Abstract
Single nucleotide polymorphisms in mitochondrial DNA, such as mitochondrial 1555 A>G (m.1555 A>G) and mitochondrial 1494 C>T (m.1494 C>T), are known to be causative mutations of nonsyndromic hearing loss following exposure to aminoglycoside antibiotics. The prevalence of the m.1555 A>G and m.1494 C>T mutations has not been reported for the general population in Japan. The purpose of this study was to investigate the prevalence of m.1555 A>G and m.1494 C>T mutations in a community-dwelling population in Japan in order to prevent aminoglycoside-induced hearing loss. We recruited participants older than 20 years of age to the Iwaki Health Promotion Project in 2014, 2015, and 2016, resulting in the recruitment of 1,683 participants. For each participant, we performed a hearing test and a genetic test for the m.1555 A>G and m.1494 C>T mutations using the TaqMan genotyping method. The m.1555 A>G mutation was detected in only 1 of the 1,683 participants (0.06%). This carrier of the m.1555 A>G mutation was a 69-year-old male with bilateral, symmetric, and high-frequency hearing loss. We provided genetic counseling and distributed a drug card advising him to avoid the administration of aminoglycoside antibiotics. In contrast, the m.1494 C>T mutation was not detected in this study population.
Subject terms: Mitochondrial genome, Mutation, Risk factors
Hearing loss: mitochondrial mutations and antibiotic use
Researchers in Japan have measured the prevalence of two hearing loss mutations associated with antibiotic use in the general population. Two mutations in mitochondrial DNA are known to cause hearing loss upon exposure to aminoglycoside antibiotics such as gentamicin. One mutation is found in 3% of Japanese hearing-impaired outpatients, but the frequency in the general population is unknown. A team led by Akira Sasaki of Hirosaki University carried out genetic and hearing tests in 1,683 community-dwelling subjects. They found one person who had one of the mutations. Although this subject had no known history of aminoglycoside antibiotic injection, he exhibited hearing loss at high frequencies. He was provided with genetic counseling and a drug use warning card. The second mutation was not found in the study population.
Introduction
The World Health Organization (WHO) estimated that over 5% of the world’s population was affected by hearing loss1. Hearing loss is associated with an array of problems, such as poor quality of life and negative outcomes related to socialization, independence, interpersonal relationships, and communication2. Hearing loss has also been reported to be a significant risk factor for dementia3. In addition, hearing loss is associated with additional costs, including health sector costs, the provision of educational support, the loss of productivity, and societal costs, worldwide1. Thus, hearing loss may result in a significant burden for both individuals and society.
Previous studies have shown that hearing loss is a common sensory disorder, affecting 1 in 1000 newborns. A total of 54–68% of hearing loss cases have been estimated to have genetic origins or to be associated with genetic predispositions4. Causative mitochondrial DNA mutations have been found in approximately 5% of patients with postlingual, nonsyndromic hearing loss5. Among the identified mitochondrial mutations, the mitochondrial 1555 A>G (m.1555 A>G) and mitochondrial 1494 C>T (m.1494 C>T) mutations have frequently been identified in patients with aminoglycoside-induced and late-onset, nonsyndromic hearing loss6,7.
Many reports have described patients with hearing loss who carry these gene mutations. The m.1555 A>G mutation was reported to occur in approximately 3% of Japanese hearing-impaired outpatients6. However, no studies have reported the prevalence of the m.1555 A>G and m.1494 C>T mutations in the general population in Japan. It is well known that in carriers of the m.1555 A>G and m.1494 C>T mutations, hearing loss can progress if aminoglycoside antibiotics are administered. Aminoglycoside antibiotics are still being used in many countries, especially for patients with tuberculosis or methicillin-resistant Staphylococcus aureus infection and Neonatal Intensive Care Unit (NICU) babies8,9.
However, aminoglycoside-induced hearing loss can be prevented by avoiding the administration of aminoglycoside antibiotics. Therefore, it is very important to investigate the population potentially at high risk for aminoglycoside antibiotic-induced hearing loss.
The purpose of this study was to investigate the prevalence of the m.1555 A>G and m.1494 C>T mutations in a community-dwelling population in Japan in order to prevent aminoglycoside-induced hearing loss.
Materials and methods
Subjects
The Iwaki Health Promotion Project is an annual, large-scale, epidemiological survey performed in the Iwaki district of Hirosaki city, Japan10. We invited all residents older than 20 years of age who live in this district to participate in this project. The data collected for this project in 2014, 2015, and 2016 were available for this study.
Data collection and genetic analysis for the present study and the Iwaki Health Promotion Project were approved by the Ethics Committee of Hirosaki University School of Medicine (Authorization numbers: 2014-014, 2014-377, 2016-028), and all subjects provided written informed consent before participating in the project.
Hearing test
Hearing tests were performed for all study participants. Air-conduction, pure-tone thresholds at octave intervals of 0.125, 0.25, 0.5, 1, 2, 4, and 8 kHz were measured by trained examiners using a diagnostic audiometer (AA-73A; Rion, Tokyo, Japan).
Validation study of the TaqMan genotyping method
To confirm the accuracy of the TaqMan genotyping method used in this study, we performed a validation study by using positive and negative control DNA samples from our previous cohort11–13. For the m.1555 A>G mutation, we performed a validation study by using 24 positive control and 24 negative control samples, and for the m.1494 mutation, we performed a validation study with one positive control sample and 24 negative control samples. All TaqMan genotyping results were consistent with the PCR-RFLP, Invader assay, and Sanger sequencing results obtained in our previous studies11–13.
Genetic test
We obtained blood samples from all participants. The presence of the m.1555 A>G and m.1494 C>T mutations was analyzed using the TaqMan genotyping method. Genomic DNA was extracted from 200 µL of a whole-blood specimen using the QIAamp 96 Blood Kit (QIAGEN, Hamburg, Germany). Then, 0.2 μM m.1494T probe, 0.2 μM m.1494C probe, 0.5μM m.1494CT forward primer and 0.5 μM m.1494CT reverse primer or 0.2 μM m.1555G probe, 0.2 μM m.1555A probe, 0.5 μM m.1555AG forward primer and 0.5 μM m.1555AG reverse primer mixed in SsoAdvanced Supermix (Bio-Rad, Hercules, CA, USA) were combined with genomic DNA and analyzed according to the manufacturer’s instructions. Primers and probes were obtained from Eurofins Genomics (Tokyo, Japan), and the sequences are presented in Table 1. A volume of 1 µL of genomic DNA was mixed with 19 µL of premix as a template (3.52–168.9 ng/reaction). Real-time polymerase chain reaction (PCR) was performed using CFX Manager 3.1 software (Bio-Rad) as follows: hot start at 95 °C for 3 min, heat denaturation at 98 °C for 10 s, and extension reaction at 63 °C for 1 min for 40 cycles using CFX 96 or CFX 384 at the 10 µL scale. Fluorescence intensity measured in 30 cycles was used for genotype discrimination.
Table 1.
List of probe and primer sequences used in this study.
| Target | Sequence |
|---|---|
| Mt1555G probe | 5′-FAM-ATG TTA CGA CTT CCT CC-MGBEQ-3′ |
| Mt1555A probe | 5′-HEX-ATG TTA CGA CTT TCT CC-MGBEQ-3′ |
| Mt1555 A>G forward primer | 5′-GGT CGA AGG TGG ATT TAG CAG TA-3′ |
| Mt1555 A>G reverse primer | 5′-AGT GTA AGT TGG GTG CTT TGT GTT AA-3′ |
| Mt1494T probe | 5′-FAM-CCG TCA CTC TCC TCA-MGBEQ-3′ |
| Mt1494C probe | 5′-HEX-CGT CAC CCT CCT CA -MGBEQ-3′ |
| Mt1494 C>T forward primer | 5′-GCC CTG AAG CGC GTA CAC-3′ |
| Mt1494 C>T reverse primer | 5′-TCC AGT ACA CTT ACC ATG TTA CGA CTT-3′ |
Results
Approximately 9,500 residents more than 20 years old live in Iwaki district, and 1,167, 1,113, and 1,148 residents participated in this project in 2014, 2015, and 2016, respectively. We excluded overlapping participants, participants without available data, and participants who did not consent to genetic testing. We performed genetic testing for a total of 1,683 subjects (Fig. 1), including 651 males and 1,032 females and ranging from 20 to 91 years of age, with an average age of 53 years.
Fig. 1. Flow chart illustrating the selection of subjects.
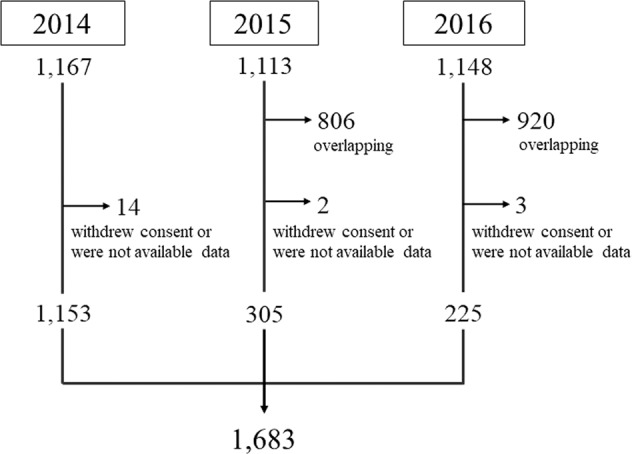
A total of 1,167, 1,113, and 1,148 individuals participated in 2014, 2015, and 2016, respectively. We excluded overlapping participants, participants with unavailable data, and participants who did not provide consent for genetic testing. We performed genetic testing for a total of 1,683 subjects.
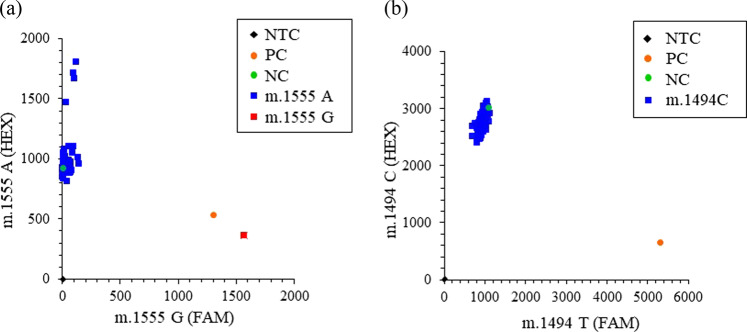
A genetic test evaluating the presence of the m.1555 A>G and m.1494 C>T mutations was performed using the TaqMan genotyping method (Fig. 2). To detect the m.1555 A>G mutation, a HEX-conjugated probe for m.1555 A and a FAM-conjugated probe for m.1555 G were mixed, and the fluorescence intensity of each was analyzed by real-time PCR. The majority of participants were genotyped as m.1555 A, and only one subject was genotyped as m.1555 A>G (Fig. 2a). In contrast, m.1494 C>T was not observed among any of the subjects in this study (Fig. 2b).
Fig. 2. Genetic testing for the mitochondrial DNA SNPs m.1555 A>G and m.1494 C>T.
a Genetic analysis of m.1555 A>G was performed using the TaqMan genotyping method. Representative data, including data for 1 patient with the m.1555 A>G mutation (red square) and participants with the m.1555 A genotype (blue squares), are shown as two-dimensional plots of m.1555 G amplification (FAM probe signal as the X-axis) and m.1555 A amplification (HEX probe signal as the Y-axis). b Representative data for the m.1494 C>T analysis are shown as a plot of m.1494 T amplification (FAM probe signal as the X-axis) and m.1494 C amplification (HEX probe signal as the Y-axis). All participants in this study were genotyped as m.1494 C (blue squares), and the m.1494 C>T mutation was not observed. The black diamond indicates the no-template control (NTC). The orange circle indicates the positive control (PC) [m.1555 A>G in (a) and m.1494 C>T in (b)]. The green circle indicates the negative control (NC) [m.1555 A in (a) and m.1494C in (b)].
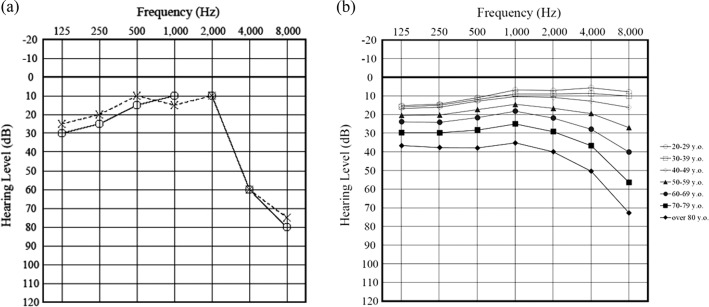
The subject with the m.1555 A>G mutation in this study was a 69-year-old male. He was diagnosed with hearing loss at a medical check-up when he was 15 years old. Although he had no known history of aminoglycoside antibiotic injection, he described working at a job with noise exposure. The audiogram pattern of this subject showed bilateral, symmetric, and high-frequency hearing loss (Fig. 3a). He has a family history of hearing loss; i.e., his elderly sister suffered from hearing loss at a young age. She was also thought to be an m.1555 A>G carrier. She has two daughters and two granddaughters who have not developed hearing loss. We counseled this individual regarding the risks associated with the use of aminoglycoside antibiotics and provided him with a warning card regarding the administration of these medicines14. We also explained to him that the m.1555 A>G mutation is maternally inherited and that his sister and her offspring could have the same gene mutation.
Fig. 3. Audiogram for the m.1555 A>G carrier in this study and generation-wise average audiograms for the subjects in this study.
a The audiogram pattern of the m.1555 A>G carrier showed bilateral, symmetric, and high-frequency (4 and 8 kHz) hearing loss. The right and left audiograms are indicated as circles on a solid line and crosses on a dashed line, respectively. b The generation-wise average audiograms for the subjects in this study. The white circle indicates 20–29 years old. The white square indicates 30–39 years old. The white diamond indicates 40–49 years old. The black triangle indicates 50–59 years old. The black circle indicates 60–69 years old. The black square indicates 70–79 years old. The black diamond indicates more than 80 years old.
Discussion
Approximately 50% of childhood hearing loss is estimated to be associated with genetic contributions, and the prevalence of the m.1555 A>G mutation is 1%4. Furthermore, the prevalence of the m.1555 A>G mutation has been reported to be 0.42–17% in individuals with hearing loss (Table 2)6,15–26. The prevalence of the m.1555 A>G mutation has been reported to be 0.08–0.7% among the general population (Table 2)27–36. In this epidemiological study, the m.1555 A>G mutation was identified in 1 out of 1,683 participants from the general population of the Iwaki district (0.06%), which is slightly lower than the frequency in previous reports. However, this estimate is important as a nonbiased frequency in a certain area where the flow of people is low. Recently, SNP information based on 4,700 healthy volunteers has become available, and the frequency of the m.1555 A>G mutation is found to be 0.15%, which is consistent with our frequency (https://jmorp.megabank.tohoku.ac.jp/202001/).
Table 2.
Prevalence of m.1555 A>G mutation in individuals with hearing loss and the general population.
| Population | Prevalence | Reference |
|---|---|---|
| Japan | 3.45% (11/319) of outpatients with HL | 6 |
| Indonesia | 5.3% (4/75) of deafness patients | 15 |
| Greece | 0.42% (2/478) of patients with NSHL | 16 |
| China | 3.96% (65/1,642) of individuals with NSHL | 17 |
| 3.96% (69/1,742) of individuals with NSHL | 18 | |
| 1.6% (7/434) of individuals with NSHL | 19 | |
| 7.5% (33/440) of individuals with HL | 20 | |
| 5.93% (39/658) of individuals with NSHL | 21 | |
| 6.03% (28/464) of individuals with NSHL | 22 | |
| 3.87% (6/155) of individuals with HL | 23 | |
| 10.17% (6/59) of individuals with NSHL | 24 | |
| 1.92% (3/156) of individuals with NSHL | 25 | |
| Spain | 17% (9/54) of deafness patients | 26 |
| United States | 0.2% (3/1,473) of general population | 27 |
| China | 0.7% (6/865) of newborns | 28 |
| 0.16% (16/10,043) of neonates | 29 | |
| 0.08% (1/1,181) of newborns | 30 | |
| 0.17% (101/58,397) of neonates | 31 | |
| Europe | 0.19% (18/9,371) of children in ALSPAC birth cohort | 32 |
| Australia | 0.21% (6/2,856) of general population > age 49 | 33 |
| Germany | 0.2% (12/7,056) of newborns | 34 |
| South Africa | 0.5% (1/204) of general population | 35 |
| Taiwan | 0.1% (1/1,017) of newborns | 36 |
| Japan | 0.06% (1/1,683) of general population | This study |
HL hearing loss, NSHL nonsyndromic hearing loss, ALSPAC Avon Longitudinal Study of Patients and Children
The m.1494 C>T mutation was not observed in any participants in this epidemiological study. The prevalence of the m.1494 C>T mutation has been reported to be 0.18–0.7% among individuals with hearing loss13,17,20,21,25,37 and 0.01–0.25% among the general population (Table 3)27,29–31. Previous studies reported that the prevalence of the m.1494 C>T mutation was very low, which agreed with the results of this study, in which the m.1494 C>T mutation was not observed among the 1,683 subjects.
Table 3.
Prevalence of m.1494 C>T mutation in individuals with hearing loss and the general population.
| Population | Prevalence | Reference |
|---|---|---|
| Japan | 0.7% (1/140) of individuals with HL | 13 |
| China | 0.18% (3/1,642) of individuals with NSHL | 17 |
| 0.45% (2/440) of individuals with HL | 20 | |
| 0.61% (4/658) of individuals with NSHL | 21 | |
| 0.64% (1/156) of individuals with NSHL | 25 | |
| 0.41% (13/3,133) of individuals with NSHL | 37 | |
| United States | 0.07% (1/1,473) of general population | 27 |
| China | 0.029% (3/10,043) of neonates | 29 |
| 0.25% (3/1,181) of neonates | 30 | |
| 0.01% (8/58,397) of neonates | 31 | |
| Japan | 0% (0/1,683) of general population | This study |
HL hearing loss, NSHL nonsyndromic hearing loss
Some common features of hearing loss have been identified among patients who carry the m.1555 A>G mutation. The audiometric patterns of patients with the m.1555 A>G mutation show bilateral, sensorineural, symmetric, and high-frequency hearing disorders. Hearing loss can also occur either with or without the administration of aminoglycoside antibiotics, and hearing loss can sometimes be progressive. However, hearing loss tends to be severe if aminoglycoside antibiotics have been administered38. In this study, the audiogram of the subject identified as a carrier of the m.1555 A>G mutation showed a bilateral, symmetric, and high-frequency hearing disorder, which corresponded to the pattern observed for mutation carriers in other studies. In Fig. 3b, we present the generation-wise average audiogram for the subjects in this study, which indicates that the hearing of the m.1555 A>G mutation carrier in this study was worse than the hearing of large numbers of noncarriers of the same generation included as subjects in this study.
Although hearing loss was relatively mild in the case under study, this subject is at a risk of progressive hearing loss if treated with aminoglycoside antibiotics. No clinically approved treatment has been reported to be effective for sensorineural hearing loss caused by the administration of aminoglycoside antibiotics; however, prescriptive, well-fitted hearing aids can improve hearing among patients with aminoglycoside-induced hearing loss38. Cochlear implantation has also been reported to be an effective therapeutic choice for patients with profound hearing loss caused by this mutation38. Hearing loss has been reported to be a significant risk factor for dementia3, and hearing loss is thought to have functional, social, emotional, and economic impacts. If carriers of m.1555 A>G and m.1494 C>T mutations are administered aminoglycoside antibiotics, then they may suffer from these disadvantages. Therefore, preventing the progression of hearing loss is an important goal. Usami et al. reported that they distributed drug use warning cards not only to patients but also to their relatives to improve avoidance of the administration of aminoglycoside antibiotics14. In our study, we performed genetic counseling and gave the participant a drug use warning card. Despite newer alternative antibiotics, aminoglycoside antibiotics are still being used worldwide8,9. Thus, the prevention of aminoglycoside administration to m.1555 A>G and m.1494 C>T mutation carriers is important, for example, through the distribution of drug cards.
We were able to identify one m.1555 A>G carrier among a community-dwelling population in the Iwaki district, and we provided genetic counseling and gave him a drug card advising the avoidance of aminoglycoside administration, which will likely contribute to the prevention of hearing loss progression.
Acknowledgements
This work was supported by JST COI [Grant Number JPMJCE1302]. The authors would like to thank all of their coworkers for their skillful contributions to the collection and management of the data for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (2019) Deafness and hearing loss. Available from: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss. Accessed 25 February 2020
- 2.Brodie A, Smith B, Ray J. The impact of rehabilitation on quality of life after hearing loss: a systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2018;275:2435–40.. doi: 10.1007/s00405-018-5100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston G, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–734.. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 4.Morton CC, Nance WE. Newborn hearing screening-a silent revolution. N. Engl. J. Med. 2006;354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs HT, et al. Mitochondrial DNA mutations in patients with postlingual, nonsyndromic hearing impairment. Eur. J. Hum. Genet. 2005;13:26–33. doi: 10.1038/sj.ejhg.5201250. [DOI] [PubMed] [Google Scholar]
- 6.Usami S, et al. Prevalence of mitochondrial gene mutations among hearing impaired patients. J. Med Genet. 2000;37:38–40. doi: 10.1136/jmg.37.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao H, et al. Maternally inherited aminoglycoside-induced and nonsyndromic deafness is associated with the novel C1494T mutation in the mitochondrial 12S rRNA gene in a large Chinese family. Am. J. Hum. Genet. 2004;74:139–152. doi: 10.1086/381133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Boeckel TP, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014;14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 9.Krzyżaniak N, Pawłowska I, Bajorek B. Review of drug utilization patterns in NICUs worldwide. J. Clin. Pharm. Therapeutics. 2016;41:612–20.. doi: 10.1111/jcpt.12440. [DOI] [PubMed] [Google Scholar]
- 10.Goto S, et al. Relationship between cognitive function and balance in a community-dwelling population in Japan. Acta Otolaryngol. 2018;138:471–474.. doi: 10.1080/00016489.2017.1408142. [DOI] [PubMed] [Google Scholar]
- 11.Abe S, Yamaguchi T, Usami S. Application of deafness diagnostic screening panel based on deafness mutation/gene database using invader assay. Genet. Test. 2007;11:333–340. doi: 10.1089/gte.2007.0002. [DOI] [PubMed] [Google Scholar]
- 12.Lu SY, et al. Factors that affect hearing level in individuals with the mitochondrial 1555A.G mutation. Clin. Genet. 2009;75:480–484. doi: 10.1111/j.1399-0004.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 13.Yano T, Nishio SY, Usami S, Deafness Gene Study C. Frequency of mitochondrial mutations in non-syndromic hearing loss as well as possibly responsible variants found by whole mitochondrial genome screening. J. Hum. Genet. 2014;59:100–106. doi: 10.1038/jhg.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usami S, Abe S, Shinkawa H, Inoue Y, Yamaguchi T. Rapid mass screening method and counseling for the 1555A->G mitochondrial mutation. J. Hum. Genet. 1999;44:304–307. doi: 10.1007/s100380050165. [DOI] [PubMed] [Google Scholar]
- 15.Malik SG, Pieter N, Sudoyo H, Kadir A, Marzuki S. Prevalence of the mitochondrial DNA A1555G mutation in sensorineural deafness patients in island Southeast Asia. J. Hum. Genet. 2003;48:480–483. doi: 10.1007/s10038-003-0056-9. [DOI] [PubMed] [Google Scholar]
- 16.Kokotas H, et al. The A1555G mitochondrial DNA mutation in Greek patients with non-syndromic, sensorineural hearing loss. Biochem. Biophys. Res. Commun. 2009;390:755–757. doi: 10.1016/j.bbrc.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, et al. Mitochondrial 12S rRNA variants in 1642 Han Chinese pediatric subjects with aminoglycoside-induced and nonsyndromic hearing loss. Mitochondrion. 2010;10:380–390. doi: 10.1016/j.mito.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu J, et al. Mitochondrial haplotypes may modulate the phenotypic manifestation of the deafness-associated 12S rRNA 1555A>G mutation. Mitochondrion. 2010;10:69–81. doi: 10.1016/j.mito.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji YB, et al. Molecular epidemiological analysis of mitochondrial DNA12SrRNA A1555G, GJB2, and SLC26A4 mutations in sporadic outpatients with nonsyndromic sensorineural hearing loss in China. Acta Otolaryngol. 2011;131:124–129. doi: 10.3109/00016489.2010.483479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Z, et al. Frequency and spectrum of mitochondrial 12S rRNA variants in 440 Han Chinese hearing impaired pediatric subjects from two otology clinics. J. Transl. Med. 2011;9:4–4. doi: 10.1186/1479-5876-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Q, et al. Genetic mutations of GJB2 and mitochondrial 12S rRNA in nonsyndromic hearing loss in Jiangsu Province of China. J. Transl. Med. 2013;11:163. doi: 10.1186/1479-5876-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu BC, et al. Analysis of common deafness gene mutations in deaf people from unique ethnic groups in Gansu Province, China. Acta Otolaryngol. 2014;134:924–929. doi: 10.3109/00016489.2014.927588. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, et al. Mutation spectrum of common deafness-causing genes in patients with non-syndromic deafness in the Xiamen Area, China. PLoS ONE. 2015;10:e0135088. doi: 10.1371/journal.pone.0135088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, Liu Q, Chen L. Screening and analysis of mutation hot-spots in deafness-associated genes among adolescents with hearing loss. Mol. Med. Rep. 2015;12:8179–8184. doi: 10.3892/mmr.2015.4475. [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, et al. GJB2, SLC26A4, and mitochondrial DNA12S rRNA hot-spots in 156 subjects with non-syndromic hearing loss in Tengzhou, China. Acta Otolaryngol. 2016;136:800–805. doi: 10.3109/00016489.2016.1164893. [DOI] [PubMed] [Google Scholar]
- 26.Bravo O, Ballana E, Estivill X. Cochlear alterations in deaf and unaffected subjects carrying the deafness-associated A1555G mutation in the mitochondrial 12S rRNA gene. Biochem. Biophys. Res. Commun. 2006;344:511–516. doi: 10.1016/j.bbrc.2006.03.143. [DOI] [PubMed] [Google Scholar]
- 27.Ealy M, Lynch KA, Meyer NC, Smith RJ. The prevalence of mitochondrial mutations associated with aminoglycoside-induced sensorineural hearing loss in an NICU population. Laryngoscope. 2011;121:1184–1186. doi: 10.1002/lary.21778. [DOI] [PubMed] [Google Scholar]
- 28.Chen G, Wang X, Fu S. Prevalence of A1555G mitochondrial mutation in Chinese newborns and the correlation with neonatal hearing screening. Int. J. Pediatr. Otorhinolaryngol. 2011;75:532–534. doi: 10.1016/j.ijporl.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, et al. Auditory screening concurrent deafness predisposing genes screening in 10,043 neonates in Gansu province, China. Int. J. Pediatr. Otorhinolaryngol. 2012;76:984–988. doi: 10.1016/j.ijporl.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Han B, et al. Newborn genetic screening for high risk deafness-associated mutations with a new Tetra-primer ARMS PCR kit. Int. J. Pediatr. Otorhinolaryngol. 2013;77:1440–1445. doi: 10.1016/j.ijporl.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, et al. Newborn hearing concurrent genetic screening for hearing impairment—A clinical practice in 58,397 neonates in Tianjin, China. Int. J. Pediatr. Otorhinolaryngol. 2013;77:1929–1935. doi: 10.1016/j.ijporl.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 32.Bitner-Glindzicz M, et al. Prevalence of mitochondrial 1555A->G mutation in European children. N. Engl. J. Med. 2009;360:640–642. doi: 10.1056/NEJMc0806396. [DOI] [PubMed] [Google Scholar]
- 33.Vandebona H, et al. Prevalence of mitochondrial 1555A->G mutation in adults of European descent. N. Engl. J. Med. 2009;360:642–644. doi: 10.1056/NEJMc0806397. [DOI] [PubMed] [Google Scholar]
- 34.Göpel W, et al. Mitochondrial mutation m.1555A>G as a risk factor for failed newborn hearing screening in a large cohort of preterm infants. BMC Pediatrics. 2014;14:210–210. doi: 10.1186/1471-2431-14-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bardien S, et al. A rapid method for detection of five known mutations associated with aminoglycoside-induced deafness. BMC Med. Genet. 2009;10:2–2. doi: 10.1186/1471-2350-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C-C, et al. Newborn genetic screening for hearing impairment: a preliminary study at a tertiary center. PLoS ONE. 2011;6:e22314. doi: 10.1371/journal.pone.0022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y, et al. Mitochondrial haplotype and phenotype of 13 Chinese families may suggest multi-original evolution of mitochondrial C1494T mutation. Mitochondrion. 2009;9:418–428. doi: 10.1016/j.mito.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Usami S, Abe S, Shinkawa H, Kimberling WJ. Sensorineural hearing loss caused by mitochondrial DNA mutations: special reference to the A1555G mutation. J. Commun. Disord. 1998;31:423–434. doi: 10.1016/S0021-9924(98)00014-8. [DOI] [PubMed] [Google Scholar]