Abstract
Leishmaniasis is the most widespread pathogenic disease in several countries. Currently, no effective vaccines are available, and the control of Leishmaniasis primarily relies on decade-old chemotherapy. The treatment for the Leishmaniasis is not up to the mark. Current therapy for Leishmaniasis is ancient and requires hospitalization for the administration. These medications are also highly toxic and resistant. β-carboline, a natural indole containing alkaloid, holds a vital position in the field of medicinal chemistry with a diversified pharmacological action. The current review focuses mainly on the anti-leishmanial effects of β-carboline analogs and their synthetic strategies, structural activity relationship studies (SAR). The past ten years alterations unveiled by β-carboline analogs present in phytoconstituents and various derivatives of synthesized analogs with the mechanism of action were briefly shortlisted and illustrated.
Keywords: Leishmaniasis, β-carboline, Synthesis, Amastigotes, Promastigotes, Phytoconstituents, Structure-activity relationship studies, Materials science, Chemistry, Natural product chemistry, Pharmaceutical chemistry, Biological sciences, Phytochemistry, Pharmaceutical science, Health sciences, Pharmacology
Leishmaniasis; β-carboline; Synthesis; Amastigotes; Promastigotes; Phytoconstituents; Structure-activity relationship studies; Materials science; Chemistry; Natural product chemistry; Pharmaceutical chemistry; Biological sciences; Phytochemistry; Pharmaceutical science; Health sciences; Pharmacology
1. Introduction
Leishmaniasis is a neglected tropical disease (NTD), endemic in 98 countries. Leishmaniasis, caused by flagellated protozoans of the genus Leishmania, is a vector-borne disease. The Leishmania parasite is transmitted by the bite of infected female phlebotomine sandflies. Around 98 species of the genera, Phlebotomus and Lutzomyia have been described as established or alleged vectors for human Leishmaniasis. Currently, there are almost 18 Leishmania species reported as pathogenic to humans (Steverding, 2017).
At present, approximately 12 million people are affected with Leishmaniasis; >350 million people are at risk of contracting the infection, and over one million new cases are reported each year. Based on the clinical manifestations exhibited in infected individuals, Leishmaniasis can be characterized into various forms like Visceral Leishmaniasis (VL) (a most deadly form of the disease, also known as Kala-azar and black fever), Cutaneous Leishmaniasis (CL) (a most common type of the disease), Mucocutaneous Leishmaniasis (ML), and Post kala-Azar dermal leishmaniasis (PKDL). VL is ranked second in mortality and fourth in morbidity among Neglected Tropical Diseases (NTDs) with 50000–90000 deaths annually and 600 million people at risk of across the globe, and 600,000–1.2 million new cases of CL in each year. In the absence of vaccines, chemotherapy and vector control are the tools for disease management (Balaña-Fouce et al., 2019), (Mark and Neglect, 2019), (DNDi, 2018) (Report, 2019). VL is characterized by weight loss, irregular bouts of fever, enlargement of spleen and liver as well as anemic condition. According to the World Health Organization report 2018 (WHO), more than 95% of cases occurred in 10 countries - China, Brazil, Ethiopia, India, Kenya, Iraq, Nepal, Somalia, South Sudan, and Sudan. VL is described as the most severe form of the disease and diagnosed by parasitological or serological tests. In CL ulcers on exposed parts of the body and skin lesions, leaving life-long scars on the body parts and severe disability of stigma, are the main features of the CL. Over 85% of the new CL cases occurred in the ten countries -Afghanistan, Algeria, Bolivia, Brazil, Colombia, Iran (the Islamic Republic of), Iraq, Pakistan, the Syrian Arab Republic and Tunisia (Al-Salem et al., 2019), (Aronson et al., 2016). ML leads to the total or partial destruction of the mucous membrane of the throat, nose, and mouth. Around 90% of cases occur in Bolivia, Brazil, Ethiopia, and Peru. In the case of both CL and ML, serological tests have limited value, and clinical manifestation with serological tests confirms the diagnosis (Zulfiqar et al., 2017). Currently, seven active clinical trials are ongoing in 20 countries, which was monitored by the Drugs for Neglected disease initiative (DNDi). DNDI-6148 (France), DNDI-0690 (UK) are ongoing in Phase-I, and GSK3186899/DDD853651 single ascending dose – safety, tolerability and pharmacokinetics was recently completed Phase-I clinical trials by United Kingdom. Thermotherapy with miltefosine combination (Colombia, Peru) was succeeded in Phase-II for cutaneous Leishmaniasis in 2019. The combination of Miltefosine in combination with paromomycin for treatment of primary VL patients in Eastern Africa (Ethiopia, Kenya, Sudan, Uganda) is currently ongoing in Phase-III and Thermotherapy, and miltefosine combination (Brazil, Panama, Peru, Bolivia) started the Phase-III clinical study (Report, 2019).
2. The lifecycle of Leishmaniasis
Leishmaniasis life cycle occurs in two stages-the human stage and the sandfly stage. The parasite exists in two forms-the amastigote form (intracellular form in the host) and the promastigote form (extracellular form in the sand fly). When the infected sand fly takes a blood meal from a healthy patient, it releases the promastigotes which are phagocytized by the macrophage cells. Then inside the macrophages, the promastigotes get converted into amastigotes and start multiplying during which the symptoms start becoming very prominent in the host, which completes the life cycle in humans. When the sand fly takes a blood meal from the person suffering from the disease, it ingests the macrophages infected with the amastigotes. The amastigotes are then converted to promastigotes in the gut of the sand fly and rapidly multiply and reach the proboscis of the sandfly completing the life cycle in the sand fly, which are then eventually injected into humans (Sunter and Gull, 2017), (Burza et al., 2018), (Zulfiqar et al., 2017).
3. Existing therapy for Leishmaniasis
Currently, no vaccine is available for the prevention or treatment of the disease. Hence drugs are the only source of treating the disease. The class of drugs that are currently used for treatment include pentavalent antimonials, Amphotericin B, Miltefosine, paromomycin, and pentamidine. Pentavalent antimonials-sodium stibogluconate and meglumine antimoniate are the first-line drugs for the treatment of leishmaniasis. Pentavalent antimonial was developed in 1945 and remained the first choice of treatment for both visceral and cutaneous Leishmaniasis in most parts of the world (Haldar et al., 2011). The pentavalent antimoniate is converted into the active form trivalent antimonite either in the macrophages or inside the parasite. The exact mechanism of action of the antimonial is still not elucidated. Amphotericin B is a polyene antifungal drug widely used to treat systemic fungal infections. Amphotericin B and pentamidine are the second line antileishmanial drugs, although they require prolonged courses of parenteral administration. Though toxicity and emerging resistance prevent the use of pentamidine, Amphotericin B has the potential to induce acute toxicity and hospitalization (Rajasekaran and Chen, 2015). Miltefosine, an alkyl phospholipid, primarily developed as an anticancer agent, is the first oral drug to be used for the treatment of leishmaniasis, which was considered a significant breakthrough in antileishmanial chemotherapy (Pinto-Martinez et al., 2018). The discovery that Miltefosine is effective against Leishmaniasis led to the identification of a new group of antiprotozoal medicines. Following clinical studies, Miltefosine was approved as ImpavidoTM and had become the first oral treatment for Leishmaniasis in some countries. Various mechanisms of actions have been proposed for miltefosine. Miltefosine was found to decrease the lipid content in membranes of promastigotes and also partial inhibition of phosphatidylethanolamine-N-methyltransferase that ultimately affects the parasite proliferation. It is useful in treating visceral and cutaneous Leishmaniasis, including antimony-resistant infections. However, this drug may not necessarily be superior to parenteral therapies for all forms of Leishmaniasis. The only clinically significant aminoglycoside with antileishmanial activity is Paramomycin (Jhingran et al., 2009), (Sundar and Chakravarty, 2013). Both visceral and cutaneous types of Leishmaniasis, can be treated with this antibiotic. However, poor and oral absorption has contributed to the emergence of parentral and topical forms of Paramomycin for the viscetal and cutaneous forms of leishmaniasis respectively. Pentamidine is an aromatic diamine currently used as the second line of the drug in antileishmanial chemotherapy. It is mainly used for the treatment of visceral leishmaniasis wherein the isothionate and methanesulphonate salts of the drug are commonly used. Its precise mechanism of action is still unexplored. However, reports suggest that it accumulates in mitochondria, where it inhibits the enzyme topoisomerase II after entering the promastigotes via polyamine and arginine transporters.
The choice of treatment also depends on the causative Leishmania species. The structures of clinically used drugs are discussed below (Figure 1). Apart from the clinically used drugs, there are many compounds currently in clinical trials. Drugs for Neglected Disease initiative (DNDi) was started with the aim of oral and safe medication for the treatment for Leishmaniasis (Maltezou, 2010a). The list of medications for the treatment of Leishmaniasis and their important roles are outlined below.
-
a.Sodium stibogluconate (Pentosam) and meglumine antimoniate:
-
✓First-line drug, available in the form of injection
-
✓Kills leishmania species by DNA fragmentation-thiol reducing reactivity
-
✓The incidence of resistance is growing, and the drug is losing activation due to widespread misuse (Maltezou, 2010b)
-
✓Pain and thrombosis on intravenous administration, intramuscular injection also painful
-
✓
-
b.Amphotericin B (AmB):
-
✓Liposomal Amphotericin B, 95% efficacy
-
✓High affinity for ergosterol, the predominant sterol of the fungal and leishmanial cell membrane
-
✓Formation of aqueous pores in the leishmanial promastigotes cell membrane that result in an osmotic change leading to the cell lysis.
-
✓Indicated for the visceral leishmaniasis.
-
✓High cost, damaging effect on kidneys (Croft and Coombs, 2003)
-
✓
-
c.Miltefosine:
-
✓A first oral drug used for the treatment of VL oral administration
-
✓Causes apoptosis in L. donovani decreased parasite proliferation effect in mitochondria as a primary target
-
✓Long terminal residence time and, contraindicated in the period of pregnancy, teratogenicity (Paris et al., 2004)
-
✓
-
d.Paromomycin:
-
✓Cures VL and CL new, simple, easily applicable
-
✓Effect on mitochondria as a primary target
-
✓Side effect like vomiting, dyspepsia, cyanosis, nephritic syndrome (Jhingran et al., 2009)
-
✓
-
e.Pentamidine:
-
✓Isothinate and methanesulphonate salts used for VL treatment
-
✓The drug enter inside L. donovani promastigotes through arginine and polyamine transport
-
✓Decline efficiency and high resistance. Toxic and causes hypoglycemia, hypotension, and nephrotoxicity (Sands et al., 1985)
-
✓
Figure 1.
Structure of clinically used anti-leishmanial drugs (1–6).
4. Introduction to β-carboline and allied structures
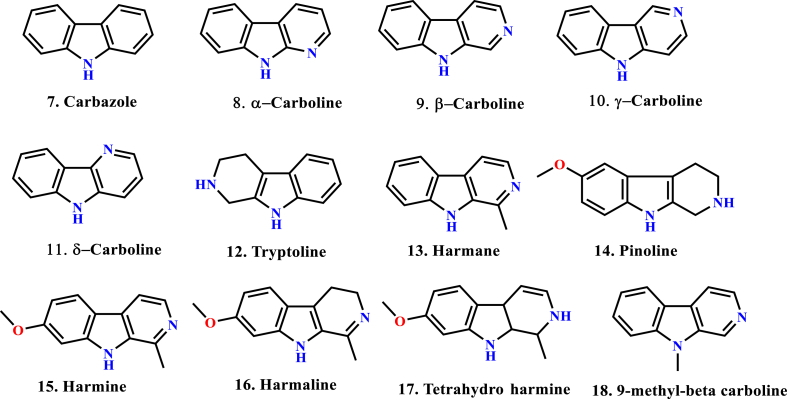
β-carboline (9H-pyrido[3,4-b]indole), also known as nor-harmane, is a nitrogen-containing heterocycle. β-carboline belongs to the group of indole alkaloids and contains a pyridine ring fused with an indole backbone (Cao et al., 2007). The structure of β-carboline is like tryptamine, with the ethylamine chain re-connected to the indole nucleus via an extra carbon atom to produce a three-ringed structure (Dewick, 2002). Since its initial discovery in the year 1841, β-carboline alkaloids have been isolated from various sources, principally plants (mainly in Rutaceae, Simaroubaceae, Amaranthaceae, Caryophyllaceae, Rubiaceaeand Zygophyllaceae), marine creatures (hydroids, bryozoans, soft corals) and marine sponges (França et al., 2014). They are also present in microorganisms, insects, food products (Piechowska et al., 2019), alcoholic beverages, tobacco smoke, as well as human tissues and body fluids. Also, β-carbolines display a wide range of unusual biological activities like anti-cancer (Samundeeswari et al., 2017), (Carvalho et al., 2017), antiviral (Brahmbhatt et al., 2010), antibacterial, antifungal (Nenaah, 2010), antileishmanial (Kam et al., 1999), antithrombotic, anti-inflammatory (Yao et al., 2011) properties, among others. They have also been reported to interact with enzymes and receptors like monoamine oxidase (Herraiz, 2007), topoisomerase I (Sobhani et al., 2002), topoisomerase II (Deveau et al., 2001), mitogen-activated protein kinase (MAPK) (Trujillo et al., 2007), cyclin-dependent kinase (CDK) (Song et al., 2004), benzodiazepine receptors (Guzman et al., 1984), 5-HT-1 receptors (Glennon et al., 1996), 5-HT-2 receptors (Audia et al., 1996) and imidazoline receptors (Glennon et al., 2004). Some important β-carboline structures are shown in Figure 2. In turn, a great deal of attention is received by the scientific community (academia and industry) to explore a chemically versatile moiety. To the best of our knowledge, nine β-carboline drugs have been commercialized to date (Dai et al., 2018) (Figure 3).
Figure 2.
Structural diversity of carbolines (7–18).
Figure 3.
Commercialized β-carboline drugs and their pharmacological activity (19–27).
5. Synthetic strategies
5.1. Tetrahydro-β-carboline (THBC) and β-carboline (β-C) ring system
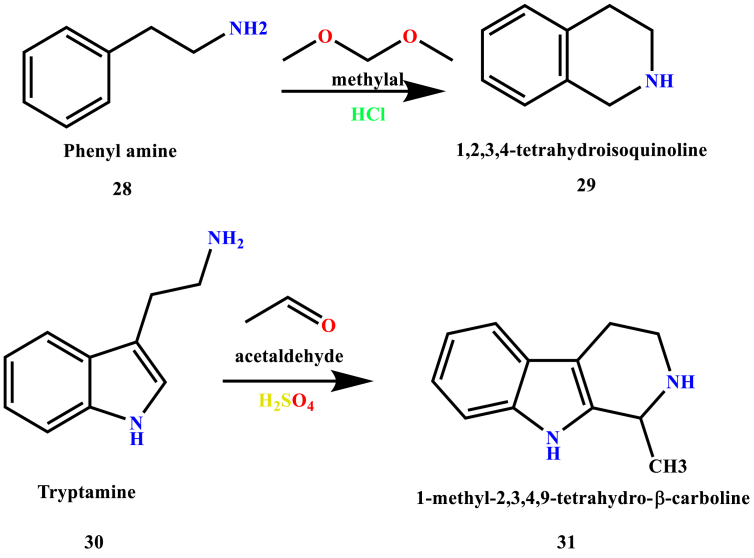
The three-cyclic tetrahydro-β-carboline (THBC) and β-carboline ring system have been considered as an essential structural element in medicinal chemistry, and it exposes a wide range of pharmacologically important alkaloids isolated from a diversity of natural sources (Cao et al., 2007). This Pictet-Spengler condensation was discovered in 1911 by Ame Pictet and Theodor Spengler when they condensed phenethylamine (28) with methylal to provide tetrahydroisoquinoline (29) (Cox and Cook, 1995). This reaction was wholly used for the synthesis of tetrahydroisoquinolines. The reaction involves two steps. In the first step, Schiff base (imine intermediate) was formed through dehydration of electron-rich β-arylamine with an aldehyde or ketone in the presence of an acid as a catalyst. In the second step, the imine undergoes 6-endo-trig cyclization which leads to the formation of THBC scaffold. However, in 1928 Tatsui synthesized and demonstrated the formation of 1-methyl-1,2,3,4-tetrahydro-β-carboline (31) from tryptamine (30) and acetaldehyde (Ascic et al., 2012), (Calcaterra et al., 2020) as shown in Scheme 1.
Scheme 1.
First reported Pictet-Spengler synthesis.
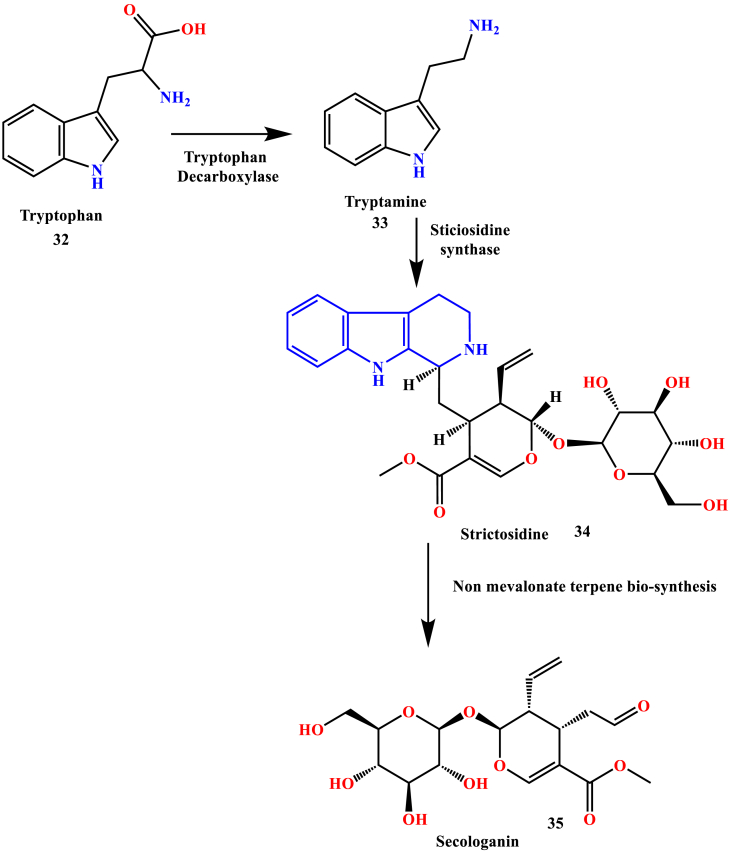
The enzyme-catalyzed Pictet-Spengler condensation of tryptamine (33) with strictosidine synthases lead to the formation of strictosidine (34). Secologanin (35) biosynthesis takes place after the strictosidine involving a lot of reaction steps, which include non-mevalonate terpene biosynthesis. This is the critical step in the biogenetic pathway of monoterpene indole alkaloids formation. The biosynthetic pathway of most of the indole alkaloids was derived from tryptophan (5) in the presence of tryptophan decarboxylase, a pyridoxal dependent enzyme (O'Connor and Maresh, 2006), (Battersby et al., 1968) depicted in Scheme 2.
Scheme 2.
Bio-synthetic pathway of indole alkaloids.
In recent years, the THBCs and β-carbolines were extensively studied, and still, many more studies are in progress. The synthetic tactics were altered, which resulted in good yields of THBCs, β-carboline, and its derivatives that were tested against various pharmacological activities like anti-cancer (Yao et al., 2019), anti-Alzheimer's (Horton et al., 2017), etc.
5.2. Microwave-assisted synthesis
Generally, the Pictet–Spengler reaction usually requires longer reaction times in the refluxing solvent. Microwave radiation significantly accelerates the reaction and yields well in a short period of time. Scott Eagon and Marc O. Anderson reported the microwave-assisted synthesis of THBC and β-carboline within 20 min. The microwave-assisted Pictet–Spengler reaction was carried out by using 1,2-dichloroethane (DCE) and trifluoroacetic acid (TFA) as solvents, and tryptamine as the starting material which resulted in the yield up to 99% (Scheme 3). After the initial THBC formation, the aromatization of resultant tetrahydro-β-carboline salts with Pd/C in EtOH under microwave radiation yielded β-carboline salts (Scheme 4) (Eagon and Anderson, 2014).
Scheme 3.
Microwave-assisted synthesis of THBC derivatives.
Scheme 4.
Microwave-assisted synthesis of β-carboline derivatives.
Wang et al. reported the synthesis of 1-substituted-1,2,3,4-tetrahydro-β-carboline in the presence of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as solvent and Li–Na as catalyst under anhydrous conditions. Pictet-Spengler reactions between tryptamine derivatives and aldehydes, or activated ketones with HFIP, could accelerated the tetrahydro-β-carbolines in high yields (99 %) upon reaction for one hour under nitrogen atmosphere at 58.6 °C (Scheme 5). The same research group also reported the synthesis of L-tryptophan methyl ester derived by THBC (Wang et al., 2014) (see Scheme 6).
Scheme 5.
Synthesis of THBC derivatives with HFIP and N2.
Scheme 6.
Synthesis of THBC methyl ester derivatives with HFIP and N2.
5.3. Various synthetic approaches
Apart from the microwave-assisted synthesis of 1,2,3,4-tetrahydro-β-carboline and β-carboline moiety, other methods like Ru (II) catalyzed C–H ortho arylation reactions of β-carboline (Rajkumar et al., 2015), Ru (II) catalyzed hydroxymethylation of β-carboline (Tokala et al., 2019) and Cu (II) mediated C–H hydroxylation of β-carboline (Bora et al., 2020), metal-free one-pot synthesis of β-carboline with N-Methyl 2- Pyrrolidone (Ramu et al., 2019), and Iodine catalyzed one-pot aromatization (Meesala et al., 2016) was also investigated. The studies disclosed that these synthetic strategies were yielded moderate to good yields after final isolations. C–H functionalization and regioselectivity of the β-carboline molecules were also investigated.
For the generalized synthesis of THBC and β-carboline derivatives, most of the research groups were reported as the initial esterification of the L-tryptophan/DL-tryptophan with the help of thionyl chloride with reflux yields the methyl/ethyl esters of the respective tryptophan. After esterification, aromatic/aliphatic aldehydes were used for the complete ring formations as THBC esters. In this stage, the isomers (cis/trans) was formed. After completion of this, oxidation of the ester derivative of the above formed THBC yields the final β-carboline compound. Singh and co-workers reported the antifungal activity of the THBC ester derivatives (cis/trans) by taking the L-tryptophan as a starting material (Scheme 7) (Singh et al., 2020).
Scheme 7.
Synthesis of THBC esters by R. Singh and co-workers.
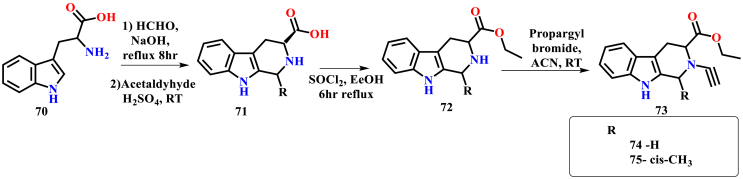
Sharma et al. reported the anti-plasmodial activity of N-substituted THBC derivatives. The synthetic strategy applied for the synthesis of THBC analogs was different from the previously reported method of Singh and co-workers. In this, initially, the THBC carboxylic acid was synthesized by refluxing L-tryptophan, formaldehyde, and sodium hydroxide for 8 h. After the THBC carboxylic acid synthesis, esterification was done by refluxing with thionyl chloride for 6 h, finally conjugated with alkynyl bromide results in the formation of titled N-substituted THBC analogs (Scheme 8) (Sharma et al., 2020).
Scheme 8.
Synthesis of THBC derivatives by Sharma and co-workers.
A convenient and highly efficient I2-catalysed method has been extended to the synthesis of highly fluorescent β-carboline C-1(3)-tethered thiazolo[4,5-c] carbazoles, naphtho[2,1-d]thiazoles, and benzothiazole derivatives. The Virender Singh research group investigated photophysical and fluorescent properties. The oxidation process of the THBC ester with both iodine and potassium permanganate was successfully implemented and yielded the titled 1-substituted-β-carboline ester up to 98% yield with L-tryptophan as the starting material. Various solvents were used in the process like dimethylformamide, tetrahydrofuran, dimethyl sulfoxide. Among these solvents, dimethyl sulfoxide in the presence of the catalytic amount of Iodine only resulted in a 98% yield (Scheme 9) (Singh et al., 2020).
Scheme 9.
Synthesis of 1-formayl β-carboline by Virender Singh and co-workers.
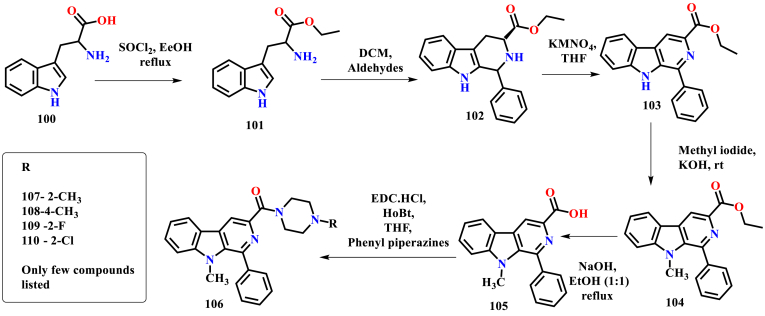
Murugesan et al. reported the synthesis and anti-leishmanial activity of the β-carboline phenyl piperazine derivatives. The initial synthesis was started by taking DL-tryptophan as a starting material. Esterification with thionyl chloride was followed by aldehyde conjugation yielded 1-substituted THBC ester. Then, oxidation with potassium permanganate in THF resulted in β-carboline ester, and alkali hydrolysis leads to the formation of β-carboline carboxylic acid. Finally, acid amide coupling in the presence of EDC. HCl and HOBt with various substituted phenyl piperazines lead to the synthesis of various 3- substituted β-carboline phenyl piperazines (Ashok et al., 2017), (Ashok et al., 2018) (Scheme 10). In the subsequent year, the same research group was also explored the successful methylation at the NH in the 9th position by using methyl iodide (Ashok et al., 2019) (Scheme 11). 1-phenyl 1,2,3,4-tetrahydro-β-carboline derivatives also reported by the same research group by taking tryptamine as a starting material. The tetrahydro-β-carboline was coupled with various acetamides in the presence of potassium carbonate, and dimethylformamide yielded up to 80% of the titled carboxamide products (Scheme 12) (Ashok et al., 2016).
Scheme 10.
Synthesis of β-carboline phenyl piperazines.
Scheme 11.
Synthesis of 9-methyl β-carboline phenyl piperazines.
Scheme 12.
Synthesis of 1,2,3,4-tetrahydro-β-carboline acetamide derivatives.
6. Biological activity
6.1. β-carboline derivatives as anti-leishmanial agents
Penta Ashok et al. reported the synthesis of piperazinyl-β-carboline-3-carboxamide derivatives and evaluated their anti-leishmanial activity against Leishmania infantum and Leishmania donovani. Among the reported derivatives, compounds 124, 125, and 126 exhibited potent inhibition of promastigotes (EC50 1.59, 1.47, and 3.73 μM, respectively) and amastigotes (EC50 1.4, 1.9 and 2.6 μM respectively) of L. infantum (Ashok et al., 2019) (see Figure 4).
Figure 4.
Structure of compounds 124, 125 and 126.
Nitin A. lunagariya et al. described the synthesis of 1,3,6-trisubstituted-β-carboline derivatives and evaluated their cytotoxic potential against four human cancer cells, namely A-549, HeLa, Hep G2, and MCF-7 as well as anti-leishmanial activity against promastigotes of L. donovani (MHOM/80/IN/Dd8). Among the studied compounds, compounds 127 and 128 were found to exhibit moderate inhibition with IC50 of 23.5 ± 9.0 and 68.0 ± 0.0 μM respectively, while compound 129 was the most active in the tested series with IC50 9.0 ± 2.8 μM, suggesting the modification at C-6 was detrimental for anti-leishmanial activity (Lunagariya et al., 2016) (see Figure 5).
Figure 5.
Structure of compounds 127, 128 and 129.
Penta Ashok et al. reported the synthesis of (1- phenyl-9H-pyrido[3,4-b] indol-3-yl) (4-phenylpiperazine-1-yl) methanone derivatives and evaluated them for inhibition activity against L. infantum and L. donovani. Amongst them, compounds 130 and 131 showed the most potent anti-leishmanial activity against the tested strains of Leishmania. The compound 130 exhibited EC50 of 3.47 ± 2.80 μM (promastigotes), 2.80 ± 0.10 μM (axenic amastigotes) and 4.00 ± 0.60 μM (intracellular amastigotes). Another compound 131 showed the EC50 2.89 ± 0.34 μM (promastigotes) and 2.80 ± 0.13 μM (axenic amastigotes) (Ashok et al., 2018) (see Figure 6).
Figure 6.
Structure of compounds 130 and 131.
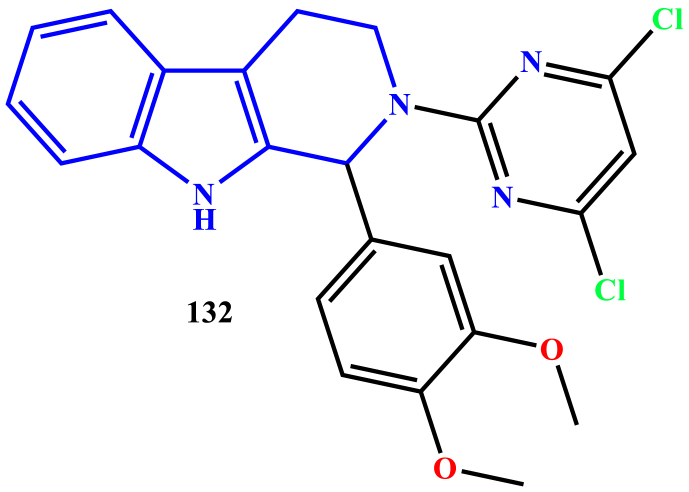
Ravi Kumar et al. reported the synthesis of 2-(pyrimidine-2-yl)-1-phenyl-2,3,4,9-tetrahydro-1H-β-carboline derivatives and evaluated for anti-leishmanial activity against L. donovani. Compound 132 exhibited significant anti-leishmanial activity with an IC50 value of 1.93 mg/ml against amastigotes (Kumar et al., 2010) (see Figure 7).
Figure 7.
Structure of compound 132.
Vikrant Singh M. Gohil et al. reported the anti-leishmanial activity of 1-aryl-β-carboline derivatives against L. donovani. Compound 133 (IC50 2.16 ± 0.26 μM) showed notable activity than the standard drug miltefosine (IC50 12.07 ± 0.82 μM) (Gohil et al., 2012) (see Figure 8).
Figure 8.

Structure of compound 133.
T. F. Stefanello et al. represented the in-vitro antileishmanial activity of N-butyl-[1-(4-methoxy) phenyl-9H-β-carboline]-3-carboxamide against L. amazonensis. The compound 134 was active against promastigote, axenic amastigote, and intracellular amastigote forms of L. amazonensis, which has high selectivity for the parasite (Stefanello et al., 2014) (see Figure 9).
Figure 9.
Structure of compound 134.
Penta Ashok et al. synthesized, and characterized of some novel tetrahydro-β-carboline derivatives against transgenic infrared fluorescent L. infantum strain. Among the tested analogs, most of the compounds exhibited potent inhibition against both promastigotes (IC50 from 1.99 ± 1.40 to 20.69 ± 0.95 μM) and amastigote (IC50 from 0.67 ± 0.05 to 4.16 ± 0.008 μM) forms of L. infantum. Moreover, compound 135 displayed the most potent and selective inhibition of parasite's amastigote form with IC50 0.67 ± 0.05 μM (Ashok et al., 2016) (see Figure 10).
Figure 10.
Structure of compound 135.
HélitoVolpato et al. studied the effects of N-butyl-1-(4-dimethylamino) phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxamide and its possible targets against L. amazonensis. The results showed that morphological and ultrastructural alterations, depolarization of the mitochondrial membrane, the loss of cell membrane integrity, and an increase in the formation of mitochondrial superoxide anions in L. amazonensis treated with compound 136 (Volpato et al., 2013) (see Figure 11).
Figure 11.
Structure of compound 136.
R. B. Pedroso et al. reported the synthesis of 1-substituted-β-carboline-3-carboxamides, 1-substituted-β-carboline-3-carboxylic acid, and screened for in-vitro activity against L. amazonensis. Compound 137, (N-benzyl-1-(4-methoxy)phenyl-9H-β-carboline-3-carboxamide) exhibited significant activity against promastigotes and axenic amastigote forms with IC50 of 2.6 and 1.0 μM, respectively (Pedroso et al., 2012) (see Figure 12).
Figure 12.
Structure of compound 137.
Shikha S. Chauhan et al., reported a detailed novel β-carboline–quinazolinone hybrids as inhibitors of L. donovani trypanothione reductase (LdTR). Among the series of analogs, compounds 138,139 and 140 revealed significant in-vitro activity against amastigotes with IC50 of 4.4, 6.0, 4.3 μM, and promastigotes with IC50 of 3.3, 4.6 and 4.8 μM, respectively along with an adequate selectivity index (SI) of >91, 36 and 24. Apart from synthesis and bio-activity, the research group also studied in-silico docking studies against the homology modeled target against the LdTR enzyme. The study results revealed that compound 138 made hydrogen bond interactions with GLU-466 and HIS-461aminoacid residues, both of which usually participate in the enzymatic process and thus may contribute to its inhibitory potential (Chauhan et al., 2015) (see Figure 13).
Figure 13.
Structure of compounds 138, 139 and140.
Lilian T. DusmanTonin et al. reported a series of 1-phenyl substituted-β-carbolines containing an N-butyl carboxamide group at C-3 of the β-carboline nucleus and evaluated in-vitro activity against epimastigote forms of Trypanosoma cruzi and promastigote forms of L. amazonensis. Two derivatives, 141 and 142, presented potent activity against both the tested parasites. The most active derivative 141 also showed high selectivity index ratio (SI) for L. amazonensis (SI = 2.084) (Tonin et al., 2010) (see Figure 14).
Figure 14.
Structure of compounds 141 and 142.
Ashok et al. reported the synthesis and anti-leishmanial activity of (4-aryl piperazine-1-yl) (1- (thiophene-2-yl)-9H-pyrido[3,4-b]indol-3-yl)methanone derivatives against both promastigotes and amastigotes of Leishmania parasites responsible for visceral (L. donovani) and cutaneous (L. amazonensis) leishmaniasis. Among the series of synthesized compounds, the compounds 143 and 144 were found to exhibit better anti-leishmanial activity than other compounds with IC50 values of 8.80 and 7.50 μM respectively, against the amastigotes of the tested strains (Ashok et al., 2017) (see Figure 15).
Figure 15.
Structure of compounds 143 and 144.
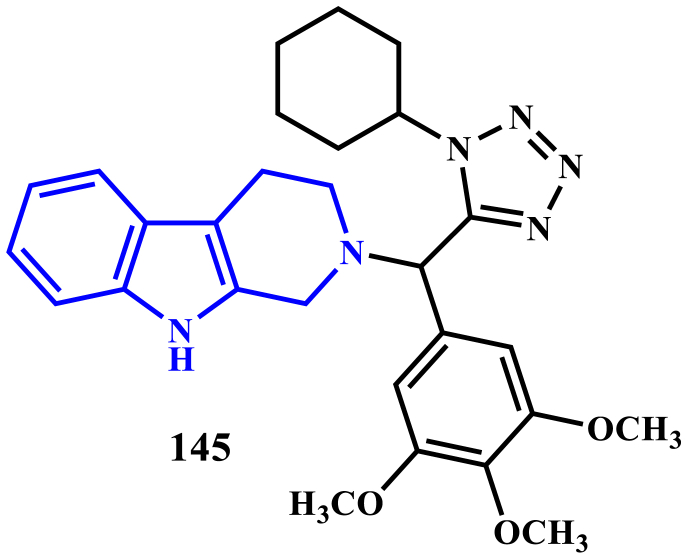
Pooja Purohit et al., detailed the synthesis of 1,2,3,4-tetrahydro-β-carboline-tetrazole derivatives using Ugi multicomponent reaction, and evaluated the compounds against Leishmaniasis. Among the screened compounds, compound 48 was found to be the most active with an IC50 value of 1.57 μM against intracellular amastigotes of L. donovani, and their activity is comparable with standard drugs miltefosine and sodium stibogluconate. Further, in-vivo evaluation of the compound 48 against L. donovani golden hamster model at a dose of 50 mg kg−1 (i.p for five days) showed 75.04 ± 7.28% inhibition of splenic parasite burden. Pharmacokinetics of compound 145 was also studied in the golden Syrian hamster followed with a 50 mg kg−1 oral dose, the compound 145 was detected in hamster serum for up to 24 h. It showed a large volume of distribution (651.8 L kg−1), high clearance (43.2 L h−1 kg−1), and long mean residence time (10 h) (Purohit et al., 2017) (see Figure 16).
Figure 16.
Structure of compound 145.
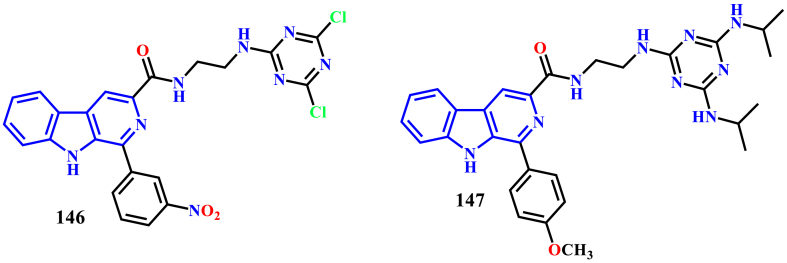
Paula Baréa et al. described the synthesis of a series of novel β-carboline-1,3,5-triazinehybrids and evaluated their in-vitro antileishmanial activity against promastigotes and amastigotes forms of L. amazonensis. Compounds 146 and 147 were found to exhibit potent activity against both amastigotes and promastigotes with IC50 values of 1.0 ± 0.1 μM and 1.2 ± 0.5 μM, respectively. Mechanism of action studies by scanning electron microscopic technique using promastigotes disclosed that the compound 147 caused alterations to the cell cycle and produced an increase in lipid storage. This increase in the lipid content may be related to the apoptotic cell death of the parasite (Baréa et al., 2018) (see Figure 17).
Figure 17.
Structure of compounds 146 and147.
Irfan Khan et al. reported the synthesis and anti-leishmanial screening of some novel β-carboline-peptide and tetrahydro-β-carbolines-peptides via natural product inspired molecular hybridization approach. Among the tested compounds, compounds 148, 149, and 150 revealed significant in-vitro anti-leishmanial activity against both promastigotes and intracellular amastigotes of L. donovani (IC50 2.43, 3.56, and 7.61 μM, respectively) than the control drug miltefosine (IC50 = 8.2 μM)with less cytotoxicity when compared with the standard drugs (sodium stibogluconate and Miltefosine). In-silico molecular docking studies of the synthesized compounds using the homology modeled LdTR indicated that the compound 149 showed significant binding free energy when compared with compound 150 (Khan et al., 2019) (see Figure 18).
Figure 18.
Structure of compounds148, 149 and 150.
6.2. Phytoconstituents with anti-leishmanial effect
Nature, as an essential source for the discovery of medicinally important compounds and the use of natural products for the treatment of various diseases/infections, is well known from the ages of (Oteng Mintah et al., 2019), (Oryan, 2015). It has been estimated that there are about 250,000 medical plant species in the world. However, the biological activities of only about 6% of them have been screened. Furthermore, only approximately 0.75% of medical herbal compounds have been studied in clinical trials. The significant merits of herbal medicine include their low cost, low incidence of serious adverse effects, and good efficacy. Various bioactive compounds like flavonoids, alkaloids, chalcones, saponins, quinolines, ligans, tannins and terpenoids present in the plant parts, crude extracts, essential oils, and other useful compounds can be a good source for discovering and producing new antileishmanial medicines (Ioset, 2008).
Renata S. Gabriel et al., isolated and investigated the effect of the β-carboline-1-propionic acid alkaloid (151) from Quassiaamara L., (Family: Simaroubaceae) against L. amazonensis and L. infatum. The alkaloid was isolated afterward, the liquid-liquid fractionation followed by chromatographic purification of the fraction Quassiaamara L., methanol extract. The isolated β-carboline-1-propionic acid alkaloid (151) exhibited anti-leishmanial activity against both promastigotes and intracellular amastigotes with 50% inhibitory concentrations ranging from 2.7 ± 0.82 to 9.4 ± 0.5 μg/ml and selectivity index >10. Furthermore, apoptotic L. amazonensis (19.5%) and L. infantum (40.4%) promastigotes were detected after 5 h incubation with the alkaloid. Moreover, the researchers also studied the inhibition effect on the production of NO by infected macrophages, and the results suggested that the alkaloid displayed a NO-independent mechanism of action for the elimination of intracellular amastigote forms (Gabriel et al., 2019) (see Figure 19).
Figure 19.

Structure of compound 151.
The harmaline (152), isolated from Peganumharmala (Family: Nitrariaceae), exhibited amastigote-specific activity (IC50 of 1.16 μM). Harmine (153) isolated from the same plant species also reduced spleen parasite load by approximately 40, 60, 70, and 80% in free, liposomal, niosomal, and nanoparticular forms respectively, when tested in micemodel (Di Giorgio et al., 2004) (see Figure 20).
Figure 20.
Structure of compounds 152 and 153.
Manzamines are unique β-carboline alkaloids isolated from Indo-Pacific sponges and characterized by a complicated nitrogen-containing polycyclic system. In 1986, Higa and co-workers first reported manzamine A from the Okinawan sponge of the genus Haliclona (Sakai et al., 1986). The full anti-leishmanial effects of the manzamine alkaloids were clearly depicted in the review of Ashok et al.,.The research group discussed the effects of the manzamine alkaloids (Figure 21) as anti-leishmanial agents, their cytotoxicity and the detailed structure-activity relationship (Ashok et al., 2015).
Figure 21.
Structure of manzamine alkaloids (154–161).
Eudistomin is also a β-carboline alkaloid isolated from the ascidians (Family: Ascidiacea). Initially, eudistomin A from Eudistomao livaceum was isolated in 1983 (Rinehart et al., 1984); eudistalbins (Adesanya et al., 1992), eudistomidins and other eudistomins (Netz and Opatz, 2015) were gradually isolated (Figure 22). The anti-leishmanial activity of these analogs was not reported. Antic cancer activity of Eudistomin H (165), extracted from Ascidian Eudistomaviride, tested against Hela cells, exhibited an IC50 of 0.49 μg/ml (Rajesh and Annappan, 2015). Antimicrobial studies of the Eudistomin H (165) revealed the zone of inhibition 7–9 mm (Rajesh and Murugan, 2019).
Figure 22.
Structure of various eudistomins (162–167).
7. Mechanism of action of β-carboline analogs
β-carbolines is known to exert their antileishmanial activity via the following possible mechanisms.
7.1. Inhibition of trypanothione reductase
The leishmanial parasite proliferates inside the macrophage cells and still protects itself from the wrathful effects of free radicals generated by the macrophage cells. Similar to the host's glutathione/glutathione reductase redox system, the parasite utilizes the trypanothione/trypanothione reductase peroxidase system to neutralize the free radicals (Figure 23).
Figure 23.
Structure of trypanothione disulphide (TS2) and glutathione disulphide (GS2).
Trypanothione TS2 is synthesized from two substrates glutathione and spermidine using the enzyme trypanothione synthetase (TS). The trypanothione (TS2) is kept in its reduced form T(SH)2 by Trypanothione Reductase (TR). T(SH)2 is, in turn, used to reduce tryparedoxin (TXNSH) into its reduced form TXN(SH)2. Tryparedoxin peroxidase (TXNP) neutralizes the free radicals generated by the macrophage cells, thus providing a favorable environment for the growth of the parasite (Chawla and Madhubala, 2010).
Trypanothione reductase is a homodimer in which each subunit is formed by three domains, namely NADPH binding domain, FAD-binding domain, and interface domain. The trypanothione binding site is placed at the interface between the FAD-binding domain and the interface domain. The active site of trypanothione reductase has an overall net negative charge to attract the positively charged trypanothione and repel the negatively charged glutathione. Cys 52, Cys 57, His 461, and Glu 466 are the essential amino acid residues that are involved in the catalytic process (Krauth-Siegel and Inhoff, 2003). Chauhan et al. designed a series of β-carboline-quinazoline hybrids as inhibitors of trypanothione reductase (Chauhan et al., 2015) (Figure 24). Compounds 138–140 were found to inhibit trypanothione reductase with good potency. Molecular docking studies were also performed to determine the binding pose of the compound in the active site of the enzyme. Compound 138 was able to occupy the active site of the enzyme and revealed hydrogen bond interactions with His 461 and Glu 466, which might have contributed to its activity. Furthermore, the compounds 138–140 also exhibited good antileishmanial activity against the promastigote and amastigote forms of L. donovani in vitro.
Figure 24.
Trypanothione peroxidase pathway. TS: Trypanothione synthase TR: Trypanothione reductase, TS2: Trypanothione (oxidized form), T(SH)2: Trypanothione (reduced form), TXNSH: Tryparedoxin (oxidized form), TXN(SH)2: Tryparedoxin (reduced form), TXNP: Tryparedoxin peroxidase.
7.2. Alterations in the cell division cycle
Baréa et al. synthesized and evaluated novel β-carboline-1,3,5-triazine hybrids against the promastigotes and amastigotes form of the leishmanial parasite (Baréa et al., 2018). Compound 147 was found to exhibit potent activity against the amastigotes. Further studies were conducted to elucidate the mechanism of action of compound 147. Electron microscopic analysis of promastigotes following 72 h of treatment with the compound 147 showed an increase in lipid-storage bodies, altercations in mitochondria, and plasma membrane. The authors postulated that the increase in lipid-storage levels was as a result of cellular stress and mitochondrial dysfunction, which ultimately culminated in cell death via apoptotic pathways.
8. Conclusion and future perspectives
8.1. Structure-activity relationship studies
Structure-activity relationship (SAR) studies are a critical key to many aspects of drug discovery, ranging from primary screening to lead optimization. A collection of molecules and their associated activities were trying to elucidate the details of one or more SARs and subsequently using that information to make structural modifications to optimize some property or activity of the particular moieties (Guha, 2013).
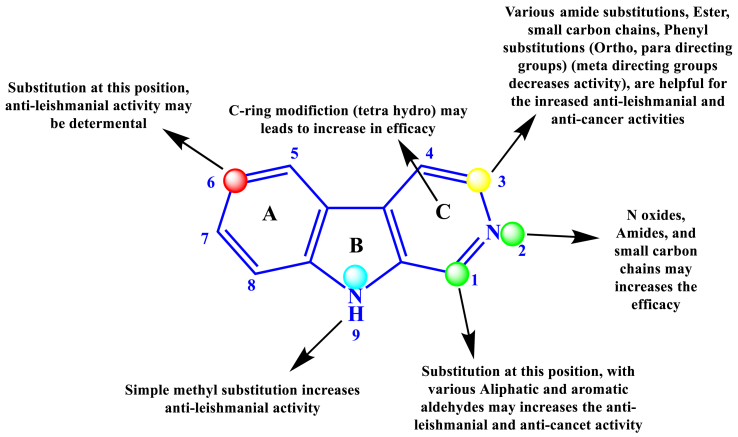
In this context, the titled β-carboline derivatives were associated with its anti-leishmanial effects. The moiety was well explored, and the library of molecules was already designed, synthesized, and many molecules are in the pipeline. The structural activity relationship pattern of this moiety helps to identify or modify the existing structure with various substitutions at possible positions to make potent analogs against the neglected tropical disease “Leishmaniasis.” The modification of the “A” ring at the 6th position with bulk group moieties decreases the anti-leishmanial activity when compared with the unsubstituted analog in the same position. Tetrahydro-β-carboline nucleus also showed significant anti-leishmanial activity. The structural modifications performed at the various positions of the “C” ring may improve the potency. Works of the literature indicated that most of the researchers focus on the “C” ring only. The modifications this “C” ring is simple to make and might lead to a drastic increase in the anti-leishmanial activity (Figure 25).
Figure 25.
Structure-activity relationship study of β-carboline associated with its anti-leishmanial activity.
On the second position of β-carboline (C ring), substitution with N-oxides, small carbon chains, amide substitutions may increase the efficacy. Substitution at the third position with numerous analogs like ortho, para directing phenyl rings, methoxy derivatives of the benzene ring, heterocyclic aldehydes like thiophene, quinoline showed significant anti-leishmanial activity. With various structural modifications at the third position of “C” ring with functional groups such as ester and acid, better anti-leishmanial and anti-cancer activities were observed. Acid derivatives with simple phenyl substitutions enhanced both the anti-cancer and anti-leishmanial activities. Substitution with meta directing functional groups at the phenyl ring (A) may decrease the anti-leishmanial efficacy. Methyl substitution at NH of ninth position (B ring) increases the anti-leishmanial activity. According to the available data on ring B, less research has been explored as an anti-leishmanial agent. To conclude, more precisely, more study may be continued on ring B.
9. Summary
In summary, antileishmanial drug discovery is still a task, despite the great efforts made during recent years. Thus, new and effective drugs should be investigated in order to overcome the toxic and side effects of decade-old existing drugs. In the last year, several candidate analogs have been deliberated to find potential new uses with the required characteristics. Unfortunately, the studies revealed less significant action on the Leishmaniasis. The recent improvements or structural modification of the β-carboline derivatives may lead to a better treatment strategy for the Leishmaniasis. The progress made so far in the research area of Leishmaniasis is less when compared against other protozoal diseases. The expansion of new synthetic drugs, along with the research on natural products, simplifies a significant approach for the discovery of new compounds against both sensitive and drug-resistant Leishmania species. Further, detailed molecular mechanistic studies may also help to improvise the development of β-carboline scaffold against leishmaniasis therapy.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was carried out under a grant from the Department of Biotechnology IndoSpain, New Delhi. (Ref. No: BT/IN/Spain/39/SM/2017–2018) and Ministry of Tribal Affairs, Government of India (Award no- 201920-NFST-TEL-01497).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors gratefully acknowledge BITS-Pilani, Pilani campus for providing the necessary facilities to do this work.
References
- Adesanya S.A., Chbani M., Pais M., Debitus C. Brominated β-carbolines from the marine tunicate eudistoma album. J. Nat. Prod. 1992;55:525–527. doi: 10.1021/np50082a025. [DOI] [PubMed] [Google Scholar]
- Al-Salem W.S., Solórzano C., Weedall G.D., Dyer N.A., Kelly-Hope L., Casas-Sánchez A., Alraey Y., Alyamani E.J., Halliday A., Balghonaim S.M., Alsohibany K.S., Alzeyadi Z., Alzahrani M.H., Al-Shahrani A.M., Assiri A.M., Memish Z., Acosta-Serrano Á. Old World cutaneous leishmaniasis treatment response varies depending on parasite species, geographical location and development of secondary infection. Parasites Vectors. 2019;12:1–9. doi: 10.1186/s13071-019-3453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson N., Herwaldt B.L., Libman M., Pearson R., Lopez-Velez R., Weina P., Carvalho E.M., Ephros M., Jeronimo S., Magill A. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the infectious diseases society of America (IDSA) and the American society of tropical medicine and hygiene (ASTMH) Clin. Infect. Dis. 2016;63:e202–e264. doi: 10.1093/cid/ciw670. [DOI] [PubMed] [Google Scholar]
- Ascic E., Hansen C.L., Le Quement S.T., Nielsen T.E. Synthesis of tetrahydro-β-carbolines via isomerization of N-allyltryptamines: a metal-catalyzed variation on the Pictet-Spengler theme. Chem. Commun. 2012;48:3345–3347. doi: 10.1039/c2cc17704h. [DOI] [PubMed] [Google Scholar]
- Ashok P., Chander S., Chow L.M.C., Wong I.L.K., Singh R.P., Jha P.N., Sankaranarayanan M. Synthesis and in-vitro anti-leishmanial activity of (4-arylpiperazin-1-yl)(1-(thiophen-2-yl)-9H-pyrido[3,4-b]indol-3-yl)methanone derivatives. Bioorg. Chem. 2017;70:100–106. doi: 10.1016/j.bioorg.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Ashok P., Chander S., Smith T.K., Prakash Singh R., Jha P.N., Sankaranarayanan M. Biological evaluation and structure activity relationship of 9-methyl-1-phenyl-9H-pyrido[3,4-b]indole derivatives as anti-leishmanial agents. Bioorg. Chem. 2019;84:98–105. doi: 10.1016/j.bioorg.2018.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok P., Chander S., Smith T.K., Sankaranarayanan M. Design, synthesis and biological evaluation of piperazinyl-β-carbolinederivatives as anti-leishmanial agents Penta. Eur. J. Med. Chem. 2018;150:559–566. doi: 10.1016/j.ejmech.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Ashok P., Chander S., Tejería A., García-Calvo L., Balaña-Fouce R., Murugesan S. Synthesis and anti-leishmanial evaluation of 1-phenyl-2,3,4,9-tetrahydro-1 H -β-carboline derivatives against Leishmania infantum. Eur. J. Med. Chem. 2016;123:814–821. doi: 10.1016/j.ejmech.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Ashok P., Lathiya H., Murugesan S. Manzamine alkaloids as antileishmanial agents: a review. Eur. J. Med. Chem. 2015;97:928–936. doi: 10.1016/j.ejmech.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Audia J.E., Evrard D.A., Murdoch G.R., Droste J.J., Nissen J.S., Schenck K.W., Fludzinski P., Lucaites V.L., Nelson D.L., Cohen M.L. Potent, selective tetrahydro-β-carboline antagonists of the serotonin 2B (5HT(2B)) contractile receptor in the rat stomach fundus. J. Med. Chem. 1996;39:2773–2780. doi: 10.1021/jm960062t. [DOI] [PubMed] [Google Scholar]
- Balaña-Fouce R., Pérez Pertejo M.Y., Domínguez-Asenjo B., Gutiérrez-Corbo C., Reguera R.M. Walking a tightrope: drug discovery in visceral leishmaniasis. Drug Discov. Today. 2019;24:1209–1216. doi: 10.1016/j.drudis.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Baréa P., Barbosa V.A., Bidóia D.L., de Paula J.C., Stefanello T.F., da Costa W.F., Nakamura C.V., Sarragiotto M.H. Synthesis, antileishmanial activity and mechanism of action studies of novel β-carboline-1,3,5-triazine hybrids. Eur. J. Med. Chem. 2018;150:579–590. doi: 10.1016/j.ejmech.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Battersby A.R., Byrne J.C., Kapil R.S., Martin J.A., Payne T.G., Arigoni D., Loew P. The mechanism of indole alkaloid biosynthesis. Chem. Commun. 1968:951–953. [Google Scholar]
- Bora D., Tokala R., John S.E., Prasanth B., Shankaraiah N. β-Carboline directed regioselective hydroxylation by employing Cu(OAc)2 and mechanistic investigation by ESI-MS. Org. Biomol. Chem. 2020;18:2307–2311. doi: 10.1039/d0ob00250j. [DOI] [PubMed] [Google Scholar]
- Brahmbhatt K.G., Ahmed N., Sabde S., Mitra D., Singh I.P., Bhutani K.K. Synthesis and evaluation of β-carboline derivatives as inhibitors of human immunodeficiency virus. Bioorg. Med. Chem. Lett. 2010;20:4416–4419. doi: 10.1016/j.bmcl.2010.06.052. [DOI] [PubMed] [Google Scholar]
- Burza S., Croft S.L., Boelaert M. Leishmaniasis. Lancet. 2018;392:951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- Calcaterra A., Mangiardi L., Monache G.D., Quaglio D., Balducci S., Berardozzi S., Iazzetti A., Franzini R., Botta B., Ghirga F. The pictet-spengler reaction updates its habits. Molecules. 2020;25 doi: 10.3390/molecules25020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R., Peng W., Wang Z., Xu A. Carboline alkaloids: biochemical and pharmacological functions. Curr. Med. Chem. 2007;14:479–500. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- Carvalho A., Chu J., Meinguet C., Kiss R., Vandenbussche G., Masereel B., Wouters J., Kornienko A., Pelletier J., Mathieu V. A harmine-derived beta-carboline displays anti-cancer effects in vitro by targeting protein synthesis. Eur. J. Pharmacol. 2017;805:25–35. doi: 10.1016/j.ejphar.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan S.S., Pandey S., Shivahare R., Ramalingam K., Krishna S., Vishwakarma P., Siddiqi M.I., Gupta S., Goyal N., Chauhan P.M.S. Novel β-carboline-quinazolinone hybrid as an inhibitor of Leishmania donovani trypanothione reductase: synthesis, molecular docking and bioevaluation. Medchemcomm. 2015;6:351–356. [Google Scholar]
- Chawla B., Madhubala R. Drug targets in leishmania. J. Parasit. Dis. 2010;34:1–13. doi: 10.1007/s12639-010-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E.D., Cook J.M. The pictet-spengler condensation: a new direction for an old reaction. Chem. Rev. 1995;95:1797–1842. [Google Scholar]
- Croft S.L., Coombs G.H. Leishmaniasis– current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19:502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Dai J., Dan W., Schneider U., Wang J. β-Carboline alkaloid monomers and dimers: occurrence, structural diversity, and biological activities. Eur. J. Med. Chem. 2018;157:622–656. doi: 10.1016/j.ejmech.2018.08.027. [DOI] [PubMed] [Google Scholar]
- Deveau A.M., Labroli M.A., Dieckhaus C.M., Barthen M.T., Smith K.S., MacDonald T.L. The synthesis of amino-acid functionalized β-carbolines as topoisomerase II inhibitors. Bioorg. Med. Chem. Lett. 2001;11:1251–1255. doi: 10.1016/s0960-894x(01)00136-6. [DOI] [PubMed] [Google Scholar]
- Dewick P.M. John Wiley & Sons, Ltd; Chichester, UK: 2002. Medicinal Natural Products: A Biosynthetic Approach, Second Edition - Dewick - Wiley Online Library, Medicinal Natural Products. [Google Scholar]
- Di Giorgio C., Delmas F., Ollivier E., Elias R., Balansard G., Timon-David P. In vitro activity of the β-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species Leishmania infantum. Exp. Parasitol. 2004;106:67–74. doi: 10.1016/j.exppara.2004.04.002. [DOI] [PubMed] [Google Scholar]
- DNDi . 2018. Disease Facctsheet: Leishmaniasis. [Google Scholar]
- Eagon S., Anderson M.O. Microwave-assisted synthesis of tetrahydro-β-carbolines and β-carbolines. Eur. J. Org Chem. 2014;2014:1653–1665. [Google Scholar]
- França P.H.B., Barbosa D.P., Da Silva D.L., Ribeiro Ê.A.N., Santana A.E.G., Santos B.V.O., Barbosa-Filho J.M., Quintans J.S.S., Barreto R.S.S., Quintans L.J., De Araújo J.X. Indole alkaloids from marine sources as potential leads against infectious diseases. BioMed Res. Int. 2014;2014:1–12. doi: 10.1155/2014/375423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel R.S., Amaral A.C.F., Lima I.C., Cruz J.D., Garcia A.R., Souza H.A.S., Adade C.M., Vermelho A.B., Alviano C.S., Alviano D.S., Rodrigues I.A. β-Carboline-1-propionic acid alkaloid: antileishmanial and cytotoxic effects. Brazil. J. Pharmacognsosy. 2019;2019:755–762. [Google Scholar]
- Glennon R.A., Grella B., Tyacke R.J., Lau A., Westaway J., Hudson A.L. Binding of β-carbolines at imidazoline I2 receptors: a structure-affinity investigation. Bioorg. Med. Chem. Lett. 2004;14:999–1002. doi: 10.1016/j.bmcl.2003.11.078. [DOI] [PubMed] [Google Scholar]
- Glennon R.A., Hong S.S., Bondarev M., Law H., Dukat M., Rakhit S., Power P., Fan E., Kinneau D., Kamboj R., Teitler M., Herrick-Davis K., Smith C. Binding of O-alkyl derivatives of serotonin at human 5-HT1Dβ receptors. J. Med. Chem. 1996;39:314–322. doi: 10.1021/jm950498t. [DOI] [PubMed] [Google Scholar]
- Gohil V.M., Brahmbhatt K.G., Loiseau P.M., Bhutani K.K. Synthesis and anti-leishmanial activity of 1-aryl-β-carboline derivatives against Leishmania donovani. Bioorg. Med. Chem. Lett. 2012;22:3905–3907. doi: 10.1016/j.bmcl.2012.04.115. [DOI] [PubMed] [Google Scholar]
- Guha R. On exploring structure activity relationships. Methods Mol. Biol. 2013;993:39–65. doi: 10.1007/978-1-62703-342-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman F., Cain M., Larscheid P., Hagen T., Cook J.M., Schweri M., Skolnick P., Paul S.M. Biomimetic approach to potential benzodiazepine. Receptor agonists and antagonists. J. Med. Chem. 1984;27:564–570. doi: 10.1021/jm00371a002. [DOI] [PubMed] [Google Scholar]
- Haldar A.K., Sen P., Roy S. Use of antimony in the treatment of leishmaniasis: current status and future directions. Mol. Biol. Int. 2011;2011:1–23. doi: 10.4061/2011/571242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herraiz T. Identification and occurrence of β-carboline alkaloids in raisins and inhibition of monoamine oxidase (MAO) J. Agric. Food Chem. 2007;55:8534–8540. doi: 10.1021/jf0719151. [DOI] [PubMed] [Google Scholar]
- Horton W., Sood A., Peerannawar S., Kugyela N., Kulkarni A., Tulsan R., Tran C.D., Soule J., LeVine H., Török B., Török M. Synthesis and application of β-carbolines as novel multi-functional anti-Alzheimer’s disease agents. Bioorg. Med. Chem. Lett. 2017;27:232–236. doi: 10.1016/j.bmcl.2016.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioset J.-R. Natural products for neglected diseases: a review. Curr. Org. Chem. 2008;12:643–666. [Google Scholar]
- Jhingran A., Chawla B., Saxena S., Barrett M.P., Madhubala R. Paromomycin: uptake and resistance in leishmania donovani. Mol. Biochem. Parasitol. 2009;164:111–117. doi: 10.1016/j.molbiopara.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam T.S., Sim K.M., Koyano T., Komiyama K. Leishmanicidal alkaloids from Kopsia griffithii. Phytochemistry. 1999;50:75–79. [Google Scholar]
- Khan I., Singh J., Kumar V., Verma V.P., Shukla M., Dhasmana A., Naruka P.S., Goswami A.K., Ameta K.L., Khan S. A versatile pre and Post Ugi modification for the synthesis of natural product inspired fused peptide-carboline scaffolds as potential anti-leishmanial agents. ChemistrySelect. 2019;4:12260–12267. [Google Scholar]
- Krauth-Siegel R.L., Inhoff O. Parasite-specific trypanothione reductase as a drug target molecule. Parasitol. Res. 2003;90:77–85. doi: 10.1007/s00436-002-0771-8. [DOI] [PubMed] [Google Scholar]
- Kumar R., Khan S., Verma A., Srivastava S., Viswakarma P., Gupta S., Meena S., Singh N., Sarkar J., Chauhan P.M.S. Synthesis of 2-(pyrimidin-2-yl)-1-phenyl-2,3,4,9-tetrahydro-1H-β- carbolinesas antileishmanial agents. Eur. J. Med. Chem. 2010;45:3274–3280. doi: 10.1016/j.ejmech.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Lunagariya N.A., Gohil V.M., Kushwah V., Neelagiri S., Jain S., Singh S., Bhutani K.K. Design, synthesis and biological evaluation of 1,3,6-trisubstituted β-carboline derivatives for cytotoxic and anti-leishmanial potential. Bioorg. Med. Chem. Lett. 2016;26:789–794. doi: 10.1016/j.bmcl.2015.12.095. [DOI] [PubMed] [Google Scholar]
- Maltezou H.C. Drug resistance in visceral Leishmaniasis. J. Biomed. Biotechnol. 2010;2010:8. doi: 10.1155/2010/617521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltezou H.C. Drug resistance in visceral leishmaniasis. J. Biomed. Biotechnol. 2010;2010:1–8. doi: 10.1155/2010/617521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark T.H.E., Neglect O.F. Cutaneous leishmaniasis - disease factsheet. DNDi. 2019:1–2. [Google Scholar]
- Meesala R., Arshad A.S.M., Mordi M.N., Mansor S.M. Iodine-catalyzed one-pot decarboxylative aromatization of tetrahydro-β-carbolines. Tetrahedron. 2016;72:8537–8541. [Google Scholar]
- Nenaah G. Antibacterial and antifungal activities of (beta)-carboline alkaloids of Peganum harmala (L) seeds and their combination effects. Fitoterapia. 2010;81:779–782. doi: 10.1016/j.fitote.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Netz N., Opatz T. Marine indole alkaloids. Mar. Drugs. 2015;13:4814–4914. doi: 10.3390/md13084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor S.E., Maresh J.J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 2006;23:532–547. doi: 10.1039/b512615k. [DOI] [PubMed] [Google Scholar]
- Oryan A. Plant-derived compounds in treatment of Leishmaniasis. Iran. J. Vet. Res. 2015;16:1–19. [PMC free article] [PubMed] [Google Scholar]
- Oteng Mintah S., Asafo-Agyei T., Archer M.-A., Atta-Adjei Junior P., Boamah D., Kumadoh D., Appiah A., Ocloo A., Duah Boakye Y., Agyare C. Medicinal plants for treatment of prevalent diseases. Pharmacogn. - Med. Plants. 2019:1–19. [Google Scholar]
- Paris C., Loiseau P.M., Bories C., Bréard J. Miltefosine induces apoptosis-like death in leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2004;48:852–859. doi: 10.1128/AAC.48.3.852-859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso R.B., Tonin L.T.D., Ueda-Nakamura T., Filho B.P.D., Sarragiotto M.H., Nakamura C.V. Beta-carboline-3-carboxamide derivatives as promising antileishmanial agents. Ann. Trop. Med. Parasitol. 2012;105:549–557. doi: 10.1179/2047773211Y.0000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechowska P., Zawirska-Wojtasiak R., Mildner-Szkudlarz S. Bioactive β-carbolines in food: a review. Nutrients. 2019;11:1–10. doi: 10.3390/nu11040814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Martinez A.K., Rodriguez-Durán J., Serrano-Martin X., Hernandez-Rodriguez V., Benaim G. Mechanism of action of Miltefosine on Leishmania donovani involves the impairment of acidocalcisome function and the activation of the sphingosine-dependent plasma membrane Ca 2+ channel. Antimicrob. Agents Chemother. 2018;62:1–10. doi: 10.1128/AAC.01614-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit P., Pandey A.K., Singh D., Chouhan P.S., Ramalingam K., Shukla M., Goyal N., Lal J., Chauhan P.M.S. An insight into tetrahydro-β-carboline–tetrazole hybrids: synthesis and bioevaluation as potent antileishmanial agents. Medchemcomm. 2017;8:1824–1834. doi: 10.1039/c7md00125h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran R., Chen Y.P.P. Potential therapeutic targets and the role of technology in developing novel antileishmanial drugs. Drug Discov. Today. 2015;20:958–968. doi: 10.1016/j.drudis.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Rajesh R.P., Annappan M. Anti-cancer effects of brominated indole alkaloid eudistomin H from marine ascidian eudistoma viride against cervical cancer cells (HeLa) Anticancer Res. 2015;35:283–294. [PubMed] [Google Scholar]
- Rajesh R.P., Murugan A. Spectroscopic identification of brominated, non-brominated alkaloids and evaluation of antimicrobial activity of Eudistomin-I, Eudistomin H, from green ascidian Eudistoma viride. J. Appl. Pharmaceut. Sci. 2019;9:116–123. [Google Scholar]
- Rajkumar S., Karthik S., Gandhi T. Ru(II)-catalyzed β-carboline directed C-H arylation and isolation of its cycloruthenated intermediates. J. Org. Chem. 2015;80:5532–5545. doi: 10.1021/acs.joc.5b00411. [DOI] [PubMed] [Google Scholar]
- Ramu S., Srinath S., Kumar A.A., Baskar B., Ilango K., Balasubramanian K.K. Metal free one pot synthesis of β-carbolines via a domino Pictet-Spengler reaction and aromatization. Mol. Catal. 2019;468:86–93. [Google Scholar]
- Report A. DNDI 2019. DNDI Annu. Rep. 2019;2019 2–2. [Google Scholar]
- Rinehart K.L., Kobayashi J., Harbour G.C., Hughes R.G., Mizsak S.A., Scahill T.A. Eudistomins C, E, K, and L, potent antiviral compounds containing a novel oxathiazepine ring from the caribbean tunicate eudistoma olivaceum. J. Am. Chem. Soc. 1984;106:1524–1526. [Google Scholar]
- Sakai R., Higa T., Jefford C.W., Bernardinelli G. Manzamine A, a novel antitumor alkaloid from a sponge. J. Am. Chem. Soc. 1986;108:6404–6405. [Google Scholar]
- Samundeeswari S., Chougala B., Holiyachi M., Shastri L., Kulkarni M., Dodamani S., Jalalpur S., Joshi S., Dixit S., Sunagar V., Hunnur R. Design and synthesis of novel phenyl -1, 4-beta-carboline-hybrid molecules as potential anti-cancer agents. Eur. J. Med. Chem. 2017;128:123–139. doi: 10.1016/j.ejmech.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Sands M., Kron M.A., Brown R.B. Pentamidine: a review. Clin. Infect. Dis. 1985;7:625–6344. doi: 10.1093/clinids/7.5.625. [DOI] [PubMed] [Google Scholar]
- Sharma B., Kaur S., Legac J., Rosenthal P.J., Kumar V. Synthesis, anti-plasmodial and cytotoxic evaluation of 1H-1,2,3-triazole/acyl hydrazide integrated tetrahydro-β-carboline-4-aminoquinoline conjugates. Bioorg. Med. Chem. Lett. 2020;30:126810. doi: 10.1016/j.bmcl.2019.126810. [DOI] [PubMed] [Google Scholar]
- Singh M., Awasthi P., Singh V. Iodine catalysed synthesis of luminescent β-carboline tethered thiazolo[4,5-c]carbazole and naphtho[2,1-d]thiazole derivatives and estimation of their light emitting properties. Eur. J. Org Chem. 2020;2020:1023–1041. [Google Scholar]
- Singh R., Jaisingh A., Maurya I.K., Salunke D.B. Design, synthesis and bio-evaluation of C-1 alkylated tetrahydro-β-carboline derivatives as novel antifungal lead compounds. Bioorg. Med. Chem. Lett. 2020;30:126869. doi: 10.1016/j.bmcl.2019.126869. [DOI] [PubMed] [Google Scholar]
- Sobhani A.M., Ebrahimi A., Mahmoudian M. An in vitro evaluation of human DNA topoisomerase-I inhibition by Peganum harmala L. seeds extract and its β-carboline alkaloids. J. Pharm. Pharmaceut. Sci. 2002;5:19–23. [PubMed] [Google Scholar]
- Song Y., Kesuma D., Wang J., Deng Y., Duan J., Wang J.H., Qi R.Z. Specific inhibition of cyclin-dependent kinases and cell proliferation by harmine. Biochem. Biophys. Res. Commun. 2004;317:128–132. doi: 10.1016/j.bbrc.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Stefanello T.F., Panice M.R., Ueda-Nakamura T., Sarragiotto M.H., Auzély-Velty R., Nakamura C.V. N -Butyl-[1-(4-Methoxy)Phenyl-9 H -β-Carboline]-3-Carboxamide prevents cytokinesis in leishmania amazonensis. Antimicrob. Agents Chemother. 2014;58:7112–7120. doi: 10.1128/AAC.03340-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steverding D. The history of Leishmaniasis. Parasites Vectors. 2017;10:82–92. doi: 10.1186/s13071-017-2028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S., Chakravarty J. Visceral leishmaniasis. Drug resist. Leishmania parasites consequences. Mol. Mech. Possible Treat. 2013;33:183–198. [Google Scholar]
- Sunter J., Gull K. Shape, form, function and Leishmania pathogenicity: from textbook descriptions to biological understanding. Open Biol. 2017;7 doi: 10.1098/rsob.170165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokala R., Bora D., Sana S., Nachtigall F.M., Santos L.S., Shankaraiah N. Ru(II)-Catalyzed regioselective hydroxymethylation of β-carbolines and isoquinolines via C-H functionalization: probing the mechanism by online ESI-MS/MS screening. J. Org. Chem. 2019;84:5504–5513. doi: 10.1021/acs.joc.9b00454. [DOI] [PubMed] [Google Scholar]
- Tonin L.T.D., Panice M.R., Nakamura C.V., Rocha K.J.P., dos Santos A.O., Ueda-Nakamura T., Costa W.F. da, Sarragiotto M.H. Antitrypanosomal and antileishmanial activities of novel N-alkyl-(1-phenylsubstituted-β-carboline)-3-carboxamides. Biomed. Pharmacother. 2010;64:386–389. doi: 10.1016/j.biopha.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Trujillo J.I., Meyers M.J., Anderson D.R., Hegde S., Mahoney M.W., Vernier W.F., Buchler I.P., Wu K.K., Yang S., Hartmann S.J., Reitz D.B. Novel tetrahydro-β-carboline-1-carboxylic acids as inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2) Bioorg. Med. Chem. Lett. 2007;17:4657–4663. doi: 10.1016/j.bmcl.2007.05.070. [DOI] [PubMed] [Google Scholar]
- Volpato H., Desoti V.C., Cogo J., Panice M.R., Sarragiotto M.H., de Silva S.O., Ueda-Nakamura T., Nakamura C.V. The effects of N-butyl-1-(4-dimethylamino)phenyl-1,2,3,4-tetrahydro- β-carboline-3-carboxamide against Leishmania amazonensis are mediated by mitochondrial dysfunction . Evidence-Based Complement. Altern. Med. 2013;2013:1–7. doi: 10.1155/2013/874367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.N., Shen S.L., Qu J. Simple and efficient synthesis of tetrahydro-β-carbolines via the Pictet-Spengler reaction in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) RSC Adv. 2014;4:30733–30741. [Google Scholar]
- Yao C., Hsieh T., Song J., Lee J. Design, synthesis and anti-cancer evaluation of β -carboline-1-one hydantoins. Futute Med. Chem. 2019;12:183–192. doi: 10.4155/fmc-2019-0276. [DOI] [PubMed] [Google Scholar]
- Yao K., Zhao M., Zhang X., Wang Y., Li L., Zheng M., Peng S. A class of oral N-[(1S,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3- carbonyl]- N′-(amino-acid-acyl)hydrazine: discovery, synthesis, in vitro anti-platelet aggregation/in vivo anti-thrombotic evaluation and 3D QSAR analysis. Eur. J. Med. Chem. 2011;46:3237–3249. doi: 10.1016/j.ejmech.2011.04.037. [DOI] [PubMed] [Google Scholar]
- Zulfiqar B., Shelper T.B., Avery V.M. Leishmaniasis drug discovery: recent progress and challenges in assay development. Drug Discov. Today. 2017;22:1516–1531. doi: 10.1016/j.drudis.2017.06.004. [DOI] [PubMed] [Google Scholar]