Abstract
Objective
Breast cancer screening aims to identify cancers in early stages when prognosis is better and treatments less invasive. We describe inter- and intra-provincial variation in the percentage of screen-detected cases under publicly funded healthcare systems and factors related to having screen- vs non-screen-detected breast cancer across five Canadian provinces.
Methods
Women aged 40+ diagnosed with incident breast cancer from 2007 to 2012 in five Canadian provinces were identified from their respective provincial cancer registries. Standardized provincial datasets were created linking screening, health administrative, and claims data. Province-specific logistic regression models were used to evaluate the association of demographic and healthcare utilization factors in each province with the odds of screen-detected cancer.
Results
There was significant inter- and intra-provincial variation by age. Screen detection ranged from 42% to 52% in ages 50–69 but women aged 50–59 had approximately 4–8% lower screen detection than those aged 60–69 in all provinces. Screening associations with income quintile and rurality varied across provinces. Those least likely to be screen-detected within a province were consistently in the lowest income quintile; OR ranged from 0.62–0.89 relative to highest income quintile/urban patients aged 50–69. Lack of visits to primary care 30 months prior to diagnosis was also consistently associated with lower odds of screen detection (OR range, 0.37–0.76).
Conclusion
Breast cancer screen detection rates in the Canadian provinces examined are relatively high. Associations with income-rurality indicate a need for greater attention and/or targeted outreach to specific communities and/or provincial regions to improve access to breast cancer screening services intra-provincially.
Keywords: Breast cancer screening, Screen-detected cancer, Multi-jurisdictional, Administrative data linkage
Résumé
Objectif
Le dépistage du cancer du sein vise à repérer les cancers aux stades précoces, quand le pronostic est meilleur et les traitements moins effractifs. Nous décrivons les écarts inter- et intraprovinciaux dans le pourcentage de cas détectés par dépistage dans les systèmes de soins de santé subventionnés par l’État, ainsi que les facteurs liés au fait d’avoir un cancer du sein détecté ou non par dépistage dans cinq provinces canadiennes.
Méthode
Les femmes de 40 ans et plus ayant reçu un diagnostic de cancer du sein incident entre 2007 et 2012 dans cinq provinces canadiennes ont été identifiées grâce au registre des cancers de leur province respective. Nous avons créé des fichiers provinciaux standardisés jumelant les données de dépistage, les données administratives sur la santé et les données sur les demandes de remboursement. Des modèles de régression logistique propres à chaque province ont servi à évaluer l’association entre les facteurs démographiques et d’utilisation des soins de santé dans la province et la probabilité de cancer détecté par dépistage.
Résultats
Nous avons observé des écarts inter- et intraprovinciaux significatifs selon l’âge. La détection par dépistage se situait entre 42 et 52 % chez les femmes de 50 à 69 ans, mais chez les femmes de 50 à 59 ans, dans toutes les provinces, elle était inférieure d’environ 4 à 8 % à celle des femmes de 60 à 69 ans. Les associations entre le dépistage et le quintile de revenu et la ruralité variaient d’une province à l’autre. Les cas les moins susceptibles d’avoir été détectés par dépistage dans une province se trouvaient uniformément dans le quintile de revenu inférieur; le rapport de cotes (RC) était de 0,62–0,89 comparativement aux patientes du quintile de revenu supérieur/vivant en milieu urbain âgées de 50 à 69 ans. L’absence de rendez-vous de soins primaires 30 mois avant le diagnostic était aussi uniformément associée à une moindre probabilité de détection par dépistage (RC, 0,37–0,76).
Conclusion
Les taux de détection du cancer du sein par dépistage dans les provinces canadiennes à l’étude sont relativement élevés. Les associations avec le revenu et la ruralité sont signes qu’une plus grande attention et/ou des activités de sensibilisation ciblées sur certaines communautés et/ou certaines régions provinciales sont nécessaires pour améliorer l’accès aux services de dépistage du cancer du sein à l’intérieur des provinces.
Mots-clés: Dépistage du cancer du sein, Cancer détecté par dépistage, Plurigouvernemental, Jumelage de données administratives
Introduction
Breast cancer screening is intended to identify cancers in early stages when prognosis is better and treatments are less invasive (Nelson et al. 2016). Breast cancer screening is a complex public health issue that has evolved over the last 30 years. Different guidelines and implementation strategies exist across the world with varying degrees of effectiveness (Shapiro et al. 1998). Although there is general consensus that breast cancer screening is important to reduce breast cancer mortality (Hanley and Njor 2018), there is less consensus regarding the age eligibility and screening frequency for women at average risk. Adherence and monitoring of quality standards for breast cancer screening programs/practices across and within countries also vary (Nelson et al. 2016; Miller et al. 2002; Tyne and Nygren 2009). All of these factors influence the ultimate effectiveness of breast cancer screening at a given jurisdictional level.
In Canada, virtually all residents have health insurance under their respective provincial and territorial governments, which also organize and deliver healthcare for residents. This structure results in inter-provincial variation in healthcare services, including access to care and potential disparities across jurisdictions (Mossialos et al. 2016). Common to all provinces, however, is that essential and preventive healthcare services are free to all residents; breast cancer screening and diagnostic work-up are covered services. Most provinces also have province-wide administrative databases that capture all ambulatory care, inpatient care, physician claims, cancer registries, breast cancer screening, and demographic data of the insured population. These databases allow conduct of provincial population-based health services research within and across regions that can help inform policy decisions (Lipscomb et al. 2013). Multi-province studies using administrative data are not common due to many factors, including differential effort in data access, availability of analytic resources and infrastructure to analyze provincial data, competing priorities for limited analytic resources, logistical complexity, and costs in both time and dollars. Such studies, however, are valuable in identifying norms as well as positive/negative variation that can then inform national, provincial, and even local policies to improve healthcare delivery and access.
The purpose of the current study was to compare overall breast cancer screening detection rates across five Canadian provinces to assess the inter- and intra-provincial variation with respect to factors related to screen- vs non-screen-detected breast cancer in the context of differing provincial screening programs. It is part of a larger study, Canadian Team to Improve Community-Based Cancer Care along the Continuum (CanIMPACT), a large multi-provincial population-based retrospective cohort study exploring issues related to inter- and intra-provincial variation in quality of care along the breast cancer care continuum (McBride et al. 2016). There are nine provinces and three territories in Canada; the five provinces included in the study accounted for approximately 69% of the Canadian population during the study period (“Tables for Population Growth: Canada, Provinces and Territories, 2010” n.d.).
Methods
Context
Table 1 provides an overview of the provincial screening programs in the five participating provinces during the study period: British Columbia (BC); Alberta (AB); Manitoba (MB); Ontario (ON); and Nova Scotia (NS) (Nova Scotia Breast Screening Program 2012; “Alberta Clinical Practice Guidelines, 2007 Update. Guidelines for the Early Detection of Breast Cancer” 2007). Key points of comparison are (1) all programs have existed for over 20 years; (2) two provinces, MB and ON, do not provide screening for women aged 40–49 via their provincial screening program; (3) all programs send letters to women aged 50–69 reminding them to get screened at their biennial date; (4) all programs recommend screening for those 70–74 or 70+, however letters are not sent to women in this age group except for in BC where letters were sent to women up to age 79; (5) in addition to formal screening programs, AB and ON have breast cancer screening available outside their respective provincial program that is performed by fee-for-service community-based radiology clinics and covered by provincial insurance; (6) in all provinces, except AB, all or the majority of screening is performed through the provincial screening program. In AB, the provincial screening program has two physical clinics, one each in the two largest cities, plus mobile units that serve rural and remote regions, similar to other provinces. Breast cancer screening conducted outside the formal screening program clinics is performed at community radiology clinics located throughout the province.
Table 1.
Overview of characteristics of each provincial screening program during their respective study period
| Breast cancer screening program characteristics |
British Columbia 2007–2010 |
Alberta 2004–2010 |
Manitoba 2007–2011 |
Ontario 2007–2012 |
Nova Scotia 2007–2012 |
|---|---|---|---|---|---|
| Year program began | 1988 | 1990 | 1995 | 1990 | 1991 (initially in Halifax only) |
| Recommended frequency? | |||||
| Age 40–49 | Patient initiative | Patient initiative | None | None | Patient initiative |
| Age 50–69 | Biennial | Biennial | Biennial | Biennial | Biennial |
| Age 70–74 | Biennial | Bienniala | Bienniala | Bienniala | Bienniala |
| Age 75+ | Biennial | Bienniala | None | Bienniala | Bienniala |
| Recall letters sent? | |||||
| Age 40–49 | Yesb | Yesb | No | No | Yesb |
| Age 50–69 | Yes | Yes | Yes | Yes | Yes |
| Age 70–74 | Yes | Yes | Yes | Yesc | No |
| Age 75+ | Yesd | No | No | Noc | No |
| Reimbursement outside of program? | No | Yesd | No | Yesd | No |
| Proportion screening performed/overseen by provincial program | 100% | Approximately 10% | 100% | 85% |
100% (as of Oct. 2008) |
| Participation rate age 50–69 | 57% | 60% | 57% | 57% | 59% |
aIf in good health
bAfter initial screen, annual recall letters sent
c2007–2010 no recall letters for age 70+ but in 2010/11 recall letters sent for age 70–74
dRecall letters not sent for age 80+ but will screen with physician referral
Study design and population
We conducted a multi-province retrospective breast cancer cohort study. All women aged 40+ diagnosed with a first-ever histologically confirmed invasive primary breast cancer (ICD-9: 174.x or ICD-O 3rd edition code C50 behaviors 2 and 3) (World Health Organization (WHO) 2000) between January 1, 2007 and December 31, 2012 (December 31, 2011 in MB; December 31, 2010 in BC; January 1, 2004 to December 31, 2010 in AB) in any of the participating provinces were identified from the respective provincial cancer registry. All cancer registries are members of the North American Association of Central Cancer Registries and routinely receive gold or silver certification, except Ontario (“NAACCR Certified Registries” n.d.). Women were excluded if they (1) did not have a valid health card number or were living outside of their home province at the time of diagnosis; (2) had a history of in situ breast cancer, as these women have an increased risk of future breast cancer so may have different screening recommendations (Sackey et al. 2016); (3) had a history of any invasive cancer other than non-melanoma skin; or (4) the diagnosis was a non-solid breast tumour, as these are not coded as breast cancers in cancer registries (“Coding Guidelines, Breast C500-509” 2007). Women under the age of 40 at the time of diagnosis and men were not included because they are not screen eligible in any of the provinces.
Data sources and data harmonization
Table 2 lists the administrative health databases used to obtain and/or create each variable of interest in the study by province. In addition to the provincial cancer registry, the following provincial databases were used to extract data of interest for the study: screening program, health insurance client registry, physician database, physician claims, hospital ambulatory care, hospital inpatient care, and the Postal Code Conversion File Plus (PCCF+) developed and maintained by Statistics Canada (“BC Cancer Agency Registry Data. V2, Population Data BC: BC Cancer Agency; 2011 [Available from: http://www.popdata.bc.ca/Data.],” n.d.; “Medical Services Plan (MSP) Payment Information File. V2, MOH (2011): British Columbia Ministry of Health; 2011 [Available from: http://www.popdata.bc.ca/Data.],” n.d.; “Consolidation File (MSP Registration & Premium Billing). V2, Population Data BC: British Columbia Ministry of Health (2011); 2011 [Available from: http://www.popdata.bc.ca/Data.],” n.d.). PCCF+ is a conversion file that links Canadian postal codes to various census data such as median household income, using the postal code or larger region to define a “neighbourhood.” To ensure variables were comparable across the five provincial cohorts, we harmonized definitions and established protocols to derive variables using comparable or similar data sources in each province as previously described (Groome et al. 2018; Jiang et al. 2015; Yuan et al. 2015). Of particular relevance is mode of diagnosis. Screening programs in BC, MB, NS, and ON routinely collect data in such a way that allows them to know whether a mammogram was conducted as a screening mammogram (i.e., asymptomatic) or diagnostic (i.e., symptomatic or in follow-up to a screening mammogram). In AB, detection mode was estimated using an algorithm applied to the provincial physician claims data, which has different codes for screening and diagnostic mammograms, that was validated against a gold standard dataset containing both test type (i.e., screening or diagnostic) and test results (e.g., positive, negative) (Yuan et al. 2015). The final algorithm has sensitivity and concordance in correct assignment of detection mode using physician claims data compared with a gold standard of approximately 94%. For the approximate 20% of screen-detected cancers in ON, diagnosed outside the provincial screening program, ON used a similar validated approach to AB to assign mode of diagnosis (Jiang et al. 2015).
Table 2.
Data sources used by each province to obtain or create each variable
| Data source | British Columbia | Alberta | Manitoba | Ontario | Nova Scotia |
|---|---|---|---|---|---|
| Provincial cancer registry |
Diagnosis date Age at diagnosis Sex Postal code at dx |
Diagnosis date Age at diagnosis Sex Postal code at dx |
Diagnosis date Age at diagnosis Sex |
Diagnosis date Sex |
Diagnosis date Age at diagnosis Sex Postal code at dx |
| Provincial screening program | Mode of diagnosis | Mode of diagnosis | Mode of diagnosis | Mode of diagnosis | |
| Health insurance plan client registry | Postal code at dx |
Postal code at dx Age at diagnosis* |
|||
| Provincial physician database | UPC index score* | UPC index score* | |||
| Provincial physician claims data |
Co-morbidity code* UPC index score* |
Mode of diagnosis* Co-morbidity score* UPC index score* |
Co-morbidity code* UPC index score* |
Mode of diagnosis* Co-morbidity code* UPC index score* |
Co-morbidity code* UPC index score* |
| Provincial hospital ambulatory care data | Co-morbidity score* | ||||
| Provincial hospital inpatient data | Co-morbidity code* | Co-morbidity score* | Co-morbidity code* |
Mode of diagnosis* Co-morbidity code* |
Co-morbidity code* |
| Postal Code Conversion File Plus from Statistics Canada |
Neighbourhood income quintile Urban/rural measure |
Neighbourhood income quintile Urban/rural measure |
Neighbourhood income quintile Urban/rural measure |
Neighbourhood income quintile Urban/rural measure |
Neighbourhood income quintile Urban/rural measure |
*Calculated or created variable using one or more datasets
Statistical analysis
The primary outcome was percentage of screen-detected breast cancers. Descriptive statistics were calculated by age decade to assess the relationship between screen-detected breast cancer and patient age including the entire 40+ age cohort to assess the impact that variation in screening age eligibility policy has on variation in proportion screen detected by age decade.
Bivariate and multivariate statistics were calculated limited to the common screen-eligibility age group, 50–69, to explore the relationship of the following factors with screen-detection: co-morbidities (using Aggregated Diagnosis Group (ADG™)) (Austin et al. 2012); neighbourhood income/rurality; and usual provider of care (UPC) (Jee and Cabana 2006). The UPC score is a typical measure used to assess patient continuity of care with their primary care physician (PCP). We calculated it by dividing the number of visits to the PCP the patient had seen the most by the total number of all PCP visits from 6 to 30 months prior to the cancer diagnosis (Jee and Cabana 2006); a minimum of three PCP visits is required to calculate the UPC (Reid et al. 2002; Ionescu-Ittu et al. 2007). We defined a UPC ≥ 0.75 as high continuity of care as a value of 1 represents perfect continuity (Liisa et al. 2004); those with 1–2 or 0 visits to a PCP (i.e., UPC was not calculable) were defined as low or non-users of PCP, respectively.
We created a single, six-level neighbourhood income-rurality variable using two variables developed by Statistics Canada and available in the 2006 Canadian Census Data: QUAIPPE, neighbourhood income levels in quintiles, and CSIZEMIZ, an urban/rural variable based on patient postal code (Wilkins and Peters 2012). We made three income levels (lowest quintile, middle three quintiles combined, and highest quintile) and two rurality levels (urban or rural). We created this joint-effect variable because we hypothesized that there may be interactions between income and rurality that vary inter-provincially.
Bivariate statistics were intended to be simply descriptive so statistical associations were not examined. We examined statistical associations using logistic regression models including all the variables of interest to examine their relationship with the likelihood of being screen-detected; adjusted odds ratios and their corresponding 95% confidence intervals are presented. All analyses were conducted using either SAS 9.2 or 9.4 (SAS Institute, Cary, NC).
Results
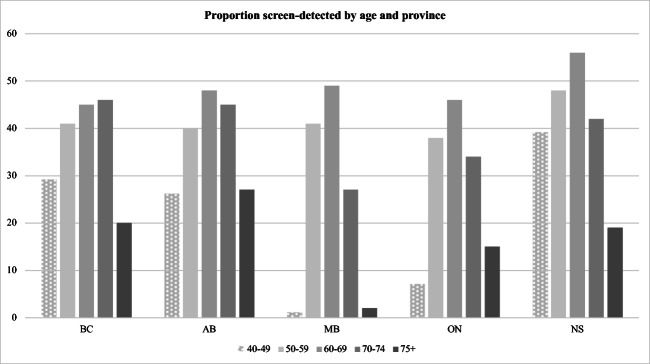
Figure 1a shows the proportion of screen-detected breast cancers in those aged 40+ by age group. The percent screen-detected by age group varies significantly within and across provinces. In all provinces except BC, women aged 50–59 had approximately 8% lower screen detection than those aged 60–69. In contrast, in BC, women aged 60–69 and 70–74 had a similar proportion of screen-detected breast cancer (45–46%) while the proportion was slightly lower for those aged 50–59 at 41%. In the three provinces that allow screening in ages 40–49, percent screen-detected ranged from a high of 39% in NS to a low of 27% in AB. Similarly, proportion screen-detected in ages 70–74 ranged from a high of 46% in BC to a low of 27% in MB.
Fig. 1.
Percentage of screen-detected breast cancers by age and province, age 40+
Table 3 shows the overall provincial proportions of screen-detected breast cancers and unadjusted relationship of age, co-morbidities, income/rurality, and UPC index with breast cancer screen detection in the common screen-eligible aged group for all the provinces, aged 50–69. Proportions screen-detected in BC, AB, MB, and ON were very similar at 42–45%; NS had a higher proportion at 52%.
Table 3.
Characteristics of women diagnosed with breast cancer and those screen-detected aged 50–69 years by province
| British Columbia | Alberta | Manitoba | Ontario | Nova Scotia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Total n = 7048 |
Screen detected n(%)= 3049(43) |
Total n = 5190 |
Screen detected n(%)= 2277(44) |
Total n = 2085 |
Screen detected n(%)= 941(45) |
Total n= 22,543 |
Screen detected n(%)= 9435(42) |
Total n = 1869 |
Screen detected n(%)= 969(52) |
| Age | ||||||||||
| 50–59 | 3407 | 41% | 2811 | 40% | 1008 | 41% | 11,278 | 38% | 843 | 48% |
| 60–69 | 3641 | 45% | 2379 | 48% | 1077 | 49% | 11,265 | 46% | 1026 | 56% |
| Co-morbidities | ||||||||||
| 0–3 ADGs | 2449 | 39% | – | – | 496 | 42% | 6219 | 40% | 431 | 40% |
| 4–5 ADGs | 1737 | 44% | 503 | 45% | 5364 | 44% | 398 | 50% | ||
| 6–7 ADGs | 1365 | 50% | 434 | 47% | 4827 | 43% | 393 | 58% | ||
| 8–9 ADGs | 870 | 43% | 332 | 44% | 3254 | 42% | 327 | 60% | ||
| 10+ ADGs | 627 | 43% | 320 | 48% | 2879 | 40% | 320 | 54% | ||
| Income-rurality | ||||||||||
| Low/rural | 189 | 45% | 229 | 39% | 96 | 42% | 578 | 48% | 123 | 50% |
| Med/rural | 620 | 40% | 723 | 40% | 385 | 48% | 1815 | 45% | 415 | 55% |
| High/rural | 207 | 41% | 217 | 41% | 147 | 48% | 582 | 44% | 125 | 59% |
| Low/urban | 1044 | 37% | 639 | 43% | 195 | 47% | 3148 | 39% | 205 | 44% |
| Med/urban | 3486 | 46% | 2399 | 45% | 945 | 44% | 11,705 | 42% | 752 | 50% |
| High/urban | 1361 | 45 | 959 | 46% | 317 | 45% | 4635 | 42% | 246 | 56% |
| Missing/unknown | 141 | 26% | 0 | 0 | 0 | 0 | 80 | 39% | 3 | 66% |
| UPC index score | ||||||||||
| 0 visits | 564 | 25% | 286 | 28% | 138 | 36% | 1707 | 35% | 106 | 24% |
| 1–2 visits | 1038 | 41% | 434 | 43% | 182 | 42% | 2647 | 41% | 45% | |
| UPC < 0.75 | 2822 | 46% | 2354 | 44% | 792 | 44% | 5910 | 41% | 224 | 52% |
| UPC > 0.75 | 2624 | 45% | 2116 | 46% | 973 | 48% | 12,279 | 43% | 1053 | 56% |
Across all provinces, those with an ADG score 0–3 and/or with no primary care visits 6–30 months prior to diagnosis had the lowest proportion of screen detection in all provinces. The range from highest to lowest proportion within each of these factors, however, varied such that intra-provincial differences were relatively small in MB and ON (4% to 6% ADGs) while differences were relatively large in BC, AB, and NS (11% to 20% ADGs).
There is also evidence for inter-provincial variation in the patterns of association of income-rurality with proportion screen-detected and intra-provincial variation in the degree of variation. The intra-provincial variation in proportion screen-detected across the income-rurality categories was highest in NS at 15%, and lowest in MB and AB (6–7%). Within the income/rurality categories, the lowest proportion of screen-detected in BC, ON, and NS were low-income urban residents, although small to moderately less than the provincial average, 3% to 8%, depending on the province. In MB, the lowest proportion was in low-income/rural patients. In AB, low- or middle-income rural residents were the least likely, about 5% less than the provincial average.
Table 4 shows the results of the logistic regression analyses for age 50–69. An odds ratio less than 1 indicates the group is less likely to be screen-detected than the corresponding reference group. Most of the observed association with co-morbidities and screen detection disappeared in the adjusted analyses in all provinces except NS where ACG groups 6–7 and 8–9 had significantly higher odds of screen detection than those in ACG 0–3 group with OR 1.58 and 1.71, respectively. Women without any visits to a primary care physician in the 2 years prior to breast cancer screening were the least likely to have screen-detected cancer in all provinces; odds ratios for screen detection ranged from 0.37 (NS) to 0.76 (ON) compared with those with UPC score greater than 0.75. Odds ratios increased with increasing UPC index in all provinces except ON; however, the trend was not statistically significant in any province.
Table 4.
Adjusted odds ratios and 95% confidence intervals of having a screen-detected breast cancer by province, age 50–69
| British Columbia | Alberta | Manitoba | Ontario | Nova Scotia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI |
| Co-morbidities | ||||||||||
| 0–3 ADGs | Reference | – | – | Reference | Reference | Reference | ||||
| 4–5 ADGs | 0.92 | 0.80–1.06 | 0.97 | 0.73–1.30 | 1.16 | 1.06–1.26 | 1.19 | 0.87–1.63 | ||
| 6–7 ADGs | 1.15 | 0.98–1.34 | 1.05 | 0.78–1.41 | 1.07 | 0.98–1.17 | 1.58 | 1.14–2.19 | ||
| 8–9 ADGs | 0.88 | 0.73–1.05 | 0.94 | 0.68–1.29 | 1.04 | 0.94–1.15 | 1.71 | 1.21–2.40 | ||
| 10+ ADGs | 0.88 | 0.71–1.05 | 1.10 | 0.80–1.53 | 0.93 | 0.84–1.03 | 1.35 | 0.96–1.90 | ||
| Income-rurality | ||||||||||
| High/urban | Reference | Reference | Reference | Reference | Reference | |||||
| Low/rural | 1.03 | 0.75–1.40 | 0.75 | 0.55–1.00 | 0.85 | 0.53–1.35 | 1.29 | 1.08–1.54 | 0.82 | 0.52–1.28 |
| Med/rural | 0.79 | 0.65–0.96 | 0.74 | 0.61–0.91 | 1.08 | 0.80–1.45 | 1.13 | 1.01–1.26 | 0.98 | 0.71–1.36 |
| High/rural | 0.85 | 0.63–1.15 | 0.79 | 0.59–1.07 | 1.07 | 0.72–1.59 | 1.08 | 0.90–1.28 | 1.16 | 0.74–1.81 |
| Low/urban | 0.72 | 0.61–0.85 | 0.88 | 0.71–1.08 | 1.03 | 0.72–1.48 | 0.89 | 0.81–0.98 | 0.62 | 0.41–0.90 |
| Med/urban | 1.03 | 0.91–1.17 | 0.97 | 0.83–1.13 | 0.91 | 0.70–1.18 | 0.99 | 0.93–1.06 | 0.80 | 0.59–1.07 |
| UPC index score | ||||||||||
| UPC > 0.75 | Reference | Reference | Reference | Reference | Reference | |||||
| 0 visits | 0.44 | 0.35–0.55 | 0.48 | 0.37–0.63 | 0.61 | 0.40–0.92 | 0.76 | 0.67–0.85 | 0.37 | 0.22–0.62 |
| 1–2 visits | 0.82 | 0.69–0.96 | 0.94 | 0.76–1.17 | 0.80 | 0.56–1.15 | 0.99 | 0.90–1.10 | 0.82 | 0.59–1.15 |
| UPC < 0.75 | 0.95 | 0.85–1.06 | 1.00 | 0.88–1.13 | 0.84 | 0.69–1.02 | 0.96 | 0.90–1.02 | 0.87 | 0.70–1.09 |
Italics indicates statistical significance: the confidence interval did not include 1.00
The patterns of association between the combined income-rurality factor and breast cancer screen detection were similar in the adjusted and unadjusted analyses in all provinces except that differences were not significant in MB or rural patients in NS and not all categories were significantly different from the reference group, high-income urban. In BC, ON, and NS, the least likely group to have screen-detected breast cancer remained the low-income urban residents (OR (95% CI): BC 0.72 (0.61–0.85); ON 0.89 (0.81–0.99); NS 0.62 (0.41–0.90)). In all provinces except ON, the group with the highest adjusted odds of screen detection were the highest income urban residents; the most likely in ON were the lowest income rural residents. In ON, point estimates for rural residents were higher than those for urban residents, although not always statistically significant. The reverse was true in AB where point estimates were higher for urban residents than rural, regardless of income level.
Discussion
We examined inter- and intra-provincial variation in proportion of screen-detected breast cancer. Inter-provincial variation is most likely due to differences in policy across the provinces; intra-provincial variation is most likely due to differences in the implementation of the policy, such as location of services and resources provided. The significant inter-provincial variation in proportion screen-detected by age in the 40+ population is driven primarily by large inter-provincial variation in the 40–49 and 75+ age groups, likely due to differences in screening policies across the provinces. Specifically, neither the MB nor ON screening programs allow screening for women aged 40–49, consistent with the 2011 breast cancer screening guidelines from the Canadian Task Force on Preventive Health Care (The Canadian Task Force on Preventive Health Care 2011), which do not recommend screening for women aged 40–49. As a result, a very small proportion of those aged 40–49 had screen-detected breast cancer in MB (1%) and ON (7%) compared with the proportion in the other three provinces (26–39%). Similar variation in policies regarding screening eligibility for age 75+ exists producing similar patterns in inter-provincial variation in this age group. The variation in provincial screening policies by age in the presence of national guidelines and in consideration of finite healthcare funds is noteworthy. Of particular note, the 2018 Canadian breast cancer screening guidelines are consistent with those published in 2011 (Klarenbach et al. 2018). Average-risk women aged 40–49 are not recommended to be screened in the updated guidelines due to the larger harm:benefit ratio created by the larger number of screens needed to detect one breast cancer case and the larger number of subsequent false positives and associated tests; women aged 75+ are not considered in national guidelines (Klarenbach et al. 2018).
Inter-provincial variation in proportion screen-detected in the 50–69 age group was minimal at 42–45%, except in NS where it was 52%, largely reflecting consistent age screening eligibility. The higher proportion screen-detected in NS is most likely explained by the fact that NS has an older population than the other provinces; the age shift translates to a higher risk population. This age-associated shift in risk can be seen by close examination of Table 3. In BC, MB, and ON, the total number of cancers are split approximately evenly between the 50–59 and 60–69 age groups. In NS, however, 55% are in the 60–69 age group (1026 out of 1869) and in AB only 45% are in the 60–69 age group (2379 out of 5190). The relatively equal participation rates in AB and NS (about 60%) translate into a 10% difference in percentage screen-detected due to the higher breast cancer risk of the NS population due to older age in NS compared with AB.
In all provinces, a larger percentage of women aged 60–69 were screen-detected compared with those aged 50–59: 8% higher in all provinces except BC which was 4% higher. This likely reflects lower participation among those aged 50–59 than 60–69. Lower participation among the younger age group may be due to higher levels of active or passive refusal rather than lower outreach of screening programs, as all the screening programs send letters of invitation to women aged 50–69. Higher non-participation among younger women may be due to differences in their interpretation of risk or because of busier work/life schedules than those 60–69. Guidelines recommend that physicians discuss the trade-offs of harms and benefits of breast cancer screening with eligible women (The Canadian Task Force on Preventive Health Care 2011; Klarenbach et al. 2018); however, it is not clear how often, or thoroughly, that is done or whether women’s interpretation of the harm:benefit ratio changes significantly from ages 50–59 to 60–69. Current evidence, however, suggests shared decision-making for breast cancer screening is not common and/or is not done particularly well (Keating and Pace 2018). Although many tools to support the breast cancer screening shared decision-making process exist (“Mammography - HealthDecision” n.d.; Gøtzsche and Jørgensen 2013; Members of the Screening and Test Evaluation Program 2013), more research efforts are needed to ensure they are understood by patients and, if so, how to successfully implement them in practice.
The significant intra-provincial variation in all provinces—except MB—related to income/rurality may reflect differences in screening implementation strategies such as service density or frequency of service availability (in the case of mobile units) relative to eligible population density. In contrast, the lack of intra-provincial variation in MB may be a result of the limited age eligibility, 50–74, which may better enable more equitable provision of screening services across the province. The lower odds of screen detection in the low-income/urban patients than their medium- and high-income urban counterparts in BC, ON, and NS suggest that targeted breast cancer screening outreach to low-income/urban women may be needed, whereas in AB, efforts to improve access in the rural/remote regions may be needed to minimize variation. Alternatively, or in addition, restricting age eligibility to those of national guidelines may also facilitate decreasing the intra-provincial variation observed in BC, AB, ON, and NS.
The patients least likely to be screen-detected in all provinces were those without any primary care visits in the 6–30 months prior to their diagnosis. The odds ratio relative to those with a UPC ≥ 0.75 ranged from 0.37 in NS to 0.76 in ON. This is consistent with the literature; patients who utilize primary care physicians are more likely to receive preventive care (Litaker and Tomolo 2007; Gorey et al. 2009). We note that the proportion of breast cancer patients who did not receive any primary care services in the 6–30 months prior to their diagnosis, however, represents a fairly small proportion of screen-eligible women in our study, ranging from 5.5% to 8% depending on the province. Lack of access to and/or utilization of primary care, therefore, has minimum impact on the attributable risk for breast cancer screen detection in these provinces.
This is the first multi-provincial population-based study of female incident invasive breast cancer patients. The inclusion/exclusion criteria, variables and derived variables, and analysis approach were standardized (Groome et al. 2018), providing reasonably comparable datasets across provinces. Apart from differences in provincial data collection, the main limitation is that we did not have patient-level data such as education, race/ethnicity, immigrant status, health literacy, screening awareness, and patient values placed on screening; some of these factors may explain some of the variation found. Another potential limitation is some variation in the overall quality of cancer registries, particularly ON not having NAACCR gold or silver certification; the potential impact on their odds ratio estimates is unknown because we do not know the reason for less than silver certification. It is likely, however, that it is due to focus on completeness and accuracy of the most common cancers, such as breast cancer, with the result that less common cancers in ON may not meet certification requirements. Differences between gold and silver certification are unlikely to impact interpretation of results for other provinces as quality requirements are high for both (“NAACCR Certification Criteria” n.d.). Although the results are specific to the five provinces included in the analysis, this study provides an example of the value of interjurisdictional comparisons in identifying areas of best practice or opportunities to improve care. Such studies identify areas in which further efforts are needed to determine reasons for poorer results and inform development of strategies and interventions for care improvement.
Conclusion
Population-based studies are vital for identifying disparities in healthcare systems. Inter-provincial variation in women younger and older than 50–69 was largely due to differences across provinces in age screen-eligibility policies even in the presence of national guidelines. Notably, inter-provincial variation was small in the 50–69 age group where screening policies are consistent across all provinces. Significant intra-provincial variation in the 50–69 age group, however, existed in all provinces except MB. Intra-provincial variation in screen detection was most significant by rurality/income groups but the pattern of variation differed across provinces. Such variation in association by rurality/income in each province except MB suggests either (1) a need for greater attention and/or targeted outreach to specific communities and/or provincial regions, or (2) provincial policy changes to be consistent with the national age guidelines to allow services focused on women with optimal benefit:harm ratio; both would improve access to breast cancer screening services for those who need them. Attention to variation in healthcare service utilization and patient outcomes is particularly important in jurisdictions in which healthcare is publicly funded.
Acknowledgements
In addition to the authors, the membership of the CanIMPACT Administrative Health Data Group who undertook this work include: Natalie Biswanger, CancerCare Manitoba, Winnipeg, Manitoba; Dongdong Li, BC Cancer Research Centre, Vancouver, British Columbia; Aisha Lofters, University of Toronto, Toronto, Ontario; Sharon Matthias, CanIMPACT Patient Advisory Committee, Alberta; Nicole Mittmann, Cancer Care Ontario, Toronto, Ontario; Rahim Moineddin, University of Toronto, Toronto, Ontario; Geoff Porter, Dalhousie University, Halifax, Nova Scotia; Dawn Powell, CanIMPACT Patient Advisory Committee, Ontario; Donna Turner, CancerCare Manitoba, Winnipeg, Manitoba; Robin Urquhart, Dalhousie University, Halifax, Nova Scotia; Bonnie Vick, CanIMPACT Patient Advisory Committee, Saskatchewan. The authors also thank Emma Shu, Marlo Whitehead, and Yan Zhang for conducting data processing and statistical analyses.
Funding and disclaimers
This work was supported by the Canadian Institutes of Health Research [grant number128272]. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funder. This study is supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and information provided by Cancer Care Ontario (CCO). The opinions, results, views, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed herein are those of the authors, and not necessarily those of CIHI. We gratefully acknowledge CancerCare Manitoba for their ongoing support and Manitoba Health for the provision of data. The results and conclusions presented are those of the authors. No official endorsement by Manitoba Health is intended or should be inferred. Nova Scotia data were provided by Health Data Nova Scotia and the Nova Scotia Department of Health and Wellness, however, the observations and opinions expressed are those of the authors and do not represent those of either Health Data Nova Scotia or the Department of Health and Wellness. Data for this study were also provided by Population Data BC and BC Cancer. All inferences, opinions, and conclusions drawn in this study are those of the authors, and do not reflect the opinions or policies of the BC Data Steward(s).(“BC Cancer Agency Registry Data. V2, Population Data BC: BC Cancer Agency; 2011 [Available from: http://www.popdata.bc.ca/Data.],” n.d.; “Medical Services Plan (MSP) Payment Information File. V2, MOH (2011): British Columbia Ministry of Health; 2011 [Available from: http://www.popdata.bc.ca/Data.],” n.d.; “Consolidation File (MSP Registration & Premium Billing). V2, Population Data BC: British Columbia Ministry of Health (2011); 2011 [Available from: http://www.popdata.bc.ca/Data.],” n.d.)
Compliance with ethical standards
Research ethics boards and privacy committees approved the study and data stewards approved data access in each province. Data sharing across provinces was not permitted by the provincial data stewards; therefore, distributed data analyses were conducted by the respective provincial teams. Data were linked at the individual level in each province through an encrypted individual health insurance number. Quality assurance and cross checks were performed on datasets during and after data linkage to ensure accuracy and completeness. None of the databases or datasets used in the study are publicly available.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marcy Winget, Email: mwinget@stanford.edu.
for the Canadian Team to Improve Community-Based Cancer Care Along the Continuum (CanIMPACT):
Natalie Biswanger, Dongdong Li, Aisha Lofters, Sharon Matthias, Nicole Mittmann, Rahim Moineddin, Geoff Porter, Dawn Powell, Donna Turner, Robin Urquhart, and Bonnie Vick
References
- Alberta Clinical Practice Guidelines, 2007 Update. Guidelines for the early detection of breast cancer. 2007. http://www.topalbertadoctors.org/cpgs/?sid=2&cpg_cats=12&cpg_info=1.
- Austin PC, Newman A, Kurdyak PA. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a population-based cohort of adults with schizophrenia in Ontario, Canada. Psychiatry Research. 2012;196(1):32–37. doi: 10.1016/j.psychres.2011.09.023. [DOI] [PubMed] [Google Scholar]
- BC Cancer Agency Registry Data. V2, Population data BC: BC Cancer Agency; 2011 [Available from: http://www.popdata.bc.ca/Data.]. n.d.
- Coding Guidelines, Breast C500-509. (2007). SEER Program Reporting and Staging Manual, 2007.
- “Consolidation File (MSP Registration & Premium Billing). V2, Population Data BC: British Columbia Ministry of Health (2011); 2011 [Available from: http://www.popdata.bc.ca/Data.].” n.d.
- Gorey KM, Luginaah IN, Holowaty EJ, Fung KY, Hamm C. Associations of physician supplies with breast cancer stage at diagnosis and survival in Ontario, 1988 to 2006. Cancer. 2009;115(15):3563–3570. doi: 10.1002/cncr.24401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gøtzsche, P. C., & Jørgensen, K. J. (2013). Screening for breast cancer with mammography. Cochrane Database of Systematic Reviews, June. 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed]
- Groome, P. A., McBride, M. L., Jiang, L., Kendell, C., Decker, K. M., Grunfeld, E., Krzyzanowska, M. K., & Winget, M. (2018). Lessons learned: it takes a village to understand inter-sectoral care using administrative data across jurisdictions. International Journal of Population Data Science. 10.23889/ijpds.v3i3.440. [DOI] [PMC free article] [PubMed]
- Hanley JA, Njor SH. Disaggregating the mortality reductions due to cancer screening: model-based estimates from population-based data. European Journal of Epidemiology. 2018;33(5):465–472. doi: 10.1007/s10654-017-0339-7. [DOI] [PubMed] [Google Scholar]
- Ionescu-Ittu R, McCusker J, Ciampi A, Vadeboncoeur A-M, Roberge D, Larouche D, Verdon J, Pineault R. Continuity of primary care and emergency department utilization among elderly people. CMAJ : Canadian Medical Association Journal = Journal de l’Association Medicale Canadienne. 2007;177(11):1362–1368. doi: 10.1503/cmaj.061615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee SH, Cabana MD. Indices for continuity of care: a systematic review of the literature. Medical Care Research and Review. 2006;63(2):158–188. doi: 10.1177/1077558705285294. [DOI] [PubMed] [Google Scholar]
- Jiang L, Gilbert J, Langley H, Moineddin R, Groome PA. Effect of specialized diagnostic assessment units on the time to diagnosis in screen-detected breast cancer patients. British Journal of Cancer. 2015;112:1744–1750. doi: 10.1038/bjc.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating NL, Pace LE. Breast cancer screening in 2018. Jama. 2018;319(17):1814. doi: 10.1001/jama.2018.3388. [DOI] [PubMed] [Google Scholar]
- Klarenbach S, Sims-Jones N, Lewin G, Singh H, Thériault G, Tonelli M, Doull M, Courage S, Garcia AJ, Thombs BD. Recommendations on screening for breast cancer in women aged 40–74 years who are not at increased risk for breast cancer. Canadian Medical Association Journal. 2018;190:E1441–E1451. doi: 10.1503/cmaj.180463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liisa, J., Upshur, R. E. G., Klein-Geltink, J. E., Leong, A., Maaten, S., Schultz, S. E., & Wang, L. (2004). Primary care in Ontario: ICES atlas. British Medical Journal, 329. 10.1136/bmj.38282.669225.AE.
- Lipscomb J, Robin Yabroff K, Hornbrook MC, Gigli A, Francisci S, Krahn M, Gatta G, et al. Comparing Cancer care, outcomes, and costs across health systems: charting the course. Journal of the National Cancer Institute. Monographs. 2013;46:124–130. doi: 10.1093/jncimonographs/lgt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litaker D, Tomolo A. Association of contextual factors and breast cancer screening: finding new targets to promote early detection. Journal of Women's Health. 2007;16(1):36–45. doi: 10.1089/jwh.2006.0090. [DOI] [PubMed] [Google Scholar]
- “Mammography - HealthDecision.” n.d. Accessed May 22, 2019. https://www.healthdecision.org/tool#/tool/mammo.
- McBride ML, Groome P, Turner D, Jorgensen M, Kendell C, Porter G, Jiang L, et al. Using Canadian administrative data to evaluate primary and oncology care of breast cancer patients post-treatment: subset of the CanIMPACT Study. Journal of Clinical Oncology. 2016;34(3_suppl):5. doi: 10.1200/jco.2016.34.3_suppl.5. [DOI] [Google Scholar]
- Medical Services Plan (MSP) Payment Information File. V2, MOH (2011): British Columbia Ministry of Health; 2011 [Available from: http://www.popdata.bc.ca/Data.]. n.d.
- Members of the Screening and Test Evaluation Program. (2013). Breast cancer screening: it’s your choice. Sydney. http://askshareknow.com.au/wp-content/uploads/2017/07/It’s-your-choice-New-information-to-help-women-aged-about-50-to-make-a-decision-includes-overdiagnosis.pdf.
- Miller AB, Teresa T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up. a randomized screening trial of mammography in women age 40 to 49 years. Annals of Internal Medicine. 2002;137(5 Part 1):305–312. doi: 10.7326/0003-4819-137-5_Part_1-200209030-00005. [DOI] [PubMed] [Google Scholar]
- Mossialos, Elias, Martin Wenzl, Robin Osborn, and Dana Sarnak. (2016). 2015 international profiles of health care systems. New York. http://www.commonwealthfund.org/~/media/files/publications/fund-report/2016/jan/1857_mossialos_intl_profiles_2015_v7.pdf?la=en.
- NAACCR Certification Criteria. n.d. Accessed November 11, 2019. https://www.naaccr.org/certification-criteria/.
- NAACCR Certified Registries. n.d. Accessed November 11, 2019. https://www.naaccr.org/certified-registries/.
- Nelson HD, Rochelle F, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Annals of Internal Medicine. 2016;164(4):244–255. doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- Nova Scotia Breast Screening Program. (2012). Nova Scotia Breast Screening Program annual report 2011. Vol. 2011. https://breastscreening.nshealth.ca/sites/default/files/sites/default/files/annual_report2016.pdf.
- Reid, R., Haggerty, J. L., & McKendry, R. (2002). Defusing the confusion: concepts and measures of continuity of healthcare. Health Services Research Foundation, 258. 10.1017/CBO9781107415324.004.
- Sackey H, Hui M, Czene K, Verkooijen H, Edgren G, Frisell J, Hartman M. The impact of in situ breast cancer and family history on risk of subsequent breast cancer events and mortality - a population-based study from Sweden. Breast Cancer Research. 2016;18(1):1–9. doi: 10.1186/s13058-016-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, S., Coleman, E. A., Broeders, M., Codd, M., de Koning, H., Fracheboud, J., Moss, S., Paci, E., Stachenko, S., & Ballard-Barbash, R. (1998). Breast cancer screening programmes in 22 countries: current policies, administration and guidelines. International Breast Cancer Screening Network (IBSN) and the European Network of Pilot Projects for Breast Cancer Screening. International Journal of Epidemiology, 27(5), 735–742 http://queens.ezp1.qub.ac.uk/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med4&AN=9839727. [DOI] [PubMed]
- Tables for population growth: Canada, provinces and territories, 2010. n.d. Accessed November 4, 2019. https://www150.statcan.gc.ca/n1/pub/91-209-x/2011001/article/11508/tbl/pop-tbl-eng.htm#a1.
- The Canadian Task Force on Preventive Health Care Recommendations on screening for breast cancer in average-risk women aged 40-74 years. CMAJ. 2011;183(17):1991–2001. doi: 10.1503/cmaj.110334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyne K, Nygren P. Screening for breast cancer: systematic evidence review update for the U. S. Preventive Services Task Force. Science. 2009;151(10):727–W242. doi: 10.1059/0003-4819-151-10-200911170-00009.Screening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins R, Peters PA. Automated geographic coding based on the Statistics Canada postal code conversion files. Health Analysis Division Statistics Canada, no. 2012;82:1–75. [Google Scholar]
- World Health Organization (WHO). (2000). International classification of diseases for oncology. World Health Organization, 240.
- Yuan Y, Li M, Yang J, Winget M. Using administrative data to estimate time to breast cancer diagnosis and percent of screen-detected breast cancers – a validation study in Alberta, Canada. European Journal of Cancer Care. 2015;24(3):367–375. doi: 10.1111/ecc.12277. [DOI] [PubMed] [Google Scholar]