Abstract
Within the reticular thalamic nucleus neurons express gamma aminobutyric acid (GABA) and these cells project to the ventral posteromedial thalamic nucleus. When GABA activity decreases the activity of excitatory cells in the ventral posteromedial nucleus would be expected to increase. In this study, we addressed the hypothesis that attenuating GABAergic cells in the reticular thalamic nucleus increases excitatory activity in the ventral posteromedial nucleus increasing varicella zoster virus (VZV) associated pain in the orofacial region. Adeno-associated virus (AAV) was infused in the reticular thalamic nucleus of Gad1-Cre rats. This virus transduced a G inhibitory designer receptor exclusively activated by designer drugs (DREADD) gene that was Cre dependent. A dose of estradiol that was previously shown to reduce VZV pain and increase GABAergic activity was administered to castrated and ovariectomized rats. Previous studies suggest that estradiol attenuates herpes zoster pain by increasing the activity of inhibitory neurons and decreasing the activity of excitatory cells within the lateral thalamic region. The ventral posteromedial nucleus was infused with AAV containing a GCaMP6f expression construct. A glass lens was implanted for miniscope imaging. Our results show that the activity of GABA cells within the reticular thalamic region decreased with clozapine N-oxide treatment concomitant with increased calcium activity of excitatory cells in the ventral posteromedial nucleus and an increased orofacial pain response. The results suggest that estradiol attenuates herpes zoster pain by increasing the activity of inhibitory neurons within the reticular thalamus that then inhibit excitatory activity in ventral posteromedial nucleus causing a reduction in orofacial pain.
Keywords: orofacial, herpes zoster, shingles, pain, estrogen, thalamus
Introduction
Gamma aminobutyric acid (GABA) is expressed in the reticular thalamic nucleus and zona incerta and the neurons within these nuclei project to the ventral posteromedial nucleus (Barbaresi et al., 1986; Nicolelis et al., 1992; Pinault and Deschenes, 1998; Bartho et al., 2002). Increased activity within the GABA positive cells would inhibit signaling to the ventral posteromedial nucleus and reduce activity of these excitatory cells. Excitatory cells within the ventral posteromedial nucleus have been shown to influence varicella zoster virus (VZV) associated orofacial pain in rats (Kramer et al., 2017a; Kramer et al., 2017b). Supporting the idea that the reticular thalamic region controls orofacial pain responses by modulating the activity of excitatory neurons in the reticular thalamic region.
VZV is responsible for herpes zoster or “shingles” pain and females report more zoster associate pain than men (Alvarez et al., 2007; Hillebrand et al., 2015). Estradiol effects neuronal activity within the thalamus, such as, increasing activity in the thalamus of stressed rats (Ueyama et al., 2006). Sex steroids alter GABA pathways to affect orofacial nociception in animal models (Puri et al., 2012; Tashiro et al., 2014) and influence the excitability of neurons by altering glutamate decarboxylase (GAD) and GABAA receptor subunit expression (Juptner et al., 1991; Noriega et al., 2010). Estradiol alters GABAergic gene expression in the thalamic region (Umorin et al., 2016) and when estradiol is high there is a reduced response to VZV induced pain. For example, a proestrus (i.e., high) dose of estradiol will decrease the VZV associated orofacial pain response (Stinson et al., 2017; Stinson et al., 2019). It is unclear the role that thalamic GABA neurons have in regulating the VZV associated pain in the presence of this high dose of estradiol.
It was hypothesized that attenuating GABAergic cells in the reticular thalamic nucleus would increase excitatory activity in the ventral posteromedial nucleus after administering a high dose of estradiol. To address this question transgenic rats having the GAD67 promoter driving Cre expression were infused with adeno-associated virus (AAV) containing a Cre dependent G inhibitory designer receptor exclusively activated by designer drugs (DREADD). Infusion of AAV within the lateral thalamic region of these transgenic rats would result in the ability to inhibit GABAergic cells in the reticular thalamic region (Lein et al., 2007). Simultaneously the ventral posteromedial thalamic nucleus was infused with AAV containing a calmodulin-dependent protein kinase II (CaMKII)-GCaMP6f construct which was followed by implantation of a glass lens for imaging with a miniscope. Estradiol was injected to maximize GABAergic activity in the reticular thalamic region (Stinson et al., 2019) and affective pain responses, as well as, calcium activity were measured in rats with VZV associated pain.
Materials and Methods
Animal husbandry
This study was carried out in accordance with the recommendations of Institutional Animal Care and Use Committee Guidebook and Texas A&M University College of Dentistry Institutional Animal Care and Use Committee. The animal protocol was approved by the Texas A&M University College of Dentistry Institutional Animal Care and Use Committee. Transgenic male (300g) and female Long Evans rats (260g) (Rat Resource and Research Center strain LE-Tg(Gad1-iCre)3Ottc, RRRC#: 00751, developed by Brandon Harvey and Jim Pickel) were kept on a 14:10 light/dark cycle. A total of 40 rats were used in these studies. The rats were given food and water ad libitum.
Treatment and Experimental groups
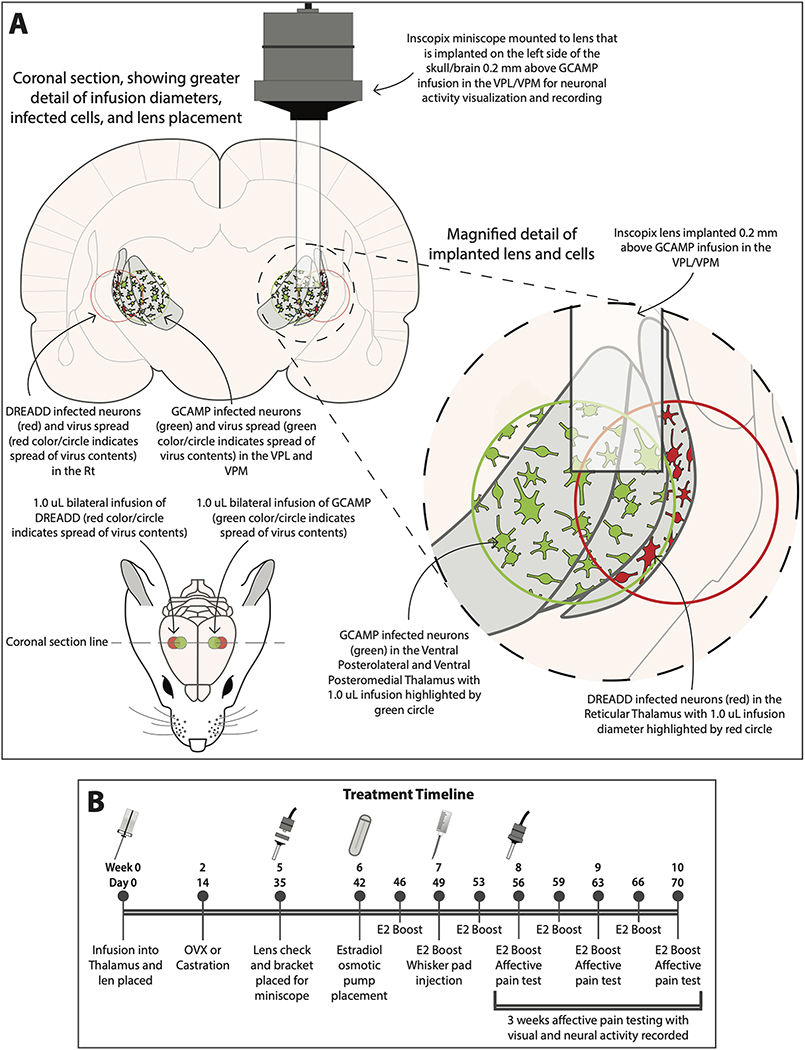
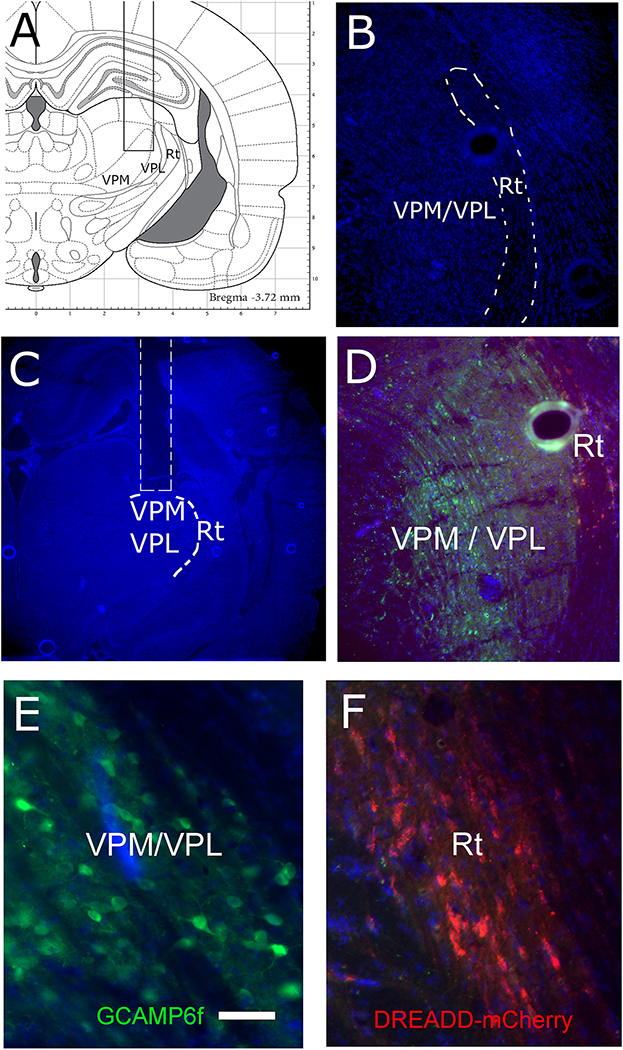
Twenty Long Evans transgenic male rats and twenty transgenic Long Evans female rats were infused bilaterally with pENN.AAV.CamKII.GCaMP6f.WPRE.SV40 (AAV1) and the DREADD construct pAAV-hSyn-DIO-hM4D(Gi)-mCherry (AAV5) (Fig. 1A). To control activity of the GABAergic population in the thalamus an inhibitory DREADD construct was infused into the reticular thalamic region (Fig. 1A, Fig. 2B, 2D and 2F). Expression of the DREADD construct was Cre dependent and infusion in the Gad1-Cre rats resulted in expression within the GABAergic population, cells could be identified by a mCherry marker (Fig. 2D and F). This inhibitory DREADD construct was activated by administration of clozapine N-oxide. Calcium activity in individual excitatory cells was quantitated after infusing a construct into the ventral posteromedial nucleus where GCaMP6f expression was controlled by the CaMKII promoter (Fig. 1A, Fig. 2D and 2E). A single glass lens was then immediately placed for the miniscope on the right side to image the ventral posteromedial nucleus (Fig. 1A, Fig. 2A and 2C). Two weeks after infusion the rats were ovariectomized or castrated, see (Fig. 1B). Five weeks after infusion a miniscope baseplate was placed above the implanted lens (Inscopix, Palo Alto, CA). Six weeks after infusion an osmotic pump was placed beneath the skin that dispensed estradiol (Fig. 1B). After pump placement, the rats were given a subcutaneous injection of estrogen or vehicle every 3 or 4 days (Fig. 1B). Seven weeks after virus infusion the left whisker pad was injected with 100 μl of MeWo cells infected with VZV (50,000–100,000 pfu/μl). An E2 boost was injected 3 hours before testing to simulate a proestrus plasma concentration (Kramer and Bellinger, 2009). Ten of the male rats and ten of the female rats received clozapine N-oxide (1mg/kg) and the other ten rats were administered with vehicle (0.9% saline). Clozapine N-oxide or vehicle were injected into the intraperitoneal space 1 hour before testing (Jendryka et al., 2019). Testing was completed once a week; weeks 8, 9 and 10 (Fig. 1B) post infusion. Groups consisted of E2/VZV/no CNO or E2/VZV/vehicle. Animal were tested for three weeks as this dose of VZV results in a significant pain response for a three week period (Kramer et al., 2017a). Following three weeks of testing the animals were sacrificed and tissue collected for molecular studies.
Figure 1.
Bilateral transduction of the thalamus with a calcium biosensor and a DREADD construct. The DREADD construct was expressed in the GABAergic neurons of the Rt, panel A. The VPM/VPL was transduced with a calcium biosensor (i.e. GCaMP6f, green cells). A 1 μl infusion spread approximately 1 mm in the thalamic region as observed from the fluorescent signal for the calcium indicator. The region transduced by the DREADD construct was estimated from the spread of the calcium indicator. In panel B a time line of surgeries and treatments is indicated in days and weeks. The “E2” indicates a boost injection of estradiol. Ventral posteromedial nucleus (VPM), ventral posterolateral nucleus (VPL) and reticular thalamic nucleus (Rt). Details of treatment are given in the materials and methods.
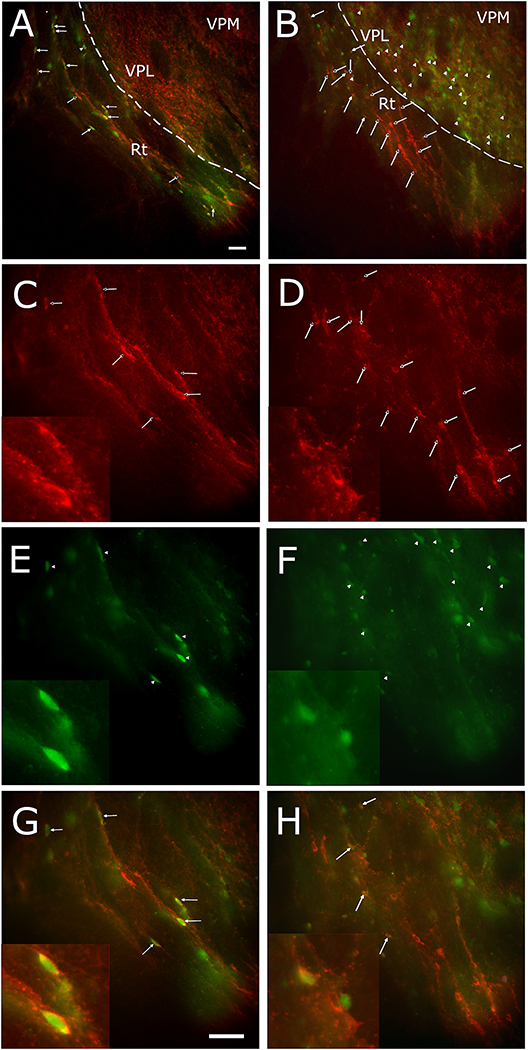
Figure 2.
Calcium biosensor GCaMP6f was expressed in the VPM/VPL and the DREADD was expressed in the Rt. Placement of the miniscope lens (rectangular region) is shown on the brain atlas (coronal section) in panel A. In panel B the reticular thalamic region is outlined on a Hoechst 33342 stained section. A representative image of the cavity for the miniscope lens (outline in white) on a coronal brain section 10 weeks after injection of the virus, blue Hoechst 33342 stain is shown in panel C. In panel D an image of the GCaMP6f expression in the VPM and mCherry expression for the DREADD construct in the Rt. In panel E a high magnification image of the GCaMP6f positive cells and in panel F a high magnification image of the mCherry positive cells. Ventral posteromedial nucleus (VPM), ventral posterolateral nucleus (VPL) and reticular thalamic nucleus (Rt). Bar equals 100 micrometers.
Infusion and lens placement
Rats were anesthetized with 2% isoflurane and an air flow of 2 liter per minute. Using sterile technique a Hamilton infusion needle (Neuros #7002) was inserted into the lateral thalamus. The brain was infused bilaterally with 1 μl of AAV1 with the GCaMP6f construct (2 X 1013 GC/ml, Addgene, Cambridge MA) at coordinates AP=3.7, midline=3.0, and depth=6.0 mm from Bregma using a flat skull and 1 μl of AAV5 with the hm4D(Gi) construct (1 X 1013 GC/ml, Addgene) at coordinates AP=3.7, midline=3.5, depth=6.0 mm (Fig. 1A). A Stoelting stereotaxic syringe pump system was used to infuse 1 μl at a rate of 50 nanoliters per minute. After infusion the needle was left in place for 5 minutes and then removed. A single glass lens (9.0 mm length by 1.0 mm diameter, Inscopix, Palo Alto, CA, Cat. # Part ID: 1050–002214) was immediately placed on the right side at coordinates AP=3.7, midline=3.0 and depth = 5.8 mm (Fig. 1A). The lens was held in place with four stainless steel screws placed within the skull and dental cement (Metabond, Parkell Inc. Edgewood, NY). hM4D(Gi) is an engineered acetylcholine Gi-protein coupled receptor that inhibits neuronal burst firing when bound by clozapine N-oxide. To affect change in neurons exclusively the modified acetylcholine Gi protein-coupled receptor was driven by the neuronal synapsin-1 promoter (hSyn). Moreover the expression of hM4D(Gi) was Cre dependent and expressed specifically in the reticular thalamic neurons due to GAD67 expressing in the reticular thalamic nucleus and not the ventral posteromedial thalamic nucleus (Lein et al., 2007).
Ovariectomization
Rats were anesthetized with 2% isoflurane in 2 liters of air per minute. The anesthetized female was placed in ventral recumbency, shaved and the dorsal surgical area swabbed with surgical scrub. Using sterile technique a 10 to 15 mm skin incision was made dorsally and bilaterally to the spine. The incision was midway between the caudal edge of the ribcage and the base of the tail. The ovary and the oviduct were exteriorized through the muscle wall incision. A sterile silk ligature was placed around the oviduct. Each ovary and part of the oviduct was removed with a single cut, the remaining tissue was replaced into the peritoneal cavity and the skin stapled.
Castration
Rats were anesthetized with 2% isoflurane in 2 liters of air per minute. The scrotal area was shaved and cleaned with betadine solution. While under anesthesia the rat was turned onto its back and the testes were pushed into the scrotum. A small incision was made on the scrotal sac and a testis was pushed close to the incision and another incision was made on the mesorchium sheath. The testis along with the epididymis were pushed out of the mesorchium sheath, the scrotum then clamped with a hemostat behind the epididymis so that the deferent duct and associated blood vessels were crushed and then the testes removed. Sometimes a suture was placed behind the crushed tissue in order to control bleeding but was found to be unnecessary in most cases. Any excess tissue that was not removed was pushed back into the scrotal sac then the procedure repeated for the other side. The scrotum was not sutured but the edges of the incision lightly pinched together so to approximate and left to drain if needed.
Pump placement and estradiol treatment
While under anesthesia, the rat was turned onto its stomach and a small incision cut into the skin at the neck. Then using a mayo scissors a pocket was made under the skin and an osmotic pump inserted. The incision was closed using 9 mm wound clips. The pump was a 28-day Alzet mini-osmotic pump (Durect Corporation, Cupertino, CA) dispensing 750 ng/6μl/day of 17β-estradiol benzoate in polyethylene glycol (Sigma, St. Louis). In addition, 2.5 μg of 17β-estradiol benzoate in 0.1 ml of sesame seed oil was injected subcutaneously every 3 or 4 days (Fig. 1B). This dose of estradiol produces a proestrus (high) dose of estradiol in female rats (Kramer and Bellinger, 2009).
Miniscope and Imaging Procedures
Five weeks after lens placement a miniscope baseplate (Inscopix part # 1050–002192) was cemented (Metabond) in place using the existing dental screws for support so that the objective lens of the miniscope would be 150–300 μm above the implanted lens. A cover (Inscopix part # 1050–002193) was placed on the baseplate to keep the lens clean. An InVoke microscope from Inscopix was placed within the bracket and images were captured and processed with Inscopix software. Inscopix Data Processing software was used to identify individual cells and events were detected using a threshold factor of 10. The number of cells with a calcium event was reported each minute. Approximately 50% of the animals that were tested behaviorally had cells that could be imaged for calcium changes with the miniscope. Animal behavior was recorded simultaneously using VLC media player software (VideoLAN) and a Microsoft LifeCam (Microsoft, Redmond, WA). Recordings were exported in the MJPEG format in Shotcut open-source software for importing into Inscopix Data Processing software.
Behavioral testing
Place Escape/Avoidance Paradigm (PEAP) testing was performed during the morning of the light phase to determine pain. To accomplish this, the rats were placed in a 30 cm X 30 cm X 30 cm acrylic box. The box has four walls and floor with the top of the box open and half the box is covered in black cloth on the outside of the acrylic. This test chamber was modeled from the PEAP test performed by the Fuchs’s laboratory (LaBuda and Fuchs, 2000). This assay was used to measure the motivation/affective aspect of pain (LaBuda and Fuchs, 2000; Baastrup et al., 2011). The PEAP test is based on the assumption that if animals escape and/or avoid a noxious stimulus, then the stimulus is aversive to the animal. Rodents being nocturnal in nature preferred to stay on the dark side when placed into the test chamber. After placing the rat in the test chamber, the rat was immediately poked with a 60-gram filament every 15 seconds on the injected side if the rat was on the dark side and on the non-injected side if it was on the light side. Because VZV was injected into the whisker pad the target region for the poking was the area below the eye and caudal to the whisker pad. This region is innervated by the second branch of trigeminal ganglion (DaSilva and DosSantos, 2012), the nerve infected by VZV injection of the whisker pad. The time spent on the dark side of the box was recorded in 5 minute bins and testing was performed for a total of 30 minutes. Testing was performed once a week. Testing was completed for three weeks (Fig. 1B). Thus, the theory behind the test is that if the rat is experiencing VZV induced pain when poked in the sensitive area it will not stay on it preferred dark side but will move to the non-preferred light side and stay there to avoid the poke. Values were given as a mean and standard error of the mean (SEM) for the ten animals in each treatment group.
Immuno-fluorescent staining
Rats were injected with 100 mg/kg ketamine and 10 mg/kg xylazine. After injection the animals were perfused with 9% sucrose followed by 4% paraformaldehyde. Fixed tissues were stored in 25% sucrose, frozen, cryo-sectioned and the 32 μm sections placed on Histobond slides (VWR international, Radnor, PA). The tissue was post-fixed for 5 minutes in 4% paraformaldehyde, rinsed and then blocked for 2 hours at room temperature with a PBS solution containing 5% normal goat serum (Sigma-Aldrich, St. Louis, MO) and 0.3% Triton-X 100. The slides were then incubated in a primary antibody solution overnight at 4°C. The primary antibody consisted of a mixture of the GAD67 antibody (Millipore clone 1G10.2, MAB5406) at a 1:500 dilution and rabbit pERK antibody (phosphorylated extracellular signal-regulated kinase, Cell Signaling Technology, Boston, MA, #4695) at a 1:150 dilution. The primary antibody was diluted with PBS, 5% BSA and 0.3% Triton X-100. After incubation in primary antibody the slides were then rinsed three times in PBS and Triton-X 100 for a total of 45 minutes and placed for 2 hours in secondary antibody and PBS and 0.3% Triton X-100. Secondary antibodies (1:500 dilution) included a mixture of goat anti-mouse 568 and goat anti-rabbit 488 (Invitrogen, Carlsbad, CA). After rinsing the slides three times in PBS and 0.3% Triton X-100 for a total of 45 min, the slides were mounted with Fluoromount-G mounting medium containing Hoechst 33342 stain (Electron Microscopy Sciences, Hatfield, PA). The fluorescent signal was imaged using a Nikon fluorescent microscope, NIS-Elements imaging software and a Photometrics CoolSnap K4 CCD camera (Roper Scientific, Inc, Duluth, GA). Controls eliminating the primary antibody showed no signal (data not shown).
Cell counts were completed by a blinded reviewer. Every other section was selected for staining. Typically three sections were counted for each animal. The slides were analyzed using Image J software, the average background for the slides within a treatment group was subtracted from the image and a fluorescent signal associated with a cell nucleus was counted as a positive cell. Counts were completed for the number of GAD67 or GAD67/pERK stained cells within a 0.125 mm2 field. Counts were completed within the ventral thalamic nuclei and cell counts from the two fields on each section were then averaged. This average count for the three sections was averaged for each animal. Values were given as a mean and standard error of the mean (SEM) for the animals in each treatment group.
Staining with GAD67 ensured we identified all GAD67 positive cells in the reticular thalamic nucleus in the event that the Cre-dependent mCherry labeling of GAD67 Cre cells was incomplete or not robust. Adjacent sections were imaged without staining and no green GCaMP6f positive cells were identified in the reticular thalamic nucleus. Thus, the green staining in the reticular thalamic nucleus was due to pERK antibody binding and not GCaMP6f expression. Animals that did not have adequate perfusion, resulting in poor identification of cells after immunostaining, were eliminated from the counts, typically one or two animals per group.
Statistics
PEAP data was analyzed with the Kruskal-Wallis test, significant main effects were followed with Dunn’s post-hoc testing. The dependent variables of calcium events and immune-fluorescent cell count data were analyzed with the non-parametric Mann-Whitney test (Prizm 5.04, GraphPad Software, La Jolla, CA).
Results
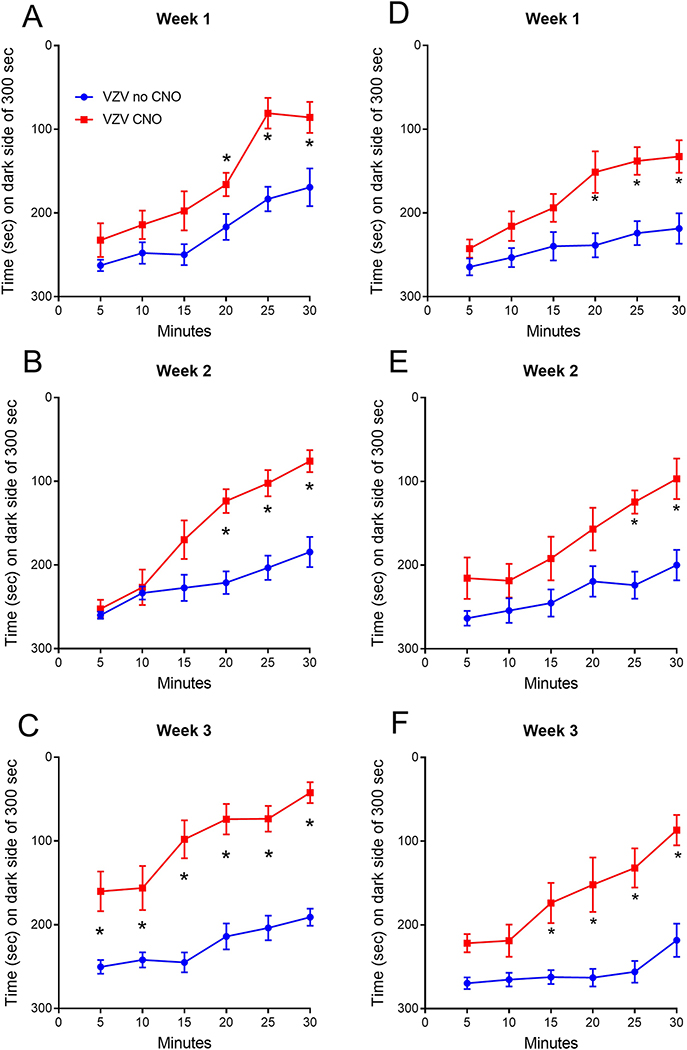
Female and male rats were given a proestrus dose of estradiol and the neuronal response was measured in a VZV associated pain model using a miniscope. Calcium imaging of excitatory neurons was completed in awake behaving animals during behavioral pain testing in the female (supplemental figure 1) and male (supplemental figure 2) rats. Four groups were tested with 10 animals in each group, the groups consisted of the females given no CNO and females given CNO, males given no CNO and males given CNO. Testing for VZV associated pain in the female rats indicated that treatment with CNO increased the response in females (Fig. 3A) during week 1 (χ3=49.5, p=0.0005) and in males (Fig. 3D) during week 1 (χ3=59.5, p<0.0001). In week 2 females also showed an increase after CNO treatment (Fig. 3B) (χ3=49.3, p=0.0006) and this increase was observed in males (Fig. 3E) (χ3=61.7, p<0.0001). Moreover, CNO treatment increased the pain response in females (Fig. 3C) during week 3 (χ3=85.7, p<0.0001) and in males (Fig. 3F) during week 3 (χ3=78.0, p<0.0001). No significant difference was observed between the female and males.
Figure 3.
Affective pain response of female and male rats after inhibition of thalamic GAD67 positive cells. PEAP testing of rats with VZV associated pain was completed after administering a proestrus dose of estradiol with and without treatment with clozapine N-oxide (CNO). A reduced amount of time spent on the dark side indicates a greater pain response. Testing was completed in female (panels A, B and C) or males (panels D, E and F) rats. Testing was completed once a week for three weeks. Asterisk indicates a significant difference (p<0.05) between groups within that testing period, there were 10 animals per group.
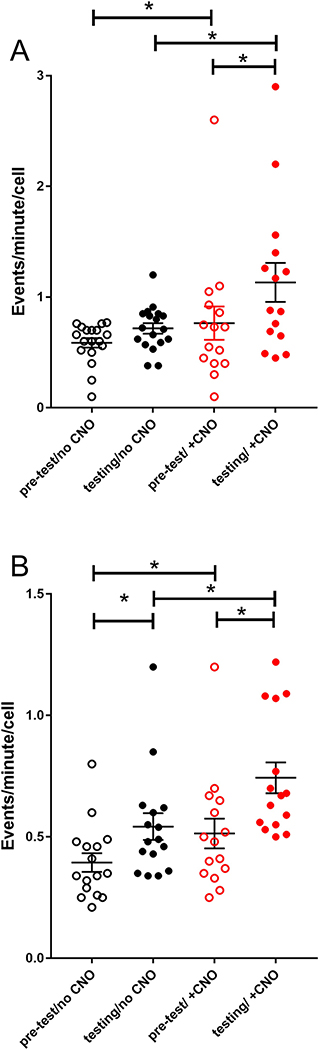
Calcium imaging of the ventral posteromedial thalamic nucleus indicated the activity of individual excitatory CaMKII neurons increased significantly during the pain testing of females (Fig. 4A) and males (Fig. 4B), comparing pre-test to testing. . Note that the data from all three weeks was combined because no significant effect was observed for an individual week. Before administering clozapine N-oxide to females (Fig. 4A) the number of calcium events in each cell before testing (pre-test/no CNO) (Mdn=0.63) was lower than during testing (testing/no CNO) (Mdn=0.72) but these differences were not significant U(N pretesting = 18, N testing = 18) = 103, p<0.06. After clozapine N-oxide treatment females showed a lower calcium activity before testing (pre-test/+CNO) (Mdn=0.73) than during testing (testing/+CNO) (Mdn=0.88) and this difference was significant U(N pretesting = 15, N testing = 15) = 65.5, p<0.05. Before administering clozapine N-oxide to males (Fig. 4B) the number of calcium events in each cell before testing (pre-test/no CNO) (Mdn=0.34) was lower than during testing (testing/no CNO) (Mdn=0.48) and these differences were significant U(N pretesting = 16, N testing = 16) = 63, p=0.013. After clozapine N-oxide treatment males showed a lower calcium activity before testing (pre-test/+CNO) (Mdn=0.48) than during testing (testing/+CNO) (Mdn=0.67) and this difference was significant U(N pretesting = 15, N testing = 15) = 46.5, p=0.005. In addition, clozapine N-oxide treatment significantly increased calcium activity during testing (compare testing/no CNO to testing/+CNO) in both females U(N no CNO = 18, N CNO = 15) = 82.5, p<0.05 and males. U(N no CNO = 16, N CNO = 15) = 50, p=0.005. No significant difference was observed between the female and males.
Figure 4.
Calcium activity in the VPM during behavioral testing. Imaging of GCaMP6f fluorescence in the VPM was completed using the miniscope. The different treatment groups include with (+ CNO) and without (no CNO) clozapine N-oxide treatment and before (pre-test) and during pain testing. Panel A is female and panel B is male data. All three weeks of testing data was combined. No significant effect was observed for individual week data. Asterisk indicates a significant difference (p<0.05) between groups.
Females had an average of 16 ± 5 GCaMP positive cells with “flashing” in a single focal plane within the field of the miniscope lens. Males had 21 ± 3 cells within the field. The difference between male and females was not significant p = 0.11.
pERK staining of the GAD67 population in the reticular thalamic region was measured in female rats (Fig. 5) and male rats (Fig. 6). Cells expressing GAD67 localize to the reticular thalamic region (Fig. 5A, B, C and D; Fig 6C and D, red cells, open arrows). pERK positive cells in the reticular thalamic region are compared before clozapine N-oxide treatment (Fig. 5A and E and 6A, green cells, arrowheads) and after clozapine N-oxide treatment (Fig. 5B and F and 6B). The number of cells that co-localized with pERK and GAD67 were greater in number before clozapine N-oxide treatment (Fig. 5A and G and 6E, yellow cells, arrows) in comparison to after clozapine N-oxide treatment (Fig. 5B and H and 6F).
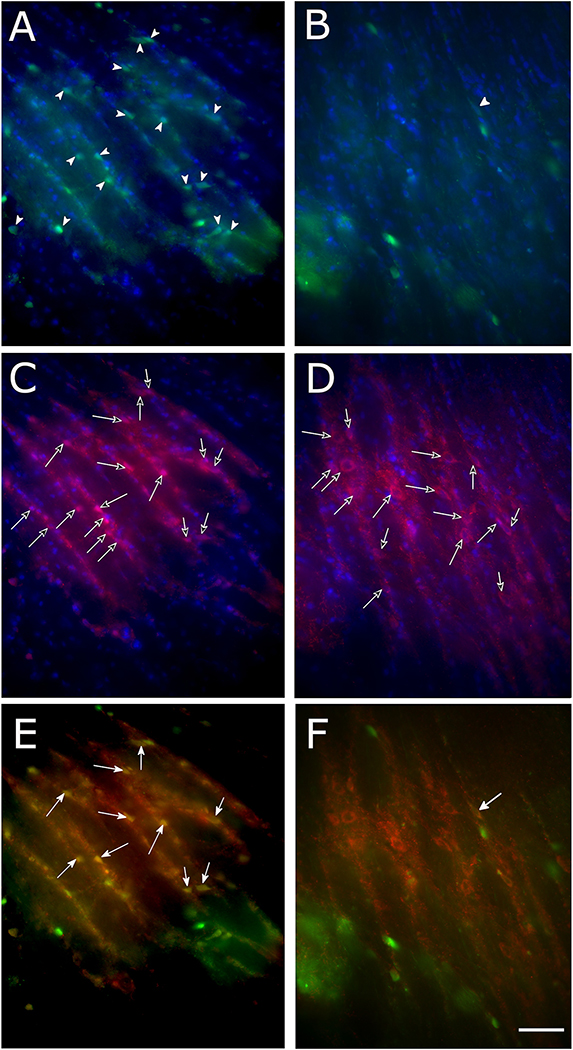
Figure 5.
pERK positive cells in the reticular thalamic nucleus (Rt) nucleus of female rats after VZV injection. Panels A and B are a low magnification image of the pERK positive cells (green, arrowheads) and the GAD67 positive cells (red, open arrows) and cells that co-localize for pERK and GAD67 (yellow, arrows). Panels C and D are a higher magnification images of the GAD67 positive cells (red cells, open arrows) and panels E and F show a higher magnification image of the pERK positive cells (green cells, arrowheads). Panels G and H show a higher magnification image of the cells that co-localize (yellow) for pERK and GAD67 (arrows). Inserts in lower left show the highest magnification image of cells. Panels A, C, E and G are for a representative animal that received no CNO treatment. Panels B, D, F and H are for an animal that received an injection of CNO. Bar is equivalent to 100 micrometers.
Figure 6.
pERK positive cells with the reticular thalamic nucleus (Rt) nucleus of male rats injected with VZV. Panels A and B show the pERK positive cells (green cells, arrowheads). Panels C and D show the GAD67 positive cells (red cells, open arrows) and panels E and F show co-localization (yellow) of pERK and GAD67 cells (arrows). Panels A, C and E are for a representative animal that received no CNO treatment. Panels B, D and F are for an animal that received and injection of CNO (clozapine N-oxide). Bar is equivalent to 100 micrometers.
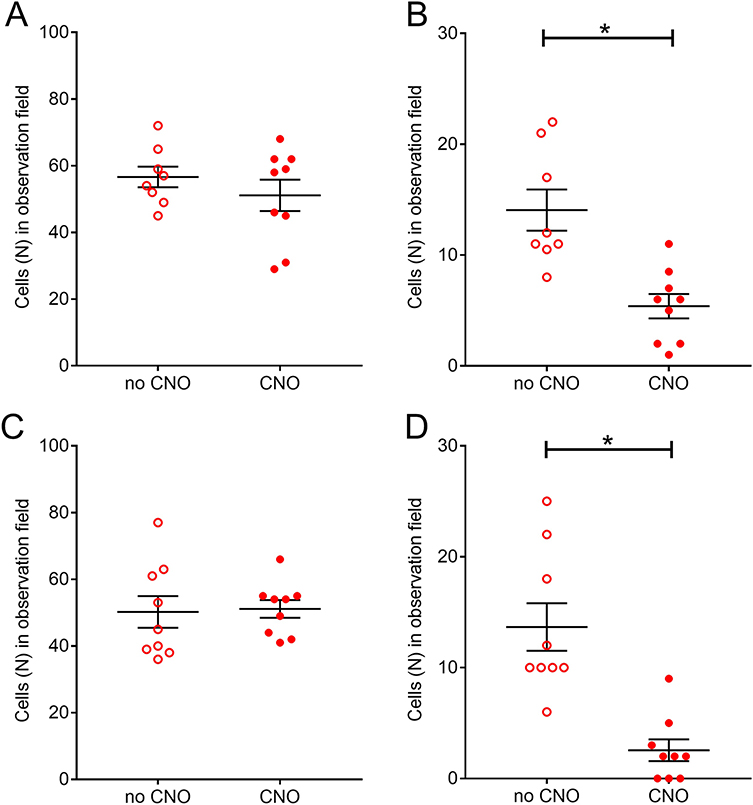
Cell counts of the number pERK positive cells in the reticular thalamic region indicated CNO treatment did not alter the total number of pERK positive cells in the reticular thalamic region in either females (Fig. 7A) or males (Fig. 7C). In contrast clozapine N-oxide significantly decrease the number of pERK positive cells co-localizing with GAD67 in both female (Fig. 7B) and male (Fig. 7D) rats. In females (Fig. 7B) clozapine N-oxide treatment (CNO) (Mdn=11.5) versus vehicle (no CNO) (Mdn=6) decreased the number of pERK/GAD67 positive cells and these differences were significant U(N no CNO = 8, N CNO = 9) = 4, p=0.0009. In males (Fig. 7D) clozapine N-oxide treatment (CNO) (Mdn=10) decreased the number of pERK/GAD67 positive cells versus vehicle (no CNO) (Mdn=1) and these differences were significant U(N no CNO = 9, N CNO = 9) = 4, p<0.0001.
Figure 7.
Number of cells positive for pERK within the reticular thalamic nucleus (Rt). Panels A and B are for female rats and panels C and D are for males. Panels A and C are for the number of pERK positive cells in the Rt. Panels B and C are the number of cells co-localizing for both pERK and GAD67 in the Rt. Animals were injected with vehicle (no CNO) or CNO (clozapine N-oxide). Asterisk indicates a significant difference (p<0.05) between groups, there were 8 or 9 animals per group.
Discussion
Do GABA cells in the reticular thalamic nucleus regulate excitatory neurons in the ventral posteromedial nucleus of the thalamus. Treatment with clozapine N-oxide inhibited activity of GABAergic cells in the reticular thalamic nucleus. Simultaneously calcium activity within the ventral posteromedial nucleus increased. This was the first demonstration of in vivo imaging of calcium activity in single neurons within the thalamus of rats. Inhibiting the GABAergic cells in the reticular thalamic nucleus increased activity of the excitable cells of the ventral posteromedial nucleus concomitant with an increased pain response. Inhibitory DREADD activation in the GABAergic population was determined in the reticular thalamic nucleus through staining for pERK. pERK levels decreased in the GAD67 positive cells but not in all cells of the reticular thalamic region. Cre dependent DREADD expression was not observed in the ventral posteromedial nucleus because GAD67 is rarely expressed in the ventral posteromedial nucleus (Lein et al., 2007). Thus, clozapine N-oxide treatment attenuated the inhibitory signals from the reticular thalamic GAD67 positive neurons and this reduced inhibitory signal increased activity of the CaMKII positive neurons within the ventral posteromedial nucleus.
Our lab previously demonstrated that a proestrus concentration of estradiol was associated with an increase in GABAergic activity in the reticular thalamic nucleus and a concomitant decrease in VZV associated pain (Stinson et al., 2019). It is unclear the role that thalamic GABA neurons have in regulating the VZV associated pain in the presence of this high dose of estradiol. In this study the model used was an animal with a high proestrus dose of estradiol and this was tested in both male and female rats. Interestingly, GABAergic control of the excitatory cells was observed in both male and female rats indicating that estradiol’s effects are not due to an inherent differences in the male and female animals (e.g., developmental differences) but to changes induced by the hormone.
Stress from having the miniscope attached is a concern, we did not see any behavioral deficits or significant alterations to the VZV associated pain response versus animals that were similarly treated in a prior study (Stinson et al., 2019). Expression of an exogenous protein within the cells, such as GCaMP, could also alter the cellular response and alter the behavioral response. Again, versus a prior study the behavioral pain response was not significantly altered comparing GCaMP infused to non-infused animals (Stinson et al., 2019) suggesting GCaMP expression did not alter the behavior and was consistent with normal neuronal function. Testing for corticosterone levels in these animals could reveal the stress effect resulting from miniscope attachment.
Calcium measurements with GCaMP can have complications resulting from overexpression causing damage to neurons. It is suggested overexpression can be identified when GCaMP expression is throughout the entire cell body. A healthy cell would only have cytoplasmic GCaMP expression with exclusion of the nucleus (Resendez et al., 2016) as shown in our studies in Figure 2. In addition GCaMP can interfere with calcium channels and signaling events (Yang et al., 2018), problems that must be considered. No overt behavioral differences was observed between these GCaMP positive rats and animals treated and tested in the same manner (Stinson et al., 2019) consistent with the idea that calcium channels and signaling was normal.
It should be noted that changes in GCaMP fluorescent signals are on a slower timescale than action potentials thus, calcium transients observed, in vivo, likely reflect changes in activity from baseline, such as bursting events, rather than absolute levels of spiking activity (i.e. frequency) (Harris et al., 2016; Theis et al., 2016). A functional connection between the anterior cingulate cortex and ventral posteromedial nucleus had been observed previously (Wang et al., 2007). Attenuation of excitatory neuronal activity in the thalamus will result in attenuation of electrical activity in the anterior cingulate cortex and reduce the pain response (Kramer et al., 2017b). This association is consistent with the idea that excitatory neurons within the ventral posteromedial nucleus modulate affective pain by altering activity in the anterior cingulate cortex. This would explain how modulating the excitable cells in this study altered the behavior in our affective/motivation pain assay. Alternatively, the reticular thalamic nucleus also projects to regions such as the parafascicular and intralaminar nuclei (Clemente-Perez et al., 2017) and because the parafascicular nucleus is important in motivational and effective pain, as tested in this study, it could also modulate the VZV associated pain response (Weigel and Krauss, 2004).
Patients with trigeminal neuropathy have decreased activity in the reticular thalamic nucleus contralateral to the pain (Moisset and Bouhassira, 2007). The reticular thalamic nucleus is comprised of inhibitory GABA neurons (Nagai et al., 1985) and these interneurons send axons back to the ventral posteromedial nucleus to regulate sensory information passing through the thalamus to the cortex (Lam and Sherman, 2011). A zoster “shingles” patient with pain had reduced thalamic activity on a PET scan (Iadarola et al., 1995). Because sensory neurons from the orofacial region project to the thalamus (Guy et al., 2005) it is likely that a reduction in inhibitory GABA signaling contributes to pain in these patients. These results are consistent with animal studies showing that a reduction in reticular thalamic activity will increase the orofacial, sensory response (Trageser et al., 2006) and that lesioning the thalamic region, including the reticular thalamic nucleus heightens pain responses (Saade et al., 1999).
Excitable neurons in the ventral posteromedial nucleus of the thalamus were imaged using GCaMP and an implanted miniscope. Simultaneously an inhibitory DREADD construct was expressed in the GAD67 population of the reticular thalamic region. Inhibition of the GAD67 positive cells increased activity of the excitable cells and increased the pain response suggesting a role for the reticular thalamus in controlling pain signals by inhibiting excitable cells within the ventral posteromedial nucleus. These events occurred after treating with a high dose of estradiol consistent with the idea that estradiol utilizes this pathway to control orofacial pain.
Supplementary Material
Highlights.
First time imaging calcium activity in vivo in the rat ventral posteromedial thalamic nucleus
Inhibition of reticular thalamic neuronal activity increased ventral posteromedial thalamic activity
Increased calcium activity in the ventral posteromedial thalamic nucleus was concomitant with an increase in pain
Acknowledgements
The authors wish to thank Mohamed Khamsi, Priscilla Hooks, Connie Tillberg and Gerald Hill for their excellent technical assistance. This study was supported by NIDCR grant DE026749 (PRKramer) and a grant from the 30, 300, 3000 Pain Research Challenge from the University of Pittsburgh Clinical and Translational Science Institute (PRKinchington). PRKinchington also acknowledges support from grant NS064022, NEI core grant EY08098 and unrestricted funds from Eye & Ear Foundation and Research to Prevent Blindness Inc. There are no conflicts of interest with this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez FK, de Siqueira SR, Okada M, Teixeira MJ, de Siqueira JT (2007), Evaluation of the sensation in patients with trigeminal post-herpetic neuralgia. J Oral Pathol Med 36:347–350. [DOI] [PubMed] [Google Scholar]
- Baastrup C, Jensen TS, Finnerup NB (2011), Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res 1370:129–135. [DOI] [PubMed] [Google Scholar]
- Barbaresi P, Spreafico R, Frassoni C, Rustioni A (1986), GABAergic neurons are present in the dorsal column nuclei but not in the ventroposterior complex of rats. Brain Res 382:305–326. [DOI] [PubMed] [Google Scholar]
- Bartho P, Freund TF, Acsady L (2002), Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci 16:999–1014. [DOI] [PubMed] [Google Scholar]
- Clemente-Perez A, Makinson SR, Higashikubo B, Brovarney S, Cho FS, Urry A, Holden SS, Wimer M, David C, Fenno LE, Acsady L, Deisseroth K, Paz JT (2017), Distinct Thalamic Reticular Cell Types Differentially Modulate Normal and Pathological Cortical Rhythms. Cell Rep 19:2130–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, DosSantos MF (2012), The role of sensory fiber demography in trigeminal and postherpetic neuralgias. J Dent Res 91:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy N, Chalus M, Dallel R, Voisin DL (2005), Both oral and caudal parts of the spinal trigeminal nucleus project to the somatosensory thalamus in the rat. Eur J Neurosci 21:741–754. [DOI] [PubMed] [Google Scholar]
- Harris KD, Quiroga RQ, Freeman J, Smith SL (2016), Improving data quality in neuronal population recordings. Nat Neurosci 19:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand K, Bricout H, Schulze-Rath R, Schink T, Garbe E (2015), Incidence of herpes zoster and its complications in Germany, 2005–2009. J Infect 70:178–186. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Max MB, Berman KF, Byas-Smith MG, Coghill RC, Gracely RH, Bennett GJ (1995), Unilateral decrease in thalamic activity observed with positron emission tomography in patients with chronic neuropathic pain. Pain 63:55–64. [DOI] [PubMed] [Google Scholar]
- Jendryka M, Palchaudhuri M, Ursu D, van der Veen B, Liss B, Katzel D, Nissen W, Pekcec A (2019), Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci Rep 9:4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juptner M, Jussofie A, Hiemke C (1991), Effects of ovariectomy and steroid replacement on GABAA receptor binding in female rat brain. J Steroid BiochemMol Biol 38:141–147. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Bellinger LL (2009), The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology 150:3680–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PR, Stinson C, Umorin M, Deng M, Rao M, Bellinger LL, Yee MB, Kinchington PR (2017a), Lateral thalamic control of nociceptive response after whisker pad injection of varicella zoster virus. Neuroscience 356:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PR, Strand J, Stinson C, Bellinger LL, Kinchington PR, Yee MB, Umorin M, Peng YB (2017b), Role for the Ventral Posterior Medial/Posterior Lateral Thalamus and Anterior Cingulate Cortex in Affective/Motivation Pain Induced by Varicella Zoster Virus. Front Integr Neurosci 11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN (2000), A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol 163:490–494. [DOI] [PubMed] [Google Scholar]
- Lam YW, Sherman SM (2011), Functional organization of the thalamic input to the thalamic reticular nucleus. J Neurosci 31:6791–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR (2007), Genome-wide atlas of gene expression in the adult mouse brain. Nature 445:168–176. [DOI] [PubMed] [Google Scholar]
- Moisset X, Bouhassira D (2007), Brain imaging of neuropathic pain. Neuroimage 37 Suppl 1:S80–88. [DOI] [PubMed] [Google Scholar]
- Nagai T, Maeda T, Imai H, McGeer PL, McGeer EG (1985), Distribution of GABA-T-intensive neurons in the rat hindbrain. J Comp Neurol 231:260–269. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Chapin JK, Lin RC (1992), Somatotopic maps within the zona incerta relay parallel GABAergic somatosensory pathways to the neocortex, superior colliculus, and brainstem. Brain Res 577:134–141. [DOI] [PubMed] [Google Scholar]
- Noriega NC, Eghlidi DH, Garyfallou VT, Kohama SG, Kryger SG, Urbanski HF (2010), Influence of 17beta-estradiol and progesterone on GABAergic gene expression in the arcuate nucleus, amygdala and hippocampus of the rhesus macaque. Brain Res 1307:28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D, Deschenes M (1998), Projection and innervation patterns of individual thalamic reticular axons in the thalamus of the adult rat: a three-dimensional, graphic, and morphometric analysis. J Comp Neurol 391:180–203. [DOI] [PubMed] [Google Scholar]
- Puri J, Vinothini P, Reuben J, Bellinger LL, Ailing L, Peng YB, Kramer PR (2012), Reduced GABA(A) receptor alpha6 expression in the trigeminal ganglion alters inflammatory TMJ hypersensitivity. Neuroscience 213:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Jennings JH, Ung RL, Namboodiri VM, Zhou ZC, Otis JM, Nomura H, McHenry JA, Kosyk O, Stuber GD (2016), Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat Protoc 11:566–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saade NE, Kafrouni AI, Saab CY, Atweh SF, Jabbur SJ (1999), Chronic thalamotomy increases pain-related behavior in rats. Pain 83:401–409. [DOI] [PubMed] [Google Scholar]
- Stinson C, Deng M, Yee MB, Bellinger LL, Kinchington PR, Kramer PR (2017), Sex differences underlying orofacial varicella zoster associated pain in rats. BMC Neurol 17:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson C, Logan SM, Bellinger LL, Rao M, Kinchington PR, Kramer PR (2019), Estradiol Acts in Lateral Thalamic Region to Attenuate Varicella Zoster Virus Associated Affective Pain. Neuroscience 414:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Bereiter DA, Thompson R, Nishida Y (2014), GABAergic influence on temporomandibular joint-responsive spinomedullary neurons depends on estrogen status. Neuroscience 259:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis L, Berens P, Froudarakis E, Reimer J, Román Rosón M, Baden T, Euler T, Tolias AS, Bethge M (2016), Benchmarking Spike Rate Inference in Population Calcium Imaging. Neuron 90:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trageser JC, Burke KA, Masri R, Li Y, Sellers L, Keller A (2006), State-dependent gating of sensory inputs by zona incerta. J Neurophysiol 96:1456–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T, Tanioku T, Nuta J, Kujira K, Ito T, Nakai S, Tsuruo Y (2006), Estrogen alters c-Fos response to immobilization stress in the brain of ovariectomized rats. Brain Res 1084:67–79. [DOI] [PubMed] [Google Scholar]
- Umorin M, Stinson C, Bellinger LL, Kramer PR (2016), Genes in the GABA Pathway Increase in the Lateral Thalamus of Sprague-Dawley Rats During the Proestrus/Estrus Phase. J Cell Physiol 231:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Chang JY, Woodward DJ, Baccala LA, Han JS, Luo F (2007), Corticofugal influences on thalamic neurons during nociceptive transmission in awake rats. Synapse 61:335–342. [DOI] [PubMed] [Google Scholar]
- Weigel R, Krauss JK (2004), Center median-parafascicular complex and pain control. Review from a neurosurgical perspective. Stereotact Funct Neurosurg 82:115–126. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu N, He Y, Liu Y, Ge L, Zou L, Song S, Xiong W, Liu X (2018), Improved calcium sensor GCaMP-X overcomes the calcium channel perturbations induced by the calmodulin in GCaMP . Nat Commun 9:1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.