Abstract
Chemoport is being routinely used to administer chemotherapy, blood, blood products, total parenteral nutrition, and also to draw blood for investigations. We started using chemoport in our institute. We use it exclusively to administer chemotherapy. We analyzed our results of chemoport usage and confirm that the rate of complications associated with chemoport usage is at par with the available literature. We also conclude that with regular use, the intra-op and post-op complications will reduce further.
Keywords: Chemoport, Complications, Wound dehiscence, Infection, Blockage
Introduction
With the continued research in medical oncology, many cancer patients are receiving multiple lines of chemotherapy. Intravenous access is a major issue for these patients. The totally implantable devices were introduced in 1980s [1].
Since then, chemoport insertion is commonly used to gain vascular access for these patients. It can be used to administer intravenous fluids, blood, blood products, parenteral nutrition, and to draw blood for investigations. Chemoport use is associated with complications. We analyzed our cases of chemoport-associated complications and discuss management of complications.
Aims and Objectives
The aim of our study was to determine the complications associated with the use of chemoport and management of the complications.
Materials and Methods
We studied the patients in our hospital who underwent chemoport insertion from May 2012 till November 2018.
The study group comprised of 98 patients who underwent chemoport insertion. Of these, 78 cases received chemotherapy for breast cancer, 12 patients had carcinoma ovary, 4 cases had colorectal cancer, 2 patients had carcinoma stomach, one patient had periampullary adenocarcinoma, and one patient had leukemia.
The chemoport insertion procedure was performed under general anesthesia in all cases. In all cases, we gained venous access through internal jugular vein. In 15 cases, we accessed the left internal jugular vein as they had undergone modified radical mastectomy on right side (Fig. 1). The reservoir was placed beneath the skin on the pectoral region, and catheter was tunneled through the subcutaneous plane into the internal jugular vein. The tip of the catheter was placed at the junction of superior vena cava and right atrium. We routinely use C-arm to confirm the position of the catheter tip. The port was used after 48 h of insertion in 87 cases. In 11 cases, the needle was inserted on-table, and the port was used for chemotherapy infusion after 24 h. We use the chemoport to administer chemotherapy only.
Fig. 1.
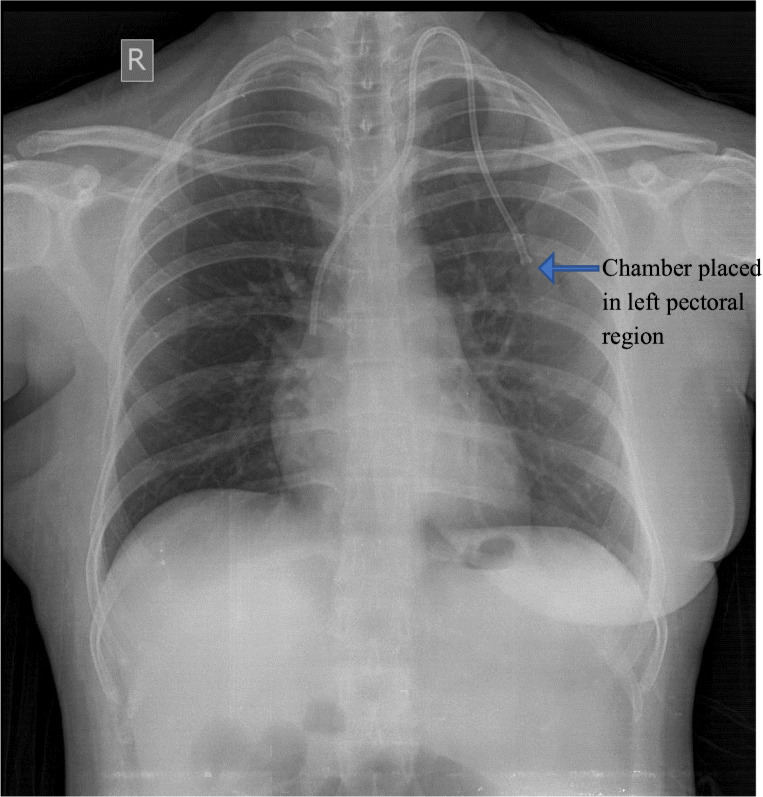
Showing placement of the port in the left pectoral region in an operated case of right side carcinoma breast
Results
The patients were followed for at least 6 months after chemoport insertion. We analyzed the complications during the procedure, post-operative period, and delayed complications as well (Table 1).
Table 1.
Complications encountered and their management
| Complications during usage | Numbers | Percentage | Consequence |
|---|---|---|---|
| Blockage | 3 | 3.06% | Removal of port |
| Port infection | 3 | 3.06% | Removal of port |
| Wound dehiscence | 4 | 4.08% | Re-suturing of the skin and continued use of port |
| Extravasation of drug | 3 | 3.06% | In 1 case, drug aspirated with syringe and needle and port used during subsequent chemo. In 2 cases, massive extravasation and port was not used subsequently |
| Reservoir rotation | 2 | 2.04% | Re-explore, untwist and continue use during subsequent chemo |
| Nil | 83 | 84.69% | – |
None of the patients had pneumothorax or intra-operative bleeding. In 3 cases, the left common carotid artery was punctured, recognized immediately. The needle was withdrawn and compression applied for 5 min. None of them developed hematoma.
Three patients developed signs of catheter infection immediately after administration of 1st cycle of chemotherapy. The organisms isolated (Table 2) included Burkholderia (P.) cepacia, Klebsiella pneumoniae, and Coagulase negative Staphylococcus species. The chemoport was removed immediately and appropriate antibiotics were administered for 2 weeks.
Table 2.
Organisms isolated and antibiotic sensitivity
| Extracted organisms | Sensitivity |
|---|---|
| Burkholderia (P.) cepacia | Levofloxacin |
| Klebsiella pneumoniae | Tigecycline |
| Coagulase negative Staphylococcus species | Vancomycin, moxifloxacin, and tetracycline |
One patient had extravasation of the chemotherapy agent during the first cycle of chemotherapy and one more patient had the same issue during the 3rd cycle. In both the cases, the port usage was withheld and the extravasated drug was aspirated percutaneously.
Three cases had wound dehiscence which was repaired surgically, and the use of chemoport continued. Two cases had reservoir rotation. The reservoir was untwisted surgically and used to administer chemotherapy.
Three patients developed catheter-related thrombosis which was managed by anticoagulants and removal of the port.
There was no mortality due to complications associated with the use of chemoport.
Discussion
Most chemotherapy drugs have significant venous toxicity and often developed venous thrombosis if administered through peripheral veins [2].
This is associated with significant pain. To overcome this, the totally implantable venous access devices were developed in the early 1980s [3].
External venous access devices are also available. These are relatively cheaper. However, TIVAPs last longer, have less infection rate, and make the patient more comfortable [4].
We do not have any experience with the external central venous access catheters. TIVADs provide easy access to administer chemotherapy drugs, blood and blood products, and parenteral nutrition to cancer patients. They are also used to draw blood for investigations and inject contrast for radiological studies [1, 5].
In our patients, we used the TIVADs exclusively for administration of the chemotherapy agents.
The use of TIVADs is definitely associated with complications if proper care is not taken during insertion of the device. Complications can occur due to long-term indwelling of the TIVADs as well. Injury to the adjacent structures during insertion gives rise to early complications [6].
Early complications include hemothorax, pneumothorax, injury to large blood vessels, air emboli, cardiac arrhythmia, and malposition of the catheter. In our study, none of the patients had early complications. This is in accordance with the recent studies which confirm decreasing incidence of early complications with improved technique and use of advanced technology during insertion of TIVADs [7].
Literature mentions arterial puncture in 2 to 4.5% cases of external venous catheterizations, resulting in arterial injury in 0.1 to 0.5% cases [8, 9]. In our series, 3 cases (i.e., 3.06%), arterial puncture was noted during the procedure and measures were taken (as described in the “Material and Methods” section) to prevent hematoma formation.
Case series reports have reported major complications such as air embolism [10], hemothorax [11], brachial plexus injury [12], and pericardial tamponade [13]. In our study, none of the patients had any of these complications.
Late complications comprise infections, venous thrombosis, extravasations of cytotoxic drugs, mechanical dysfunction of the catheter, and skin necrosis [14, 15].
In our series, wound dehiscence was the most common delayed complication experienced in 4.06% of the cases. This was probably because of thin skin flaps created during the preparation for chamber placement. The other common complication in our series was catheter-related thrombosis (3.06%). Literature mentions catheter-related thrombosis between 1 and 5% [16]. Low-molecular weight heparin was immediately started and TIVAD removed as they were non-functional.
TIVAD-related infection was noted in 3.06% cases. Literature mentions TIVAD-related infection up to 4.8% [8]. The causes explained for infection include frequent access, administration of total parenteral nutrition, chronic steroid use, neutropenia, thrombosis, and metastatic disease [17–19]. The incidence of infection is higher for hematologic malignancy as compared with solid tumors [20]. Intravenous antibiotics were administered as per the culture sensitivity. The TIVAD was removed in all 3 cases as they continued to have fever.
Conclusion
We conclude that use of TIVADs is safe. The intra-op and immediate post-op complication rates have decreased with increased use of TIVADs. The long-term complications still remain a challenge to overcome.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kumar M. Vinchurkar, Email: vkumar_007@yahoo.com
Preeti Maste, Email: drpreetikv79@gmail.com.
Manoj D. Togale, Email: zapmanojtogale@yahoo.com
Vishwanath M. Pattanshetti, Email: drvmshetti@gmail.com
References
- 1.Vescia S, Baumgärtner AK, Jacobs VR, Kiechle-Bahat M, Rody A, Loibl S, et al. Management of venous port systems in oncology: a review of current evidence. Ann Oncol Off J Eur Soc Med Oncol. 2008;19(1):9–15. doi: 10.1093/annonc/mdm272. [DOI] [PubMed] [Google Scholar]
- 2.Ignatov A, Hoffman O, Smith B, Fahlke J, Peters B, Bischoff J, et al. An 11-year retrospective study of totally implanted central venous access ports: complications and patient satisfaction. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2009;35(3):241–246. doi: 10.1016/j.ejso.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K, Cozzi E Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982; 92(4):706–712 [PubMed]
- 4.Di Carlo I, Cordio S, La Greca G, Privitera G, Russello D, Puleo S, Latteri F Totally implantable venous access devices implanted surgically: a retrospective study on early and late complications. Arch Surg 2001; 136(9):1050–1053 [DOI] [PubMed]
- 5.de Oliveira EB, Reis MA, Avelar TM, Vieira SC. Totally implantable central venous catheters for chemotherapy: experience with 793 patients. Rev Col Bras Cir. 2013;40(3):186–190. doi: 10.1590/S0100-69912013000300004. [DOI] [PubMed] [Google Scholar]
- 6.Tabatabaie O, Kasumova GG, Eskander MF, Critchlow JF, Tawa NE, Tseng JF. Totally implantable venous access devices: a review of complications and management strategies. Am J Clin Oncol. 2017;40(1):94–105. doi: 10.1097/COC.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 7.Granziera E, Scarpa M, Ciccarese A, Filip B, Cagol M, Manfredi V, et al. Totally implantable venous access devices: retrospective analysis of different insertion techniques and predictors of complications in 796 devices implanted in a single institution. BMC Surg. 2014;14:27. doi: 10.1186/1471-2482-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaghal A, Khalife M, Mukherji D, El Majzoub N, Shamseddine A, Hoballah J, et al. Update on totally implantable venous access devices. Surg Oncol. 2012;21(3):207–215. doi: 10.1016/j.suronc.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Ezaru CS, Mangione MP, Oravitz TM, Ibinson JW, Bjerke RJ (2009) Eliminating arterial injury during central venous catheterization using manometry. Anesth Analg 109(1):130–134 [DOI] [PubMed]
- 10.Vesely TM. Air embolism during insertion of central venous catheters. J Vasc Interv Radiol JVIR. 2001;12(11):129–125. doi: 10.1016/S1051-0443(07)61554-1. [DOI] [PubMed] [Google Scholar]
- 11.Innami Y, Oyaizu T, Ouchi T, Umemura N, Koitabashi T. Life-threatening hemothorax resulting from right brachiocephalic vein perforation during right internal jugular vein catheterization. J Anesth. 2009;23(1):135–138. doi: 10.1007/s00540-008-0696-1. [DOI] [PubMed] [Google Scholar]
- 12.Porzionato A, Montisci M, Manani G. Brachial plexus injury following subclavian vein catheterization: a case report. J Clin Anesth. 2003;15(8):582–586. doi: 10.1016/j.jclinane.2003.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Collier PE, Goodman GB. Cardiac tamponade caused by central venous catheter perforation of the heart: a preventable complication. J Am Coll Surg. 1995;181(5):459–463. [PubMed] [Google Scholar]
- 14.Narducci F, Jean-Laurent M, Boulanger L, El Bédoui S, Mallet Y, Houpeau JL, et al. Totally implantable venous access port systems and risk factors for complications: a one-year prospective study in a cancer centre. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2011;37(10):913. doi: 10.1016/j.ejso.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Granic M, Zdravkovic D, Krstajic S, Kostic S, Simic A, Sarac M, et al. Totally implantable central venous catheters of the port-a-cath type: complications due to its use in the treatment of cancer patients. J BUON Off J Balk Union Oncol. 2014;19(3):842–846. [PubMed] [Google Scholar]
- 16.Geerts W. Central venous catheter-related thrombosis. Hematol Am Soc Hematol Educ Prog. 2014;2014:306–311. doi: 10.1182/asheducation-2014.1.306. [DOI] [PubMed] [Google Scholar]
- 17.Lebeaux D, Fernández-Hidalgo N, Chauhan A, Lee S, Ghigo JM, Almirante B, Beloin C. Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis. 2014;14(2):146–159. doi: 10.1016/S1473-3099(13)70266-4. [DOI] [PubMed] [Google Scholar]
- 18.Chang L, Tsai JS, Huang SJ, Shih CC. Evaluation of infectious complications of the implantable venous access system in a general oncologic population. Am J Infect Control. 2003;31(1):34–39. doi: 10.1067/mic.2003.29. [DOI] [PubMed] [Google Scholar]
- 19.Chen IC, Hsu C, Chen YC, Chien SF, Kao HF, Chang SY, et al. Predictors of bloodstream infection associated with permanently implantable venous port in solid cancer patients. Ann Oncol Off J Eur Soc Med Oncol. 2013;24(2):463–468. doi: 10.1093/annonc/mds468. [DOI] [PubMed] [Google Scholar]
- 20.Samaras P, Dold S, Braun J, Kestenholz P, Breitenstein S, Imhof A, Renner C, Stenner-Liewen F, Pestalozzi BC. Infectious port complications are more frequent in younger patients with hematologic malignancies than in solid tumor patients. Oncology. 2008;74:237–244. doi: 10.1159/000151393. [DOI] [PubMed] [Google Scholar]


