, Capaldi Phillips ED, Wilkie LM. Teaching children to like and eat vegetables. Appetite. 2015;93:75–84. doi: 10.1016/j.appet.2015.06.016 [DOI] [PubMed] [Google Scholar]
- 74.Yee AZH, Lwin MO, Ho SS. The influence of parental practices on child promotive and preventive food consumption behaviors: A systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2017;14(1):47. doi: 10.1186/s12966-017-0501-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kachurak A, Davey A, Bailey RL, Fisher JO. Daily Snacking Occasions and Weight Status Among US Children Aged 1 to 5 Years. Obesity. 2018;26(6):1034–1042. doi: 10.1002/oby.22172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flatt JP, Ravussin E, Acheson KJ, Jequier E. Effects of dietary fat on postprandial substrate oxidation and on carbohydrate and fat balances. J Clin Invest. 1985. doi: 10.1172/JCI112054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abbott WGH, Howard BV, Christin L, et al. Short-term energy balance: Relationship with protein, carbohydrate, and fat balances. Am J Physiol - Endocrinol Metab. 1988. [DOI] [PubMed] [Google Scholar]
- 78.Rising R, Alger S, Boyce V, et al. Food intake measured by an automated food-selection system: Relationship to energy expenditure. Am J Clin Nutr. 1992. doi: 10.1093/ajcn/55.2.343 [DOI] [PubMed] [Google Scholar]
- 79.Schutz Y, Flatt JP, Jequier E. Failure of dietary fat intake to promote fat oxidation: A factor favoring the development of obesity. Am J Clin Nutr. 1989. doi: 10.1093/ajcn/50.2.307 [DOI] [PubMed] [Google Scholar]
- 80.Zurlo F, Lillioja S, Esposito-Del Puente A, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol Metab. 2017;259(5):E650–E657. doi: 10.1152/ajpendo.1990.259.5.e650 [DOI] [PubMed] [Google Scholar]
- 81.Flatt JP. McCollum award lecture, 1995: Diet, lifestyle, and weight maintenance. Am J Clin Nutr. 1995;62(4):820–836. doi: 10.1093/ajcn/62.4.820 [DOI] [PubMed] [Google Scholar]
- 82.Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA - J Am Med Assoc. 2014;311(21):2167–2168. doi: 10.1001/jama.2014.4133 [DOI] [PubMed] [Google Scholar]
- 83.Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “calories in, calories out.” JAMA Intern Med. 2018;178(8):1098–1103. doi: 10.1001/jamainternmed.2018.2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: A meta-analysis. JAMA - J Am Med Assoc. 2014;312(9):923–933. doi: 10.1001/jama.2014.10397 [DOI] [PubMed] [Google Scholar]
- 85.Nordmann AJ, Nordmann A, Briel M, et al. Effects of Low-Carbohydrate vs Low-Fat Diets on Weight Loss and Cardiovascular Risk Factors. Arch Intern Med. 2006;166(3):285–293. doi: 10.1001/archinte.166.3.285 [DOI] [PubMed] [Google Scholar]
- 86.Ebbeling CB, Swain JF, Feldman HA, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA - J Am Med Assoc. 2012;307(24):2627–2634. doi: 10.1001/jama.2012.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ebbeling CB, Feldman HA, Klein GL, et al. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363:k4583. doi: 10.1136/bmj.k4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hall KD, Chen KY, Guo J, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016. doi: 10.3945/ajcn.116.133561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone Diets for weight loss and heart disease risk reduction: A randomized trial. J Am Med Assoc. 2005;293(1):43–53. doi: 10.1001/jama.293.1.43 [DOI] [PubMed] [Google Scholar]
- 90.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: The A to Z weight loss study: A randomized trial. J Am Med Assoc. 2007;297(9):969–977. doi: 10.1001/jama.297.9.969 [DOI] [PubMed] [Google Scholar]
- 92.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–241. [DOI] [PubMed] [Google Scholar]
- 93.Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: An endocrine society scientific statement. Endocr Rev. 2018. doi: 10.1210/er.2017-00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hall KD, Bemis T, Brychta R, et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab. 2015;22(3):427–436. doi: 10.1016/j.cmet.2015.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pagoto SL, Appelhans BM. A call for an end to the diet debates. JAMA - J Am Med Assoc. 2013;310(7):687–688. doi: 10.1001/jama.2013.8601 [DOI] [PubMed] [Google Scholar]
- 96.Gardner CD, Trepanowski JF, Gobbo LCD, et al. Effect of low-fat VS low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion the DIETFITS randomized clinical trial. JAMA - J Am Med Assoc. 2018;319(7):667–679. doi: 10.1001/jama.2018.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Freedman MR, King J, Kennedy E. Popular diets: a scientific review. Obes Res. 2001;9(Supplement 1):1S–40S. [DOI] [PubMed] [Google Scholar]
- 98.Ludwig DS, Ebbeling CB, Heymsfield SB. Improving the quality of dietary research. JAMA - J Am Med Assoc. 2019. doi: 10.1001/jama.2019.11169 [DOI] [PubMed] [Google Scholar]
- 99.Rebello C, Greenway FL, Dhurandhar NV. Functional foods to promote weight loss and satiety. Curr Opin Clin Nutr Metab Care. 2014;17(6):596–604. doi: 10.1097/MCO.0000000000000110 [DOI] [PubMed] [Google Scholar]
- 100.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One. 2009. doi: 10.1371/journal.pone.0004515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Church TS, Earnest CP, Thompson AM, et al. Exercise without weight loss does not reduce C-reactive protein: The INFLAME study. Med Sci Sports Exerc. 2010;42(4):708–716. doi: 10.1249/MSS.0b013e3181c03a43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thomas DM, Bouchard C, Church T, et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? an energy balance analysis. Obes Rev. 2012. doi: 10.1111/j.1467-789X.2012.01012.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Myers CA, Johnson WD, Earnest CP, et al. Examination of mechanisms (E-MECHANIC) of exercise-induced weight compensation: Study protocol for a randomized controlled trial. Trials. 2014. doi: 10.1186/1745-6215-15-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martin CK, Johnson WD, Myers CA, et al. Effect of different doses of supervised exercise on food intake, metabolism, and non-exercise physical activity: The E-MECHANIC randomized controlled trial. Am J Clin Nutr. 2019. doi: 10.1093/ajcn/nqz054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ross R, Dagnone D, Jones PJH, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: A randomized, controlled trial. Ann Intern Med. 2000;133(2):92–103. doi: 10.7326/0003-4819-133-2-200007180-00008 [DOI] [PubMed] [Google Scholar]
- 106.Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: A randomized controlled trial. Obes Res. 2004;12(5):789–798. doi: 10.1038/oby.2004.95 [DOI] [PubMed] [Google Scholar]
- 107.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009. doi: 10.1249/MSS.0b013e3181949333 [DOI] [PubMed] [Google Scholar]
- 108.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56(4):441–447. doi: 10.1016/j.pcad.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spetter MS. Current state of the use of neuroimaging techniques to understand and alter appetite control in humans. Curr Opin Clin Nutr Metab Care. 2018;21(5):329–335. doi: 10.1097/MCO.0000000000000493 [DOI] [PubMed] [Google Scholar]
- 110.Forcano L, Mata F, de la Torre R, Verdejo-Garcia A. Cognitive and neuromodulation strategies for unhealthy eating and obesity: Systematic review and discussion of neurocognitive mechanisms. Neurosci Biobehav Rev. 2018;87:161–191. doi: 10.1016/j.neubiorev.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 111.Dalton B, Campbell IC, Schmidt U. Neuromodulation and neurofeedback treatments in eating disorders and obesity. Curr Opin Psychiatry. 2017. doi: 10.1097/YCO.0000000000000361 [DOI] [PubMed] [Google Scholar]
- 112.Spetter MS, Malekshahi R, Birbaumer N, et al. Volitional regulation of brain responses to food stimuli in overweight and obese subjects: A real-time fMRI feedback study. Appetite. 2017;112:188–195. doi: 10.1016/j.appet.2017.01.032 [DOI] [PubMed] [Google Scholar]
- 113.Kohl SH, Veit R, Spetter MS, et al. Real-time fMRI neurofeedback training to improve eating behavior by self-regulation of the dorsolateral prefrontal cortex: A randomized controlled trial in overweight and obese subjects. Neuroimage. 2019. doi: 10.1016/j.neuroimage.2019.02.033 [DOI] [PubMed] [Google Scholar]
- 114.Bartholdy S, Musiat P, Campbell IC, Schmidt U. The potential of neurofeedback in the treatment of eating disorders: A review of the literature. Eur Eat Disord Rev. 2013;21(6):456–463. doi: 10.1002/erv.2250 [DOI] [PubMed] [Google Scholar]
- 115.Bray GA, Ryan DH. Update on obesity pharmacotherapy. Ann N Y Acad Sci. 2014. doi: 10.1111/nyas.12328 [DOI] [PubMed] [Google Scholar]
- 116.Khera R, Murad MH, Chandar AK, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events. JAMA. 2016. doi: 10.1001/jama.2016.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 118.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 2002;341(12):879–884. doi: 10.1056/nejm199909163411204 [DOI] [PubMed] [Google Scholar]
- 119.Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes, Obes Metab. 2017;19(9):1242–1251. doi: 10.1111/dom.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tschöp MH, Finan B, Clemmensen C, et al. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metab. 2016;24(1):51–62. doi: 10.1016/j.cmet.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 121.Schauer P, Bhatt DL, Kirwan JP, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376(7):641–651. doi: 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: A randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2(7):545–552. doi: 10.1016/S2213-8587(14)70066-X [DOI] [PubMed] [Google Scholar]
- 123.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: The diabetes surgery study randomized clinical trial. JAMA - J Am Med Assoc. 2013;309:2240–2249. doi: 10.1001/jama.2013.5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240(3):416–424. doi: 10.1097/01.sla.0000137343.63376.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. [DOI] [PubMed] [Google Scholar]
- 126.Sjöström L, Narbro K, Sjöström CD, et al. Effects of Bariatric Surgery on Mortality in Swedish Obese Subjects. N Engl J Med. 2007;357(8):741–752. doi: 10.1056/nejmoa066254 [DOI] [PubMed] [Google Scholar]
- 127.Aminian A, Zajichek A, Arterburn DE, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA - J Am Med Assoc. 2019;44195:1–12. doi: 10.1001/jama.2019.14231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ionut V, Bergman RN. Mechanisms responsible for excess weight loss after bariatric surgery. J Diabetes Sci Technol. 2011;5(5):1263–1282. doi: 10.1177/193229681100500536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mathes CM, Bohnenkamp RA, le Roux CW, Spector AC. Reduced sweet and fatty fluid intake after Roux-en-Y gastric bypass in rats is dependent on experience without change in stimulus motivational potency. Am J Physiol Integr Comp Physiol. 2015;309(8):R864–R874. doi: 10.1152/ajpregu.00029.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ahmed K, Penney N, Darzi A, Purkayastha S. Taste changes after bariatric surgery: a systematic review. Obes Surg. 2018;28(10):3321–3332. doi: 10.1007/s11695-018-3420-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gorski M, Roberto C. Public health policies to encourage healthy eating habits: recent perspectives. J Healthc Leadersh. 2015;7:81. doi: 10.2147/JHL.S69188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mayne SL, Auchincloss AH, Michael YL. Impact of policy and built environment changes on obesity-related outcomes: A systematic review of naturally occurring experiments. Obes Rev. 2015. doi: 10.1111/obr.12269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brownell KD, Roberto CA. Strategic science with policy impact. Lancet. 2015;385(9986):2445–2446. doi: 10.1016/s0140-6736(14)62397-7 [DOI] [PubMed] [Google Scholar]
- 134.Roberto CA, Lawman HG, Levasseur MT, et al. Association of a Beverage Tax on Sugar-Sweetened and Artificially Sweetened Beverages with Changes in Beverage Prices and Sales at Chain Retailers in a Large Urban Setting. JAMA - J Am Med Assoc. 2019. doi: 10.1001/jama.2019.4249 [DOI] [PubMed] [Google Scholar]
- 135.Wadhera D, Capaldi Phillips ED, Wilkie LM, Boggess MM. Perceived recollection of frequent exposure to foods in childhood is associated with adulthood liking. Appetite. 2015;89(1):22–32. doi: 10.1016/j.appet.2015.01.011 [DOI] [PubMed] [Google Scholar]


