Abstract
Pancreas is an exceptionally rare location for desmoid tumors. There are very few case reports of pancreatic fibromatosis in the English radiology literature. We present a case of a 45-year-old male with a mixed solid and cystic desmoid tumor of the pancreas which was surgically resected and was followed by recurrence in the mesentery. This will be the first case report of pancreatic desmoid with documented recurrence of fibromatosis in the mesentery which was also surgically resected and confirmed on pathology. In this case report, we discuss this entity's radiological findings with pathology correlation, clinical findings and management along with literature review.
Keywords: Desmoid, Fibromatosis, Pancreas, Locally aggressive
Introduction
Desmoid tumors of the pancreas are rare, the first case was described by Wilson in 1956 [1]. Desmoid fibromatoses are benign but locally aggressive fibroblastic proliferations which are characterized by a variable and sometimes unpredictable clinical course [2]. Although pancreatic fibromatosis is a rare entity, the radiologists and clinicians need to be aware of it and consider it in the differential diagnosis of solid and partially cystic pancreatic lesions. Pancreatic fibromatosis can closely resemble pancreatic adenocarcinoma on imaging and clinical presentation, but it has much more favorable prognosis.
Case presentation
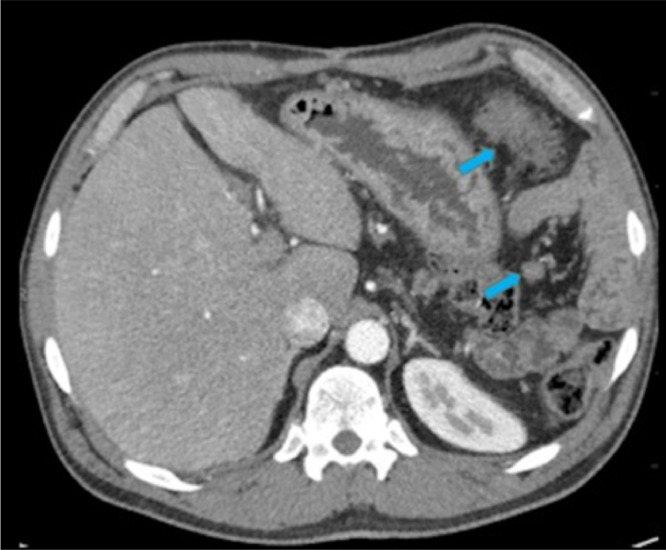
A 45-year-old Caucasian male presented with periumbilical pain, nausea, vomiting, and loss of appetite. Laboratory evaluation showed normal liver function tests, (CEA) levels, amylase, and lipase. Subsequently the patient underwent contrast enhanced CT of the abdomen and pelvis (Portal venous phase iv contrast enhanced [125 mL of Isovue 300] volume acquisition 64 detector CT [Phillips Brilliance 64] acquired at 1 mm slice thickness, 120 kVp, 269-500 mAs), which showed a large, heterogenous mass arising from the body of the pancreas. The mass was predominantly solid with a small cystic component and was seen to abut the stomach and duodenum with loss of intervening fat planes (Figs. 1, 2). There was moderate to severe narrowing of the portal venous confluence and adjacent segments of the splenic vein and superior mesenteric vein (Figs. 1, 2). The pancreatic duct in the tail was not dilated and there was no distal glandular atrophy. CT guided biopsy was performed, and pathology was compatible with pancreatic fibromatosis. The patient underwent an extensive surgery comprising radical resection of the mass with distal pancreatectomy, splenectomy, partial gastrectomy, duodenectomy of the 4th portion of duodenum, and resection of portal vein with interposition of deep femoral vein graft from superior mesenteric vein to portal vein. Due to the densely adherent nature of the tumor to the aforementioned structures, the surgery was technically difficult. Final pathology confirmed the diagnosis of desmoid type pancreatic fibromatosis. On gross examination (Fig. 3), the tumor was 12 × 10 × 10 cm in size and the tumor margin was 0.5 cm from the pancreatic resection margin. The surface of the tumor appeared tan brown to hemorrhagic and smooth to irregular/ rough with adhesions. Pancreatic parenchyma was seen in the cut sections. The histology of the mass (Fig. 4A, B) contained zones that resembled usual desmoid-type fibromatosis, as well as other areas with thick collagen bands, resembling keloidal collagen. The latter has been reported in particular in mesenteric fibromatosis lesions. The neoplasm was involving parenchyma of the pancreas, and serosa and connective tissue of stomach. It had low to intermediate cellularity and was made up of a uniform population of spindle cells with attenuated cytoplasm and oval slender nuclei with pointed cells. No atypical mitotic figures or necrosis was seen. Immunohistochemical staining (Fig. 4C, D) demonstrated strong nuclear staining with beta-catenin, favoring fibromatosis. Smooth muscle actin was weakly positive, CD34 was patchy positive, and C-Kit was negative. The molecular evaluation of the sample did not demonstrate any coding variant.
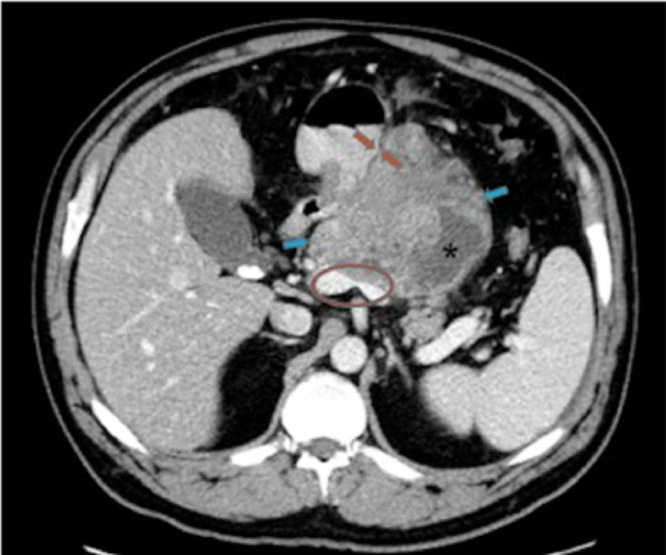
Fig. 1.
Enhanced axial CT image at the level of pancreas demonstrates a 10 cm heterogenous, predominantly solid mass (blue arrows) with small cystic component (*) arising from the pancreatic body with loss of fat planes between the mass and the adjacent stomach (red arrows). The distal pancreatic duct is not dilated. The mass is causing marked narrowing of portal confluence and splenic vein (red circle).
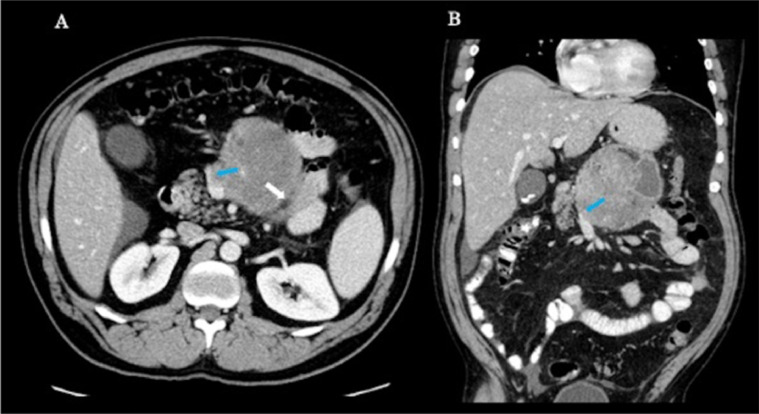
Fig. 2.
Enhanced axial (A) and coronal (B) CT images demonstrate that the mass is abutting distal fourth portion of the duodenum (white arrow) and is narrowing the superior mesenteric vein (blue arrows).
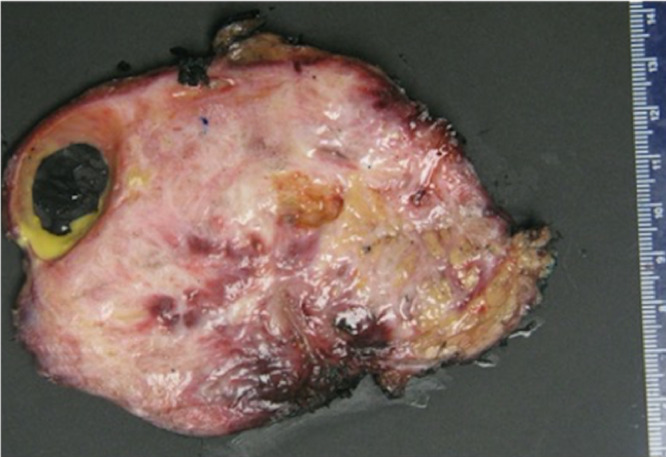
Fig. 3.
On gross pathology examination, the removed tumor was 12 × 10 × 10 cm in size. The cut surface of the tumor was ill circumscribed, firm and gritty, heterogenous with tan white to pink fleshy areas. A cystic area with smooth lining and filled with yellowish material (3 × 2 × 1 cm) was also identified.
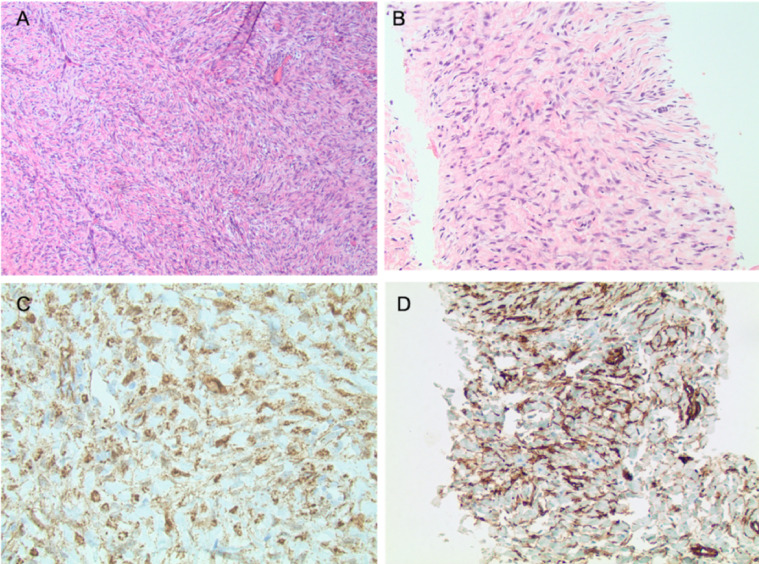
Fig. 4.
A and B: Slides stained with Hematoxylin and Eosin stain with 4x (A) and 10x (B) magnification) demonstrates intermediate cellularity with a uniform population of spindle cells with attenuated cytoplasm and oval slender nuclei with pointed cells. Bundles of collagen fibers resembling keloidal collagen are seen interspersed between the neoplastic cells. No atypical mitotic figures or necrosis is seen. C and D: Immunohistochemical staining demonstrates strong nuclear staining with beta-catenin (C), favoring fibromatosis. CD34 (D) is patchy positive.
Post operatively, patient did well initially, but on surveillance imaging, there was recurrence of fibromatosis with multiple nodular soft tissue masses in the gastro colic ligament and pericolic fat in the left upper quadrant which were increasing in size (measuring up to 5 cm) on follow-up imaging despite treatment with Sulindac (Fig. 5). Second line treatment was pursued with Sorafenib which helped with disease stabilization. After few months, the patient developed acute calculous cholecystitis, and was taken to the operating room. Along with cholecystectomy, he underwent omental and mesenteric nodule resection, pathology of the nodules confirmed desmoid fibromatosis. The gall bladder was inflamed, and the gall bladder wall also showed focal involvement with fibromatosis. Patient continues to receive Sorafenib and is doing well and receiving surveillance imaging.
Fig. 5.
Surveillance CT at 12 months after resection of the primary tumor demonstrates nodular soft tissue masses in the anterior left upper quadrant and in the surgical bed (blue arrows).
Discussion
Desmoid tumors (DTs) are rare, locally aggressive but benign soft tissue neoplasms which rarely metastasize but have high propensity to local aggressive expansion to adjacent tissues, resulting in high rates of morbidity.
It is rare for desmoid to arise in the pancreas. Incorporating the findings of two comprehensive single institutional studies of pancreatic mesenchymal tumors performed by Hongkai et al. and Kim et al. it was found that desmoid tumor was the most common benign mesenchymal pancreatic tumor, followed by solitary fibrous tumor [3]. The mean age of presentation is 36 years old and there is slight female to male predilection for abdominal DTs [4]. Pancreatic DTs are most commonly sporadic but trauma and genetic factors, such as Familial Adenomatous Polyposis syndrome (FAP) and Gardner syndrome, were previously described as potential risk factors [5,6]. Tail is the most common site for pancreatic DTs [4].
Clinically, patients remain asymptomatic for extended periods of time and once symptomatic, vague abdominal pain is the most common presentation. Nausea and vomiting and weight loss are the other common presenting symptoms. They may occasionally present with acute abdomen secondary to complications such as intestinal obstruction, caused by mass effect or local invasion.
Contrast-enhanced CT or MRI are the preferred imaging modalities. While Fluorodeoxyglucose (18F) FDG-PET may aid in differentiating DT from the other hypermetabolic pancreatic malignancies, it has no role in the evaluation of disease progression. The most common CT finding is a solid enhancing mass in the distal body or tail of the pancreas, as in our case. It may occasionally present as cystic or complex cystic-solid mass, mimicking a pancreatic pseudocyst or a cystic pancreatic neoplasm [4,5,7,8]. There are few reported cases of pancreatic head or diffuse pancreatic involvement [4,9]. Local invasion to adjacent vasculature causing pseudoaneurysms which is commonly seen in pancreatitis or organs involvement may be encountered, which is usually seen in pancreatic adenocarcinoma [10].
The main differential diagnosis for an entity with the aforementioned imaging findings is pancreatic adenocarcinoma. However, common associated findings seen in adenocarcinoma are pancreatic ductal dilatation, distal pancreatic atrophy and regional lymphadenopathy, that are absent in DT. There are case reports of pancreatic fibromatosis presenting as a predominantly cystic mass, in which case pancreatic pseudocyst and cystic pancreatic neoplasm are the main differential diagnoses.
The Desmoid Tumor Working Group suggested active treatment for this condition in the patients with sporadic (beta-catenin mutated versus wild type) and FAP associated desmoids [2]. For this reason, it is strongly recommended to perform mutational analysis in these patients. Either APC loss or CTNNB1 mutation can lead to DT development [11]. The options of active treatment in these patients are surgery, radiotherapy and systemic treatment. Penel et al. compared initial surgical management with initial observation and did not see any significant difference between the two groups. The location of DT however had a major prognostic factor in their study [12]. The Desmoid Tumor Working Group came up with a treatment algorithm after reviewing the available literature, where they proposed “active surveillance” for the initial 1-2 years of diagnosis and proceed to further treatment in case of disease progression. The treatment options available are surgery (wide local resection), Medical treatment and Radiotherapy. The available medical therapies are: Antihormonal therapies, Nonsteroidal anti-inflammatory drugs, Tyrosine kinase inhibitors, and low dose conventional chemotherapeutic regimens.
Local recurrence has been reported post-surgery, mainly in cases associated with FAP or Gardner syndrome. There are two case reports of pancreatic desmoid tumor treated with nonsteroidal anti-inflammatory drug (NSAID) and complete response was reported in one of them [9,13].
The patient we present here underwent an extensive surgery comprising radical resection of the mass with distal pancreatectomy, splenectomy, partial gastrectomy, duodenectomy of the 4th portion of duodenum, and portal vein reconstruction with right in situ femoral vein. The surgery for pancreatic DTs can be technically challenging given the adherent and locally aggressive nature of the tumor. On surveillance imaging, our patient had a recurrent DT in the mesentery. The patient was initially started on NSAIDs, but the lesions continued to slowly enlarge and subsequently, Tyrosine Kinase inhibitor (Sorafenib) was started, and the disease was seen to stabilize. Patient underwent cholecystectomy for cholecystitis, during surgery the omental and mesenteric nodules were also excised and confirmed to represent fibromatoses on pathology. The patient continues to be on Sorafenib and routine surveillance imaging and is doing ewell.
Conclusion
Pancreatic fibromatosis is a rare, locally aggressive neoplasm with relatively favorable prognosis which can be misinterpreted as pancreatic adenocarcinoma on imaging. Familiarity of radiologists and clinicians with this entity is essential as it is a benign neoplasm and needs to be included in the differential diagnosis in the proper setting.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Informed Consent
Verbal consent taken for the case report publication from the patient. A reasonable individual would be unlikely to object to publication as no personal information has been provided and if required informed consent with documentation can be obtained when publication is accepted for publication.
Availability of data and material
Not applicable.
Footnotes
Conflicts of Interest: The authors declare that they have no competing or conflicts of interests.
Acknowledgment: None.
Financial Disclosures: None.
References
- 1.Wilson E. Desmoid tumor resembling a pseudo-pancreatic cyst in a male. Br Med J. 1956;2(4999):982‐983. doi: 10.1136/bmj.2.4999.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desmoid Tumor Working Group The management of desmoid tumours: a joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer. 2020;127:96–107. doi: 10.1016/j.ejca.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Yu S, Wang W, Cheng Y, Xiao Y, Lu Z. Primary mesenchymal tumors of the pancreas in a single center over 15 years. Oncol Lett. 2016;12(5):4027‐4034. doi: 10.3892/ol.2016.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia C, Tian B, Dai C, Wang X, Bu X, Xu F. Idiopathic desmoid-type fibromatosis of the pancreatic head: case report and literature review. World J Surg Oncol. 2014;12:103. doi: 10.1186/1477-7819-12-103. Published 2014 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu B, Zhu LH, Wu JG, Wang XF, Matro E, Ni JJ. Pancreatic solid cystic desmoid tumor: case report and literature review. World J Gastroenterol. 2013;19(46):8793‐8798. doi: 10.3748/wjg.v19.i46.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mourra N, Ghorra C, Arrive L. An unusual solid and cystic pancreatic tumor in a 20-year-old woman. Desmoid Tumor. 2015;149(3):e5‐e6. doi: 10.1053/j.gastro.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 7.Słowik-Moczydłowska Ż, Rogulski R, Piotrowska A, Małdyk J, Kluge P, Kamiński A. Desmoid tumor of the pancreas: a case report. J Med Case Rep. 2015;9:104. doi: 10.1186/s13256-015-0591-y. Published 2015 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saida K, Miyazaki O, Matsuoka K, Watanabe T, Fujino A, Nosaka S. Pancreatic desmoid tumor in a 4-year-old male with hemihypertrophy. J Pediatr Surg Case Rep. 2015;3:244–347. [Google Scholar]
- 9.Wang YC, Wong JU. Complete remission of pancreatic head desmoid tumor treated by COX-2 inhibitor-a case report. World J Surg Oncol. 2016;14(1):190. doi: 10.1186/s12957-016-0944-z. Published 2016 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan U, Puri SK, Jain N, Garg L, Kapoor A, Gupta N. Percutaneous thrombin injection under sonographic guidance for exclusion of non-catheterizable post-pancreatitis pseudoaneurysm of the superior mesenteric artery: a minimally invasive and expeditious treatment option. J Med Ultrason (2001) 2016;43(2):295‐299. doi: 10.1007/s10396-015-0687-4. [DOI] [PubMed] [Google Scholar]
- 11.Le Guellec S, Soubeyran I, Rochaix P, Filleron T, Neuville A, Hostein I. CTNNB1 mutation analysis is a useful tool for the diagnosis of desmoid tumors: a study of 260 desmoid tumors and 191 potential morphologic mimics. Mod Pathol. 2012;25(12):1551‐1558. doi: 10.1038/modpathol.2012.115. [DOI] [PubMed] [Google Scholar]
- 12.Penel N, Le Cesne A, Bonvalot S, Giraud A, Bompas E, Rios M. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: A nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer. 2017;83:125‐131. doi: 10.1016/j.ejca.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka K, Yoshikawa R, Yanagi H, Gega M, Fujiwara Y, Hashimoto-Tamaoki T. Regression of sporadic intra-abdominal desmoid tumour following administration of non-steroidal anti-inflammatory drug. World J Surg Oncol. 2008;6:17. doi: 10.1186/1477-7819-6-17. Published 2008 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.