Abstract
Evaluation of the efficacy of the combination of radical surgery, hyperthermic intraperitoneal chemotherapy (HIPEC), and adjuvant systemic chemotherapy (ACT) in reducing gastric cancer progression in patients with resectable serosa-invasive gastric cancer in a single institution. In 2015–2016, 19 patients with gastric cancer (stage IIB-IIIC) were included in the trial. The trial protocol comprised radical surgery, HIPEC (cisplatin 50 mg/m2 + doxorubicin 50 mg/m2, 42 °C, 1 hour), and 1–8 cycles of ACT (oxaliplatin 100 mg/m2 administered on day 1 of each cycle and oral capecitabine 1000 mg/m2 (or tegafur 10–15 mg/kg) administered twice daily on days 1–14 of each cycle with an interval of 7 days between cycles). Following the ACT treatment, the patients were divided into 2 subgroups—those who underwent up to 6 ACT cycles (1–6 cycles, subgroup ≤ 6–8 patients) and those who underwent 7–8 ACT cycles (subgroup > 6–11 patients). Three-year metastasis-free survival (MFS) for the > 6 subgroup was 91 ± 9%. With a follow-up median of 17 months, 3-year MFS for the ≤ 6 subgroup was not reached − plog-rank = 0.003. The trial showed that in managing advanced gastric cancer patients (pT4a-4bN0-3 M0) by supplementing radical surgery with ACT-enhanced hyperthermic intraperitoneal chemotherapy, ACT proved to be highly effective when administered in its full mode of 7–8 cycles compared with its truncated variant of 1–6 cycles.
Keywords: Gastric cancer, Intraoperative intraperitoneal hyperthermic chemotherapy, Adjuvant chemotherapy
Introduction
Tumor invasion extending beyond the stomach wall into the regional lymph node despite a radical surgery treatment may be regarded as an initial stage of a systemic gastric cancer (GC) progression resulting not only in the development of peritoneal dissemination but also in the onset of distant metastasеs. Although the administration of HIPEC reduces the frequency and risks of carcinomatosis [1], it has no effect on the distant metastasеs development because of its primarily loco-regional effect [2], thus reflecting poorly on long-term treatment outcomes, so it is obviously logical to target distant metastasеs development by administering a multimodal treatment combining radical surgery (gastrectomy or subtotal gastric resection with D2 lymphadenectomy) with HIPEC and ACT [1, 3]. This consideration buttressed by positive outcomes of a similar multimodal approach in the treatment of ovarian cancer [4] prompted us to launch this study.
Materials and Methods
The study is based on the results of treating 19 GC patients (R. Borrmann type III-IV with preoperatively established performance status of 0-I ECOG and tumor stage of pT4N+ without esophagus involvement), aged 39–65 (median age of 57.4 ± 5.8), comprising 12 men and 7 women, who underwent a multimodal treatment in 2015–2016 at the N.N. Alexandrov National Cancer Center of Belarus. Resectability was determined preoperatively by means of computed tomography and ultrasonography. Exclusion criteria comprised metastatic disease (M1), New York Heart Association class III-IV, history of active infectious disease or myocardial infarction over the previous 6 months.
Treatment protocol included (1) either radical gastrectomy or distal subtotal gastric resection in combination with D2 lymphadenectomy; (2) HIPEC in accordance with our previously designed regimen (cisplatin 50 mg/m2 + doxorubicin 50 mg/m2 administered at 42 °С for 1 h) [5]; and (3) 8 cycles of ACT consisting of intravenous oxaliplatin 100 mg/m2 administered on day 1 of each cycle and oral capecitabine 1000 mg/m2 administered twice daily on days 1–14 of each cycle with an interval of 7 days between cycles (14 patients) or tegafur 10–15 mg/kg twice daily on days 1–14 of each cycle with an interval of 7 days between cycles (5 patients). The patients who underwent this multimodal treatment made up the HIPEC+ACT group. Patient characteristics are summed up in Table 1.
Table 1.
Patient characteristics
| Characteristics | Number of patients by characteristic |
|---|---|
| Males/females | 12 (63.2) / 7 (36.8) |
| Age, years ± SE | 57.4 ± 1.3 |
| pT | |
| pT4a | 14 (73.7) |
| pT4b | 5 (26.3) |
| pN | |
| pN0 | 5 (26.3) |
| pN1 | 7 (36.8) |
| pN2 | 2 (10.5) |
| pN3 | 5 (26.3) |
| G | |
| GI | 1 (5.3) |
| GII | 6 (31.6) |
| GIII | 12 (63.2) |
| GIV | 0 |
The trial was approved by the Ethics Committee of the N.N. Alexandrov National Cancer Center. Written informed consent was obtained from all the eligible patients before their trial entry.
Assessment of toxicities was performed by means of CTCAE version 4.03.
Surgical treatment consisted of total or partial (distal subtotal resection) gastrectomy with free margins (R0 resection) and D2 lymphadenectomy. Five patients underwent combined resection. HIPEC was conducted for 1 h by means of a Thermochem™ НТ-1000 automatic device (Thermasolutions, Inc., Pittsburgh, PA, USA) after completing gastrectomy/alimentary tract reconstruction and wound closure. The inflow catheter (32F) was inserted under the left hemidiaphragm. Three outflow catheters (32F) were positioned in the true and false pelvises in the subhepatic area with probes for temperature control mounted on the tips of the inflow and outflow catheters.
Follow-up consisted of detailed clinical examinations, laboratory tests (blood count, hepatic function), and periodic diagnostic imaging (chest radiography, ultrasonography, CT) performed every 3 months during the first year after the treatment and every 6 months during the second and subsequent years. Metachronous peritoneal dissemination such as massive ascites, enhanced nodules located in the abdominal or pelvic cavity, and abnormal wall thickness of the intestine, was monitored by performing CT and ultrasonography and also by second-look laparoscopy and peritoneal biopsy every year after the treatment or where there was a suspicion of gastric cancer progression. Metachronous peritoneal dissemination, hematogenous, and distant lymph node metastases (paraaortic, mesenteric, and extraabdominal lymph nodes) were classified as distant metastases (DM).
End Points and Statistical Analysis
Metastasis-free survival (MFS) was used for evaluating the efficacy of the proposed treatment modality. MFS was calculated from the date of the diagnosis to the first event (distant metastases (DM) or death from any cause). The survival rate from the date of diagnosis until the event of interest or loss of contact was assessed using the Kaplan-Meier estimator. The comparative analysis of the MFS was performed using the Mantel-Cox log-rank test. The statistical analysis was conducted by means of the R statistical package (R Project for Statistical Computing, http://www.r-project.org), version 3.4, library survival [6].
Results
Eleven out of 19 patients completed the scheduled treatment course. The other 8 patients were administered from 1 to 6 ACT cycles: 4 of them refused to participate further in the trial, 2 patients, while undergoing ACT therapy, were diagnosed with an early disease relapse resulting from a non-radical R1 surgery treatment (tumor growth at the esophageal resection margin), and 2 patients developed asthenia, nausea, and decreased appetite after undergoing 3 ACT cycles that were viewed as ACT-induced toxicities. These patients continued to receive ACT after being transferred to oral fluoropyrimidine therapy.
No post-HIPEC grade III-IV toxicities according to CTCAE 4.03 were detected. Owing to the absence of these toxic complications ACT was administered as initially scheduled. Post-ACT biochemical indicators showed statistically significant changes in the levels of (a) urea, AST, and ALT when comparing their levels prior to the ACT treatment with those after the completion of cycle 8; (b) glucose, AST, ALT, and potassium when comparing their pre-surgery levels with those after the completion of cycle 8. Comparison of other biochemical indicators with their initial values showed no statistical significance within the study time limits.
As regards ACT-related side effects, those were mainly hematological and metabolic toxicities, but no grade III-IV complications were observed (Table 2).
Table 2.
Toxicities observed during the proposed ACT treatment according to СТСАЕ criteria, version 4.03
| Toxicity | Toxicity grade absolute count, % | ||
|---|---|---|---|
| I | II | III–V | |
| Neurotoxicity | – | – | – |
| Cardiotoxicity | – | – | – |
| Skin toxicity (hand-foot syndrome) | 1 (5.0%) | – | – |
| Allergy | 1 (5.0%) | – | – |
| Gastrointestinal toxicity | |||
| Nausea | 4 (21.1%) | 2 (10.5%) | – |
| Vomiting | 1 (5.0%) | 1 (5.0%) | – |
| Diarrhea | – | – | – |
| Hematological toxicity | |||
| Hemoglobin | 9 (47.4%) | 2 (10.5%) | – |
| Leucopenia | – | – | – |
| Lymphopenia | 1 (5.0%) | 2 (10.5%) | – |
| Thrombocytopenia | 2 (10.5%) | 1 (5.0%) | – |
| Neutropenia | 3 (15.8%) | – | – |
| Metabolic toxicity | |||
| AST | 1 (5.0%) | – | – |
| ALT | 1 (5.0%) | – | – |
| Bilirubenia | – | – | – |
| Creatinine | – | – | – |
| Constitutional symptoms | 1 (5.0%) | 1 (5.0%) | – |
The above data testify to a satisfactory tolerability of the proposed ACT regimen that allowed, firstly, to carry out the chemotherapy treatment plan in full and on schedule, and secondly, to perform ACT in combination with radical surgery and HIPEC without detriment to the patients’ health. That could possibly be attributed to a reduced oxaliplatin dosage of 100 mg/m2 in our ACT regimen compared with previously reported studies of similar design [7, 8].
Follow-up median in the present study was 36 months; follow-up median prior to the onset of disease progression was 14 months. Peritoneal cancer dissemination was diagnosed in one patient 6.3 months after surgery. The frequency and characteristics of disease progression are summed up in Table 3.
Table 3.
Frequency and characteristics of disease progression after combined HIPEC+ACT administration
| Characteristics of disease progression | TNM | Total n = 19 (100%) | ||
|---|---|---|---|---|
| T4a-bN0, n = 5 (26.3%) | T4a-bN1, n = 7 (36.8%) | T4a-bN2–3, n = 7 (36.8%) | ||
| Peritoneal cancer dissemination | 0 | 0 | 1 (14.3) | > 0.99 |
| Progression (without peritoneal dissemination) | 0 | 3 (42.9) | 3 (42.9) | 0.278 |
| Distant metastasis of a different localization | ||||
| Liver metastasis | 0 | 1 (14.3) | 0 | > 0.99 |
| Metastasis in non-regional lymph nodes | 0 | 2 (28.6) | 1 (14.3) | 0.747 |
| Bone metastasis | 0 | 1(14.3) | 3 (42.9) | 0.381 |
| Death from cancer progression | 0 | 0 | 2 (28.6) | 0.304 |
At the time of writing this article (January of 2020), 12 patients remained alive without any signs of disease progression. pN0 patients showed no signs of disease progression within the above-stated follow-up time frames. Disease progression in patients with metastases in the regional lymph node showed a predominantly DM development.
For purposes of comparison, the control group included patients who underwent surgery/HIPEC treatment in our previously conducted randomized study [5].
In terms of long-term treatment outcomes, there was no difference between MFS for the HIPEC-only control group patients (n = 68) and that for the entire HIPEC+ ACT group patients (n = 19) who underwent the proposed combined treatment − plog-rank = 0.412; 3-year MFS was 48.6 ± 6.4% in the HIPEC-only control group and 66.7 ± 11.1% in the HIPEC+ACT group. However, given the fact that the HIPEC+ACT group was not homogeneous in terms of the number of ACT cycles administered to patients, we conducted an in-group analysis of MFS in relation to the number of administered ACT cycles. In view of the currently accepted optimal time-frame of no less than 6 months for administering fluoropyrimidine-based ACT [9], the patients in the HIPEC+ACT group were divided into 2 subgroups—those who underwent up to 6 ACT cycles (1–6 cycles, subgroup ≤6–8 patients) and those who underwent 7–8 ACT cycles (subgroup >6–11 patients). Three-year MFS for the > 6 subgroup was 91 ± 9%. With a follow-up median of 17 months, 3-year MFS for the ≤ 6 subgroup was not reached − plog-rank = 0.003 (Fig. 1). of administered ACT cycles
Fig. 1.
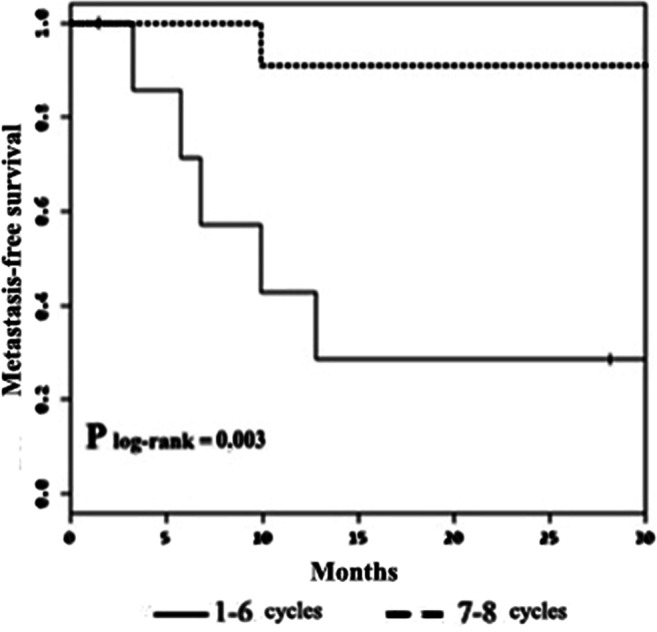
Post-HIPEC+ACT metastasis-free survival rate in relation to the number of administered ACT cycles
Comparison of MFS for the > 6 HIPEC+ACT subgroup with that for the HIPEC-only group (within the same follow-up time) also points to benefits to be gained from the HIPEC+ACT combination providing an adequate number of ACT cycles is administered. Thus, 3-year MFS for the > 6 HIPEC+ACT subgroup was 91.0 ± 9.0% while that for the HIPEC-only control group was 48.6 ± 6.4 − plog-rank = 0.025 (Fig. 2).
Fig. 2.
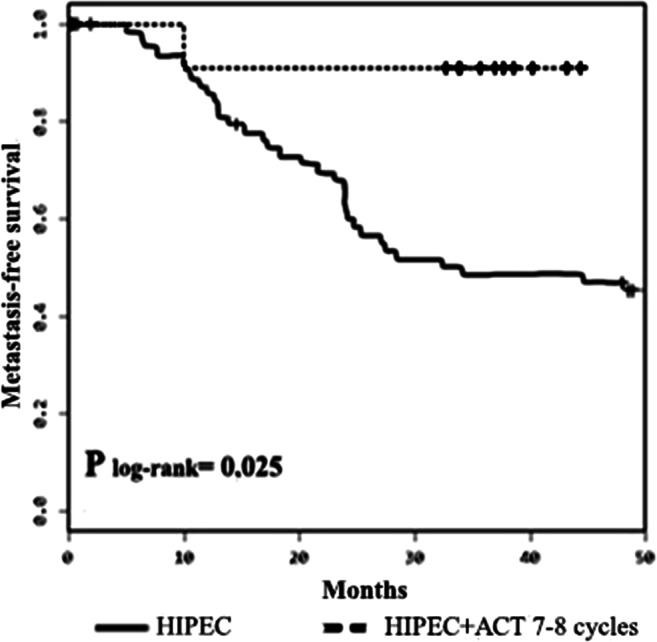
Metastasis-free survival rate after administering HIPEC and HIPEC+7–8 ACT cycles
As it is evident from the above, the administration of HIPEC in combination with ACT of an optimal duration comprising 7–8 cycles allows to attain statistically significantly improved long-term results of treating serosa-invasive gastric cancer.
Discussion
Among the none-too-many works discussing the combined application of systemic and intrapertoneal chemotherapy, there are some publications that highlight the potentialities of this integrated method of GC treatment. Way back in 2000, Zuo Y. et al. [10] reported their statistically significant results of improving 3-year MFS by employing a multimodal GC treatment (radical surgery + HIPEC + ACT)—up to 83% against 61% in the control group (radical surgery + ACT). C. Shi et al. (2011) [11] also mentioned a longer overall 5-year survival rate attained after using a combination of radical surgery + intraperitoneal chemotherapy + ACT compared with radical surgery only – 60.4% vs. 42.9% (p = 0.001), and likewise, progression-free survival – 60.5% vs. 46.2% (p = 0.001).
To date, there are three known methods of combining HIPEC and ACT mentioned in literature. The most radical of them, in our view, is a method combining perioperative SCT and HIPEC advanced among others by Costa W.L. Jr. et al. (2012) [12] who used three perioperative SCT cycles of DCF (docetaxel 75 mg/m2, cisplatin 75 mg/m2, and continuous intravenous infusion of 5-fluorouracil 750 mg/m2 for 5 days), followed by radical surgery and HIPEC (mytomicin C at 34 mg/m2 for 90 min), and finally, three more postoperative cycles of DCF. Disease progression was diagnosed to occur in 3 out of 10 patients (with 2 of them also developing tumor dissemination in peritoneum) within a follow-up period from 4 to 15 months. Seven patients remained disease-free with 2 of them—more than 4 years of follow-up observations.
The second method was based on the combination of intraperitoneal chemotherapy and ACT. Employing this method, Xue S.-L. et al. (2012) achieved improvements in long-term results of patient management by combining intraperitoneal chemotherapy (intraperitoneal delivery of 5-flurorouracil 600 mg/m2 on days 4 and 5 and cisplatin 40 mg/m2 on day 5) and 6 post-surgery ACT cycles (oxaliplatin 85 mg/m2 on day 1 followed by leucovorin 200 mg/m2 and 5-flurorouracil 450 mg/m2 on days 1–3) [3].
The third method sought to improve HIPEC efficacy by performing intraoperative systemic chemotherapy. This method was used in GASTRICHIP randomized and multicenter phase III studies and consisted of a radical surgery followed by systemic chemotherapy (5-fluorouracil of 400 mg/m2 and leucovorin of 10 mg/m2) administered 15 min prior to the start of a HIPEC procedure (oxaliplatin of 250 mg/m2 and glucose of 5%/2 L/m2 at a perfusate temperature of 42°-43 °C for 30 min) [13].
The present study employed the second method combining HIPEC and postoperatively administered ACT. ACT drug combination included capecitabine (or tegafur) and oxaliplatin. The choice of this combination was prompted primarily by its lower toxicity profile as confirmed by some authors [14, 15]. It was reported, in particular, that in comparison with cisplatin, the use of oxaliplatin led to fewer hematological complications. For example, according to Cunningham D. et al. (2008) [16], administration of ACT based on the combination of epirubicin/5-FU/oxaliplatin led to fewer occurrences of grade III-IV neutropenea, creatinine increase, and alopecia (p = 0.003) compared with a similar mix that used cisplatin instead of oxaliplatin. Hand-foot syndrome, the most frequently occurring complication, was shown to be directly associated with capecitabine dosage. For example, Lee J. et al. (2012) [8] reported that hand-foot syndrome frequency increased from 10.6 to 13.7% when the capecitabine dosage was raised from 1750 to 2000 mg/m2. Neutropenea is the second most frequent complication with a frequency of occurrence from 18.8% in a study conducted by G.M. Kim et al. (2012) [17] to 60.5% observed by Noh S.H. et al. (2014) in their study [7]. Literature survey shows that most of complications are manageable and can be corrected by modifying drug dosages [7, 8]. This observation was also confirmed by the present study.
Alongside toxicity profile, no less important consideration in designing the ACT regimen was to improve long-term treatment outcomes. In pursuance of this goal, the design of the ACT regimen incorporated the techniques employed by other researchers who reported improved long-term results of treatment by combining oral fluoropyrimidines with oxaliplatin [7, 9, 18]. For example, I.H. Kim et al. (2018) [9] reported about the possibility of obtaining satisfactory survival results by using a combination of capecitabine/oxaliplatin with oxaliplatin 10 mg/m2 in the treatment of resectable GC. A comparative analysis of efficacy of S-1 and capecitabine regimens conducted by these researchers showed a higher efficacy of the latter regimen, especially with regard to treating stage IIIB-C gastric cancer including prevention of peritoneal dissemination: progression-free survival rates were lower for the S-1 regimen – 65.8% vs. 68.9% (p = 0.019) for the IIIB stage, and 48.4% vs. 66.7% (р = 0,002) for the IIIC stage [9]. Noh S.H. et al. (2014) [7] achieved a statistically significant improvement in relapse-free survival rates in the combined treatment group from 59% (control group) to 74% (р < 0.0001). In the opinion of these authors, such results could be attributed to a relatively low occurrence of side effects (in comparison with other ACT designs) comparable with that of 5-FU regimens [19], and the absence of post-treatment lethal complications. In addition to this, it could be presumed that another possible factor that contributed to higher survival rates achieved by Noh S.H. et al. (2014) [7] was the application a metronomic chemotherapy regimen that allowed to suppress the growth of subclinical DM, and as a consequence, to improve treatment results. From this standpoint, oral fluoropyrimidines are more convenient to apply than their parenteral forms and are more preferable for patients [20]. Furthermore, their use excludes complications associated with peripheral vein catheterization.
With a view to generally improving the tolerability of the administered multimodal treatment and preventing the development of complications, the oxaliplatin dosage in the present study was reduced to 100 mg/m2 compared with similar studies [7, 18]. It allowed to improve the toxicity profile without compromising the cancer treatment potential of the proposed ACT design.
Overall, our study confirmed published research data about the safety and practicability of combining oral fluoropyrimidines (capecitabine or tegafur) with oxaliplatin in the adjuvant regime as well as the possibility of their use jointly with radical surgery and HIPEC. However, it should be specifically stated that the application of the proposed capecitabine/oxaliplatin-based ACT regimen can be successful only when administered during an optimal length of time which should be not less than 6 months (7–8 cycles). This conclusion is also confirmed by other studies [18]. Other benefits of the proposed ACT regimen based on oral chemotherapeutic drugs include reduction in labor input and lower treatment costs, fewer psycho-emotional stresses, and greater convenience for patients.
The results of the present study confirm the potentiality of complimenting radical surgery with HIPEC and ACT in improving survival outcomes of patients with locally advanced pT4a-4bN0-3 M0 gastric cancer and highlight the importance of developing an integrated approach to the prevention of disease progression after performing radical surgery.
Conclusions
The administration of adjuvant systemic chemotherapy employing oxaliplatin 100 mg/m2 on day 1 of each cycle in combination with capecitabine 1000 mg/m2 or tegafur 10–15 mg/kg twice daily on days 1–14 of each cycle was accompanied mainly by a grade I-II hematological toxicity (CTCAE, version 4.03). The frequency and the degree of intensity of toxic reactions were lower than those cited in reports on analogous adjuvant treatment modalities employing other dosage strategies thereby attesting to a more patient-friendly and effective performance of the proposed integrated treatment modality.
The administration of 1 to 6 ACT cycles (oxaliplatin and capecitabine or tegafur) in combination with radical surgery and HIPEC is unpractical due to a statistically significant decline in 3-year metastasis-free survival compared with that achieved after administering HIPEC jointly with 7–8 ACT cycles of the same design − plog-rank = 0.003.
The combination of radical surgery, intraoperative intraperitoneal hyperthermic chemoperfusion (cisplatin 50 mg/m2 and doxorubicin 50 mg/m2), and 7–8 ACT cycles (oxaliplatin and capecitabine or tegafur) allowed to raise the 3-year survival rate to up to 91.0 ± 9.0% (plog-rank = 0.025) compared with 48.6 ± 6.4% for patients who underwent a combined surgery/HIPEC only.
Acknowledgments
The authors thank all patients, coordinators, and investigators who participated in the study.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (include name of committee + reference number) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
M. Yu. Reutovich, Email: mihail_revtovich@yahoo.com
O. V. Krasko, Email: krasko@newman.bas-net.by
O. G. Sukonko, Email: olre_minsk@mail.ru
References
- 1.Sugarbaker PH. Adjuvant intraperitoneal chemotherapy for advanced primary gastric cancer. Scand J Surg. 2006;95(4):270–273. doi: 10.1177/145749690609500410. [DOI] [PubMed] [Google Scholar]
- 2.Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, Yonemura Y, Ansaloni L. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol. 2014;40:12–26. doi: 10.1016/j.ejso.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Xue S-L, Su H-F, Hu X-Q, et al. Adjuvant combined systemic chemotherapy and intraperitoneal chemotherapy for locally advanced gastric cancer. Oncol Lett. 2012;4:1309–1314. doi: 10.3892/ol.2012.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA, Gynecologic Oncology Group Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 5.Reutovich MY, Krasko OV, Sukonko OG. Hyperthermic intraperitoneal chemotherapy in serosa-invasive gastric cancer patients. Eur J Surg Oncol. 2019;45(12):2405–2411. doi: 10.1016/j.ejso.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Therneau T (2015) _A package for survival analysis in S_. version 2.38. https://CRAN.R-project.org/package=survival. Accessed 12 Dec 2018.
- 7.Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ, CLASSIC trial investigators Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomized controlled trial. Lancet. 2014;15(12):1389–1396. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, Bae JM, Ahn YC, Sohn I, Jung SH, Park CK, Kim KM, Kang WK. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30(3):268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 9.Kim IH, Park SS, Lee CM, Kim MC, Kwon IK, Min JS, Kim HI, Lee HH, Lee SI, Chae H. Efficacy of adjuvant S-1 versus XELOX chemotherapy for patients with gastric cancer after D2 lymph node dissection: a retrospective, multi-center observational study. Ann Surg Oncol. 2018;25(5):1176–1183. doi: 10.1245/s10434-018-6375-z. [DOI] [PubMed] [Google Scholar]
- 10.Zuo Y, Xu M, Shen D, et al. Postoperative intraperitoneal hyperthermic chemoperfusion combined with intravenous chemotherapy for 82 advanced gastric cancer patients. Zhonghua Zhong Liu Za Zhi. 2004;26(4):247–249. [PubMed] [Google Scholar]
- 11.Shi C, Yang B, Chen Q, Yang J, Fan N. Retrospective analysis of adjuvant intraperitoneal chemotherapy effect prognosis of resectable gastric cancer. Oncology. 2011;80(5–6):289–295. doi: 10.1159/000329075. [DOI] [PubMed] [Google Scholar]
- 12.Costa WL, Jr, Coimbra FJ, Ribeiro HS, et al. Safety and preliminary results of perioperative chemotherapy and hyperthermic intraperitoneal chemotherapy (HIPEC) for high-risk gastric cancer patients. World J Surg Oncol. 2012;10:195. doi: 10.1186/1477-7819-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glehen O, Passot G, Villeneuve L, Vaudoyer D, Bin-Dorel S, Boschetti G, Piaton E, Garofalo A. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer. 2014;14:183. doi: 10.1186/1471-2407-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porschen RJ, Arkenau HT, Kubicka S, Greil R, Seufferlein T, Freier W, Kretzschmar A, Graeven U, Grothey A, Hinke A, Schmiegel W, Schmoll HJ, AIO Colorectal Study Group Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO colorectal study group. J Clin Oncol. 2007;25(27):4217–4223. doi: 10.1200/JCO.2006.09.2684. [DOI] [PubMed] [Google Scholar]
- 15.Cho EK, Lee WK, Im SA, Lee SN, Park SH, Bang SM, Park DK, Park YH, Shin DB, Lee JH. A phase II study of epirubicin, cisplatin and capecitabine combination chemotherapy in patients with metastatic or advanced gastric cancer. Oncology. 2005;68(4–6):333–340. doi: 10.1159/000086972. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom Capecitabine and Oxaliplatin for advanced Esophagogastric Cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 17.Kim GM, Jeung HC, Rha SY, Kim HS, Jung I, Nam BH, Lee KH, Chung HC. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer. 2012;48(4):518–526. doi: 10.1016/j.ejca.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Fuse N, Bando H, Chin K, Ito S, Yoshikawa T, Tsuburaya A, Terashima M, Kawashima Y, Fukunaga T, Gotoh M, Emi Y, Yoshida K, Oki E, Takahashi S, Kuriki H, Sato K, Sasako M. Adjuvant capecitabine plus oxaliplatin after D2 gastrectomy in Japanese patients with gastric cancer: a phase II study. Gastric Cancer. 2017;20(2):332–340. doi: 10.1007/s10120-016-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sehnake KJ, Sugarbaker PH, Yoo D. Neutropenia following perioperative intraperitoneal chemotherapy. Tumori. 1999;85(1):41–46. doi: 10.1177/030089169908500109. [DOI] [PubMed] [Google Scholar]
- 20.Borner MM, Schoffski P, de Wit R, et al. Patient preference and pharmacokinetics of oral modulated UFT vs intravenous fluorouracil and leucovorin: a randomized crossover trial in advanced colorectal cancer. Eur J Cancer. 2002;38(3):349–358. doi: 10.1016/S0959-8049(01)00371-9. [DOI] [PubMed] [Google Scholar]


