Abstract
Phyllodes tumors (PT) are rare fibroepithelial lesions, about 0.3–0.5% of all breast tumors. This study is an evaluation of patient characteristics, clinicopathologic features, diagnostic tools, therapeutic options, risk factors for recurrence, and distant metastasis and follow-up findings in patients with PTs. One hundred twenty-seven patients with pathologically proved PTs in the National Cancer Institute, Cairo University, Egypt, from January 2011 to January 2016 were reviewed and analyzed. Sixty patients presented with benign PTs (47.2%), 34 had borderline PTs (26.8%), and 33 had malignant PTs (26%). The mean follow-up period was approximately 36 months; local recurrence occurred in 34 patients, 9 benign cases (14.5%), 11 borderlines (32.4%), and 14 malignant PTs (42.4%). Mastectomy was the most commonly used surgery in recurrent cases (61.4%). Axillary staging was performed in 31 cases (24.4%); only 2 cases showed positive nodal metastasis (6.5%) and were of the malignant subtype. Distant metastasis occurred in 12 patients, 4 with borderline PTs, and 8 with malignant PTs. The most common site for metastasis was the lungs and bones. Adjuvant radiotherapy was applied in 9 patients, 2 in borderline phyllodes, and 7 in malignant phyllodes; post-radiotherapy recurrence occurred in 5 malignant phyllodes patients. Chemotherapy was employed in 10 metastatic patients (4 with borderline and 6 with malignant phyllodes); excision with clear margins is important to reduce the local recurrence. Routine axillary staging should not be done. The adjuvant radiation therapy is still controversial. Local recurrence can develop even after appropriate surgery. Therefore, close follow-up is mandatory.
Keywords: Breast, Egypt, Phyllodes tumor
Introduction
Phyllodes tumors (PT) are rare fibroepithelial lesions that comprise 0.3–0.5% of all breast tumors in women. The peak age for this tumor is around 45–49 years [9]. PT was first defined as a giant type of fibroadenoma in 1774 [24]. In 1981, the World Health Organization (WHO) adopted the phyllodes tumor terminology [38]. PTs were classified as benign, borderline, or malignant based on histologic tumor characteristics (cellular atypia, stromal overgrowth, tumor margins, tumor necrosis, and mitotic count) ([26]). Axillary lymph nodes can be identified clinically and by imaging, in up to 10–15% of patients but less than 1% had pathological positive nodes [31]. Mammography and ultrasonography are the main imaging modalities for diagnosis. The sensitivity of core needle biopsy (CNB) in diagnosing PTs is 63% while that with (FNAC) is 40% [37]. Wide Local Excision (WLE), with a margin of at least 1 cm is the surgery of choice (S. [21]). Re-excision is indicated when necessary to reduce recurrence rates following excision with inadequate margins. The local recurrence rates following WLE are 8% for benign PTs and 21–36% for borderline and malignant tumors [6]. Adjuvant radiotherapy (RT) role is controversial. Radiotherapy is unnecessary for benign PTs. However, available data indicate that RT will reduce recurrence after breast conserving resection for borderline or malignant PTs. There is less agreement about the role of adjuvant RT when wide margins ⩾ 1 cm can be obtained (S. [21]). Adjuvant chemotherapy should be administered only for a minority of patients with large, high-risk or recurrent malignant tumors, and only after a thorough discussion about the risks and benefits of treatment ([21]). Hormone therapy is not effective for PTs despite the presence of positive hormone receptors in the epithelial component of some of these tumors [34].The survival rate for malignant PTs is reported as approximately 60–80% at 5 years [32]. Metastatic disease has been reported in 13–40% of patients. Metastases frequently involve the lungs, with a mean overall survival of about 30 months [16].
The aim of the study
The aim of the study was to evaluate the clinicopathologic features, diagnostic tools, therapeutic options to the breast and axilla, and their outcomes and risk factors associated with recurrence and distant metastasis in patients with phyllodes tumor.
Patients and Methods
Study Design
This is a retrospective cohort study that included all patients diagnosed as PTs of the breast who were treated between January 2011 and January 2016 at the National Cancer Institute, Cairo University, Egypt. The following data were retrieved and analyzed:
Age at presentation, menopausal status, clinical data including symptoms or signs, radiological data including results of sonomammography, CT scan, bone scan, preoperative histopathology, methods of biopsy, and type of surgery.
The final pathology report data including tumor size, tumor type, tumor grade, axillary lymph nodes if present, and surgical margin.
Type of adjuvant therapy, local recurrence, distant metastasis, follow-up period, survival data, and recurrence rate.
Statistical Methods
Data management and statistical analysis were performed using Statistical Package for Social Sciences (SPSS) vs. 21. Numerical data were summarized using means and standard deviations or medians and ranges. Categorical data were summarized as percentages. A comparison between the 3 groups was done using chi square test and Fisher’s exact test when appropriate. Kaplan and Meier procedure was used to estimate the overall survival rates and disease-free survival rates, and comparisons between the different prognostic factors were done using the Logrank test.
Overall survival rates were calculated from the date of diagnosis to the date of death. Living patients or patients lost to follow-up were censored on last known alive date, while disease-free survival was calculated from date of achieving remission to date of progression, relapse, or death whichever occurred first.
Results
This review included 127 patients pathologically proven to have PT of the breast and treated at the National Cancer Institute in Egypt during the period from January 2011 to January 2016.
Demographic Clinical and Radiological Characteristics: (Table 1)
Table 1.
Characteristics of the three pathological types separately
| Benign | Borderline | Malignant | ||||||
|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | p value | ||
| Family history | Negative | 57 | 95.0 | 31 | 91.2 | 26 | 78.8 | 0.045 |
| Positive | 3 | 5.0 | 3 | 8.8 | 7 | 21.2 | ||
| Menopausal status | Post | 5 | 8.3 | 9 | 26.5 | 15 | 45.5 | < 0.001 |
| Pre | 55 | 91.7 | 25 | 73.5 | 18 | 54.5 | ||
| Laterality | Bilateral | 1 | 1.7 | 0 | 0.0 | 1 | 3.0 | 0.647 |
| Left | 32 | 14 | 41.2 | 16 | 48.5 | |||
| Right | 27 | 45.0 | 20 | 58.8 | 16 | 48.5 | ||
| Multicentricity | Multiple | 5 | 8.3 | 3 | 8.8 | 2 | 6.1 | 0.901 |
| Single | 55 | 91.7 | 31 | 91.2 | 31 | 93.9 | ||
| Radiology | B2 | 2 | 4.7 | 0 | 0.0 | 0 | 0.0 | < 0.001 |
| B3 | 33 | 76.7 | 11 | 33.3 | 3 | 10.0 | ||
| B4 | 8 | 18.6 | 20 | 60.6 | 22 | 73.3 | ||
| B5 | 0 | 0.0 | 2 | 6.1 | 5 | 16.7 | ||
| Surgery | NA | |||||||
| Lumpectomy | 34 | 56.7 | 3 | 8.8 | 2 | 6.1 | ||
| WLE | 22 | 36.7 | 21 | 61.8 | 14 | 42.4 | ||
| MRM | 2 | 3.3 | 4 | 11.8 | 12 | 36.4 | ||
| SM | 1 | 1.7 | 5 | 14.7 | 5 | 15.2 | ||
| SSM | 1 | 1.7 | 1 | 2.9 | 0 | 0.0 | ||
| Axillary dissection | No | 55 | 91.7 | 24 | 70.6 | 17 | 51.5 | < 0.001 |
| Yes | 5 | 8.3 | 10 | 29.4 | 16 | 48.5 | ||
| LN status | Negative | 5 | 100.0 | 10 | 100.0 | 14 | 87.5 | NA |
| Positive | 0 | 0.0 | 0 | 0.0 | 2 | 12.5 | ||
| Adjuvant RTH | No | 60 | 100.0 | 32 | 94.1 | 26 | 78.8 | 0.001 |
| Yes | 0 | 0.0 | 2 | 5.9 | 7 | 21.2 | ||
| CTH | No | 60 | 100.0 | 30 | 88.2 | 27 | 81.8 | 0.005 |
| Yes | 0 | 0.0 | 4 | 11.8 | 6 | 18.2 | ||
All patients were females with a mean age at diagnosis of 39 years (range 13–77). Mean age for benign cases was 33 years, for borderline cases it was 43 years, and for malignant cases it was 46 years. Ninety-eight patients (77.2%) were premenopausal and 29 (22.8%) were postmenopausal. All patients presented with a breast mass, in 21 cases, the tumor was huge (>T3) involving almost the whole breast. Four cases presented with tumor ulceration, 6 with pain, and 2 with nipple discharge. Median clinical tumor size for benign cases was 4 cm (range 2–25 cm), for borderline cases it was 7 cm (range from 2 to 20 cm), and for malignant cases it was 6 cm (range 3–27 cm). The most frequent site for the tumor was the upper outer quadrant (UOQ) (45.6%). Most of PT lesions were single while multicentric lesions were observed in 10 patients (7.9%). Mammography and/or ultrasonography were employed in 105 patients, 2 were reported as BIRADS 2, 47 as BIRADS 3, 50 as BIRADS 4, and 7 as BIRADS 5. The diagnosis was confirmed by CNB in the majority of cases (n = 76, 82.6%), and suspicion of PT was reported in 50 patients (65.07%). FNAC was performed in only 16 patients (17.4%), and suspicion of PT was reported in 2 patients only.
Initial Management and Histopathological Features: (Table 1)
Initially various surgical procedures were performed including lumpectomy (30.7%), wide local excision (WLE) (44.9%) (Fig. 1), simple mastectomy (SM) (8.7%), modified radical mastectomy (MRM) (14.2%), and only 2 cases underwent skin sparing mastectomy (SSM) with immediate reconstruction. Sixty patients presented with benign phyllodes tumor (47.2%), 34 had borderline phyllodes tumor (26.8%), and 33 had malignant phyllodes tumor (26%). Table 1 shows the characteristics of the three pathological types separately. The median pathologic tumor size for benign cases was 4 cm (range 1–24 cm), for borderline cases it was 7.3 cm (range from 2.5–18 cm), and for malignant cases it was 6.5 cm (range from 2.3–43 cm). Surgical axillary staging was performed in 31 cases, due to large palpable lymph nodes intra-operatively, (24.4%) and only 2 cases showed positive nodal metastasis (6.5%) and were of the malignant subtype. Adjuvant radiotherapy was given to 9 patients, 2 with borderline phyllodes, and 7 with malignant phyllodes; however, post-radiotherapy recurrence occurred in 5 malignant phyllodes patients. Adjuvant chemotherapy was employed in 10 patients (4 with borderline and 6 with malignant phyllodes); all of them showed metastasis in the lungs and/or bones.
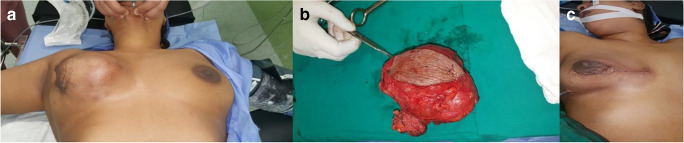
Fig. 1.
(A) A case of large right-side benign PT of the breast, managed by WLE in the National Cancer Institute. (B) The specimen sent for pathological assessment. (C) Post-operative view of the patient. The final pathology report showed circumscribed mass 10*8 cm, negative surgical margins; after 3 years follow-up, patient remained recurrence free
Local Recurrence and Distant Metastases in Relation to Surgical Intervention (Table 2)
Table 2.
Relation of different studied factors in correlation with local recurrence
| Recurrence | ||||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| No. | % | No. | % | p value | ||
| Age group | < 40 yrs. | 49 | 76.6 | 15 | 23.4 | 0.645 |
| ≥ 40 yrs. | 46 | 73.0 | 17 | 27.0 | ||
| Family history | Neg. | 89 | 78.1 | 25 | 21.9 | 0.012 |
| Pos. | 6 | 46.2 | 7 | 53.8 | ||
| Menopausal status | Post. | 22 | 75.9 | 7 | 24.1 | 0.881 |
| Pre. | 73 | 74.5 | 25 | 25.5 | ||
| Site of the tumor | LIQ | 6 | 85.7 | 1 | 14.3 | 0.634 |
| LOQ | 9 | 90.0 | 1 | 10.0 | ||
| Retro areolar | 9 | 75.0 | 3 | 25.0 | ||
| UIQ | 14 | 77.8 | 4 | 22.2 | ||
| UOQ | 39 | 68.4 | 18 | 31.6 | ||
| Whole breast | 17 | 81.0 | 4 | 19.0 | ||
| Laterality | Left | 51 | 82.3 | 11 | 17.7 | 0.070 |
| Right | 43 | 68.3 | 20 | 31.7 | ||
| Multicentricity | Multiple | 8 | 80.0 | 2 | 20.0 | 0.693 |
| Single | 87 | 74.4 | 30 | 25.6 | ||
| Pathological size | ≤ 5 cm | 49 | 75.4 | 16 | 24.6 | 0.877 |
| > 5 cm | 46 | 74.2 | 16 | 25.8 | ||
| Surgical margin | Negative | 53 | 84.1 | 10 | 15.9 | < 0.001 |
| Positive | 4 | 36.4 | 7 | 63.6 | ||
| Closed | 7 | 38.9 | 11 | 61.1 | ||
| Axillary dissection | No | 79 | 82.3 | 17 | 17.7 | 0.001 |
| Yes | 16 | 51.6 | 15 | 48.4 | ||
| Adjuvant RTH | No | 91 | 77.1 | 27 | 22.9 | 0.030 |
| Yes | 4 | 44.4 | 5 | 55.6 | ||
| CTH | No | 94 | 80.3 | 23 | 19.7 | < 0.001 |
| Yes | 1 | 10.0 | 9 | 90.0 | ||
| Behavior | Benign | 53 | 88.3 | 9 | 14.5 | 0.003 |
| Borderline | 23 | 67.6 | 11 | 32.4 | ||
| Malignant | 19 | 57.6 | 14 | 42.4 | ||
Local recurrence occurred in 34 patients, 9 benign cases (14.5%), 11 borderline (32.4%), and 14 malignant phyllodes (42.4%). Local recurrence was managed by WLE in 12 cases, SM in 10 cases, MRM in 6 cases, and SSM with immediate reconstruction in 3 cases.
Out of the 9 benign PT recurrent cases, 3 progressed to malignant and 3 progressed to borderline phyllodes. In borderline phyllodes recurrent cases, 4 progressed to malignant phyllodes and 1 progressed to carcinosarcoma. The histopathology of recurrence in all malignant phyllodes cases was similar to the original pathology.
Several factors were studied in correlation with local recurrence as shown in (Table 2), the most important was surgical margin (p value < 0.001) as cases with negative margins showed lower recurrence rates (15.9%) while those with positive or close margins showed higher recurrence rates (63.6% and 61.1%), respectively. Histological subtype of the tumor was also a significant factor for local recurrence as benign cases showed lower recurrence rates (14.5%) compared with borderline (32.4%) and malignant phyllodes cases (42.4%).
Distant metastasis occurred in 12 patients (9.4%), 4 with borderline phyllodes, and 8 with malignant phyllodes. The most common site for metastasis was in the lungs. Other areas for metastasis included bones, iliac nodes, chest wall, and mediastinal nodes.
Overall Survival Rate (OSR)
Due to the short-term follow-up period, in addition to the large number of cases who were lost to follow-up, median follow-up period in our cohort was 36 months.
The 3-year OSR for the three groups was 76.6%; however, addition of benign cases greatly affected the OSR, so we excluded the benign cases from survival analysis.
The 3-year overall survival for borderline and malignant PTs was 85.5% and 49.8%, respectively.
In malignant PT, negative surgical margins and addition of adjuvant radiotherapy were the only factors that significantly affected the OSR (p < 0.012 and < 0.012, respectively), while other factors like age, size of the tumor, axillary lymph node dissection, and adjuvant chemotherapy did not affect the OSR. Table 3 illustrates an analysis of different prognostic factors in relation to OSR in malignant phyllodes.
Table 3.
Analysis of different prognostic factors in relation to OSR in malignant phyllodes
| Malignant overall survival rate | Median(m) | ||||
|---|---|---|---|---|---|
| Factors | N | 2 yrs. | 3 yrs. | (95% CI) | p value |
| All | 33 | 84.0 | 49.8 | 36.5(18.2–54.8) | NA |
| Age group (yrs.) | |||||
| < 40 yrs. | 11 | 81.8 | 46.8 | 36.5(15.2–57.8) | 0.995 |
| ≥ 40 yrs. | 22 | 84.6 | 52.9 | 54.8(29.9–55.2) | |
| Family history | |||||
| Negative | 26 | 84.4 | 57.7 | 42.6(30.4–54.8) | 0.234 |
| Positive | 7 | 80.0 | NA | 28.4(3.3–53.5) | |
| Menopause | |||||
| Post | 15 | 84.0 | 42.0 | 29.4(10.9–47.8) | 0.981 |
| Pre | 18 | 83.0 | 52.8 | 42.6(21.5–63.6) | |
| Multicentricity | |||||
| Multiple | 2 | 100 | 100 | 42.5 | NV |
| Single | 31 | 82.8 | 46.6 | 36.5(23.8–49.3) | |
| Pathological size | |||||
| ≤ 5 cm | 13 | 84.6 | 50.8 | 36.5(27.8–45.2) | 0.327 |
| > 5 cm | 20 | 84.0 | 58.8 | 42.6(14.1–71.3) | |
| Axillary LN dissection | |||||
| No | 17 | 88.2 | 55.1 | NA | 0.408 |
| Yes | 16 | 80.2 | 44.6 | 29.4(27.8–31.0) | |
| Surgical margin | |||||
| Negative | 19 | 92.9 | 77.4 | 51.7(19.6–83.8) | 0.012 |
| Positive | 6 | 83.3 | 41.7 | 63.5(21.7–51.3) | |
| Closed | 8 | 62.5 | 25.0 | 27.4(1.0–58.3) | |
| Adjuvant radiotherapy | |||||
| No | 26 | 79.2 | 23.5 | 29.4(28.4–30.4) | 0.012 |
| Yes | 7 | 100 | 100 | 51.8(37.9–65.5) | |
| Adjuvant chemotherapy | |||||
| No | 27 | 80.3 | 64.2 | 42.5 | 0.302 |
| Yes | 6 | 100 | 20.0 | 29.4(28.3–30.5) | |
In borderline phyllodes, the surgical margins did not affect overall survival; however, adjuvant radiotherapy was given in only 2 cases and showed 100% OSR in 3 years. Other factors were not shown to be significant in correlation with OSR. Table 4 illustrates an analysis of different prognostic factors in relation to OSR in borderline phyllodes.
Table 4.
Analysis of different prognostic factors in relation to OSR in borderline phyllodes
| Borderline overall survival rate | Median (m) | ||||
|---|---|---|---|---|---|
| Factors | n | 2 yrs. | 3 yrs. | (95% CI) | p value |
| All | 34 | 100 | 85.5 | 54.8(32.5–77.1) | NA |
| Age group (yrs.) | |||||
| < 40 yrs. | 15 | 100 | 75.0 | 39.6(10.7–68.3) | 0.619 |
| ≥ 40 yrs. | 19 | 100 | 100 | 54.8 | |
| Family history | |||||
| Negative | 31 | 100 | 100 | 54.8 | NV |
| Positive | 3 | 100 | 66.7 | 78.5 | |
| Menopause | |||||
| Post | 9 | 100 | NA | NA | 0.545 |
| Pre | 25 | 100 | 83.3 | 54.8 | |
| Multicentricity | |||||
| Multiple | 3 | 100 | 100 | 78.5 | NV |
| Single | 31 | 100 | 83.3 | 54.8 | |
| Pathological size | |||||
| ≤ 5 cm | 12 | 100 | 66.7 | 39.5(31.5–47.6) | 0.070 |
| >5 cm | 22 | 100 | 100 | 54.8 | |
| Axillary LN dissection | |||||
| No | 24 | 100 | 75.0 | 54.8 | 0.619 |
| Yes | 10 | 100 | 100 | 78.5 | |
| Surgical margina | |||||
| Negative b | 22 | 100 | 100 | NA | NV |
| Positive | 4 | 100 | 100 | 39.0 | |
| Closed | 7 | 100 | 75.0 | 78.0 | |
| Radiotherapy | |||||
| No | 32 | 100 | 83.3 | 54.8(25.7–83.8) | NV |
| Yes | 2 | 100 | 100 | 39.5 | |
| Chemotherapy | |||||
| No | 30 | 100 | 100 | NA | NV |
| Yes | 4 | 100 | 75.0 | 39.5 | |
Disease-Free Survival (DFS)
Three-year disease-free survival for borderline and malignant phyllodes was 59% and 42.3%, respectively. In borderline phyllodes, DFS for cases with negative margins and close margins were 80.9% and 66.7%, respectively, while 4 cases with positive margins developed relapse in less than a year. Type of surgery and addition of radiotherapy did not affect DFS. Table 5 illustrates an analysis of different prognostic factors in relation to DFS in borderline phyllodes. In malignant phyllodes, negative surgical margin was the most important factor affecting the DFS (p < 0.007) while the addition of radiotherapy did not significantly affect the DFS. Also, it was shown that the type of surgery affected the DFS in malignant PTs favoring mastectomy over WLE and lumpectomy (p < 0.001). Table 6 illustrates an analysis of different prognostic factors in relation to DFS in malignant phyllodes.
Table 5.
Analysis of different prognostic factors in relation to DFS in borderline phyllodes
| Borderline DFS rate | Median (m) | |||||
|---|---|---|---|---|---|---|
| Factors | N | 1 yr. | 2 yrs. | 3 yrs. | (95% CI) | p value |
| All | 34 | 68.8 | 68.8 | 59.0 | 40.6(18.8–62.4) | NA |
| Age group (yrs.) | ||||||
| < 40 yrs. | 15 | 69.2 | 69.2 | 69.2 | 40.6(1.0–89.9) | 0.799 |
| ≥ 40 yrs. | 19 | 67.8 | 67.8 | 45.2 | 28.4 | |
| Laterality* | ||||||
| Left | 14 | 77.8 | 77.8 | 58.3 | NA | 0.471 |
| Right | 20 | 71.3 | 64.2 | 64.2 | 40.6(1.0–81.3) | |
| Family history | ||||||
| Negative | 31 | 68.8 | 68.8 | 55.1 | 42.6 | NV |
| Positive | 3 | 66.7 | 66.7 | 66.7 | 40.6(1–97.0) | |
| Menopause | ||||||
| Post | 9 | 100 | 100 | NA | NA | 0.061 |
| Pre | 25 | 62.7 | 62.7 | 52.2 | 41.2 | |
| Multicentricity | ||||||
| Multiple | 3 | 100 | 100 | 100 | 40.6 | NV |
| Single | 31 | 66.0 | 66.0 | 55.0 | 42.6(21.9–63.3) | |
| Pathological size | ||||||
| ≤ 5 cm | 12 | 53.0 | 53.0 | 53.0 | 42.6 | 0.090 |
| > 5 cm | 22 | 87.1 | 80.4 | 67.0 | 40.6(22.8–58.3) | |
| Axillary dissection | ||||||
| No | 24 | 76.9 | 69.9 | 46.6 | 28.4 | 0.646 |
| Yes | 10 | 66.7 | 66.7 | 66.7 | 40.6(1–90.5) | |
| Surgical margin | ||||||
| Negative | 22 | 80.9 | 80.9 | 80.9 | NA | NV |
| Positive | 4 | NA | NA | NA | 5(1.7–8.4) | |
| Closed | 7 | 66.7 | 66.7 | 66.7 | 21.7(1–83.2) | |
| Radiotherapy | ||||||
| No | 32 | 75.4 | 70.4 | 60.3 | 40.6(18.7–62.5) | NV |
| Yes | 2 | 50.0 | 50.0 | NA | 6.1 | |
| Chemotherapy | ||||||
| No | 30 | 72.1 | 72.1 | 72.1 | 42.6(1.0–85.5) | NV |
| Yes | 4 | 50.0 | 50.0 | 25.0 | 6.0(1.0–29.1) | |
| Type of surgery | ||||||
| Mastectomy | 10 | 84.6 | 66.6 | 44.4 | 32.5(5.1–59.6) | < 0.001 |
| Lumpectomy | 3 | NA | NA | NA | 1.0 | |
| WLE | 21 | 42.1 | 42.1 | 42.1 | 11.1(3.6–18.7) | |
Table 6.
Analysis of different prognostic factors in relation to DFS in malignant phyllodes
| Malignant DFS rate | Median (m) | |||||
|---|---|---|---|---|---|---|
| Factors | N | 1 yr. | 2 yrs. | 3 yrs. | (95% CI) | p value |
| All | 33 | 66.3 | 52.9 | 42.3 | 32.5(3.4–61.5) | NA |
| Age group (yrs.) | ||||||
| < 40 yrs. | 11 | 70.7 | 58.9 | 39.3 | 32.5(1.0–68.1) | 0.815 |
| ≥ 40 yrs. | 22 | 57.2 | 50.1 | 50.1 | NA | |
| Laterality* | ||||||
| Left | 16 | 70.9 | 63.0 | 63.0 | 44.6(1.0–103.1) | 0.119 |
| Right | 16 | 61.1 | 40.7 | NA | 17.2(4.6–29.8) | |
| Family history | ||||||
| Negative | 26 | 69.7 | 57.5 | 46.0 | 32.5(5.1–59.8) | 0.320 |
| Positive | 7 | 53.6 | NA | NA | 12.1(2.3–22.1) | |
| Menopause | ||||||
| Post | 15 | 65.2 | 55.9 | NA | NA | 0.869 |
| Pre | 18 | 67.6 | 51.5 | 41.2 | 32.5(5.1–59.9) | |
| Multicentricity | ||||||
| Multiple | 2 | 100 | NA | NA | 17.2 | NV |
| Single | 31 | 63.7 | 54.2 | 43.4 | 32.5(1.0–64.4) | |
| Pathological size | ||||||
| ≤ 5 cm | 13 | 55.1 | 44.1 | 44.1 | 12.2(9.3–15.1) | 0.305 |
| > 5 cm | 20 | 73.3 | 58.7 | 44.0 | 32.5(0.5–64.7) | |
| Axillary dissection | ||||||
| No | 17 | 61.4 | 61.4 | 61.4 | NA | 0.784 |
| Yes | 16 | 71.6 | 47.7 | 31.8 | 17.2(1.0–38.3) | |
| Surgical margin | ||||||
| Negative | 19 | 79.4 | 71.5 | 71.5 | 44.6 | 0.007 |
| Positive | 6 | 41.7 | 20.8 | 20.8 | 6.1(1.0–12.4) | |
| Closed | 8 | 50.0 | 33.3 | NA | 3.1(1.0–13.9) | |
| Metastasis | ||||||
| No | 25 | 72.0 | 65.5 | 49.1 | 32.5 | 0.049 |
| Yes | 8 | 37.5 | 25.0 | 25.0 | 9.1(1–21.7) | |
| Radiotherapy | ||||||
| No | 26 | 60.5 | 54.5 | 54.5 | NA | 0.826 |
| Yes | 7 | 85.7 | 57.1 | 42.9 | 32.5(1.0–71.7) | |
| Chemotherapy | ||||||
| No | 27 | 69.4 | 57.3 | 42.9 | 32.5(1.0–63.9) | 0.125 |
| Yes | 6 | 33.3 | 33.3 | 33.3 | 3.1(1.0–11.9) | |
| Type of surgery | ||||||
| Mastectomy | 17 | 84.6 | 66.6 | 44.4 | 32.5(5.0–59.6) | < 0.001 |
| Lumpectomy | 2 | NA | NA | NA | 1 | |
| WLE | 14 | 42.1 | 42.1 | 42.1 | 11.1(3.6–18.7) | |
Discussion
Phyllodes tumor is an uncommon breast neoplasm usually occurring in women aged 35–50 years; however, malignant PTs have a slightly older age presentation than others [4]. This correlates with our findings where the mean age at diagnosis was 39 years, with malignant PT showing an older presentation (median age 46 years). Although these tumors have an average size of 5 cm, lesions of up to 40 cm have been reported [36]. The association between tumor size and malignancy is controversial; however, rapid growth may be detected in malignant tumors [4].
In this study, the mean pathological tumor size was 4 cm, which is consistent with other studies [4], and the largest PT was 43 cm in diameter, which was diagnosed as malignant PT. In addition, the median size of borderline PTs was higher than that of the others, and the mean tumor sizes of benign, borderline, and malignant PTs were 4 cm, 7.3 cm, and 6.5 cm, respectively, and the difference was found statistically significant. The upper outer quadrant of the breast is the most frequent location of PT, and both sides are often equally affected. Multifocality and bilaterality are seen infrequently [5]. These findings correlated with ours since in this study population, right breasts were almost equally affected as left breasts (63 and 62 cases, respectively), and the most common tumor location was the upper outer quadrant (45.6%). Additionally, both multifocality and bilaterality were found in a very small number of patients, which is similar to previous reports. In general, PT is difficult to diagnose using imaging methods due to the lack of specific radiologic characteristics, and it is often confused with fibroadenoma, cysts, and well-circumscribed carcinoma [2]. With US, the majority of PTs are described as well-defined hypoechoic oval lesions surrounded by a capsule or pseudocapsule. Contrary to fibroadenomas, several sonographic findings including heterogeneous internal structure with irregular margins, septae, lobulation, and the absence of microcalcifications were reported to be associated with PT [23]. In addition, increased intralesional vascularity with Doppler US is a frequent feature of these tumors [11]. However, no specific color Doppler US finding was found to help differentiate PT from FA, or benign PTs from malignant [14]. Mammography also has limited diagnostic value in differentiating PT and other benign breast lesions [32]. In the present study, US was used as the first-step imaging method in most patients, and mammography was performed in patients aged more than 35 years. The sonographic and mammographic findings were similar to the literature since no specific radiologic feature was identified to differentiate the histologic subtypes of PTs; however, most borderline and malignant PTs were classified as BIRADS 4 and/or 5. In recent years, several studies regarding the potential role of magnetic resonance imaging (MRI) in the diagnosis of PT have been published; the most important of which was one by Korean investigators who reported that tumor size, several US, and MRI findings can be used to help determine preoperatively the histologic grade of breast PTs. They concluded that when a patient presents with a progressively enlarging, painless breast mass, MRI should be recommended first [31]. However, MRI was not used in the diagnostic examinations of our patients, because of economic reasons. FNAC, CNB, incisional, and excisional biopsies can be used in the preoperative histopathologic diagnosis of PT. Distinction of benign PT from cellular fibroadenoma (FA) and malignant PT from spindle cell metaplastic carcinoma and primary breast sarcoma are the main problematic issues in the histopathologic evaluation [33]. CNB is considered more reliable than FNAC in obtaining a correct diagnosis because it can provide specific histopathologic findings. However, its sensitivity was reported as approximately 65% in the definitive diagnosis of PT [37]. In this study, histologic diagnosis was confirmed by CNB in the majority of cases, with an approximately 65.07% diagnostic accuracy for PT. This was in accordance with other studies that found CNB to be a valuable tool in the differential diagnosis of PT and FA, with high specificity and sensitivity rates [14]. In our experience, CNB played an important role in the preoperative histopathologic diagnosis of PT whereas FNAC was used in 16 patients and suspicion of PT was reported in only 2 of these. Excisional biopsy was preferred in cases that were strongly considered as FA or another benign lesion in the preoperative clinical and radiologic evaluations. Wide local surgical excision, with removal of tumor with at least 1-cm clear microscopic margins, is the primary treatment of PT [39] while mastectomy may be needed in patients with large malignant tumors or those with inappropriate tumor-breast tissue ratio [27]. In the present study, WLE was the most commonly performed initial surgery (44.1%), while mastectomy was most commonly resorted to in recurrent cases (61.4%). Axillary dissection is not recommended as a part of routine surgical treatment because PT mainly spreads via a hematogenous route and nodal involvement is extremely rare. However, axillary dissection may be considered in patients with malignant PT who have axillary metastasis [32]. In our study, surgical axillary staging was performed in 31 cases (24.4%) and only 2 cases showed positive nodal metastasis (6.5%) and these were of the malignant subtype. Several recent studies showed that benign PTs with positive margins or less than 1-cm clear margin may not require re-excision; however, such patients should be closely followed up because of their high local recurrence risk which may reach up to 15% [17, 35]. In the 22 cases of benign PTs treated by WLE, 3 recurrences occurred, 2 of which progressed, one to borderline and the other to malignant PT. Among the 4 benign cases that were treated with mastectomy, only 1 recurred. From these data, we observed that there is no difference in the recurrence rate of benign phyllodes tumors that were initially excised with positive or < 1-cm margins when they are subjected to observation alone or repeat wider excision, concluding that “watch and wait” policy does appear to be safe and can be accorded to benign PTs that have been initially enucleated without margins. On the other hand, there was a debate in several studies regarding the relationship between width of surgical margins in relation to local recurrence and 5-year disease-free survival in cases of borderline and malignant PTs. In a study of 67 borderline and malignant PTs from the Mayo Clinic, it was found that the extent of surgical excision had no impact on disease-free survival [19]. In another study by the same investigators that included a series of 33 cases, they found no relationship was found between width of surgical margin and disease recurrence [19]. In concordance with these findings, an analysis of 164 cases by Jang et al. [16] had revealed no significant local control advantage conferred by wide (at least 10 mm) margins over narrower margins. These results were controversial to those reported by a study of 40 cases from the Massachusetts General Hospital that found that post-excision recurrences were confined to cases with positive margins, or margins of < 10 mm. Following re-excision with a 10-mm clearance, patients remained recurrence-free [22].
In a recent Korean study of 285 cases, the benefit of a second excision following initial ‘inadequate’ (< 10 mm) clearance was evaluated. Tumor size and mitotic activity were found to be independently prognostic of local recurrence, whereas margin status and surgical procedure were not. On the basis of these findings, the group proposed that wide margins, if necessary via re-excision, should be the goal in treating small (< 50 mm) tumors with high mitotic activity (> 10 mitoses/10 HPFs), as these tumors constituted a distinct group associated with a significant (55.6%) local recurrence rate [39].
Moreover, a retrospective review of 44 Asian cases found no cases of local recurrence in benign tumors treated with simple excision (enucleation), regardless of margin status, after a mean follow-up of 47.6 months. Hence, a benign PT diagnosed after representative sampling of an excision specimen may be conservatively handled even when positive margins are encountered [35]. Conversely, malignant PTs are associated with a recurrence rate of 29.6% [31], with metastases and death being observed in 22%, [18] underscoring the need to recognize this subset of aggressive PTs for complete surgical eradication. In our study, tumor-free surgical margins were the most important factor reducing risk of local recurrence in cases of borderline and malignant PTs and affected both OS and DFS rates. However, in cases of malignant PTs, those treated with mastectomy showed better DFS rates. The local recurrence rate in our study population was 14.5%, 32.4%, and 42.4% for benign, borderline, and malignant PTs, respectively. This was higher than those reported in the literature [18].
Distant metastasis (DM) can be seen in 10% of cases, which most often affects the lungs and bones [3]. In our case series, DM were detected in 12 patients (9.4%), 8 with malignant PT, and 4 with borderline PTs and the most common site for metastasis were the lungs.
There is no global consensus on the role of adjuvant radiotherapy and chemotherapy in the management of PT [29]. However, application of radiotherapy to the breast after surgery for borderline and malignant PTs was shown to reduce the risk of local recurrence, without any significant survival benefit [15]. Therefore, adjuvant radiotherapy should be considered in patients with borderline and malignant PT on an individualized basis [33]. An analysis of 3120 malignant cases from the US National Cancer Data Base showed a pronounced increase in the use of radiotherapy where it increased from 9.5% (1998–1999) to 19.5% (2008–2009), which, although being associated with reduced local recurrence, had no impact on disease-free or overall survival [15]. Another study found adjuvant radiotherapy to be beneficial in patients with adverse features (e.g., bulky tumors, close or positive surgical margins, hypercellular stroma, high nuclear pleomorphism, high mitotic rate, presence of necrosis, and increased vascularity within the tumor and tumor recurrence) but it concluded that its use was sill controversial [10]. In another study included cases collected from by the Rare Cancer Network between 1971 and 2003, concluded that adjuvant radiotherapy for borderline and malignant tumors yielded superior 10-year local control rates (86% with radiation versus 59% without radiation), but no survival benefit [8]. These results were consolidated by another study of malignant PTs, in which patients were subjected to radiation only if tumor-free margins were < 10 mm, whereas no adjuvant therapy was administered if margins were wide (≥ 10 mm). The two conservatively treated groups showed identical 5-year disease-free survival rates [25].
A recent study which included 46 patients who were treated with a margin negative breast-conserving resection of borderline and malignant PTs followed by adjuvant radiotherapy concluded that margin negative resection combined with adjuvant radiotherapy is very effective therapy for local control of borderline and malignant PTs. The local recurrence rate with adjuvant radiotherapy was significantly less than that observed in reported patients treated with margin negative resection alone [7].
In this study, adjuvant radiotherapy was employed in 9 patients, 2 in borderline phyllodes, and 7 in malignant phyllodes; however, post-radiotherapy recurrence occurred in 5 patients, all of them were of the malignant subtype. Addition of radiotherapy did not affect DFS. Although there have been no randomized clinical trials on the role of systemic therapy in malignant PTs, various chemotherapy regimens containing doxorubicin, dacarbazine, cisplatin, isophosphamide, and etoposide are generally recommended for patients with malignant and/or metastatic disease [28]. Nevertheless, adjuvant chemotherapy was reported to have no beneficial effect on patient survival [20]. In this study, when chemotherapy was employed in 10 patients (4 with borderline and 6 with malignant phyllodes), all of them showed metastasis in the lungs and/or bones and it had no effect on patient survival. The 5-year overall survival rates in patients with benign and malignant PTs in the MD Anderson series was 91% and 82%, respectively [12]. In another study, the 3-year survival of benign/borderline tumors was 100% in 6 patients, while the 3-year survival rate was reported as and 53.4% in 13 patients with malignant PT [13]. In a study of 15 malignant PTs, Suzuki et al. [30] reported the 5-year survival rate after primary surgery as 10%. In another study, the 5-year disease-free survival was reported to be approximately 90% for the patients with benign tumors, 70% for the patients with borderline, and 60% for malignant PTs [1]. In our study, the 3-year OSR for the three groups was 76.6%; however, addition of benign cases greatly affected the OSR so we excluded the benign cases from survival analysis. The 3-year OSR for borderline and malignant PTs was 85.5% and 49.8% respectively. On the other hand, we found the 3-year disease-free survival for borderline and malignant phyllodes to be 59% and 42.3%, respectively.
Conclusion
PT has non-specific clinical and radiologic findings and can easily be confused with other similar breast masses, particularly FA. Total excision with adequate clear margins is of great importance to reduce the risk of local recurrence. Routine axillary staging should not be done. The role of adjuvant radiation therapy is still controversial; however, it should be always kept in mind that local recurrence can develop even after appropriate surgery for all histologic subtypes of PT. Therefore, these patients should be closely followed up at regular intervals after surgery.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abdalla HM, Sakr MA. Predictive factors of local recurrence and survival following primary surgical treatment of phyllodes tumors of the breast. J Egypt Natl Cancer Inst. 2006;18:125–133. [PubMed] [Google Scholar]
- 2.Abdulcadir D, Nori J, Meattini I, Giannotti E, Boeri C, Vanzi E, Vezzosi V, Bianchi S. Phyllodes tumors of the breast diagnosed as B3 category on image-guided 14-gauge core biopsy: analysis of 51 cases from a single institution and review of the literature. Eur J Surg Oncol. 2014;40:859–864. doi: 10.1016/j.ejso.2014.02.222. [DOI] [PubMed] [Google Scholar]
- 3.Acar T, Tarcan E, Hacıyanlı M, Kamer E, Peşkersoy M, Yiğit S, Gür Ö, Cin N, Sarı AA, Tatar F. How to approach phyllodes tumors of the breast? Ulus Cerrahi Derg. 2015;31:197–201. doi: 10.5152/UCD.2015.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atalay C, Kınaş V, Çelebioğlu S. Analysis of patients with phyllodes tumor of the breast. Ulus Cerrahi Derg. 2014;30:129–132. doi: 10.5152/UCD.2014.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrio AV, Clark BD, Goldberg JI, Hoque LW, Bernik SF, Flynn LW, Susnik B, Giri D, Polo K, Patil S, van Zee KJ. Clinicopathologic features and long-term outcomes of 293 phyllodes tumors of the breast. Ann Surg Oncol. 2007;14:2961–2970. doi: 10.1245/s10434-007-9439-z. [DOI] [PubMed] [Google Scholar]
- 6.Barth RJ, Wells WA, Mitchell SE, et al. A prospective multi-institutional study of adjuvant radiotherapy after resection of malignant phyllodes tumors. Ann Surg Oncol. 2009;16:2288–2294. doi: 10.1245/s10434-009-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barth RJ, Richard J., Wells, et al. (2016) A Prospective, Multi-Institutional Study of Adjuvant Radiotherapy After Resection of Malignant Phyllodes Tumors. Pubmed central [DOI] [PMC free article] [PubMed]
- 8.Belkacemi Y, Bousquet G, Marsiglia H, et al. Phyllodes tumor of the breast. Int J Radiat Oncol Biol Phys. 2008;70:492–500. doi: 10.1016/j.ijrobp.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 9.Calhoun KE, Lawton J, Kim JN, C D, et al. Diseases of the breast. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 781–792. [Google Scholar]
- 10.Chaney AW, Pollack A, McNeese MD, et al. Primary treatment of cystosarcoma phyllodes of the breast. Cancer. 2000;89:1502–1511. doi: 10.1002/1097-0142(20001001)89:7<1502::AID-CNCR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 11.Chao T, Lo Y, Chen S, Chen M. Phyllodes tumors of the breast. Eur Radiol. 2003;13:88–93. doi: 10.1007/s00330-002-1370-x. [DOI] [PubMed] [Google Scholar]
- 12.Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, Strom EA, McNeese MD, Kuerer HM, Ross MI, Singletary SE, Ames FC, Feig BW, Sahin AA, Perkins GH, Schechter NR, Hortobagyi GN, Buchholz TA. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22:2303–2312. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 13.Confavreux C, Lurkina A, Mitton N, et al. Sarcomas and malignant phyllodes tumors of the breast – a retrospective study. Eur J Cancer. 2006;42:2715–2721. doi: 10.1016/j.ejca.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 14.Gatta G, Iaselli F, Parlato V, di Grezia G, Grassi R, Rotondo A. Differential diagnosis between fibroadenoma, giant fibroadenoma and phyllodes tumour: sonographic features and core needle biopsy. Radiol Med. 2011;116:905–918. doi: 10.1007/s11547-011-0672-y. [DOI] [PubMed] [Google Scholar]
- 15.Gnerlich JL, Williams RT, Yao K, Jaskowiak N, Kulkarni SA. Utilization of radiotherapy for malignant phyllodes tumors: analysis of the National Cancer Data Base, 1998–2009. Ann Surg Oncol. 2014;21:1222–1230. doi: 10.1245/s10434-013-3395-6. [DOI] [PubMed] [Google Scholar]
- 16.Jang JH, Choi M-Y, Lee SK, Kim S, Kim J, Lee J, Jung SP, Choe JH, Kim JH, Kim JS, Cho EY, Lee JE, Nam SJ, Yang JH. Clinicopathologic risk factors for the local recurrence of phyllodes tumors of the breast. Ann Surg Oncol. 2012;19:2612–2617. doi: 10.1245/s10434-012-2307-5. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Kim J-Y, Kim DH, Jung WH, Koo JS. Analysis of phyllodes tumor recurrence according to the histologic grade. Breast Cancer Res Treat. 2013;141:353–363. doi: 10.1007/s10549-013-2684-x. [DOI] [PubMed] [Google Scholar]
- 18.Lakhani SR, Ellis IO, Schnitt SJ, et al. WHO classification of tumors of the breast. 4. Lyon: International Agency for Research on Cancer; 2012. International Agency for Research on Cancer, World Health Organization; p. 240. [Google Scholar]
- 19.Lin C-C, Chang H-W, Lin C-Y, Chiu CF, Yeh SP. The clinical features and prognosis of phyllodes tumors: a single institution experience in Taiwan. Int J Clin Oncol. 2013;18:614–620. doi: 10.1007/s10147-012-0442-4. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald OK, Lee CM, Tward JD, Chappel CD, Gaffney DK. Malignant phyllodes tumor of the female breast: association of primary therapy with cause-specific survival from the surveillance, epidemiology, and end results (SEER) program. Cancer. 2006;107:2127–2133. doi: 10.1002/cncr.22228. [DOI] [PubMed] [Google Scholar]
- 21.Mallick S, Joshi NP, Roy S, et al. Malignant and borderline phyllodes tumor of breast treated with a multi-modality approach in a tertiary cancer care centre in North India South Asian. J Cancer. 2016;5(1):1–3. doi: 10.4103/2278-330X.179696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangi AA, Smith BL, Gadd MA, Tanabe KK, Ott MJ, Souba WW. Surgical management of phyllodes tumors. Arch Surg. 1999;134:487–492. doi: 10.1001/archsurg.134.5.487. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy E, Kavanagh J, O’Donoghue Y, et al. Phyllodes tumors of the breast: radiological presentation, management and follow-up. Br J Radiol. 2014;87:20140239. doi: 10.1259/bjr.20140239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra SP, Tiwary SK, Mishra M et al (2013) Phyllodes tumor of breast: a review article. ISRN Surg:361–469 [DOI] [PMC free article] [PubMed]
- 25.Mituś J, Reinfuss M, Mituś JW, Jakubowicz J, Blecharz P, Wysocki WM, Skotnicki P. Malignant phyllodes tumor of the breast: treatment and prognosis. Breast J. 2014;20:639–644. doi: 10.1111/tbj.12333. [DOI] [PubMed] [Google Scholar]
- 26.Rosen PP (2001) Rosen’s breast pathology, 2nd edn, New York, Lippincott William Wikins
- 27.Salvadori B, Cusumano F, Del Bo R, et al. Surgical treatment of phyllodes tumors of the breast. Cancer. 1989;62:2532–2536. doi: 10.1002/1097-0142(19890615)63:12<2532::AID-CNCR2820631229>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.Santiago Chinchilla A, Ramos Font C, Custodio Rebollo Aguirre A, et al. Malignant phyllodes tumor of the breast with lymph node metastases shown by FDG PET-CT. Rev Esp Med Nucl. 2010;29:314–315. doi: 10.1016/j.remn.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Spitaleri G, Toesca A, Botteri E, Bottiglieri L, Rotmensz N, Boselli S, Sangalli C, Catania C, Toffalorio F, Noberasco C, Delmonte A, Luini A, Veronesi P, Colleoni M, Viale G, Zurrida S, Goldhirsch A, Veronesi U, de Pas T. Breast phyllodes tumor: a review of literature and a single center retrospective series analysis. Crit Rev Oncol Hematol. 2013;88:427–436. doi: 10.1016/j.critrevonc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki-Uematsu S, Shiraishi K, Ito T, Adachi N, Inage Y, Taeda Y, Ueki H, Ohtani H. Malignant phyllodes tumor composed almost exclusively of a fibrosarcomatous component: a case report and review of malignant phyllodes tumors with metastases. Breast Cancer. 2010;17:218–224. doi: 10.1007/s12282-009-0099-7. [DOI] [PubMed] [Google Scholar]
- 31.Tan H, Zhang S, Liu H, et al. Imaging findings in phyllodes tumors of the breast. Eur J Radiol. 2012;81:62–69. doi: 10.1016/j.ejrad.2011.01.085. [DOI] [PubMed] [Google Scholar]
- 32.Tan PH, Thike AA, Tan WJ, Thu MMM, Busmanis I, Li HH, Chay WY, Tan MH, The Phyllodes Tumour Network Singapore Predicting clinical behaviour of breast phyllodes tumors: a nomogram based on histological criteria and surgical margins. J Clin Pathol. 2012;65:69–76. doi: 10.1136/jclinpath-2011-200368. [DOI] [PubMed] [Google Scholar]
- 33.Tan BY, Acs G, Apple SK, Badve S, Bleiweiss IJ, Brogi E, Calvo JP, Dabbs DJ, Ellis IO, Eusebi V, Farshid G, Fox SB, Ichihara S, Lakhani SR, Rakha EA, Reis-Filho JS, Richardson AL, Sahin A, Schmitt FC, Schnitt SJ, Siziopikou KP, Soares FA, Tse GM, Vincent-Salomon A, Tan PH. Phyllodes tumors of the breast: a consensus review. Histopathology. 2016;68:5–21. doi: 10.1111/his.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Telli ML, Horst KC. Phyllodes tumors of the breast: natural history, diagnosis and treatment. J Natl Compr Cancer Netw. 2007;5:324–330. doi: 10.6004/jnccn.2007.0027. [DOI] [PubMed] [Google Scholar]
- 35.Teo JY, Cheong CS-J, Wong CY. Low local recurrence rates in young Asian patients with phyllodes tumors: less is more. ANZ J Surg. 2012;82:325–328. doi: 10.1111/j.1445-2197.2012.06045.x. [DOI] [PubMed] [Google Scholar]
- 36.Testori A, Meroni S, Errico V, Travaglini R, Voulaz E, Alloisio M. Huge malignant phyllodes breast tumor: a real entity in a new era of early breast cancer. World J Surg Oncol. 2015;13:81. doi: 10.1186/s12957-015-0508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward ST, Jewkes AJ, Jones BG, Chaudhri S, Hejmadi RK, Ismail T, Hallissey MT. The sensitivity of needle core biopsy in combination with other investigations for the diagnosis of phyllodes tumors of the breast. Int J Surg. 2012;10:527–531. doi: 10.1016/j.ijsu.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization . Histologic Typing of Breast Tumors. 2. Geneva: WHO; 1981. [Google Scholar]
- 39.Yom CK, Han W, Kim S-W, Park SY, Park IA, Noh DY. Reappraisal of conventional risk stratification for local recurrence based on clinical outcomes in 285 resected phyllodes tumors of the breast. Ann Surg Oncol. 2015;22:2912–2918. doi: 10.1245/s10434-015-4395-5. [DOI] [PubMed] [Google Scholar]