Abstract
Objectives
Left atrial (LA) function is an important marker of hemodynamic status in cardiac amyloidosis (CA), and its characterization may provide relevant prognostic information. We sought to assess the prevalence and prognostic impact of LA dysfunction by cardiac magnetic resonance (CMR) in patients with CA.
Methods
We performed CMR in 80 consecutive patients with CA, including 38 with AL (47%) and 42 with ATTR (53%). LA function was assessed by acquiring short axis cine steady-state free precession (SSFP) covering the entire chamber. The atrial emptying fraction (AEF) was calculated as the ratio between the difference of LA maximal and minimal volume to LA maximal volume, expressed as percentage. Severe atrial dysfunction was defined as AEF ≤ 14%.
Results
Mean AEF was 18% (13–35%). Overall, AEF ≤ 14% was present in 19 patients (24%), including 21% of those in sinus rhythm (SR) with no history of atrial fibrillation (AF). After a median of 3 years (IQR 2–4), 36 patients (44%) died of cardiac causes. Patients with AEF ≤ 14% showed increased cardiac mortality, with an independent OR of 4.2 (95 IC 2.1–8.2, P < 0.0001). Of note, AEF ≤ 14% was the stronger independent predictor of cardiac death. Patients in SR with AEF ≤ 14% had worse outcome than those with AF.
Conclusions
Severe impairment of LA contractile function was present in three-quarters of patients with CA, and was prevalent irrespective of CA etiology, both in the presence and absence of AF. Severe LA dysfunction was associated with an independent 4-fold increase in risk for cardiac death at three years.
Keywords: Cardiac amyloidosis, Cardiac magnetic resonance, Prognosis, Left atrial function
1. Introduction
Cardiac amyloidosis (CA) is characterized by extracellular accumulation of fibrillary proteins within the myocardium, leading to progressive infiltration and functional impairment [1]. CA begins with a subclinical stage characterized by mild and non-specific cardiac symptoms [2]. Progression of amyloid deposition causes marked thickening of the cardiac walls leading to subsequent stages of heart failure with preserved systolic function (HFpEF) and different grades of diastolic dysfunction. The end-stage of CA is characterized by congestive HF with biventricular systolic impairment, atrial fibrillation (AF) or sinus node dysfunction and atrioventricular block [3].
While the ventricular manifestations of CA dominate the clinical picture, the disease is known to affect the heart as a whole, including the left atrium (LA), interatrial septum and valvular apparatus. Amyloid deposition has been documented in the atrial walls and may create dysfunction [4]. LA remodeling and functional impairment, although different in etiology and molecular substrates, is a well-established epiphenomenon of many cardiac diseases, including pathophysiologic models resembling CA, such as hypertrophic cardiomyopathy (HCM). In HCM, both LA size and systolic function represent important predictors of outcome [5]. However, whether this concept may be applied to CA is still unresolved. The issue has a number of potential implications in clinical practice, ranging from screening and stratifying for disease severity to evaluating likelihood of AF. Cardiac magnetic resonance (CMR) is today the gold standard for the evaluation of LA size and function. The present study aimed to exploit the potential of CMR in CA patients to 1) evaluate the prevalence of LA dysfunction through the analysis of LA volume\time curve, both in patients in sinus rhythm and AF, and 2) evaluate its independent clinical and prognostic.
2. Methods
We enrolled 80 consecutive patients (mean age 70 ± 12 years, 56 males) with an established diagnosis of CA: 38 patients with systemic AL amyloidosis (47%); 13 with genetic amyloidosis due to ATTR mutations (16%); 29 with wild type ATTR amyloidosis (37%). Diagnosis of AL amyloidosis was confirmed by biopsy of abdominal fat pad or of an involved organ. Amyloid deposits demonstrated typical Congo Red birefringence under polarized light and were characterized as AL type by immunohistochemistry on optic or immunoelectron microscopy or proteomics analysis according to the time of diagnosis. A monoclonal light chain of the same isotype (κ or λ) as that found in the amyloid deposits needed to be detected in serum and/or urine by immunofixation and by circulating FLC quantitation. A diagnosis of ATTR was made after evidence of documentation of Congo-red staining and apple-green birefringence under cross-polarized light in at least one involved organ followed by TTR deposits typing by immunohistochemistry on optic or immunoelectron microscopy or proteomics analysis according to the time of diagnosis in a positive tissue biopsy; (b) non-invasive documentation of intense cardiac uptake (visual score 2 or 3) on bone tracer scintigraphy with 99mTc-HMDP in the absence of either an abnormal serum free light chain ratio or a monoclonal protein in the serum or urine by immunofixation [6]. Diagnosis of CA was confirmed by myocardial biopsy (Congo-red staining combined with polarized light) and/or by the presence of CMR morphologic and post-contrast criteria of CA [7] or cardiac 99mTc-HMDP retention. The study was approved by the institutional ethical committee, and all subjects gave their written informed consent.
All patients underwent a thorough clinical, biohumoral, electrocardiographic and echocardiographic evaluation at enrollment. Presence and the severity of dyspnea was classified by New York Heart Association (NYHA) functional class. Biomarkers included serum creatinine, troponine I and plasma NT-proBNP assay. Patients with contraindications to CMR and those with an estimated glomerular filtration rate (eGFR) < 30 ml/min, by Cockroft Gault formula were excluded from the study.
2.1. CMR protocol
CMR was performed with a dedicated 1.5-T (Signa Artist, General Electrics Healthcare, Milwaukee, Wisconsin) with a 16-channel cardiac phased array coil. Short-axis cine images were acquired orthogonal to main LV axis covering both LV and LA, using a steady-state free precessing FIESTA (fast imaging employing steady-state acquisition) pulse sequence with the following parameters: 30 phases, slice thickness 8 mm, no gap, 8 views per segment, number of excitation 1, field of view 40 cm, phase field of view 1, 224 × 224 matrix, voxel dimensions 1.78 × 1.78 × 8 mm, reconstruction matrix 256 × 256, 45° flip angle, repetition time/echo time equal to 3.5/1.5, and a bandwidth of 125 KHz. After cine imaging, 0.2 mmol/kg Gadoteridol (Prohance, Bracco Imaging) were administered and late gadolinium enhancement (LGE) images acquired approximately 10 after the administration of the contrast medium in a full set of short-axis views in the same plane of cine images. The following parameters were used: field of view 35–40 cm, slice thickness 8 mm, no gap between each slice, repetition time 4.6 ms, echo time 1.3, 20° flip angle, matrix 224 × 224, reconstruction matrix 256 × 256, number of excitations 1. A modified Look-Locker Imaging (MOLLI) pulse sequence with 3(3)3(3)5 pattern was used to evaluate native T1 in 3 (basal, middle and apical) short axis slices.
2.2. Image analysis and definition of atrial emptying fraction (AEF)
Analysis of CMR images was performed, using a commercially available research software package (CMR42, Circle). Endocardial and epicardial contours of LV and RV myocardium were traced in the end-diastolic and end-systolic phases. End-diastolic and end-systolic volume indexes, biventricular mass and mass index were measured as previously described [8]. The presence of typical LGE pattern for CA was evaluated by three expert level-III EACVI accredited observers, blinded to each other [9].
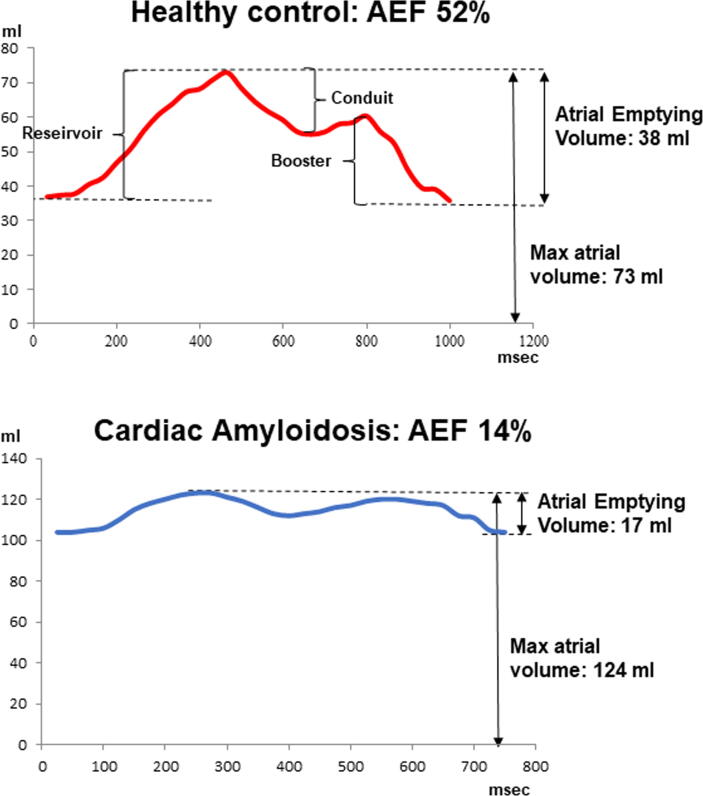
Parameters of LA function were evaluated as previously reported [8]. Briefly, LA endocardial contours were automatically traced in every cine frame to obtain volumetric data of all cardiac phases. Manual correction was performed when needed. Volumetric data were plotted against time (in ms) generating a LA volume over time (V/t) curve (Fig. 1). Through the analysis of the atrial V/t curves, atrial emptying volume was measured as the difference between the maximal and minimal atrial volumes. The atrial emptying fraction (AEF) was then measured as the ratio between atrial emptying volume and maximal atrial volume, expressed in %. The atrial reservoir, conduit and booster volumes were measured in ml. As reported in the previously mentioned study [8] in healthy controls AEF was > 36% and this was considered the cut-off for preserved LA function. Using this threshold, patients were divided in those with preserved and those with impaired LA function (AEF ≤ 36%). Patients with impaired LA function, were further divided in 3 groups based on tertiles of AEF found in our cohort: severe (AEF ≤ 14%), moderate (>14% to ≤20%), and mild dysfunction (>20%−≤36%).
Fig. 1.
Left atrial Volume\Time curves: Examples of left atrial volume\time curve in a healthy control, upper panel and in a patient with cardiac amyloidosis.
2.3. Clinical follow-up and study end-point
All patients were followed-up after CMR. A clinical questionnaire was compiled by an expert physician during periodic ambulatory visitations in each hospital, by contacting their relatives by telephone, by a general practitioner, or by consulting the office of vital statistics at the patient’s place of residence. The end-point of the study was death due to cardiac causes (heart failure or sudden cardiac death).
2.4. Statistical analysis
Values are presented as the mean ± SD or as the median and interquartile range (IQR) for variables with normal and non-normal distributions, respectively. Values with non-normal distribution according to the Kolmogorov-Smirnov test were logarithmically transformed for parametric analysis. Qualitative data are expressed as percentages. Categorical variables by the Chi-square or by the Fisher exact test. Continuous variables were compared by the ANOVA with Bonferroni correction when needed, or with Wilcoxon non-parametric test as appropriate. ROC curve analysis was used to found the more accurate threshold of AEF for predicting cardiac death. The Kaplan-Meier time-to-event method was employed to calculate and compare longitudinal curves between groups. Univariate and Multivariate COX regression analysis (stepwise) was used to explore the impact of each significant variable to predict the occurrence of cardiac death). The risk of multicollinearity among the covariates was evaluated by the variance inflation factor (VIF). VIF values were <10 for all the variable included in the multivariable models, indicating low risk of multicollinearity. A p-value < 0.05 was considered statistically significant.
3. Results
A total of 80 patients with mean age 70 ± 12 years, 56 males (69%) were enrolled in the study, including 57 patients (71%) in SR and 23 (29%) in AF. Of these 80 patients, 19 had severe LA dysfunction (AEF ≤ 14%), 21 moderate (AEF > 14% to ≤20%), and 20 mild dysfunction (AEF > 20%−≤36%); the remaining 20 patients had normal AEF values (Table 1). Notably, AEF ≤ 14% was found in 12 of the 57 (21%) patients in sinus rhythm. Patients with severe LA dysfunction (AEF ≤ 14%) had higher NT-pro-BNP values, higher wall thickness and atrial volume than those with preserved LA function. An inverse relation was found between AEF and NT-pro-BNP (R2 0.24, p = 0.015). Patients with aTTR amyloidosis were older (64 ± 12 vs 76 ± 10 years, p < 0.001), had higher wall thickness (14 ± 4 vs 17 ± 5 mm, p = 0.007), higher LV mass index (88 ± 27 vs 121 ± 38 g/m2, p < 0.001) and greater atrial maximal volume (120 ± 33 vs 140 ± 42 ml/m2, p < 0.02) than those with AL. However, AEF was not significantly different between AL and aTTR.
Table 1.
Prevalence and clinical correlates of left atrial (LA) systolic dysfunction in 80 patients with cardiac amyloidosis.
| Overall population | Severe LA dysfunction | Moderate LA dysfunction | Mild LA dysfunction | Preserved LA function | p value | |
|---|---|---|---|---|---|---|
| AEF | ≤ 14% | 15–≤20% | 20–≥ 36% | > 36% | ||
| n. | 80 | 19 | 21 | 20 | 20 | |
| AL/ATTR | 38/42 | 10 (53%)/9 (47%) | 6 (29%)/15 (71%) | 9 (45%)/11 (55%) | 13 (65%)/7 (35%) | 0.10. |
| Age (y) | 70 ± 12 | 71 ±12 | 73 ±10 | 73 ±8 | 63 ±15 | <0.05 |
| Males n (%) | 56 (69%) | 14 (70%) | 17 (81%) | 16 (80%) | 9 (45%) | 0.051 |
| Height (cm) | 169 ± 8 | 169 ±8 | 170 ±6 | 171 ±6 | 167 ±10 | 0.35 |
| Weight (Kg) | 73 ± 12 | 74 ±11 | 74 ±12 | 72 ±14 | 70 ±13 | 0.74 |
| BSA (m2) | 1.83 ± 0.18 | 1.85 ±0.15 | 1.86 ±0.16 | 1.84 ±0.2 | 179 ±0.19 | 0.64 |
| NYHA I | 14 | 0 | 4 (20%) | 5 (28%) | 5 (25%) | 0.07 |
| NYHA II | 37 | 4 (20%) | 9 (43%) | 9 (43%) | 15 (75%) | <0.05 |
| NYHA ≥III | 29 | 15 (80%) | 8 (37%) | 6 (29%) | 0 | <0.05 |
| NT-proBNP (pg/ml) | 1984 (859–8185) | 9531(2022–11251) 4 | 1901(920–4775) | 2207(9585–9659) | 624(521–856) 1 | <0.05 |
| Troponine I hs (pg/dl) | 66 (47–101) | 100(95–106) | 50(33–66) | 51(34–65) | 51 (34–51) | 0.11 |
| Atrial fibrillation | 23(28%) | 8(42%) | 9(43%) | 4(20%) | 2(10%) | 0.048 |
| IVS (mm) | 16 ± 5 | 19 ±54 | 17 ±44 | 16 ±44 | 11 ±31, 2, 3 | <0.001 |
| PW (mm) | 12 ± 4 | 15 ±34 | 13 ±34 | 12 ±34 | 8 ±21, 2, 3 | <0.001 |
| LVEF (%) | 58 ± 11 | 56 ±11 | 53 ±84 | 58 ±13 | 65 ±102 | <0.05 |
| LVEDVi (ml/m2) | 75 (63–91) | 81 ±38 | 87 ±27 | 78 ±20 | 81 ±29 | 0.79 |
| LV SV (ml/m2) | 31 (23–40) | 82 ±25 | 88 ±29 | 81 ±17 | 91 ±19 | 0.52 |
| Mass index (g/m2) | 105 ± 41 | 119 ±414 | 119 ±324 | 109 ±474 | 72 ±241, 2, 3 | <0.001 |
| LGE specific for CA diagnosis n(%) | 53 (65%) | 12 (65%) | 18 (86%) | 13 (65%) | 13 (65%) | 0.06 |
| WMSI | 1 (1–1.35) | 1.34 ±0.4 | 1.30 ±0.4 | 1.18 ±0.4 | 1.10 ±0.3 | 0.15 |
| Maximal Atrial volume (ml) | 122 (95–160) | 162 ±514 | 151 ±474 | 128 ±424 | 83 ±221, 2, 3 | <0.01 |
| Minimal Atrial Volume (ml) | 101 (63–144) | 149 ±473, 4 | 128 ±403, 4 | 95 ±331, 2, 4 | 45 ±151, 2, 3 | <0.01 |
AEF, atrial emptying fraction; AL, light chain amyloidosis; ATTR, transthyretin amyloidosis; NYHA, New York Heart Association; BSA, body surface area; IVS, interventricular septum; PW, posterior wall; LVEF, left ventricle ejection fraction; LV EDVi, left ventricle end diastolic volume index; LV SV, left ventricle stroke volume; LGE, late gadolinium enhancement; WMSI, wall motion score index.
significant p value vs severe LA dysfunction.
significant p value vs Moderate LA dysfunction.
significant p value vs Mild LA dysfunction.
significant p value vs Preserved LA function.
3.1. Clinical Follow-up
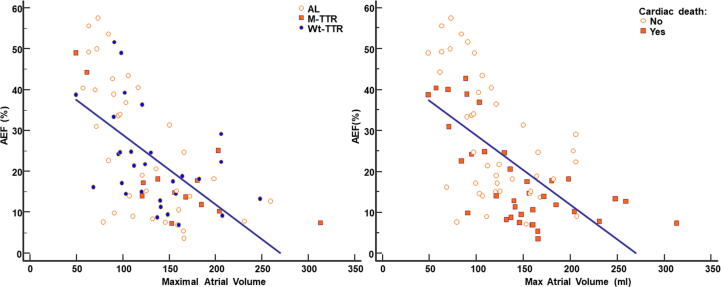
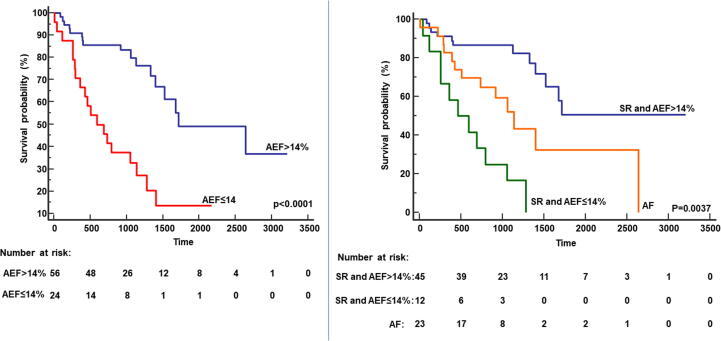
During a median follow up of 3 (2–5) years, 36 patients died (44%) of cardiac causes. At baseline, patients with cardiac death, compared to survivors, had higher TnI-HS values 95 vs 52 pg/dl); p < 0.05), lower RVEF (54%vs 60%; p < 0.05), higher LA maximal (146 vs 120 ml; p < 0.05) and minimal volume (124 vs 90 ml; p < 0.005), lower LA reservoir (18 vs 28 ml; p < 0.005) and booster volumes (12 vs 18 ml; p < 0.005) and lower AEF (14 vs24; p < 0.001). At ROC curve analysis, AEF predicted cardiac death with an AUC of 0.72 (95% CI 0.60–0.82; p = 0.0002). The best AEF threshold for cardiac death prediction was ≤14% (sensitivity 60%, specificity 86%). The relation between AEF and the maximal atrial volume is showed in Fig. 2. Kaplan Meier survival analysis identified impaired survival in patients with AEF ≤ 14% compared to patients with AEF > 14% (Log rank p < 0.0001) as depicted in Fig. 3 (left panel). At univariate Cox regression analysis, NYHA class, NT-proBNP, troponin I, RVEF, maximum atrial volume, AEF and AEF ≤ 14% were associated with cardiac death (Table 2). Multivariable Cox regression analysis was performed in two different models, the first including AEF as continuous variable, the second with AEF ≤ 14% as dichotomous parameter. In the first model NYHA class and AEF were independent predictors of cardiac death, in the second model NYHA class and AEF ≤ 14% were independent predictors.
Fig. 2.
Relation between the atrial emptying fraction (AEF) and the maximal atrial volume: A significant inverse relation was found between AEF and the maximal atrial volume (r = −0.61, p < 0.0001), but some patients had low AEF with only mild atrial dilation. In left panels patients were divided by the type of amyloidosis (AL, mutated-aTTR, wild type-aTTR). In right panel, patients were divided by cardiac death or not.
Fig. 3.
Prognostic differences between patients with and without atrial dysfunction. In left panel, Kaplan Meier curve demonstrating that patient with impaired left atrial function with atrial emptying fraction (AEF) ≤ 14% had worse prognosis than those with better function. In right panel, Kaplan Meier curve demonstrating that patient with atrial fibrillation had worse prognosis than those with sinus rhythm and atrial emptying fraction (AEF) > 14%, but the worst prognosis is showed in patient with sinus rhythm and AEF ≤ 14%.
Table 2.
Cox Logistic regression analysis for the risk of hard cardiac events.
|
Univariate |
|||
|---|---|---|---|
| HR | 95% CI | p value | |
| Age | 1.8 | 0.8–7.4 | 0.08 |
| NYHA Class (≥III) | 2.3 | 1.4–3.8 | 0.001 |
| HS Troponine I | 1.05 | 1.2–3.02 | 0.017 |
| NT-proBNP | 1.4 | 1.2–4.5 | 0.004 |
| AL/TTR | 1.18 | 0.6–2.3 | 0.6 |
| LV EF | 1.74 | 0.87–3.5 | 0.12 |
| LV EDVi | 1.5 | 0.61–3.9 | 0.36 |
| LV mass index | 1.9 | 0.98–3.8 | 0.06 |
| RVEF (%) | 0.97 | 0.95–0.99 | 0.013 |
| RV EDVi | 1.16 | 0.46–2.9 | 0.73 |
| LA maximal Volume | 1.01 | 1–1.01 | 0.0008 |
| AEF | 0.94 | 0.91–0.97 | 0.0009 |
| AEF ≤ 14% | 4.1 | 2.08–8.1 | <0.0001 |
| Model with AEF (continuous) |
Multivariate |
||
| HR | 95% CI | p value | |
| NYHA Class (≥III) | 2.4 | 1.2–4.7 | 0.012 |
| NT-proBNP | |||
| RVEF (%) | |||
| LA maximal Volume | |||
| AEF | 0.9 | 0.9–0.98 | 0.005 |
| Model with AEF ≤ 14% (dichotomic) |
Multivariate |
||
| HR | 95% CI | p value | |
| NYHA Class (≥III) | 2.9 | 1.5–5.7 | 0.002 |
| NT-proBNP | |||
| RVEF (%) | |||
| LA maximal Volume | |||
| AEF ≤ 14% | 4.0 | 2.0–7.9 | <0.0001 |
LV, left ventricle; RV, right ventricle; EDVi, end-diastolic volume index; EF, ejection fraction; LA, left atrium; AEF, atrial ejection fraction, HS, High-sensitivity.
Only parameters with p value <0.05 at univariate were included in multivariate analysis.
3.2. Sinus rhythm vs atrial fibrillation
Among patients in SR, Kaplan Meier survival analysis showed that the presence of AEF ≤ 14% was associated to worse prognosis than those with AEF > 14% (Log rank < 0.0001, Fig. 3, right panel). In contrast, among patients in AF, no significant difference in survival was found between AEF > 14% and AEF ≤ 14%. Interestingly, patients in SR and AEF ≤ 14% had higher risk of cardiac death than those with AF: 1- year risk of cardiac death, respectively 42% (95% CI 40–43) vs 17% (95% CI 16–18), p < 0.0001; 3-year risk, respectively 75% (95% CI 72–78) vs 42% (95%CI 40–43), p < 0.0001.
4. Discussion
The main findings of the present study can be summarized as follows: a) left atrial dysfunction is common in CA patients, and can be severe even in the presence of stable SR; b) when severe, i.e. AEF ≤ 14%, atrial dysfunction is associated with a > 4-fold increase in cardiac mortality, representing a powerful independent predictor of risk; c) the prognosis of CA patients in SR with AEF ≤ 14% is worse than that of patients in AF. Severe LA dysfunction, defined as AEF ≤ 14% was a strong independent predictor of cardiac death. Patients with AEF ≤ 14% had a 32% risk at 1 year and a 61% risk at 3 years. The identification of AEF by CMR is a simple, accurate and reproducible parameter that can facilitate risk stratification and prognosis assessment in patients with AL and ATTR CA. Furthermore, phasic measurement of LA function using feature-tracking CMR add important information regarding LA fibrosis and provide additional insight for procedural outcomes and stroke risk stratification [23]. In HF patients, Pellicori et al [21] found that left atrial function measured by CMR was an important marker of cardiac dysfunction and cardiovascular outcome. In that study, AEF was associated with increasing plasma concentrations of NT-proBNP and a higher incidence of AF and was a predictor of adverse outcome independently of other parameters of cardiac dysfunction. Mohty et al [22] observed an association of AEF with severity of cardiac involvement, functional status and presence of LGE in patients with AL CA.
LA is a “victim” of CA for a number of reasons. First, the deposition of amyloid proteins causes an increase in LV stiffness and diastolic dysfunction that determines LA dilatation, and ultimately precipitates AF and electromechanical dysfunction. Second, amyloid directly affects the LA wall reducing atrial contractility and contributing to the impairment of LV filling. Henein et al. analyzed LA strain by 2D echocardiography in 46 patients with CA [16]. They found that, despite normal chamber size, LA myocardial deformation function was depressed, suggesting that infiltration with amyloid fibrils could limit LA distension and primarily affecting the atrial booster phase.
Notably, although severe atrial dysfunction was more prevalent (48%) among CA patients in AF, it was far from rare among in sinus rhythm (involving one in five or 21%). While patients in SR and preserved atrial function expectedly showed more favorable prognosis than those in AF, patients with SR and severe atrial dysfunction had the worst outcome of all. Although the small sample size warrants caution in its interpretation, this finding suggests that LA dysfunction per se carries sufficient hemodynamic burden to cause clinical deterioration without the mediation of AF; this may also reflect the fact that loss of atrial contribution in these stages of CA is irrelevant and does not carry adverse consequences when lost to AF: The other explanation is that severe atrial dysfunction in SR might be a marker of severe atrial amyloid deposition inducing atrial standstill with preserved electrical conduction, in a sort of regional electromechanical dissociation reflecting particularly severe cardiac involvement. This observation deserves further investigation as it largely counters what is seen in other forms of heart failure and cardiomyopathies including HCM. Finally, severe atrial dysfunction in the presence of SR has important management implications, including a potentially high cardioembolic risk even in the absence of AF. The fact that we did not observe stroke or other embolic complications does not exclude this possibility, as shown in previous studies on CA patients in sinus rhythm [18], [19], [20].
Some study limitations should be mentioned. First, the study population patients was relatively small (n = 80) and allowed limited power for subanalyses (including different CA etiologies). Second, we did not employ dedicated software for atrial function assessment such as, feature tracking. The choice to evaluate atrial function using only atrial volume was aimed at obtaining an easily reproducible parameter available with every clinical CMR scanner without the need for complex and expensive post-processing software.
5. Conclusions
Severe impairment of LA contractile function was present in three-quarters of patients with CA, and was prevalent irrespective of CA etiology, both in the presence and absence of AF. Severe LA dysfunction was associated with an independent 4-fold increase in risk for cardiac death at three years. AEF is easily obtainable and should be considered routinely in CA patients undergoing CMR.
CRediT authorship contribution statement
Giovanni Donato Aquaro: Conceptualization, Methodology, Formal analysis, Writing - original draft. Sofia Morini: Investigation. Chrysanthos Grigoratos: Investigation. Giulia Taborchi: Investigation. Gianluca Di Bella: Conceptualization. Raffaele Martone: Investigation. Elisa Vignini: Investigation. Michele Emdin: Investigation. Iacopo Olivotto: Writing - review & editing. Federico Perfetto: Writing - review & editing. Francesco Cappelli: Supervision, Writing - review & editing.
Acknowledgements
The authors report no relationships that could be construed as a conflict of interest
References
- 1.Falk R.H., Dubrey S.W. Amyloid heart disease. Prog. Cardiovasc. Dis. 2010;52:347–361. doi: 10.1016/j.pcad.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Banypersad S.M., Moon J.C., Whelan C., Hawkins P.N., Wechalekar A.D. Updates in cardiac amyloidosis: a review. J. Am. Heart Assoc. 2012;1 doi: 10.1161/JAHA.111.000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Bella G., Pizzino F., Minutoli F., Zito C., Donato R., Dattilo G., Oreto G., Baldari S., Vita G., Khandheria B.K., Carerj S. The mosaic of the cardiac amyloidosis diagnosis: role of imaging in subtypes and stages of the disease. Eur. Heart J. Cardiovasc. Imaging. 2014;15:1307–1315. doi: 10.1093/ehjci/jeu158. [DOI] [PubMed] [Google Scholar]
- 4.Nochioka K., Quarta C.C., Claggett B., Roca G.Q., Rapezzi C., Falk R.H., Solomon S.D. Left atrial structure and function in cardiac amyloidosis. Eur. Heart J. Cardiovasc. Imaging. 2017;18:1128–1137. doi: 10.1093/ehjci/jex097. [DOI] [PubMed] [Google Scholar]
- 5.Maron B.J., Haas T.S., Maron M.S., Lesser J.R., Browning J.A., Chan R.H., Olivotto I., Garberich R.F., Schwartz R.S. Left atrial remodeling in hypertrophic cardiomyopathy and susceptibility markers for atrial fibrillation identified by cardiovascular magnetic resonance. Am. J. Cardiol. 2014;113(8):1394–1400. doi: 10.1016/j.amjcard.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 6.Gillmore J.D., Maurer M.S., Falk R.H. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016 Jun 14;133(24):2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 7.Aquaro G.D., Pugliese N.R., Perfetto F., Cappelli F., Barison A., Masci P.G., Passino C., Emdin M. Myocardial signal intensity decay after gadolinium injection: a fast and effective method for the diagnosis of cardiac amyloidosis. Int. J. Cardiovasc. Imaging. 2014;30:1105–1115. doi: 10.1007/s10554-014-0436-6. [DOI] [PubMed] [Google Scholar]
- 8.Aquaro G.D., Camastra G., Monti L., Lombardi M., Pepe A., Castelletti S., Maestrini V., Todiere G., Masci P., di Giovine G., Barison A., Dellegrottaglie S., Perazzolo Marra M., Pontone G., Di Bella G. Reference values of cardiac volumes, dimensions, and new functional parameters by MR: A multicenter, multivendor study. J. Magn. Reson. Imaging. 2017 Apr;45(4):1055–1067. doi: 10.1002/jmri.25450. [DOI] [PubMed] [Google Scholar]
- 9.Quarta G., Aquaro G.D., Pedrotti P., Pontone G., Dellegrottaglie S., Iacovoni A., Brambilla P., Pradella S., Todiere G., Rigo F., Bucciarelli-Ducci C., Limongelli G., Roghi A., Olivotto I. Cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy: the importance of clinical context. Eur. Heart J. Cardiovasc. Imaging. 2018 Jun 1;19(6):601–610. doi: 10.1093/ehjci/jex323. [DOI] [PubMed] [Google Scholar]
- 16.Hausfater P., Costedoat-Chalumeau N., Amoura Z., Cacoub P., Papo T., Grateau G., Leblond V., Godeau P., Piette J.C. AL cardiac amyloidosis and arterial thromboembolic events. Scand. J. Rheumatol. 2005;34:315–319. doi: 10.1080/03009740510015203. [DOI] [PubMed] [Google Scholar]
- 18.Dubrey S., Pollak A., Skinner M., Falk R.H. Atrial thrombi occurring during sinus rhythm in cardiac amyloidosis: evidence for atrial electromechanical dissociation. Br. Heart J. 1995;74:541–544. doi: 10.1136/hrt.74.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henein M.Y., Suhr O.B., Arvidsson S., Pilebro B., Westermark P., Hörnsten R., Lindqvist P. Reduced left atrial myocardial deformation irrespective of cavity size: a potential cause for atrial arrhythmia in hereditary transthyretin amyloidosis. Amyloid. 2018 Mar;25(1):46–53. doi: 10.1080/13506129.2018.1430027. [DOI] [PubMed] [Google Scholar]
- 20.Mints Yuliya Y., Doros Gheorghe, Berk John L., Connors Lawreen H., Ruberg Frederick L. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail. 2018 Oct;5(5):772–779. doi: 10.1002/ehf2.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellicori P., Zhang J., Lukaschuk E., Joseph A.C., Bourantas C.V., Loh H., Bragadeesh T., Clark A.L., Cleland J.G. Left atrial function measured by cardiac magnetic resonance imaging in patients with heart failure: clinical associations and prognostic value. Eur. Heart J. 2015 Mar 21;36(12):733–742. doi: 10.1093/eurheartj/ehu405. [DOI] [PubMed] [Google Scholar]
- 22.Mohty D., Petitalot V., Magne J., Fadel B.M., Boulogne C., Rouabhia D., ElHamel C., Lavergne D., Damy T., Aboyans V., Jaccard A. Left atrial function in patients with light chain amyloidosis: A transthoracic 3D speckle tracking imaging study. J. Cardiol. 2018;71:419–427. doi: 10.1016/j.jjcc.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Habibi M., Lima J.A., Khurram I.M., Zimmerman S.L., Zipunnikov V., Fukumoto K., Spragg D., Ashikaga H., Rickard J., Marine J.E., Calkins H., Nazarian S. Association of left atrial function and left atrial enhancement in patients with atrial fibrillation: cardiac magnetic resonance study. Circ. Cardiovasc. Imaging. 2015 Feb;8(2) doi: 10.1161/CIRCIMAGING.114.002769. [DOI] [PMC free article] [PubMed] [Google Scholar]