Abstract
Purpose
To use pathologic indicators to determine which patients benefit from postmastectomy radiation therapy (PMRT) for breast cancer after neoadjuvant chemotherapy (NACT) and total mastectomy (TM).
Patients and methods
We enrolled 4236 patients with breast invasive ductal carcinoma who received NACT followed by TM. Cox regression analysis was used to calculate hazard ratios (HRs) and confidence intervals; independent predictors were controlled for or stratified in the analysis.
Results
After multivariate Cox regression analyses, the adjusted HRs derived for PMRT for all-cause mortality were 0.65 (0.52–0.81, P < 0.0001) and 0.58 (0.47–0.71, P < 0.0001) in postchemotherapy pathologic tumor stages T2–4 (ypT3–4) and postchemotherapy pathologic nodal stages N2–3 (ypN2–3), respectively. Moreover, adjusted HRs derived for PMRT with all-cause mortality were 0.51 (0.38–0.69, P < 0.0001), 0.60 (0.40–0.88, P = 0.0096), and 0.64 (0.48–0.86, P = 0.0024) in pathological stages IIIA, IIIB, and IIIC, respectively. Additionally, the PMRT group showed significant locoregional control irrespective of the pathologic response, even ypT0, ypN0, or pathological complete response (pCR), compared with the No-PMRT group. The multivariate analysis showed no statistical differences between the PMRT and No-PMRT groups for distant metastasis-free survival in any pathologic response of ypT0–4, ypN0–3, and pathologic American Joint Committee on Cancer stages pCR to IIIC.
Conclusion
For patients with breast cancer ypT3–4, ypN2–3, or pathologic stages IIIA–IIIC receiving NACT and TM, benefit from PMRT if it is associated with OS benefits, regardless of the clinical stage of the disease. Compared with No-PMRT, PMRT improved locoregional recurrence-free survival, even pCR, in patients with breast cancer receiving NACT and TM.
Keywords: Breast cancer, Postmastectomy radiation therapy, Survival, Pathologic response, Neoadjuvant chemotherapy
Abbreviations: PMRT, postmastectomy radiation therapy; T, tumor; N, nodal; ypT, postchemotherapy pathologic tumor stages; ypN, postchemotherapy pathologic nodal stages; OS, overall survival; LRR, locoregional recurrence; DM, distant metastasis; NACT, neoadjuvant chemotherapy; TM, total mastectomy; HRs, hazard ratios; CI, confidence interval; IDC, invasive ductal carcinoma; TCRD, Taiwan Cancer Registry database; AJCC, American Joint Committee on Cancer; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; CCI, Charlson comorbidity index; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; pCR, pathological complete response
Highlights
-
•
No large-scale study has estimated detailed outcome patterns of postmastectomy radiation therapy stratified based on postchemotherapy pathologic stages in breast cancer receiving neoadjuvant chemotherapy and total mastectomy.
-
•
We used pathologic indicators to determine which patients benefit from PMRT for breast cancer after NACT and TM.
-
•
For patients with breast cancer ypT3–4, ypN2–3, or pathologic stages IIIA–IIIC receiving NACT and TM, PMRT should be performed if it is associated with survival benefits, regardless of their clinical stages.
1. Introduction
Most patients with locally advanced breast cancer, and some with early-stage disease, particularly with triple negative or human epidermal growth factor receptor 2 (HER2) positive status, are treated with neoadjuvant chemotherapy (NACT) [1,2]. The goal of the treatment is to induce a tumor response before surgery and enable breast conservation [1,2]. Moreover, NACT provides information regarding response to therapy that may be useful in the future if the disease recurs. NACT results in long-term distant disease-free survival and overall survival (OS) comparable with those achieved with primary surgery followed by adjuvant systemic therapy [3,4]. However, the choice between breast conservation and total mastectomy (TM) after NACT is dependent on the pathologic response and patients’ breast size in relation to residual tumor size [5,6]. Therefore, the surgical approach to the primary tumor depends on the size of the tumor and breast [5,6]. Asian women have relatively smaller breasts compared with women in Western countries [7]. Thus, TM rates among women receiving NACT in Asia have been high [8]. Therefore, the number of Taiwanese patients with breast cancer receiving NACT followed by TM is high [9]. The effect of postchemotherapy pathologic tumor stages (ypT), postchemotherapy pathologic nodal stages (ypN), or overall pathologic American Joint Committee on Cancer (AJCC) stages would be valuable for further adjuvant treatment in Taiwan or Asia because most patients in Taiwan still receive TM after NACT [8,9].
Postmastectomy radiation therapy (PMRT) has two potential benefits, namely a decrease in the rate of locoregional recurrence (LRR) and increases in long-term breast cancer-specific survival and OS for certain patient populations (one or more of the following: involvement of axillary lymph nodes, a tumor size of more than 5 cm, and invasion of the cancer to skin or pectoral fascia) [[10], [11], [12], [13]]. These benefits have been consistently reported in multiple studies [[10], [11], [12], [13]]. Decisions on who should receive PMRT depend on the baseline risk for recurrence, such as women who have >3 involved lymph nodes, 1–3 involved lymph nodes, or high-risk primary tumors [[10], [11], [12], [13]]. However, the indications of PMRT for patients who received neoadjuvant therapy have been controversial, especially in patients receiving TM [14,15]. LRR benefits have been presented in patients with any degree of residual macroscopic nodal disease after NACT with PMRT because retrospective evidence suggests that recurrence is high in such patients [16]. PMRT has been offered to patients with residual breast disease (ypT1–4), although the threshold to omit PMRT in such patients is lower than that for patients with residual nodal (ypN1–3) disease [16,17]. Evidence with ypT or ypN as indicators is insufficient for determining further PMRT, and a combination of ypT and ypN as indicators has not been considered for determining further PMRT.
Until now, no detailed outcome analysis is available regarding PMRT for breast cancer after NACT and TM depending on different pathologic responses and stratification based on ypT, ypN, and overall pathologic AJCC stages. In our study, we estimated the detailed outcomes of OS, LRR, and distant metastasis (DM) in PMRT for breast cancer status after NACT and TM with various pathologic responses of ypT, ypN, or overall pathologic AJCC stages. Moreover, we prefer using pathologic indicators to determine conditions for PMRT for breast cancer after NACT and TM.
2. Patients and methods
In this study, we established a cohort of breast cancer using data from the Taiwan Cancer Registry database (TCRD). The final cohort eligible for further analysis consisted of 4236 patients (2917 and 1319 patients in PMRT and No-PMRT, respectively). We enrolled patients with breast invasive ductal carcinoma (IDC) diagnosis between January 1, 2007 and December 31, 2015. The follow-up duration was from the index date (the date of breast cancer diagnosis) to December 31, 2016. The Cancer Registry database of the Collaboration Center of Health Information Application contains detailed cancer-related information of patients, including the clinical stage, treatment modalities, pathological data, radiation techniques, irradiation doses, hormone receptor status, HER2 status, and chemotherapy regimens used [[18], [19], [20], [21], [22], [23], [24], [25], [26]]. In the study, we included PMRT of both the chest wall and regional nodes with a minimum of 50 Gy. Patients with no evidence of lymph node involvement prior to or during NACT, or those who had negative needle biopsies of any suspicious nodes at diagnosis, should undergo post-NACT sentinel lymph node biopsy (SLNB). If the SLNB post-treatment is positive, surgeons in Taiwan suggest proceeding with axillary lymph node dissection (ALND). Our protocols were reviewed and approved by the Institutional Review Board of Taipei Medical University. The diagnoses of the enrolled patients were confirmed through their pathological data, and patients who received a new diagnosis of breast IDC were confirmed to have no other cancer. Patients with a diagnosis of breast IDC receiving NACT followed by TM, age ≥20 years, and AJCC clinical cancer stage I–IV were included. Moreover, the AJCC clinical staging was recorded in the TCRD. The breast cancer stages were based on AJCC, seventh edition. Patients with metastasis, missing sex data, age <20 years, nonstandard PMRT, unclear differentiation of tumor grade, unclear pathologic response, missing estrogen receptor (ER), progesterone receptor (PR) status, missing HER2 status, and unclear staging were excluded. Furthermore, we excluded patients with unclear NACT regimen, fewer than four cycles of NACT, ill-defined nodal surgery (neither SLNB nor ALND), and nonrecorded hospital type [27] (academic center or community hospitals) in our cohort. ER or PR positive was defined as ≥ 1% of tumor cells demonstrating positive nuclear staining through immunohistochemistry [28], and HER2 positive was defined as immunohistochemistry score 3+ or fluorescence in situ hybridization ratio ≥ 2 [27,29]. Finally, we enrolled patients with breast IDC receiving NACT followed by TM and categorized them into the following groups according to the treatment modality to compare their outcomes: group 1 (control group), consisting of patients who did not receive PMRT, and group 2 (case group), consisting of patients who received PMRT. Index date means the date met inclusion criteria and also the start of follow-up. The index date was the date of breast cancer diagnosis. Comorbidities were scored using the Charlson comorbidity index (CCI) [30,31]. Only comorbidities observed 6 months before the index date were included; comorbidities were identified and included according to the main International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes for the first admission or more than two repeated main diagnosis codes for visits to the outpatient department.
After adjustment for confounders, the time-dependent Cox proportional method was used to model the time from the index date to all-cause mortality, LRR, and DM among patients who underwent PMRT or No-PMRT. In the multivariate analysis, hazard ratios (HRs) were adjusted for PMRT, age, diagnosis year, CCI scores, tumor differentiation, AJCC clinical stages, ypT, ypN, NACT regimen, nodal surgery, ER/PR, HER2 status, and hospital type. The effects of PMRT on OS, LRR-free survival, and DM-free survival in multivariable Cox regression analysis, in patients who received NACT and TM with or without PMRT, were stratified according to ypT, ypN, or pathologic AJCC stages. Stratified analyses in different pathologic T or N stages were performed to evaluate the OS, LRR, and DM risk associated with PMRT or No-PMRT; furthermore, in the multivariate analysis, we used age, diagnosis year, CCI scores, tumor differentiation, AJCC clinical stages, ypT, ypN, NACT regimen, nodal surgery, ER/PR, HER2 positive, and hospital type. All analyses were performed using SAS (version 9.3; SAS, Cary, NC, USA). A two-tailed value of p < 0.05 was considered statistically significant.
3. Results
The final cohort eligible for further analysis consisted of 4236 patients (2917 and 1319 patients in groups 1 and 2, respectively). The patient characteristics are summarized in Table 1. No statistical differences were noted between the PMRT and No-PMRT groups in terms of age, tumor grade, and ER/PR status (Table 1). The number of patients receiving PMRT in 2011–2015 was higher than that in 2007–2010. In the PMRT group, the number of patients with breast cancer with AJCC clinical stages III–IV was high. Few patients with pathological complete response (pCR) received PMRT. Moreover, most patients with breast cancer receiving PMRT received NACT and TM irrespective of the pathologic response. Patients receiving PMRT included those with advanced residual T or N stages. Most patients in the PMRT group received ALND as nodal surgery. Most patients receiving NACT with a taxane-based regimen received PMRT. The PMRT group mostly consisted of HER2-positive patients. Most patients receiving PMRT were treated in nonacademic hospitals (Table 1).
Table 1.
Characteristics of patients with breast cancer who received neoadjuvant chemotherapy followed by total mastectomy stratified into PMRT and No-PMRT groups.
| Variable | TM |
|||
|---|---|---|---|---|
| PMRT (N = 2917) | No-PMRT (N = 1319) | p | ||
| Age | Mean (SD) | 51.3 (10.3) | 52.0 (10.9) | 0.1108 |
| Median (IQR: Q1, Q3) | 51 (44,58) | 51 (44,59) | ||
| 20–49 | 1301 (69.8%) | 562 (30.2%) | 0.2263 | |
| 50+ | 1616 (68.1%) | 757 (31.9%) | ||
| Diagnosis year | 2007–2010 | 956 (63.2%) | 556 (36.8%) | <0.0001 |
| 2011–2015 | 1961 (72.0%) | 763 (28.0%) | ||
| CCI scores | 0 | 2423 (69.9%) | 1042 (30.1%) | 0.0065 |
| 1 | 350 (64.1%) | 196 (35.9%) | ||
| 2+ | 144 (64.0%) | 81 (36.0%) | ||
| Differentiation | Well | 185 (6.3%) | 86 (6.5%) | 0.9504 |
| Moderate | 1505 (51.6%) | 690 (52.3%) | ||
| Poor | 1227 (42.1%) | 543 (41.2%) | ||
| AJCC clinical stages | I | 66 (57.9%) | 48 (42.1%) | <0.0001 |
| II | 995 (77.7%) | 285 (22.3%) | ||
| III | 959 (58.2%) | 690 (41.8%) | ||
| IV | 897 (75.2%) | 296 (24.8%) | ||
| ypT | ypT0 | 197 (60.2%) | 130 (39.8%) | <0.0001 |
| ypT1 | 749 (64.1%) | 419 (35.9%) | ||
| ypT2 | 1163 (68.6%) | 532 (31.4%) | ||
| ypT3–4 | 808 (77.2%) | 238 (22.8%) | ||
| ypN | ypN0 | 822 (71.6%) | 326 (28.4%) | <0.0001 |
| ypN1 | 1291 (84.6%) | 235 (15.4%) | ||
| ypN2–3 | 66 (57.9%) | 48 (42.1%) | <0.0001 | |
| yp pathologic AJCC stage | pCR | 154 (56.0%) | 121 (44.0%) | <0.0001 |
| IA | 277 (50.5%) | 272 (49.5%) | ||
| IB | 36 (65.5%) | 19 (34.5%) | ||
| IIA | 448 (53.6%) | 388 (46.4%) | ||
| IIB | 456 (71.3%) | 184 (28.8%) | ||
| IIIA–IIIC | 1546 (82.2%) | 335 (17.8%) | ||
| NACT regimen | Taxanes | 1176 (78.0%) | 331 (22.0%) | <0.0001 |
| Anthracycline | 772 (59.2%) | 533 (40.8%) | ||
| Both | 833 (73.1%) | 306 (26.9%) | ||
| Neither | 136 (47.7%) | 149 (52.3%) | ||
| Nodal surgery | ALND | 2104 (70.3%) | 890 (29.7%) | <0.0001 |
| SLNB | 813 (65.5%) | 429 (34.5%) | ||
| ER/PR | Negative | 1401 (68.2%) | 653 (31.8%) | 0.3726 |
| Positive | 1516 (69.5%) | 666 (30.5%) | ||
| HER2 | Negative | 1876 (67.2%) | 915 (32.8%) | 0.0013 |
| Positive | 1041 (72.0%) | 404 (28.0%) | ||
| Hospital level | Academic/research facility | 1595 (62.8%) | 946 (37.2%) | <0.0001 |
| Others | 1322 (78.0%) | 373 (22.0%) | ||
PMRT, postmastectomy radiation therapy; T, tumor; N, nodal; NACT, neoadjuvant chemotherapy; TM, total mastectomy; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; pCR, pathological complete response; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; SD, standard deviation; CCI, Charlson comorbidity index; AJCC, American Joint Committee on Cancer; ypT, postchemotherapy pathologic tumor stages; ypN, postchemotherapy pathologic nodal stages; IQR, interquartile range.
According to the multivariate Cox regression analysis, PMRT was a significantly independent predictor of OS and LRR but a nonsignificant predictor of DM (Table 2, Table 3, Table 4). Both univariate and multivariate Cox regression analyses indicated that No-PMRT, CCI ≥2, poor differentiation, AJCC clinical stages III–IV, and pathologic residual tumor (ypT1–4) or nodal (ypN1–3) stages are poor prognostic factors for OS (Table 2). Well-differentiated tumor grade, namely ypT0, ypN0, or ER/PR positive, was an independent good prognostic factor for OS. In addition, according to a multivariate analysis, poor prognostic factors for LRR were No-PMRT, poor differentiation of tumor grade, AJCC clinical stages III–IV, residual ypT1–4 or ypN1–3, and ER/PR positive status (Table 3). Table 4 shows that AJCC clinical stage IV, poor differentiation of tumor grade, ypT2–4, ypN1–3, and HER2 positive status were poor prognostic factors for DM. According to both univariate and multivariate Cox regression analyses, the adjusted HRs (95% confidence interval [CI]) of PMRT and No-PMRT were 0.71 (0.56–0.77), 0.51 (0.41–0.58), and 0.91 (0.77–1.21) for all-cause mortality, LRR, and DM, respectively.
Table 2.
Multivariate analysis of all-cause mortality in patients with breast cancer who received neoadjuvant chemotherapy followed by total mastectomy.
| All-cause mortality |
||||
|---|---|---|---|---|
| HR | (95% CI) | p value | ||
| PMRT | No | Ref | 0.0001 | |
| Yes | 0.71 | (0.56–0.77) | ||
| Age | 20–49 | Ref | 0.59 | |
| 50+ | 1.02 | (0.89–1.16) | ||
| Diagnosis year | 2007–2010 | Ref | 0.88 | |
| 2011–2015 | 0.97 | (0.89–1.11) | ||
| CCI scores | 0 | Ref | 0.0004 | |
| 1 | 0.91 | (0.73–1.11) | ||
| 2+ | 1.54 | (1.24–1.90) | ||
| Differentiation | Poor | Ref | <0.0001 | |
| Moderate | 0.73 | (0.66–0.86) | ||
| Well | 0.43 | (0.32–0.61) | ||
| AJCC clinical stages | I | Ref | <0.0001 | |
| II | 1.86 | (0.92–2.88) | ||
| III | 2.08 | (1.29–3.77) | ||
| IV | 2.80 | (1.44–3.75) | ||
| ypT | ypT0 | Ref | <0.0001 | |
| ypT1 | 1.59 | (1.11–2.32) | ||
| ypT2 | 1.79 | (1.22–1.98) | ||
| ypT3–4 | 2.59 | (2.01–3.70) | ||
| ypN | ypN0 | Ref | <0.0001 | |
| ypN1 | 1.44 | (1.16–1.84) | ||
| ypN2–3 | 2.33 | (2.01–2.77) | ||
| NACT regimen | Anthracycline | Ref | 0.39 | |
| Taxanes | 1.10 | (0.93–1.29) | ||
| Both | 1.04 | (0.87–1.20) | ||
| Neither | 1.13 | (0.89–1.37) | ||
| Nodal surgery | SLNB | Ref | 0.88 | |
| ALND | 1.07 | (0.89–1.33) | ||
| ER/PR | Negative | Ref | <0.0001 | |
| Positive | 0.65 | (0.55–0.74) | ||
| HER2 positive | Negative | Ref | 0.88 | |
| Positive | 1.02 | (0.88–1.14) | ||
| Hospital level | Academic | Ref | 0.29 | |
| Others | 0.91 | (0.82–1.07) | ||
HR, hazard ratio; CI, confidence interval; PMRT, postmastectomy radiation therapy; T, tumor; N, nodal; NACT, neoadjuvant chemotherapy; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; CCI, Charlson comorbidity index; AJCC, American Joint Committee on Cancer; ypT, postchemotherapy pathologic tumor stages; ypN, postchemotherapy pathologic nodal stages.
Table 3.
Multivariate analysis of locoregional recurrence in patients with breast cancer who received neoadjuvant chemotherapy followed by total mastectomy.
| Locoregional recurrence |
||||
|---|---|---|---|---|
| HR | (95% CI) | p value | ||
| PMRT | No | Ref | <0.0001 | |
| Yes | 0.51 | (0.41–0.58) | ||
| Age | 20–49 | Ref | 0.28 | |
| 50+ | 0.93 | (0.84–1.06) | ||
| Diagnosis year | 2007–2010 | Ref | 0.43 | |
| 2011–2015 | 1.05 | (0.92–1.18) | ||
| CCI scores | 0 | Ref | 0.54 | |
| 1 | 1.03 | (0.91–1.26) | ||
| 2+ | 1.16 | (0.90–1.50) | ||
| Differentiation | Poor | Ref | 0.0081 | |
| Moderate | 0.88 | (0.75–0.94) | ||
| Well | 0.64 | (0.46–0.88) | ||
| AJCC clinical stages | I | Ref | <0.0001 | |
| II | 1.25 | (0.76–1.97) | ||
| III | 1.52 | (1.01–2.34) | ||
| IV | 1.85 | (1.17–2.89) | ||
| ypT | ypT0 | Ref | <0.0001 | |
| ypT1 | 1.61 | (1.15–2.29) | ||
| ypT2 | 1.81 | (1.29–2.51) | ||
| ypT3–4 | 2.48 | (1.70–3.24) | ||
| ypN | ypN0 | Ref | 0.0013 | |
| ypN1 | 1.40 | (1.16–1.72) | ||
| ypN2–3 | 2.22 | (1.84–1.93) | ||
| NACT regimen | Anthracycline | Ref | 0.19 | |
| Taxanes | 1.03 | (0.96–1.09) | ||
| Both | 1.10 | (0.94–1.30) | ||
| Neither | 1.12 | (0.98–1.65) | ||
| Nodal surgery | SLNB | Ref | 0.44 | |
| ALND | 1.29 | (0.93–1.80) | ||
| ER/PR positive | Negative | Ref | 0.22 | |
| Positive | 1.03 | (0.93–1.27) | ||
| HER2 positive | Negative | Ref | <0.0001 | |
| Positive | 1.56 | (1.34–1.70) | ||
| Hospital level | Academic | Ref | 0.59 | |
| Others | 1.02 | (0.90–1.16) | ||
HR, hazard ratio; CI, confidence interval; PMRT, postmastectomy radiation therapy; T, tumor; N, nodal; NACT, neoadjuvant chemotherapy; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; CCI, Charlson comorbidity index; AJCC, American Joint Committee on Cancer; ypT, postchemotherapy pathologic tumor stages; ypN, postchemotherapy pathologic nodal stages.
Table 4.
Multivariate analysis of distant metastasis in patients with breast cancer who received neoadjuvant chemotherapy followed by total mastectomy.
| Distant metastasis |
||||
|---|---|---|---|---|
| HR | (95% CI) | p value | ||
| PMRT | No | Ref | 0.33 | |
| Yes | 0.91 | (0.77–1.21) | ||
| Age | 20–49 | Ref | 0.37 | |
| 50+ | 0.89 | (0.88–1.21) | ||
| Diagnosis year | 2007–2010 | Ref | 0.45 | |
| 2011–2015 | 0.93 | (0.73–1.16) | ||
| CCI scores | 0 | Ref | 0.1386 | |
| 1 | 1.22 | (0.87–1.69) | ||
| 2+ | 1.43 | (0.23–1.89) | ||
| Differentiation | Poor | Ref | 0.0039 | |
| Moderate | 0.78 | (0.39–0.85) | ||
| Well | 0.69 | (0.35–0.79) | ||
| AJCC clinical stages | I | Ref | 0.0048 | |
| II | 1.29 | (0.97–1.69) | ||
| III | 1.34 | (0.82–2.20) | ||
| IV | 1.77 | (1.19–2.39) | ||
| ypT | ypT0 | Ref | <0.0001 | |
| ypT1 | 1.90 | (0.94–3.66) | ||
| ypT2 | 2.73 | (1.45–5.51) | ||
| ypT3–4 | 4.41 | (2.21–7.87) | ||
| ypN | ypN0 | Ref | <0.0001 | |
| ypN1 | 1.19 | (1.11–1.59) | ||
| ypN2–3 | 1.28 | (1.07–2.90) | ||
| NACT regimen | Anthracycline | Ref | 0.89 | |
| Taxanes | 0.98 | (0.74–1.39) | ||
| Both | 1.04 | (0.78–1.30) | ||
| Neither | 1.01 | (0.72–1.46) | ||
| Nodal surgery | SLNB | Ref | 0.1098 | |
| ALND | 1.08 | (0.91–1.43) | ||
| ER/PR positive | Negative | Ref | 0.35 | |
| Positive | 1.13 | (0.90–1.44) | ||
| HER2 positive | Negative | Ref | <0.0001 | |
| Positive | 1.80 | (1.39–2.21) | ||
| Hospital level | Academic | Ref | 0.26 | |
| Others | 0.87 | (0.69–1.12) | ||
HR, hazard ratio; CI, confidence interval; PMRT, postmastectomy radiation therapy; T, tumor; N, nodal; NACT, neoadjuvant chemotherapy; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; CCI, Charlson comorbidity index; AJCC, American Joint Committee on Cancer; ypT, postchemotherapy pathologic tumor stages; ypN, postchemotherapy pathologic nodal stages.
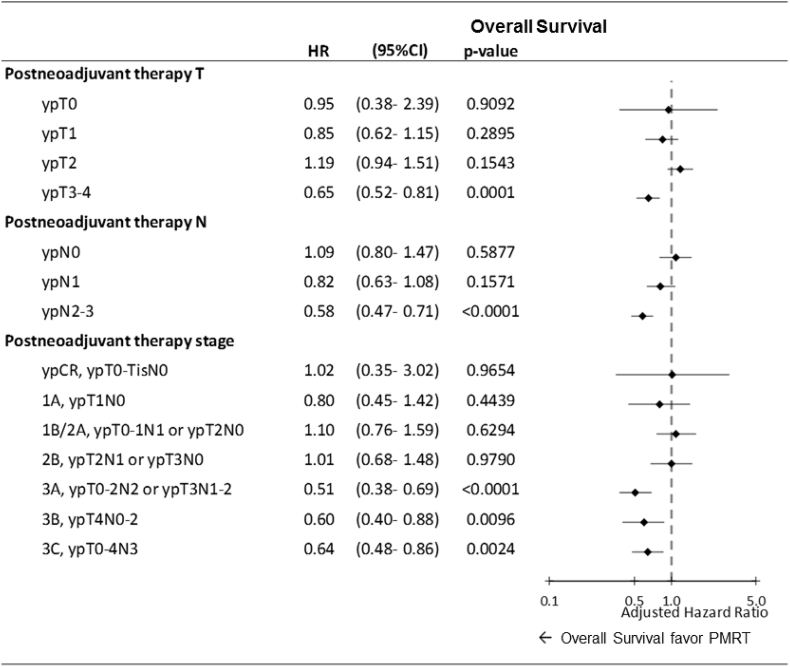
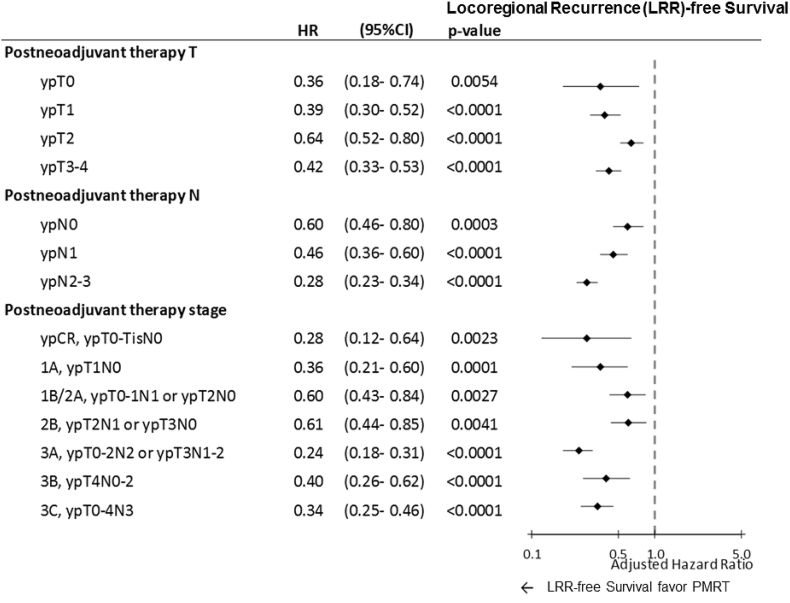
For stratified pathologic T (ypT0–4), pathologic N (ypN0–3), or pathologic AJCC stages, multivariate Cox regression analyses revealed that PMRT was a significant independent predictor of improved OS in patients with breast cancer who received NACT and TM with pathologic ypT3–4, ypN2–3, or pathologic AJCC stage IIIA–IIIC (Fig. 1). Adjusted HRs for PMRT for all-cause mortality were 0.65 (0.52–0.81) and 0.58 (0.47–0.71) in ypT3–4 and ypN2–3, respectively (Fig. 1). Moreover, adjusted HRs for PMRT for all-cause mortality were 0.51 (0.38–0.69), 0.60 (0.40–0.88), and 0.64 (0.48–0.86) in pathological AJCC stages IIIA, IIIB, and IIIC, respectively (Fig. 1). Additionally, PMRT showed significant locoregional control irrespective of the pathologic response, even ypT0, ypN0, or pCR, compared with the No-PMRT group (Fig. 2). The adjusted HRs (95% CI) of the PMRT group to No-PMRT group for LRR-free survival were 0.36 (0.18–0.74), 0.39 (0.30–0.52), 0.64 (0.52–0.80), 0.42 (0.33–0.53), 0.60 (0.46–0.80), 0.46 (0.36–0.60), and 0.28 (0.23–0.34) in ypT0, ypT1, ypT2, yoT3–4, ypN0, ypN1, and ypN2–3, respectively (Fig. 2). The adjusted HRs of LRR-free survival derived for PMRT for breast cancer after NACT and TM were 0.28 (0.12–0.64), 0.36 (0.21–0.60), 0.690(0.43–0.84), 0.61 (0.44–0.85), 0.24 (0.18–0.31), 0.40 (0.26–0.62), and 0.34 (0.25–0.46) in patients with pathologic AJCC stage pCR, stage IA, IB–IIA, IIB, IIIA, IIIB, and IIIC, respectively (Fig. 2). A multivariate analysis revealed no statistical differences between PMRT and No-PMRT groups for DM-free survival in any pathologic response of ypT0–4, ypN0–3, and pathologic AJCC stages pCR to IIIC (Supplemental Figure 1).
Fig. 1.
Impact of PMRT on overall survival in multivariate Cox regression analysis for patients who received total mastectomy with or without PMRT. Adjusted hazard ratio: All variables presented in Table 2 were used in the multivariate analysis. HR, hazard ratio; CI, confidence interval; PMRT, postmastectomy radiation therapy; T, tumor; N, nodal.
Fig. 2.
Impact of PMRT on locoregional recurrence-free survival in multivariate Cox regression analysis for patients who received total mastectomy with or without PMRT. Adjusted hazard ratio: All variables presented in Table 2 were used in the multivariate analysis. HR, hazard ratio; CI, confidence interval; PMRT, postmastectomy radiation therapy; T, tumor; N, nodal.
4. Discussion
PMRT has been prevalent in patients with breast cancer receiving NACT and TM in recent years (Table 1). However, the definitive indications of adjuvant PMRT are controversial in these patients [14,15]; clinical stages did not provide convincing evidence for performing PMRT in patients with breast cancer who have received NACT and TM [[32], [33], [34], [35], [36], [37], [38]]. Because controversy exists regarding clinical stages for indicating PMRT [14,15,[32], [33], [34], [35], [36], [37], [38]], pathologic tumor or nodal stages might be important basic references for further PMRT in patients with breast cancer receiving NACT and TM. Therefore, we focused on the pathologic stages after NACT as indicators for performing further PMRT in these patients.
According to Table 1, the clinical stage or pathologic stages were more advanced in the PMRT group than those in the No-PMRT group. Patients in the PMRT group had higher CCI scores than did those in the No-PMRT group (Table 1). Advanced clinical stages, pathologic stages, and higher CCI scores were poor prognostic factors for OS or LRR in patients with breast cancer after NACT and TM (Table 2, Table 3). Although there were more patients with advanced stages or high CCI scores in the PMRT group compared with in the No-PMRT group, OS was superior in the PMRT group compared with the No-PMRT group. The survival benefits of OS in the PMRT group were only underestimated and null to the hypothesis. PMRT leads to improved OS and LRR, and the conclusions could not be overturned.
According to Table 2, the AJCC clinical stage was an independent poor prognostic factor of OS, especially in stages III–IV. In addition, clinical stage III–IV was a poor prognostic factor for LRR (Table 3), and clinical stage IV was a poor prognostic factor for DM (Table 4). The clinical stage is an important factor indicating the risk of OS, LRR, and DM (Table 2, Table 3, Table 4). Our findings were compatible with previous studies [[32], [33], [34], [35], [36], [37], [38]]. Retrospective data of women with clinical stage III receiving PMRT have indicated improved local control even for patients who had a pCR to NACT [32,34,37,38]. In one retrospective study consisting of >670 women treated with NACT followed by TM, PMRT was associated with a significantly low rate of LRR at 10 years (22% versus 11%) and a low risk of death from breast cancer (HR 0.5, 95% CI 0.34–0.71) [34]. Among the 46 patients who presented with clinical stage III or IV and achieved a pCR with NACT, PMRT was associated with a reduced 10-year rate of LRR (3% versus 33% among patients not receiving PMRT). By contrast, other retrospective data have suggested that certain patients who achieve a pCR with NACT have low rates of LRR following TM without PMRT [14,15]. The conclusions are conflicting regarding the need for PMRT in patients with breast cancer who received NACT and TM, especially pCR [14,15]. For example, a large retrospective study of 3000 women treated with mastectomy with or without PMRT revealed that PMRT was associated with a modest reduction in 10-year LRR (10.3% versus 12.6% among patients who did not receive PMRT), with predictors of recurrence being clinical node involvement prior to NACT and tumor size > 5 cm [15]. Patients lacking these features were at low risk of LRR [15]. Taken together, whether PMRT is advantageous for patients with breast cancer after NACT and TM based on clinical stages is still debatable. Thus, pathologic findings might be crucial indicators of PMRT. Our study showed that PMRT improves OS in patients with ypT3–4, ypN2–3, or pathologic stage IIIA–IIIC compared with the No-PMRT group, and clinical stages were adjusted (Fig. 1). Regardless of clinical stages, we recommend that PMRT is necessary for patients with breast cancer who received NACT and TM with ypT3–4, ypTN1–3, or pathologic stages IIIA–IIIC, and PMRT could result in greater OS than could No-PMRT.
Other predictors of OS in these patients with breast cancer who received NACT and TM are also presented in Table 2, with poor differentiation, CCI ≥2, and ER/PR negative being poor prognostic factors for OS. No study has shown that poor differentiation, CCI ≥2, and ER/PR negative are poor prognostic factors in breast cancer after NACT and TM, but previous studies have considered high CCI scores [39], ER/PR negative [40], and poor tumor differentiation [[41], [42], [43]] as poor prognostic factors for OS, DM, or LRR in patients with breast cancer who received various treatments. Our study showed that ER/PR negative, CCI ≥2, or poor tumor differentiation are poor prognostic factors for OS in patients with breast cancer receiving NACT and TM (Table 2). In addition, poor differentiation and HER2 positive status are poor prognostic factors for LRR (Table 3), and our outcomes were similar to those of previous studies in different treatments for breast cancer [41,44]. Moreover, poor differentiation and HER2 positive status were high risk factors for DM (Table 4), and our findings were compatible with those of other studies in different treatments for breast cancer [42,45]. Thus, poor differentiation was a poor prognostic factor for OS, LRR, and DM; HER2 positive was a poor prognostic factor for LRR and DM; and CCI ≥2 and ER/PR positive were poor prognostic factors for OS. Furthermore, our study showed that not only were clinical stages, pathologic stages, ypT, and ypN significant factors for survival but also poor differentiation, CCI ≥2, and HER2 positive status were poor prognostic factors for survival (Table 2, Table 3, Table 4).
According to Table 2, Table 3, Table 4, pathologic stages are significant factors for PMRT in patients with breast cancer receiving NACT and TM. The effects of PMRT on OS, LRR-free survival, and distant metastasis-free survival in multivariable Cox regression analysis for patients who received NACT and TM with or without PMRT were analyzed (Fig. 1, Fig. 2 and Supplemental Figure 1). After the adjustment of all predictors mentioned in Table 2, PMRT was found to be superior for OS in patients with breast cancer receiving NACT and TM with ypT3–4, ypN1–3, and pathologic AJCC stages IIIA–IIIC compared with the No-PMRT group. Our findings suggest that PMRT might be necessary for patients with breast cancer receiving NACT and TM with ypT3–4, ypN2–3, or pathologic stages IIIA–IIIIC. Thus, PMRT is not required for patients with breast cancer receiving NACT and TM with pCR, early pathologic stages IA–IIB, ypT0–2, or ypN0–1 regardless of clinical stages or other predictors (Fig. 1). Moreover, PMRT is significantly superior for LRR-free survival in patients with breast cancer receiving NACT and TM with pCR, ypT0–4, or ypN0–3 (Fig. 2). Our findings were compatible with some retrospective studies, indicating that PMRT is beneficial for lowering LRR irrespective of the pathologic response [[32], [33], [34], [35], [36], [37], [38]]. In addition, PMRT is not significant for the reduction of DM risk in patients with breast cancer receiving NACT and TM (Supplemental Figure 1). Our findings suggest that PMRT associated with improved OS should be a necessary factor for ypT3–4, ypN2–3, or pathologic stage IIIA–IIIC patients with breast cancer receiving NACT and TM regardless of clinical stages. PMRT could improve LRR-free survival, even pCR, in patients with breast cancer receiving NACT and TM compared with No-PMRT (Fig. 2).
The strength of our study is that it is the largest cohort study in Taiwan to estimate the detailed outcomes of PMRT for patients with breast cancer, including OS, LRR, and DM, depending on the pathologic response of ypT, ypN, or pathologic stages. The PMRT treatment and NACT regimens were relatively homogenous in our study. Scarce studies have estimated the effects of PMRT for detailed outcomes of OS, LRR, and DM in patients with breast cancer receiving NACT and TM and adjustment of all predictors including clinical stages. In our study, poor prognostic factors for OS in these patients were no PMRT, advanced clinical stages III–IV before NACT, poor differentiation, ypT1–4, ypN1–3, CCI ≥2, ER/PR negative, and HER2 positive status (Table 2). Multivariate Cox regression analysis for patients who received NACT and TM with or without PMRT revealed that PMRT led to superior OS in ypT3–4, yN1–3, or stage IIIA–IIIC irrespective of clinical stages and other predictors (Fig. 1). Our study is the first to estimate the OS, LRR, and DM of PMRT for patients with breast cancer receiving NACT and TM with different ypT, ypN, and overall AJCC pathological stages. The beneficial effects of PMRT were improved OS and LRR-free survival compared with the No-PMRT group based on the multivariate analysis.
This study has some limitations. First, because all patients with breast IDC were Asian, the corresponding ethnic susceptibility compared with non-Asian populations remains unclear; hence, our results should be cautiously extrapolated to non-Asian populations. However, no evidence demonstrates the differences in outcomes of PMRT for patients with breast cancer receiving NACT and TM between Asian and non-Asian populations. Second, the diagnoses of all comorbid conditions were based on ICD-9-CM codes. Nevertheless, the Taiwan Cancer Registry Administration randomly reviews charts and interviews patients to verify the accuracy of the diagnoses, and hospitals with outlier chargers or practices may be audited and subsequently heavily penalized if malpractice or discrepancies are identified. Third, to prevent the creation of several subgroups, various neoadjuvant treatments were not categorized separately during the analyses. Thus, the effects of different neoadjuvant treatments remain unclear. Fourth, the selection bias in the study were patients in the PMRT group had higher CCI scores than did those in the No-PMRT group. However, there were more patients with advanced stages or high CCI scores in the PMRT group compared with in the No-PMRT group, OS was superior in the PMRT group compared with the No-PMRT group. The survival benefits of OS in the PMRT group were only underestimated and null to the hypothesis. PMRT leads to improved OS and LRR, and the conclusions could not be overturned. Accordingly, to obtain crucial information on population specificity and disease occurrence, a large-scale randomized trial comparing carefully selected patients undergoing suitable treatments is essential. Finally, the TCRD does not contain information regarding dietary habits, socioeconomic status, or body mass index, all of which may be risk factors for mortality. However, considering the magnitude and statistical significance of the observed effects in this study, these limitations are unlikely to affect the conclusions.
5. Conclusions
In patients with breast cancer type ypT3–4, ypN2–3, or pathologic stage IIIA–IIIC receiving NACT and TM, benefit from PMRT if it is associated with improved OS. Compared with No-PMRT, PMRT improved LRR-free survival, even pCR, in patients with breast cancer receiving NACT and TM.
Ethics approval and consent
Our protocols were reviewed and approved by the Institutional Review Board of Taipei Medical University (TMU-JIRB No. 201712019).
Consent for publication
Not applicable.
Availability of data and material
The datasets supporting the study conclusions are included within this manuscript and its additional files.
Author contributions
Conception and Design: Jiaqiang Zhang, MD, PhD; Chang-Yun Lu, MD; Chien-Hsin Chen, MD; Szu-Yuan Wu, MD, MPH, PhD, Financial Support: Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital, supports Szu-Yuan Wu’s work (Funding Number: 10908 and 10909). Collection and Assembly of Data: Chang-Yun Lu, MD; Ho-Min Chen, MS; Szu-Yuan Wu, MD, MPH, PhD∗, Data Analysis and Interpretation: Ho-Min Chen, MS; Szu-Yuan Wu, MD, MPH, PhD∗, Administrative Support: Szu-Yuan Wu∗, Manuscript Writing: Jiaqiang Zhang, MD, PhD; Chien-Hsin Chen, MD; Szu-Yuan Wu, MD, MPH, PhD, Final Approval of Manuscript: All authors.
Condensed abstract
No large-scale study has estimated detailed outcome patterns of postmastectomy radiation therapy (PMRT) stratified based on postchemotherapy pathologic tumor or nodal stages (ypT and ypN, respectively) for overall survival (OS), locoregional recurrence, or distant metastasis in patients with breast cancer receiving neoadjuvant chemotherapy (NACT) and total mastectomy (TM). We used pathologic indicators to determine which patients benefit from PMRT for breast cancer after NACT and TM. For patients with breast cancer ypT3–4, ypN2–3, or pathologic stages IIIA–IIIC receiving NACT and TM, PMRT should be performed if it is associated with OS benefits, regardless of their clinical stages. Compared with No-PMRT, PMRT improved locoregional recurrence-free survival and even pathological complete response in patients with breast cancer receiving NACT and TM.
Declaration of competing interest
The authors have no potential conflicts of interest to declare. The datasets supporting the study conclusions are included within the manuscript.
Acknowledgments
Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital, supports Szu-Yuan Wu’s work (Funding Number: 10908 and 10909).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.08.017.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Teshome M., Hunt K.K. Neoadjuvant therapy in the treatment of breast cancer. Surg Oncol Clin. 2014;23:505–523. doi: 10.1016/j.soc.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarneri V., Dieci M.V., Barbieri E., Piacentini F., Omarini C., Ficarra G. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol. 2013;24:2990–2994. doi: 10.1093/annonc/mdt364. [DOI] [PubMed] [Google Scholar]
- 3.Thompson A.M., Moulder-Thompson S.L. Neoadjuvant treatment of breast cancer. Ann Oncol. 2012;23(Suppl 10):x231–x236. doi: 10.1093/annonc/mds324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cance W.G., Carey L.A., Calvo B.F., Sartor C., Sawyer L., Moore D.T. Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg. 2002;236:295–302. doi: 10.1097/01.SLA.0000027526.67560.64. discussion -3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J.H., Feig B.A., Hsiang D.J., Butler J.A., Mehta R.S., Bahri S. Impact of MRI-evaluated neoadjuvant chemotherapy response on change of surgical recommendation in breast cancer. Ann Surg. 2009;249:448–454. doi: 10.1097/SLA.0b013e31819a6e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murugappan K., Saboo A., Kuo L., Ung O. Paradigm shift in the local treatment of breast cancer: mastectomy to breast conservation surgery. Gland Surg. 2018;7:506–519. doi: 10.21037/gs.2018.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maskarinec G., Meng L., Ursin G. Ethnic differences in mammographic densities. Int J Epidemiol. 2001;30:959–965. doi: 10.1093/ije/30.5.959. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y., Liao M., He L., Zhu C. Comparison of breast-conserving surgery with mastectomy in locally advanced breast cancer after good response to neoadjuvant chemotherapy: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Insurance Administration . 2015. Ministry of Health and welfare, Taiwan, R.O.C. 2017. [Google Scholar]
- 10.Clarke M., Collins R., Darby S., Davies C., Elphinstone P., Evans V. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 11.Danish Breast Cancer Cooperative G., Nielsen H.M., Overgaard M., Grau C., Jensen A.R., Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol : Off J Am Soc Clin Oncol. 2006;24:2268–2275. doi: 10.1200/JCO.2005.02.8738. [DOI] [PubMed] [Google Scholar]
- 12.Ragaz J., Olivotto I.A., Spinelli J.J., Phillips N., Jackson S.M., Wilson K.S. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 13.Ebctcg, McGale P., Taylor C., Correa C., Cutter D., Duane F. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang E.H., Tucker S.L., Strom E.A., McNeese M.D., Kuerer H.M., Hortobagyi G.N. Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:351–357. doi: 10.1016/j.ijrobp.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 15.Mamounas E.P., Anderson S.J., Dignam J.J., Bear H.D., Julian T.B., Geyer C.E., Jr. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol : Off J Am Soc Clin Oncol. 2012;30:3960–3966. doi: 10.1200/JCO.2011.40.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J., Mao K., Jiang S., Jiang W., Chen K., Kim B.Y. The role of postmastectomy radiotherapy in clinically node-positive, stage II-III breast cancer patients with pathological negative nodes after neoadjuvant chemotherapy: an analysis from the NCDB. Oncotarget. 2016;7:24848–24859. doi: 10.18632/oncotarget.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rusthoven C.G., Rabinovitch R.A., Jones B.L., Koshy M., Amini A., Yeh N. The impact of postmastectomy and regional nodal radiation after neoadjuvant chemotherapy for clinically lymph node-positive breast cancer: a National Cancer Database (NCDB) analysis. Ann Oncol. 2016;27:818–827. doi: 10.1093/annonc/mdw046. [DOI] [PubMed] [Google Scholar]
- 18.Chang C.L., Tsai H.C., Lin W.C., Chang J.H., Hsu H.L., Chow J.M. Dose escalation intensity-modulated radiotherapy-based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiother Oncol. 2017 doi: 10.1016/j.radonc.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Chang W.W., Hsiao P.K., Qin L., Chang C.L., Chow J.M., Wu S.Y. Treatment outcomes for unresectable intrahepatic cholangiocarcinoma: nationwide, population-based, cohort study based on propensity score matching with the Mahalanobis metric. Radiother Oncol. 2018 doi: 10.1016/j.radonc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Chen T.M., Lin K.C., Yuan K.S., Chang C.L., Chow J.M., Wu S.Y. Treatment of advanced nasopharyngeal cancer using low- or high-dose concurrent chemoradiotherapy with intensity-modulated radiotherapy: a propensity score-matched, nationwide, population-based cohort study. Radiother Oncol. 2017 doi: 10.1016/j.radonc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y.K., Hsieh M.C., Chang C.L., Chow J.M., Yuan K.S., Wu A.T.H. Intensity-modulated radiotherapy with systemic chemotherapy improves survival in patients with nonmetastatic unresectable pancreatic adenocarcinoma: a propensity score-matched, nationwide, population-based cohort study. Radiother Oncol. 2018 doi: 10.1016/j.radonc.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y.K., Hsieh M.C., Wang W.W., Lin Y.C., Chang W.W., Chang C.L. Outcomes of adjuvant treatments for resectable intrahepatic cholangiocarcinoma: chemotherapy alone, sequential chemoradiotherapy, or concurrent chemoradiotherapy. Radiother Oncol. 2018 doi: 10.1016/j.radonc.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Yen Y.C., Hsu H.L., Chang J.H., Lin W.C., Chang Y.C., Chang C.L. Efficacy of thoracic radiotherapy in patients with stage IIIB-IV epidermal growth factor receptor-mutant lung adenocarcinomas who received and responded to tyrosine kinase inhibitor treatment. Radiother Oncol. 2018 doi: 10.1016/j.radonc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Lin W.C., Ding Y.F., Hsu H.L., Chang J.H., Yuan K.S., Wu A.T.H. Cancer; 2017. Value and application of trimodality therapy or definitive concurrent chemoradiotherapy in thoracic esophageal squamous cell carcinoma. [DOI] [PubMed] [Google Scholar]
- 25.Yen Y.C., Chang J.H., Lin W.C., Chiou J.F., Chang Y.C., Chang C.L. Effectiveness of esophagectomy in patients with thoracic esophageal squamous cell carcinoma receiving definitive radiotherapy or concurrent chemoradiotherapy through intensity-modulated radiation therapy techniques. Cancer. 2017;123:2043–2053. doi: 10.1002/cncr.30565. [DOI] [PubMed] [Google Scholar]
- 26.Wu S.Y., Fang S.C., Shih H.J., Wen Y.C., Shao Y.J. Mortality associated with statins in men with advanced prostate cancer treated with androgen deprivation therapy. Eur J Canc. 2019;112:109–117. doi: 10.1016/j.ejca.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 27.Bahreini F., Soltanian A.R., Mehdipour P. A meta-analysis on concordance between immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) to detect HER2 gene overexpression in breast cancer. Breast Cancer. 2015;22:615–625. doi: 10.1007/s12282-014-0528-0. [DOI] [PubMed] [Google Scholar]
- 28.Hammond M.E., Hayes D.F., Dowsett M., Allred D.C., Hagerty K.L., Badve S. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol : Off J Am Soc Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fehrenbacher L., Cecchini R.S., Geyer C.E., Jr., Rastogi P., Costantino J.P., Atkins J.N. NSABP B-47/NRG Oncology phase III randomized trial comparing adjuvant chemotherapy with or without Trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol : Off J Am Soc Clin Oncol. 2020;38:444–453. doi: 10.1200/JCO.19.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charlson M., Szatrowski T.P., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 31.Chen J.H., Yen Y.C., Yang H.C., Liu S.H., Yuan S.P., Wu L.L. Curative-intent aggressive treatment improves survival in elderly patients with locally advanced head and neck squamous cell carcinoma and high comorbidity index. Medicine (Baltim) 2016;95:e3268. doi: 10.1097/MD.0000000000003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang E.H., Tucker S.L., Strom E.A., McNeese M.D., Kuerer H.M., Buzdar A.U. Postmastectomy radiation improves local-regional control and survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J Clin Oncol : Off J Am Soc Clin Oncol. 2004;22:4691–4699. doi: 10.1200/JCO.2004.11.129. [DOI] [PubMed] [Google Scholar]
- 33.Abdel-Wahab M., Wolfson A., Raub W., Mies C., Brandon A., Morrell L. The importance of postoperative radiation therapy in multimodality management of locally advanced breast cancer: a phase II trial of neoadjuvant MVAC, surgery, and radiation. Int J Radiat Oncol Biol Phys. 1998;40:875–880. doi: 10.1016/s0360-3016(97)00897-3. [DOI] [PubMed] [Google Scholar]
- 34.Buchholz T.A., Tucker S.L., Masullo L., Kuerer H.M., Erwin J., Salas J. Predictors of local-regional recurrence after neoadjuvant chemotherapy and mastectomy without radiation. J Clin Oncol : Off J Am Soc Clin Oncol. 2002;20:17–23. doi: 10.1200/JCO.2002.20.1.17. [DOI] [PubMed] [Google Scholar]
- 35.Ring A., Webb A., Ashley S., Allum W.H., Ebbs S., Gui G. Is surgery necessary after complete clinical remission following neoadjuvant chemotherapy for early breast cancer? J Clin Oncol : Off J Am Soc Clin Oncol. 2003;21:4540–4545. doi: 10.1200/JCO.2003.05.208. [DOI] [PubMed] [Google Scholar]
- 36.Panades M., Olivotto I.A., Speers C.H., Shenkier T., Olivotto T.A., Weir L. Evolving treatment strategies for inflammatory breast cancer: a population-based survival analysis. J Clin Oncol : Off J Am Soc Clin Oncol. 2005;23:1941–1950. doi: 10.1200/JCO.2005.06.233. [DOI] [PubMed] [Google Scholar]
- 37.McGuire S.E., Gonzalez-Angulo A.M., Huang E.H., Tucker S.L., Kau S.W., Yu T.K. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1004–1009. doi: 10.1016/j.ijrobp.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce L.J., Lippman M., Ben-Baruch N., Swain S., O’Shaughnessy J., Bader J.L. The effect of systemic therapy on local-regional control in locally advanced breast cancer. Int J Radiat Oncol Biol Phys. 1992;23:949–960. doi: 10.1016/0360-3016(92)90899-s. [DOI] [PubMed] [Google Scholar]
- 39.Land L.H., Dalton S.O., Jensen M.B., Ewertz M. Impact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990-2008. Breast Canc Res Treat. 2012;131:1013–1020. doi: 10.1007/s10549-011-1819-1. [DOI] [PubMed] [Google Scholar]
- 40.Onitilo A.A., Engel J.M., Greenlee R.T., Mukesh B.N. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medina-Franco H., Vasconez L.O., Fix R.J., Heslin M.J., Beenken S.W., Bland K.I. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg. 2002;235:814–819. doi: 10.1097/00000658-200206000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoppmann S.F., Bayer G., Aumayr K., Taucher S., Geleff S., Rudas M. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004;240:306–312. doi: 10.1097/01.sla.0000133355.48672.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elston C.W. The assessment of histological differentiation in breast cancer. Aust N Z J Surg. 1984;54:11–15. doi: 10.1111/j.1445-2197.1984.tb06677.x. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Angulo A.M., Litton J.K., Broglio K.R., Meric-Bernstam F., Rakkhit R., Cardoso F. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol : Off J Am Soc Clin Oncol. 2009;27:5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duchnowska R., Dziadziuszko R., Czartoryska-Arlukowicz B., Radecka B., Szostakiewicz B., Sosinska-Mielcarek K. Risk factors for brain relapse in HER2-positive metastatic breast cancer patients. Breast Canc Res Treat. 2009;117:297–303. doi: 10.1007/s10549-008-0275-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the study conclusions are included within this manuscript and its additional files.