Abstract
Objectives
Breast cancer incidence has fluctuated considerably in Canada, with recent reductions in rates among screening-eligible women. However, incidence of early-onset and pre-menopausal breast cancer is understudied. We examined age-specific trends in breast cancer incidence between 1971 and 2015, as well as possible trends by birth cohort.
Methods
Incidence data were collected from the National Cancer Incidence Reporting System and the Canadian Cancer Registry, and annual percent changes were estimated using the Joinpoint Regression Program. Five-year birth cohort models were fit using the National Cancer Institute’s web tool.
Results
Breast cancer incidence among women under age 40 has increased since 2000, while incidence under 50 has remained stable. Rates of post-menopausal breast cancer declined sharply and have recently plateaued. More recent birth cohorts are at a non-significantly increased risk of breast cancer compared with the reference, with an increasing upward trend.
Conclusions
Rates of breast cancer may be increasing among younger women, and there is suggestive evidence that more recent birth cohorts are at increased risk of the disease. More research is needed into the risk factors for pre-menopausal breast cancer to support primary prevention efforts in this area.
Electronic supplementary material
The online version of this article (10.17269/s41997-020-00305-6) contains supplementary material, which is available to authorized users.
Keywords: Breast neoplasms, Incidence, Canada, Age of onset, Pre-menopause
Résumé
Objectifs
L’incidence du cancer du sein fluctue considérablement au Canada, avec des baisses récentes des taux chez les femmes admissibles au dépistage. Par contre, l’incidence du cancer du sein à début précoce et préménopausique est insuffisamment étudiée. Nous avons examiné les tendances selon l’âge de l’incidence du cancer du sein entre 1971 et 2015, ainsi que les éventuelles tendances par cohorte de naissance.
Méthode
Les données sur l’incidence proviennent du Système national de déclaration des cas de cancer et du Registre canadien du cancer, et les pourcentages de changement annuel ont été estimés à l’aide du programme de régression Joinpoint. Des modèles de cohorte de naissance de 5 ans ont été adaptés à l’aide d’un outil en ligne de l’Institut national du cancer.
Résultats
L’incidence du cancer du sein chez les femmes de moins de 40 ans augmente depuis 2000, tandis qu’elle est stable chez les femmes de moins de 50 ans. Après avoir diminué abruptement, les taux de cancer du sein postménopausique ont plafonné récemment. Les cohortes de naissance plus récentes présentent un risque accru non significatif de cancer du sein comparativement aux cohortes de référence, avec un mouvement de hausse.
Conclusions
Les taux de cancer du sein peuvent sembler croître chez les jeunes femmes, et les données portent à croire que les cohortes de naissance plus récentes présentent un risque accru pour cette maladie. Il faudrait pousser la recherche sur les facteurs de risque de cancer du sein préménopausique pour appuyer les démarches de prévention primaire dans ce domaine.
Mots-clés: Tumeurs du sein, Incidence, Canada, Âge de début, Préménopause
Introduction
Breast cancer is the most common malignancy among women in Canada, accounting for an estimated 25% of all cancers among women and 13% of all cancers diagnosed in 2017 (Canadian Cancer Statistics Advisory Committee 2018). Breast cancer detection and treatment have improved dramatically in recent decades, with mortality due to breast cancer decreasing by over 40% since 1986 (Zakaria and Shaw 2019). Despite steady improvements in breast cancer-related mortality, there have been considerable fluctuations in breast cancer incidence in Canada for several decades, which has been attributed to a variety of healthcare-related factors and lifestyle exposures that are associated with breast cancer etiology (Canadian Cancer Statistics Advisory Committee 2018).
The vast majority of breast cancer cases are diagnosed among post-menopausal women, but almost 20% occur among those under 50 years of age (Canadian Partnership Against Cancer 2017) and 4–5% among those before age 40 (Canadian Cancer Statistics Advisory Committee 2017). Although there is a lower proportion of pre-menopausal women with breast cancer, the disease can manifest more aggressively in this population. Young women are more likely to be diagnosed at a later stage, to have triple-negative or hormone receptor-negative type disease, and to have a poorer prognosis (Anastasiadi et al. 2017; Shoemaker et al. 2018). Compared with post-menopausal breast cancer, pre-menopausal breast cancer is less well understood and there is a significant research gap examining this population.
The objective of the current study is to examine breast cancer incidence trends by age groups with a focus on different age groups related to screening guidelines (> and < 50 years of age) to determine whether there have been changes in the age distribution and age-related breast cancer incidence burden in Canada. Here, we present breast cancer incidence data from 1971 to 2015 representing nine aggregated 5-year periods, with additional analysis by birth cohort.
Methods
Incidence data on historical breast cancer (ICD-0-3 codes C50.0-50.6, C50.8-50.9) were collected from the National Cancer Incidence Reporting System (1969–1992) and the Canadian Cancer Registry (CCR) (1992–2015). The CCR compiles data on cancer cases in all Canadian provinces and territories, and as reporting of cancer cases is a legislated responsibility, the registry is of high quality.
Trends in incidence rates were examined for women below 50 years of age (age 20–49), 50–74 years of age, and below 40 years of age. Annualized percent change (APC) incidence rates were estimated with the Joinpoint Regression Program (version 4.5.0.1, National Cancer Institute), as described in a previous publication (Brenner et al. 2017). In short, log-transformed incidence rates analyzed with permutation analysis were fit with joined lines; there could be at least 0 and at most 4 joinpoints, and the best fitting model was selected.
NCI’s web tool was used to fit birth cohort models (Rosenberg et al. 2014). Cohort models were selected because changes in risk by birth cohort can indicate changes in exposure prevalence, or the emergence of new risk factors, over different generations. Input data included cases and population size for 14 5-year age groups and 8 5-year periods. Cohort effects are presented as incidence rate ratios with 1938 as the reference cohort; adjustments were made for age. The first cohort was born in 1888, and the most recent cohort was born in 1988.
Results
Young-onset and pre-menopausal breast cancer
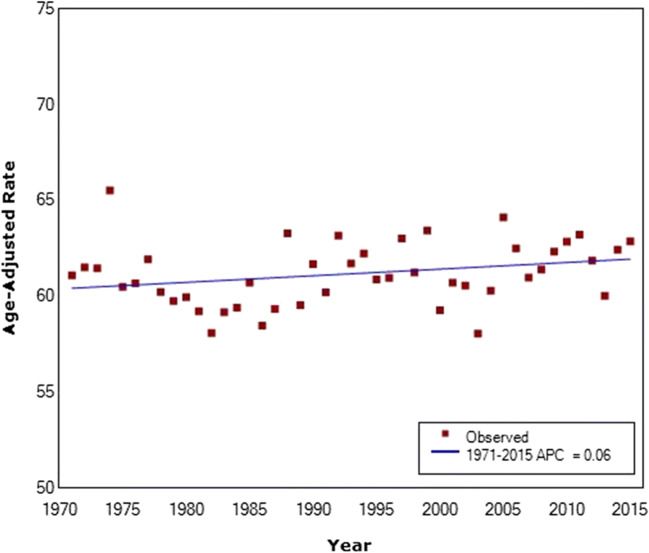
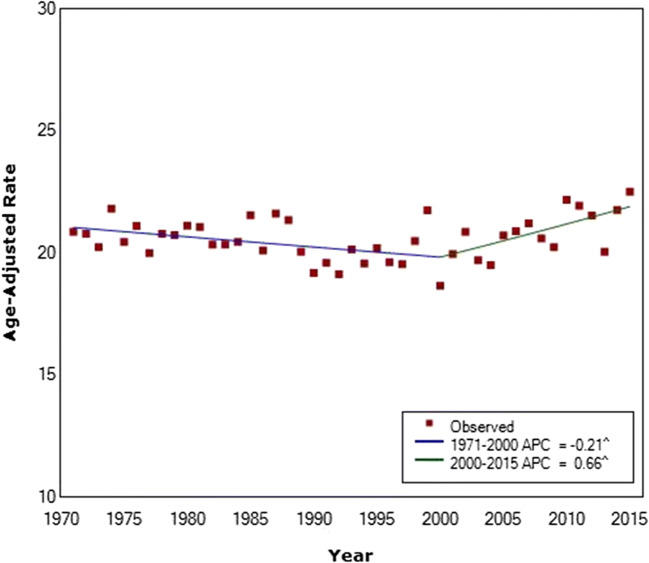
For women under the age of 50 (age 20–49), there has been no significant change in incidence since 1971 (APC = 0.06) (Fig. 1). Women under the age of 40 have a considerably lower incidence rate than women above the age of 50, and there was a decrease in the incidence of these younger women between 1971 and 2000 (APC = − 0.21) (Fig. 2, Supplementary Table 1). However, between 2000 and 2015, incidence has increased significantly, with higher rates in 2015 than in 1971 (APC = 0.66).
Fig. 1.
Breast cancer incidence in Canada among women under the age of 50 from 1971 to 2015. Average annual percent change given in legend. Final selected model includes 0 joinpoints
Fig. 2.
Breast cancer incidence in Canada among women under the age of 40 from 1971 to 2015. Average annual percent changes given in legend. Final selected model includes one joinpoint. ^ indicates that the APC is significantly different from zero at the alpha = 0.05 level
Breast cancer among women age 50–74
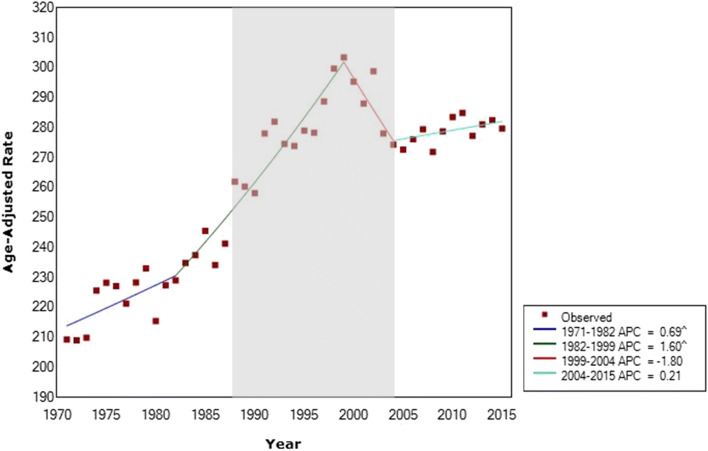
Among women aged 50–74, incidence increased significantly since the start of the analytical period (1971) to 1999 (Fig. 3). Between 1971 and 1982, the APC was 0.69, and between 1982 and 1999, the APC was 1.60. After 1999, incidence decreased greatly, with an APC of − 1.80 until 2004. Incidence has remained relatively stable between 2004 and 2015 (APC = 0.21). The shaded area in Fig. 3 indicates the timeframe when organized mammography was initiated in Canadian provinces (Canadian Partnership Against Cancer 2013), where women above the age of 50 have consistently been a target age group. Incidence of breast cancer in women above 50 years of age began increasing prior to organized mammography and continued to increase to the peak in 1999, by which time all provinces and territories had screening programs except the Northwest Territories and Nunavut.
Fig. 3.
Breast cancer incidence in Canada among women age 50–74 from 1971 to 2015. Average annual percent changes (APC) given in legend. Final selected model includes 3 joinpoints. Shaded area represents the period of organized mammography implementation in Canada. The first screening program began in 1988 and the final program in 2004. ^ indicates that the APC is significantly different from zero at the alpha = 0.05 level
Cohort effects
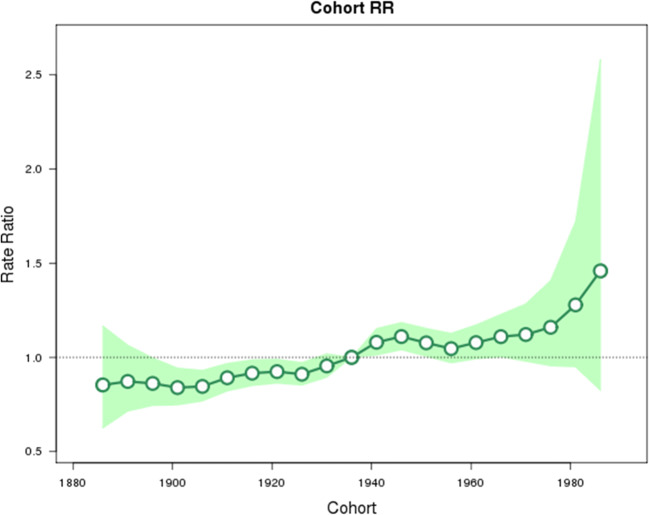
The analysis by birth cohort suggests a trend of increasing breast cancer incidence among the more recent cohorts of all ages (Fig. 4). Cohorts born after the reference year (1936) all have rate ratios trending upward, but birth years after 1951 are all non-significant and the most recent cohorts (1978, 1983, and 1988) have wide, non-significant confidence intervals.
Fig. 4.
Rate ratio of breast cancer in Canada by birth cohort. Cohorts from 1888 to 1988, with 1936 as the reference cohort. Shaded area represents 95% confidence intervals
Discussion
These results provide additional evidence that the rate of post-menopausal breast cancer is decreasing in Canada, but incidence may be increasing among younger women. Additionally, the analysis by birth cohort suggests that more recent cohorts may be at higher risk than cohorts born earlier, though the results are not significant and should be interpreted with caution. As previous analyses have noted, the decreases in post-menopausal breast cancer rates occurred in the early 2000s, which corresponds to the decreased use of hormone replacement therapy among post-menopausal women at this time (De et al. 2010). Similar trends have been noted in other study populations (Baeyens-Fernandez et al. 2018; DeSantis et al. 2015), and a previous Canadian analysis found decreasing incidence for post-menopausal women and stable or slightly increasing incidence for women under age 50 (Zakaria and Shaw 2019). The results among younger women suggest a changing risk environment or risk factor profiles that may increase susceptibility to breast cancer.
Organized screening with mammography was initiated provincially in Canada, starting in British Columbia in 1988 (Canadian Partnership Against Cancer 2013). By 2004, all Canadian provinces and territories had established breast cancer screening programs, with the exception of Nunavut. Incidence of cancer typically increases temporarily following the initiation of a screening program, particularly the incidence of early-stage cancers (Bleyer and Welch 2012). Incidence may also increase after the organized screening begins because of over-diagnoses among women who may not have been diagnosed otherwise (Welch 2009; Miller et al. 2014). While it is difficult to correlate breast cancer incidence with screening programs in Canada due to the nature of a provincial roll-out, the results from our study do show an increase in incidence in the 1980s and 1990s among the screening-eligible population. However, the increase begins prior to the initiation of organized screening programs in Canada. It is possible that there was opportunistic mammography occurring before organized programs were developed, but participation rates in mammography have historically been low in Canada even with these organized programs (Shields and Wilkins 2009), so it is unlikely that the increase can be attributed to screening alone.
Despite the observation of increasing rates among younger age groups and more recent cohorts, additional screening in this population is not recommended. There is evidence that screening younger women for breast cancer will result in more harm than benefits. The Canadian Task Force on Preventive Health Care estimates that 30% of women at average risk between the ages of 40 and 49 will receive a false-positive result, which may result in unnecessary and invasive diagnostic procedures (Klarenbach et al. 2018). Therefore, future efforts should be focused on research into determinants for pre-menopausal breast cancer as a distinct disease from post-menopausal breast cancer. Additionally, given that the results of our analysis by birth cohort were suggestive of an increased risk of breast cancer for those born in most recent decades, future research should continue to examine these trends.
Risk factors for pre- and post-menopausal women differ greatly, and those among young women are not well understood. However, risk factors for post-menopausal breast cancer have been examined extensively. Recent reports from Canada found that 28% of post-menopausal breast cancer cases could be prevented through changes in modifiable behaviours (Poirier et al. 2019; Labreche et al. 2019). High BMI is a known risk factor for post-menopausal breast cancer; recent results showed that 5% of post-menopausal breast cancer cases in Canada in 2015 were attributable to excess body weight (Brenner et al. 2019). This translates to approximately 1000 cases attributable to this exposure, which is a considerable burden of cancer cases. In the United States, an analysis from the Nurses’ Health Study estimated that almost 19% of all post-menopausal breast cancer cases can be attributed to excess BMI, which is the highest attributable risk of any modifiable factor (Tamimi et al. 2016). This association with excess BMI is not found for pre-menopausal breast cancers (Chen et al. 2016; Shoemaker et al. 2018). Other well-established modifiable factors include hormone therapy use, alcohol consumption, and low physical activity (American Cancer Society 2017). In general, the prevalence of these behaviours has increased in recent decades, which may have played a role in the incidence rates that increase over time in the screening-eligible age groups of this analysis.
Increased breast cancer risk among pre-menopausal women may involve reproductive, genetic, or immunologic components. Cancer susceptibility genes, such as BRCA 1 and 2 mutations, are associated with a dramatically increased risk of breast and ovarian cancer, especially prior to menopause (Paul and Paul 2014). Testing for BRCA1/2 mutations is generally initiated based on personal or family history of breast cancer in Canada, though some women may carry mutations unknowingly. Guidelines for screening are based on both the patient’s age and her carrier status (Klarenbach et al. 2018; Scaranelo 2012). Reproductive factors such as nulliparity, late age at first birth (30 years of age and older), and oral contraceptive use also confer a slightly higher risk of pre-menopausal breast cancer, while risk reductions were found for women with high BMI, late age at menarche, and at least three full-term births (Nelson et al. 2012).
The upward incidence trends reported here may be the result of changing fertility and reproductive factors among Canadian women. The fertility rate in Canada has been declining since 2009, and the average age at first birth has been climbing for decades, from 23.5 in 1966 to 29.2 years of age in 2016 (Provencher et al. 2018). Additionally, women are having fewer full-term pregnancies than in past decades, with the fertility rate in Canada falling from 2.26 births per woman in 1970 to 1.60 births per woman in 2016 (Provencher et al. 2018). These changes in fertility patterns can affect the risk of breast cancer, because pregnancy may alter female hormone concentrations and mammary tissues in a protective manner (Kotsopoulos et al. 2018). Additionally, there has been a decline in the age at menarche among girls in high-income countries, including Canada (Harris et al. 2008). This trend increases the levels of estrogen in the female body at a younger age, and as age of menopause has remained in the range of average (Costanian et al. 2018), those levels remain elevated for a longer time.
The risk factors for pre-menopausal status have challenging implications for primary prevention and public health practice. With the exception of oral contraceptive use, these are either non-modifiable risk factors or non-actionable risk factors from the perspective of health professionals. However, they may be useful in risk assessment to determine women who could benefit from early screening. A more comprehensive risk assessment tool that is based on current evidence of consistent risk factors would help to identify those women who may require early screening or ongoing surveillance for breast cancer.
As treatment options improve for women with breast cancer in Canada, mortality has decreased significantly among those who develop the disease. However, the results of this study show that more Canadian women are developing breast cancer at a young age and that women born in recent cohorts are at greater risk. Given that breast cancer at a younger age is associated with worse outcomes, these results are troubling. With this evidence, steps should be taken to ensure that women at higher risk are identified. Additionally, more effort should be placed on the risk factors for young-onset and pre-menopausal breast cancer and potential options for primary prevention.
Electronic supplementary material
(DOCX 12 kb)
Acknowledgements
Darren Brenner is supported by a Canadian Cancer Society Capacity Development Award in Prevention (#703917).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- American Cancer Society . Breast cancer: facts & figures 2017-2018. Atlanta, GA: American Cancer Society Inc.; 2017. [Google Scholar]
- Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: an overview. Updates in Surgery. 2017;69(3):313–317. doi: 10.1007/s13304-017-0424-1. [DOI] [PubMed] [Google Scholar]
- Baeyens-Fernandez JA, Molina-Portillo E, Pollan M, Rodriguez-Barranco M, Del Moral R, Arribas-Mir L, et al. Trends in incidence, mortality and survival in women with breast cancer from 1985 to 2012 in Granada, Spain: a population-based study. BMC Cancer. 2018;18(1):781. doi: 10.1186/s12885-018-4682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. The New England Journal of Medicine. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- Brenner DR, Poirier AE, Ruan Y, Hebert LA, Grevers X, Walter SD, et al. Estimates of the current and future burden of cancer attributable to excess body weight and abdominal adiposity in Canada. Preventive Medicine. 2019;122:49–64. doi: 10.1016/j.ypmed.2019.03.014. [DOI] [PubMed] [Google Scholar]
- Brenner DR, Ruan Y, Shaw E, De P, Heitman SJ, Hilsden RJ. Increasing colorectal cancer incidence trends among younger adults in Canada. Preventive Medicine. 2017;105:345–349. doi: 10.1016/j.ypmed.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Canadian Cancer Statistics Advisory Committee (2017). Canadian Cancer statistics 2017. Toronto, ON: Canadian Cancer Society.
- Canadian Cancer Statistics Advisory Committee . Canadian cancer statistics 2018. Toronto, ON: Canadian Cancer Society; 2018. [Google Scholar]
- Canadian Partnership Against Cancer . Organized breast cancer screening programs in Canada: report on program performance in 2007 and 2008. Toronto: Canadian Partnership Against Cancer; 2013. [Google Scholar]
- Canadian Partnership Against Cancer . Breast cancer in Canada. Ottawa, ON: Public Health Agency of Canada; 2017. [Google Scholar]
- Chen GC, Chen SJ, Zhang R, Hidayat K, Qin JB, Zhang YS, et al. Central obesity and risks of pre- and postmenopausal breast cancer: a dose-response meta-analysis of prospective studies. Obesity Reviews. 2016;17(11):1167–1177. doi: 10.1111/obr.12443. [DOI] [PubMed] [Google Scholar]
- Costanian C, McCague H, Tamim H. Age at natural menopause and its associated factors in Canada: cross-sectional analyses from the Canadian Longitudinal Study on Aging. Menopause. 2018;25(3):265–272. doi: 10.1097/GME.0000000000000990. [DOI] [PubMed] [Google Scholar]
- De P, Neutel CI, Olivotto I, Morrison H. Breast cancer incidence and hormone replacement therapy in Canada. Journal of the National Cancer Institute. 2010;102(19):1489–1495. doi: 10.1093/jnci/djq345. [DOI] [PubMed] [Google Scholar]
- DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiology, Biomarkers & Prevention. 2015;24(10):1495–1506. doi: 10.1158/1055-9965.EPI-15-0535. [DOI] [PubMed] [Google Scholar]
- Harris MA, Prior JC, Koehoorn M. Age at menarche in the Canadian population: secular trends and relationship to adulthood BMI. The Journal of Adolescent Health. 2008;43(6):548–554. doi: 10.1016/j.jadohealth.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Klarenbach S, Sims-Jones N, Lewin G, Singh H, Theriault G, Tonelli M, et al. Recommendations on screening for breast cancer in women aged 40-74 years who are not at increased risk for breast cancer. CMAJ. 2018;190(49):E1441–E1451. doi: 10.1503/cmaj.180463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsopoulos J, Gronwald J, Lynch HT, Eisen A, Neuhausen SL, Tung N, et al. Age at first full-term birth and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Breast Cancer Research and Treatment. 2018;171(2):421–426. doi: 10.1007/s10549-018-4822-y. [DOI] [PubMed] [Google Scholar]
- Labreche F, Kim J, Song C, Pahwa M, Ge CB, Arrandale VH, et al. The current burden of cancer attributable to occupational exposures in Canada. Preventive Medicine. 2019;122:128–139. doi: 10.1016/j.ypmed.2019.03.016. [DOI] [PubMed] [Google Scholar]
- Miller AB, Wall C, Baines CJ, Sun P, To, T. Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HD, Zakher B, Cantor A, Fu R, Griffin J, O’Meara ES, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Annals of Internal Medicine. 2012;156(9):635–648. doi: 10.7326/0003-4819-156-9-201205010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Paul S. The breast cancer susceptibility genes (BRCA) in breast and ovarian cancers. Frontiers in Bioscience (Landmark Ed) 2014;19:605–618. doi: 10.2741/4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier AE, Ruan Y, Volesky KD, King WD, O’Sullivan DE, Gogna P, et al. The current and future burden of cancer attributable to modifiable risk factors in Canada: summary of results. Preventive Medicine. 2019;122:140–147. doi: 10.1016/j.ypmed.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Provencher C, Milan A, Hiallman S, D’Aoust C. Report on the demographic situation in Canada - fertility: overview, 2012 to 2016. Ottawa, ON: Statistics Canada; 2018. [Google Scholar]
- Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiology, Biomarkers & Prevention. 2014;23(11):2296–2302. doi: 10.1158/1055-9965.EPI-14-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaranelo A. Breast screening with magnetic resonance imaging. Canadian Medical Association Journal. 2012;184(16):E877. doi: 10.1503/cmaj.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields M, Wilkins K. An update on mammography use in Canada. Health Reports. 2009;20(3):7–19. [PubMed] [Google Scholar]
- Shoemaker ML, White MC, Wu M, Weir HK, Romieu I. Differences in breast cancer incidence among young women aged 20-49 years by stage and tumor characteristics, age, race, and ethnicity, 2004-2013. Breast Cancer Research and Treatment. 2018;169(3):595–606. doi: 10.1007/s10549-018-4699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamimi RM, Spiegelman D, Smith-Warner SA, Wang M, Pazaris M, Willett WC, et al. Population attributable risk of modifiable and nonmodifiable breast cancer risk factors in postmenopausal breast cancer. American Journal of Epidemiology. 2016;184(12):884–893. doi: 10.1093/aje/kww145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch HG. Overdiagnosis and mammography screening. BMJ. 2009;339:b1425. doi: 10.1136/bmj.b1425. [DOI] [PubMed] [Google Scholar]
- Zakaria D, Shaw A. Trends in mammography, hormone replacement therapy, and breast cancer incidence and mortality in Canadian women. Cancer Causes & Control. 2019;30(2):137–147. doi: 10.1007/s10552-019-1127-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 12 kb)