Highlights
-
•
Prostate and oligometastases patients were most common first sites treated.
-
•
Conventional professional roles and responsibilities will evolve in the future.
-
•
Thresholds for on-line adaptive radiotherapy remain undefined.
Keywords: Adaptive radiotherapy, Current practice, Professional roles, Workflow
Abstract
Background and purpose
The uptake of new technologies has varied internationally and there have often been barriers to implementation. On-line adaptive radiotherapy (ART) promises to improve patient outcome. This survey focuses on the implementation phase of delivering ART and professional roles and responsibilities currently involved in the workflow and changes which may be expected in the future.
Materials and methods
A 38 question survey included aspects on current practice; professional responsibilities; benefits and barriers; and decision making and responsibilities. For the purposes of the questionnaire and paper, ART was considered where tumour and /or organs at risk were contoured and re-planning was performed on-line. The questionnaire was electronically distributed via radiotherapy networks.
Results
Nineteen international responses were received. Europe (n = 11), United States of America (n = 4); Canada (n = 2), Australia (n = 1) and Hong Kong (n = 1). The majority of centres started using ART in either 2018 (n = 7) or 2019 (n = 6). Four centres started treating with ART between 2015 and 2017, and the first was in 2014. Centres initially treated prostate and oligometastases patients, expanding to treat prostate, oligometastases, pancreas and rectum. The majority of centres were working in conventional roles, however moving towards radiographers taking more responsibility in contouring organs at risk (OAR), target and dosimetry. The three most important criteria chosen by medical doctors to determine if ART should be used were overall gross anatomy changes of target and OAR, target not covered by planning target volume (PTV) and OAR close to the high dose area. There was no clear consensus on the minimum improvement in dose to target or reduction in dose to OAR to warrant adaption.
Conclusion
On-line ART has been implemented successfully internationally. Initial practice maintains conventional professional roles and responsibilities, however there is trend to changing roles for the future. There is little consensus regarding the triggers of adaption.
Introduction
Adaptive Radiotherapy (ART) was first defined in 1997 as ‘a radiation treatment process where the treatment plan can be modified using a systematic feedback of measurements’ [1]. Until recently this process has been completed off-line, either by re-scanning and re-planning [2] or by using treatment verification images to create a new plan [3], [4]. Initial clinical implementation of online adaption of treatment has been restricted to selecting the best fit planning target volume (PTV) from a library of pre prepared plans (‘plan of the day’) [5], [6]. However, the ability to deliver adaptive radiotherapy on-line has been realised with the introduction of combined Magnetic Resonance imaging (MRI) scanners and radiotherapy treatment units (Co 60 and Linac) with an integrated adaptive workflow (On-line MRIgRT) [7], [8].
Historically, the uptake and implementation of new radiotherapy technologies and techniques has varied internationally, often taking longer than ideal to become routine clinical practice with barriers such as staff resources and training cited. This has been illustrated with intensity modulated radiotherapy (IMRT) where uptake in centres was less than 50% in UK, ten years after first implementation [9]. A similar pattern was seen in many European countries and Australia [10], [11]. A key barrier to implementation cited was the lack of trained staff, in addition to the lack of specific funding for IMRT [12], [13]. Investment in human resources including education and training has also been identified as a requirement for implementation of high technologic solutions for example stereotactic radiosurgery (SRS) [12] and 3D IGRT techniques [14], [15], [16].
On-line MRIgRT promises to revolutionise radiotherapy delivery by compensating for patient and tumour changes on a daily basis and has shown to be feasible [17]. However, the increase in treatment time coupled with the number of staff involved at the time of treatment will limit the uptake [18]. Only 6% of adaptive treatments were delivered by online daily replanning in a recently published survey and although human resources were identified as the main barrier there was no detail regarding the professionals involved in the workflow [19]. To thoroughly investigate the potential of on-line MRIgRT wide implementation is essential. To achieve this, the human resources required and potential barriers for treatment delivery must be identified to prepare new users and the radiotherapy community. This is the first survey to focus on the delivery of on-line MRIgRT. The professional roles and responsibilities currently involved in the workflow and changes which may be expected in the future are determined. The thresholds for decision to treat with ART and potential barriers of using MRIgRT will also be investigated. As such, a baseline measure regarding clinical use of MRIgRT will be established, the result of which will inform and aide future training for implementation
Method
The survey was designed to capture current MRIgRT practice and after the initial introductory questions (Q1 to Q3), was divided into four sections.
-
(i)
Current practice (Q4 to Q10)
-
(ii)
Professional Responsibilities (Q11 to Q19)
-
(iii)
Benefits and Barriers (Q20 to Q21)
-
(iv)
Decision making and criteria - Physicist/Radiographer/RTT (Q22 to Q27) and Medical Doctor (Q28 to Q38) perspective
The survey was piloted to physicists, radiographers and medical doctors in the UK, asking to comment on clarity of questions and the time taken to complete. This process was repeated 3 times when a consensus was reached regarding question wording. All respondents completed the questionnaire in 10 minutes or less.
The final questionnaire (Appendix 1) consisted of a total of 37 questions with 21 questions in sections 1–3 covering tumour sites treated, professional roles in treatment delivery, perceived benefits of ART and the barriers to implementation. Section 4 (6 questions for physicists and radiographers and 10 questions for medical doctors) investigated opinions regarding when ART should be used, and the importance of criteria used when determining to treat with ART. The questionnaire was distributed between November 2019 and February 2020 to centres with clinical Hybrid MR guided radiotherapy systems (based on either Cobalt or Linac technology from 1 of 2 manufacturers MRIdian systems (ViewRay, Inc., CA) or Unity MR-linac (Elekta AB, Stockhom, Sweden) via email. Unity MR-linac users were identified via the Elekta consortium and MRIdian system users were identified through publications and networks.
For the purposes of the questionnaire and paper, ART was considered where tumour and /or organs at risk were contoured and replanning was performed on-line (On-line MRIgRT). For Unity MR-linac (Elekta AB, Stockhom, Sweden) users this was considered as ‘Adapt to Shape’ [14].
A ‘table shift’, similar to conventional IGRT techniques, requires adjusting the table to correct for translational shifts in anatomy can be performed on MRIdian systems (ViewRay, Inc., CA) whereas a dose shift requires adapting the multi leaf collimator leaves according to the translational corrections and is performed on Unity MR-linac where table shifts are not possible.
Questions 20 and 21 were analysed by allocating a score of 4 to very important, 3 to fairly important, 2 to important, 1 to slightly important and −1 to not at all important. The median and range of scores for each question were determined.
Results
Respondents (Q1 to Q4)
66 respondents replied from nineteen international centres based in Europe (n = 11), United States of America (n = 4); Canada (n = 2), Australia (n = 1) and Hong Kong (n = 1). Thirteen of the 14 centres using Unity MR-linac clinically at the time of questionnaire distribution and six centres using MRIdian (5 Linac and 1 Cobalt) responded,
A total of 21 medical doctors, 15 physicists (including 1 dosimetrist) and 29 radiographers responded with a median of 3 (range 1–8) respondents per centre. Where more than one respondent had completed sections 1 and 2, the most comprehensive set of answers were used for that centre. Where there were discrepancies in answers this was either followed up by contacting the centre or an average of the answer used.
Current practice (Q5 to Q10)
The peak year of implementation was in 2018 when seven centres started treating with ART, followed by six centres in 2019 (Q5). The first treatment was in 2014 and subsequently four centres (3 ViewRay and one Unity) started treating with ART between 2015 and 2017. A table shift (MRidian users) or a dose shift (Unity users) was used as treatment option by seven centres, prior to ART by five centres and subsequent to ART by nine centres (Q6). Two centres selected more than one option.
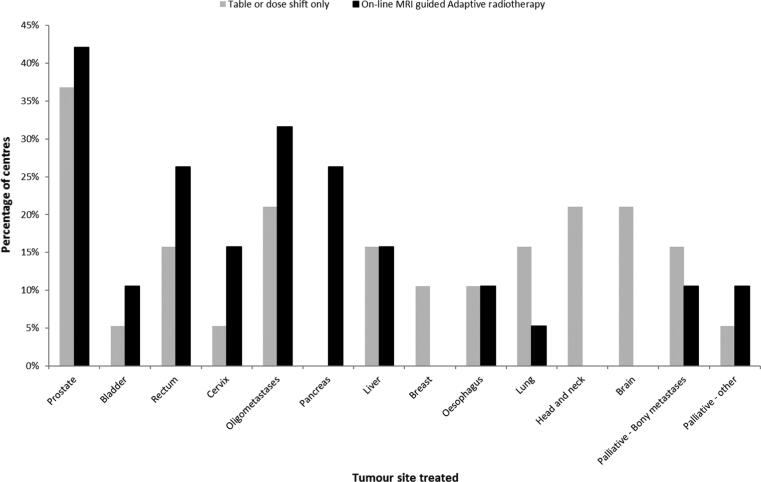
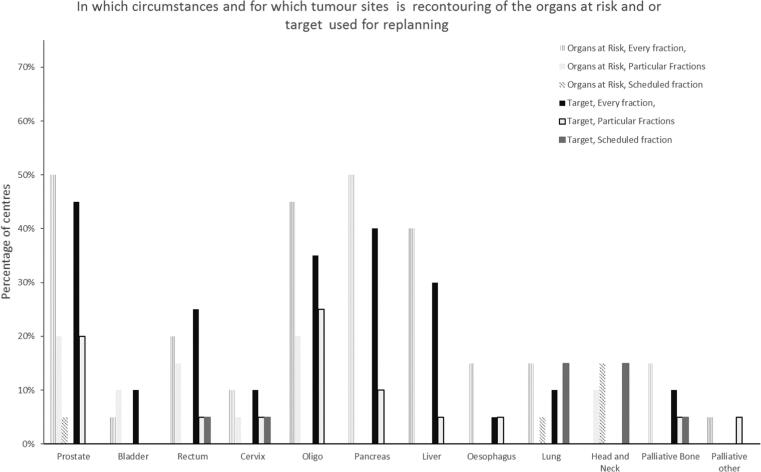
Prostate cancer was the most common treatment site to be initially treated on MR-Linac/Co60 systems (42% of centres), followed by oligometastases (15%) and liver (10%). Other tumour sites treated were brain, rectum and pancreas (Q7). After initial implementation, the tumour sites most commonly treated were prostate, oligometastases, pancreas and rectum(Q8) (Fig. 1), with the target most often contoured in prostate cancer patients and OAR in pancreas and oligometastases treatments (Figure 2, Q9 and 10)). (Fig. 2).
Fig. 1.
The percentage of centres treating each tumour site using MR-Linac/Co60 systems. On-line MRI guided radiotherapy- tumour and /or organs at risk were contoured and replanning was performed on-line. Table or Dose shift Only - A ‘table shift’ requires adjusting the table to correct for translational shifts in anatomy and can be performed on MRIdian systems. A dose shift requires adapting the multi leaf collimator leaves according to the translational corrections and is performed on Unity MR-linac where table shifts are not possible.
Fig. 2.
Percentage of centres recontouring target or organs at risk for each tumour site.
Professional responsibilities
The median (range) of the number of professionals required and available to treat in each centre was similar across professions (Q11 and Q14; Table 1). The skill mix available influenced the number required, for example radiographers were also dosimetrists in three centres which reduced the number of physicists required. One centre also commented that it was dependant on tumour site treated for example, for prostate and oligometastases only three radiographers were required,(Q14). One centre indicated that the highest number available for selection on the survey (n = 10) was not large enough to reflect their staff numbers. Three centres reported the staffing numbers had changed since starting (Q12), one centre required one less doctor and physicist, one centre, one less doctor and the third centre reported an increase of three doctors, one physicists and one dosimetrist although commented that the staff rotated each day (Q13).
Table 1.
Number of professionals required to treat with on-line MRI guided adaptive radiotherapy T and total number of professional available in team (*one centre indicated that 10 was not a high enough number to reflect numbers and another added 12 RTT’s in comments).
| Professional | Number currently required to treat Median (range) |
Total number in team Median (range) |
|---|---|---|
| Physicist | 1 (0–5) | 5 (3–10) |
| Dosimetrist | 0 (0–4) | 2 (0–7) |
| Radiographer | 2 (1–5) | 6 (3–12) |
| Doctor | 1 (0–5) | 5 (2–10) |
The majority of centres were working in conventional roles with radiographers alone being responsible for patient set-up (100%), image acquisition (94%) and image registration (76%) (Q15). Medical doctors alone were responsible for contouring the tumour in 76% of centres with medical doctor and radiographer responsible in remaining centres. Contouring organs at risk varied between medical doctors alone (41%) , medical doctor and radiographer (18%), radiographer alone (18%) and medical doctor physicist and radiographer (6%). In 64% of centres physicists were responsible for plan creation whilst the remaining centres radiographers/RTT’s completed the plan. Plan checking was predominantly performed by physicists alone in 52% of centres but in the remaining centres it was either medical doctor, physicists and radiographer/RTT’s (18%) , medical doctor and physicist (18%), medical doctor and radiographer/RTT (6%) or radiographer/RTTalone (6%), (Q15). Medical doctors were the most common profession to make the decision to treat with the adapted plan (41%) with the multidisciplinary team of all professions next (24%) , medical doctors and physicists (18%) and . e radiographers alone and physicists alone in 6% of centres.
Three centres had already changed roles since implementation (Q16). In one centre, radiographers/RTT’s were performing the image registration rather than the doctor, in another plan creating and checking. The third, one of the most experienced centres which started treatment in 2017, had progressed to not requiring the medical doctor to be present for prostate, oligometastases and rectum patients treatments, (Q17). A training and implementation programme, which included contouring test cases with one–one review with a clinician, had enabled this change and the doctor was available on call. In the future, 18% of centres envisaged radiographers/RTTs alone and 6% medical doctors and radiographers/RTTs, contouring the target, organs at risk and making the decision to replan /recontour (Q19). Plan checking (12% of centres) and plan creation (6% of centres) were the next most common areas of role expansion for radiographers/RTTs.
Benefits and barriers of implementation
Improvement in patient outcome was the most important driver for implementing ART with nearly 90% of respondents answering as such (Q20). However closer team working was also seen as important aspect and although job satisfaction was rated as important it was the least of the three aspects.
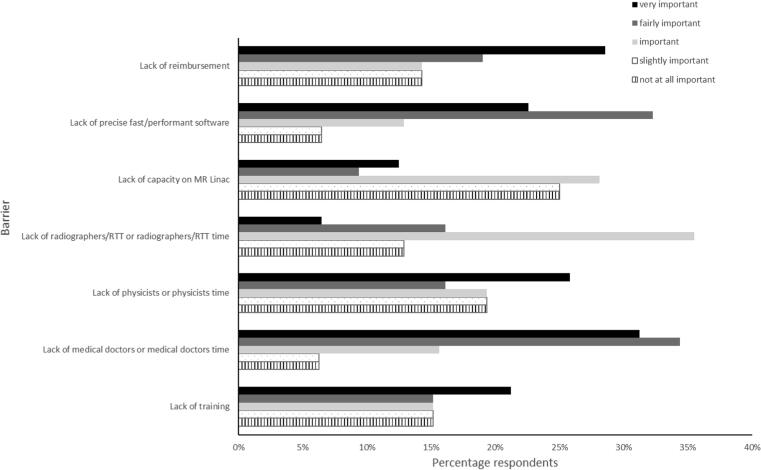
The highest median score of ‘fairly important’ for barriers to implementing ART was attributed to lack of medical doctors and the need for precise/fast software (Q21). Staffing of other professions were the next two important (physicists followed by lack of radiographers), followed by lack of reimbursement and lack of training. Lack of capacity on the MR-Linac/Co60 systems was rated as ‘slightly important’, the least important barrier (Fig. 3).
Fig. 3.
Barriers to implementing or increasing the use of on-line MRI guided ART.
Decision making and criteria - Physicist/Radiographer/RTT and medical doctor perspective
Tumour site specialties
The most common tumour sites physicists and radiographers indicated expertise were prostate (58%) and pancreas (31%). Less than 9% were experts in rectum, oligometastases, liver, palliative, lung, breast and oesophagus, (Q22).The 21 medical doctors who responded indicated speciality in the following tumour sites: Oligometastases (n = 11), rectum (n = 10) and prostate (n = 8) were the most common followed by bladder (n = 7), liver (n = 6), oesophagus (n = 6) and pancreas (n = 5). The remaining tumour sites specialities were head and neck, lung, palliative, breast and cervix. When asked their primary site speciality, the most common was prostate (28%) and pancreas (14%) (Q28)
When asked if they would prefer to use ART regardless of anatomy changes, 44% of medical doctors agreed whilst the remainder would not. A higher number of radiographers and physicists (65%) indicated they would prefer to use ART regardless of anatomy changes (Q23 and Q32).
In routine practice on C arm Linacs, all but two respondents stated that medical doctors saw and approved the treatment plan prior to treatment (Q29). However on the MR-Linac/Co60 systems the most common responses were either ‘approving the plan every day’ or ‘only if constraints weren’t met’ with eight doctors responding as such for each option. Three responded ‘when present treating’, three ‘on request’ and one, ‘once a week’. Two respondents mentioned that because the technology was new the plan was reviewed every day or prior to next fraction, indicating a potential future change in practice (Q30 and Q31)
Criteria in decision to treat with ART
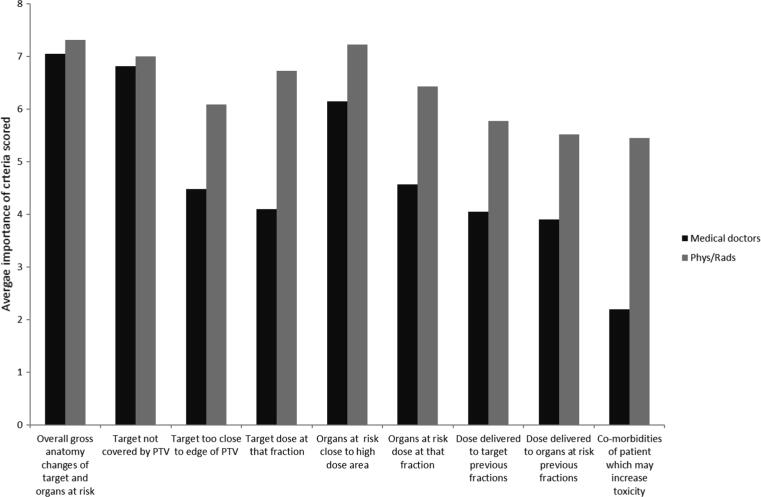
When asked to score nine criteria (where one was least important, and nine most important) to determine if ART should be used, medical doctors scored ‘Overall gross anatomy changes of target and OAR’, ‘target not covered by PTV’ and ‘OAR close to high dose area’ (mean score 7.1, 6.8 and 6.1 respectively) as most important with < 4 in importance (range 2.2–7.1) attributed other factors (Q33). Physicists and radiographers were less clear in rating the importance of criteria, although ‘overall gross anatomy changes of target and OAR’, ‘target not covered by PTV’ and ‘OAR close to high dose area’ were rated as highly (7.3, 7 and 7.2) there was much less distinction between each criteria; range 5.5 to 7.3 (Q24: Fig. 4).
Fig. 4.
The importance of the criteria considered when making the decision to use on-line MRI guided ART where 0 is least important and 8 is most important.
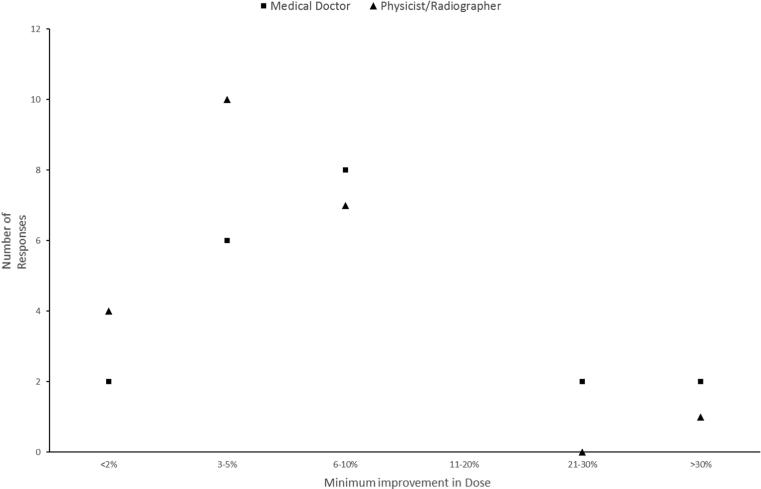
Overall Medical doctors responded slightly higher doses than physicists/radiographers (6–10% and 11–20% as opposed to < 2% and 3–5%) required for the minimum improvement in dose to the tumour volume believed to justify treating with on-line MRI guided ART (Fig. 5) .
Fig. 5.
The minimum improvement in dose to the target that would justify treating with on-line MRI guided ART.
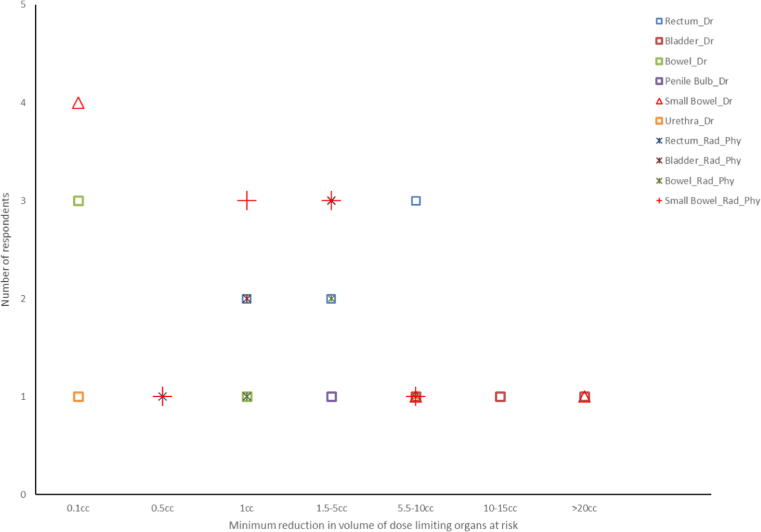
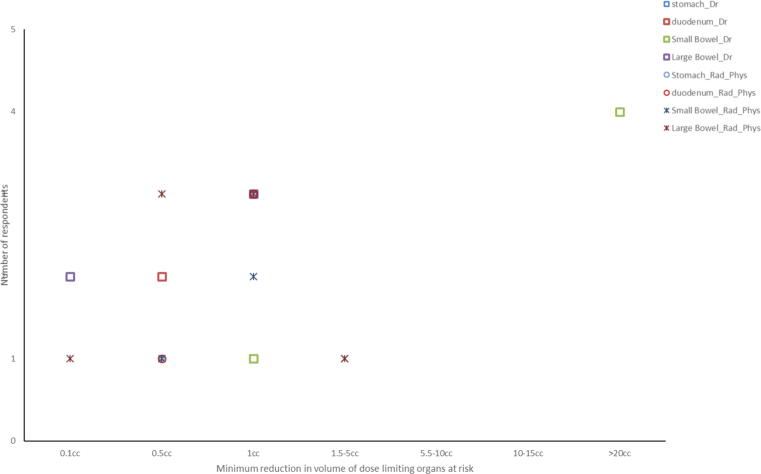
To provide a like for like comparison of professions when deciding the minimum reduction in volume of ‘dose limiting organs at risk (OAR)’ receiving the maximum dose permitted that would justify treating with on line MRI guided ART pancreas and prostate tumour site responses were analysed because the majority of physicists and radiographers/RTTs chose either prostate (58%) or pancreas (31%) as their preferred tumour target and it was most common preferred tumour sites chose by medical doctors (Q28). No clear consensus emerged (Fig. 6a, Fig. 6b) .
Fig. 6a.
The minimum reduction in volume of ‘dose limiting organs at risk’ receiving the maximum dose permitted that would justify treating with on-line MRI guided ART when treating prostate cancer.
Fig. 6b.
The minimum reduction in volume of ‘dose limiting organs at risk’ receiving the maximum dose permitted that would justify treating with on-line MRI guided ART when treating pancreas cancer.
Discussion
Current practice
By investigating current practice of MRIgRT amongst early adopters of the technology, we hope to have improved the understanding of the initial implementation of MRIgRT and the requirements for staffing. This paper also provides a baseline report for comparison with future developments.
The most common tumour sites treated, prostate, pancreas and oligometastases, may have been an indication of treating tumour sites where centres are experienced whilst gaining confidence with the system, for example prostate cancer, versus tumours sites which have either begun to show benefit for example pancreas [20] or sites which promise to be of benefit, for example oligometastases [21]. Caution is needed in interpreting this data as this may be a reflection of the MR-Linac/Co60 systems technologies used. It is noteworthy that the majority of the centres which treated pancreas used Viewray systems, which were the minority of centres in this survey and is a work in progress for the majority of Unity centres . Early reports of implementation from single centres demonstrated a shift over time, with an increase in abdominal tumours and reduction in pelvic malignancies [22].
It was clear that the decision-making process is very complex. This is reflected in two respondents who found it difficult to align current practice with the questions. One comment was that the decision to use a dose shift, re-contour and re-plan varied within a tumour site and was dependant on other criteria for example fractionation, proximity of organ at risks. The other concern was that the dose shift plans delivered sub optimal plans [23], and when this changed the decisions would be different.
Professional responsibilities
Though conventional professional roles dominated current practice a trend towards radiographer/RTT led MRIgRT was a common theme. The Netherlands was the leading country with radiographer led contouring and dosimetry. Elsewhere contouring of OAR was more frequently performed by non-medical staff than contouring of the target, reflecting conventional practice where dosimetrists/radiographers/physicists often contour OAR. Bearing in mind contouring is the weakest link in the chain in the radiotherapy pathway, the best way forward to make the workflow more efficient whilst maintaining or improving quality must be considered. Artificial intelligence (AI) could have an important role here and is under intense investigation at present [24], [25]. However, a human element will most probably remain, and the role and responsibilities will need to be defined.
Many radiographers in the Netherlands are also dosimetrists, whilst this is less common in other European countries; Australia and New Zealand have similar skill mix structures which could influence the workflow and uptake. It was interesting to note that, even though radiographers were taking responsibility for contouring plan checking and creation, the decision to treat with an adaptive plan still lay with medical doctor in all but three centres.
Whilst plan approval continues to be a medical doctor’s responsibility, one solution to avoid doctors being present on the MR-Linac/Co60 systems, would be a process that relies on whether the dose constraints have been met, with automated procedures checking for anomalies, for example hot spots or dose inhomogeneity. The approval process initially, may be more stringent because of introducing a new technology but once confidence is gained and thresholds can be set, guidance with protocols on acceptability could be transferred to the dosimetrist or radiographer. However, this may involve a change of practice because all but two respondents stated that the clinical plan was always reviewed prior to conventional treatments on the C arm linacs.
Drivers and barriers
Not surprisingly improvement in patient outcome was seen as the most important driver towards uptake of this technology. This potential of improved patient outcome has been postulated but needs to be translated into clinical trials and evidence, most likely needing multi-centre collaboration [26].
Closer team working was also noted as important as MRIgRTrequires a wide multi professional team for successful implementation. Although this is resource intensive it may provide a forum for informal conversations to take place which would not happen in a sequential workflow and may improve collaborations and enhance job satisfaction. Moving forward, it will be important to determine and define the most effective workflow for each centre and tumour site, similar to SABR and IGRT treatments, which will also require contribution from the whole team.
The speed and effectiveness of software and lack of staff are currently seen as the main barriers. The treatment times for ART are generally more than 40 minutes, contouring, optimisation and treatment delivery, if gating used, contributing the majority of the time involved [27]. Therefore, any time saving in exporting /importing images or plan optimisation would be of benefit. Software development was also highlighted as an issue in early IMRT treatments was achieved [28]. Solving these two issues would increase the number of patients able to have access and treatment. Issues of capacity and reimbursement may then become of greater importance. However, to create an effective reimbursement programme will require evidence, which will require more resources to increase capacity. The resolution of these issues will vary internationally and may become more prominent as technology becomes increasingly available and treatments move from a research and development environment to a more routine clinical service.
Decision making and criteria
Criteria in decision to treat with ART
Consensus was gained in the main criteria for treating ART from a medical perspective (Overall gross anatomy changes of target and OAR; target not covered by PTV; OAR close to high dose area). This correlated with the fact that the majority of medical doctors did not feel ART should be used regardless of anatomical changes. The difference in opinion from the physicists and radiographers may have been because of the enthusiasm for using new technology or because it has less impact on other clinical commitments.
The dose thresholds for improving outcome and using ART varied between professionals and centres which emphasises the need for multi-centre trials to provide the evidence linking anatomical and/or dosimetric changes to outcome. Otherwise ART may be inefficiently utilized, used when not necessary or not used when necessary. It will also be important for consistent decision to be made between professionals as roles and responsibilities change therefore setting of thresholds for guidance on actions and decision making will be essential. The approach is likely to be tumour site specific and dependant on the predicted variability of anatomy. For example, ART has been shown to have more dosimetric effect early in treatment course in head and neck cancers [29] whereas changes in anatomy in pancreas and bladder patient is less predictable and can occur at any stage [29], [30].
Limitations
As mentioned above the number of centres using Unity (15) outweighed the number of centres with MRidain systems (5), therefore results may be skewed. However, the workflow of both systems involves similar tasks which may make the results may be generisable. It is also to be noted that the survey was created and piloted in the UK which may have contributed to bias in the language and meaning.
Conclusion
Though MRIgRT has been in clinical use since 2014 most centres have started using this technology in the last three years. The most common patients treated initially are patients with prostate cancer, pancreas cancer and oligometastases. Faster software and staff resources were the main barriers to implementing ART. The survey has demonstrated that although conventional workflows and professional responsibilities are currently being used to deliver ART, this is already changing. The most efficient and effective process to implement the current pathway onto MR-Linac/Co60 systems systems needs to be defined to aid further implementation. To do so will require reconsidering the workflow, professional responsibilities and implementing training programmes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This report is independent research funded by the National Institute for Health Research and Health Education England (HEE/ NIHR ICA Programme Senior Clinical Lectureship, Dr Helen McNair, ICA-SCL-2018-04-ST2-002) and supported by the NIHR Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care. Research at The Institute of Cancer Research is supported by Cancer Research UK under Programme C33589/A28284. The Institute of Cancer Research is part of the Elekta MR-linac Consortium. We also acknowledge Elekta MR-Linac Research Consortium for assistance in distributing the survey.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tipsro.2020.08.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Yan D., Vicini F., Wong J. Adaptive radiation therapy. Phys Med Biol. 1997;42:123–132. doi: 10.1088/0031-9155/42/1/008. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz D.L., Garden A.S., Shah S.J., Chronowski G., Sejpal S., Rosenthal D.I. Adaptive radiotherapy for head and neck cancer–dosimetric results from a prospective clinical trial. Radiother Oncol. 2013;106(1):80–84. doi: 10.1016/j.radonc.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Posiewnik M., Piotrowski T. A review of cone-beam CT applications for adaptive radiotherapy of prostate cancer. Phys Med. 2019 Mar;59:13–21. doi: 10.1016/j.ejmp.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Kong VC, Taylor A, Rosewall T. Adaptive Radiotherapy for Bladder Cancer-A Systematic Review. J Med Imaging Radiat Sci. 2017 Jun;48(2):199-206. [DOI] [PubMed]
- 5.Hafeez S., McDonald F., Lalondrelle S., McNair H., Warren-Oseni K., Jones K. Clinical Outcomes of Image Guided Adaptive Hypofractionated Weekly Radiation Therapy for Bladder Cancer in Patients Unsuitable for Radical Treatment. Int J Radiat Oncol Biol Phys. 2017 May 1;98(1):115–122. doi: 10.1016/j.ijrobp.2017.01.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel E, Tsang Y, Baker A, Callender J, Hafeez S, Hall E, et al. Quality assuring “Plan of the day” selection in a multicentre adaptive bladder trial: Implementation of a pre-accrual IGRT guidance and assessment module. Clin Transl Radiat Oncol. 2019 Jul 24; 19:27-32. doi: 0.1016/j.ctro.2019.07.006. [DOI] [PMC free article] [PubMed]
- 7.Mutic S., Dempsey J.F. The Viewray system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196e199 doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Lagendijk J.J., Raaymakers B.W. Van Vulpen M. The magnetic resonance imaging linac system. Semin Radiat Oncol. 2014;24:207e209 doi: 10.1016/j.semradonc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Mayles W.P.M. Survey of the Availability and Use of Advanced Radiotherapy Technology in the UK. Clin Oncol (R Coll Radiol) 2010;22:636–642. doi: 10.1016/j.clon.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Grau C., Defourny N., Malicki J., Dunscombe P., Borras J.M., Coffey M. Radiotherapy equipment and departments in the European countries: final results from the ESTRO-HERO survey. Radiother Oncol. 2014 Aug;112(2):155–164. doi: 10.1016/j.radonc.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Duchesne G.M., Grand M., Kron T. Trans Tasman Radiation Oncology Group: Development of the Assessment of New Radiation Oncology Technology and Treatments (ANROTAT) Framework. J Med Imaging Radiat Oncol. 2015;59(3):363–370. doi: 10.1111/1754-9485.12255. [DOI] [PubMed] [Google Scholar]
- 12.AlDuhaiby E.Z., Breen S., Bissonnette J.P., Sharpe M., Mayhew L., Tyldesley S. A national survey of the availability of intensity-modulated radiation therapy and stereotactic radiosurgery in Canada. Radiat Oncol. 2012;7(7):18. doi: 10.1186/1748-717X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mell L.K., Mehrotra A.K., Mundt A.J. Intensity-modulated radiation therapy use in the U.S 2004. Cancer. 2005;104:1296–1303. doi: 10.1002/cncr.21284. [DOI] [PubMed] [Google Scholar]
- 14.Padayachee J., Loh J., Tiong A., Lao L. National survey on image-guided radiotherapy practice in New Zealand. J Med Imaging Radiat Oncol. 2018;62(2):262–269. doi: 10.1111/1754-9485.12682. [DOI] [PubMed] [Google Scholar]
- 15.National Radiotherapy Implementation Group Report. Image Guided Radiotherapy (IGRT) Guidance for Implementation and use. National Health Service, 2012.
- 16.Nabavizadeh N., Elliott D.A., Chen Y., Kusano A.S., Mitin T., Thomas C.R., Jr Image guided radiation therapy (IGRT) practice patterns and IGRT’s impact on workflow and treatment planning: results from a national survey of American Society for Radiation Oncology members. Int J Radiat Oncol Biol Phys. 2016;94:850–857. doi: 10.1016/j.ijrobp.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Bertelsen A.S., Schytte T., Møller P.K., Mahmood F., Riis H.L., Gottlieb K.L. First clinical experiences with a high field 1.5 T MR linac. Acta Oncol. 2019;58(10):1352–1357. doi: 10.1080/0284186X.2019.1627417. [DOI] [PubMed] [Google Scholar]
- 18.Lamb J, Cao M, Kishan A, Agazaryan N, Thomas DH, Shaverdian N, Yang Y, Ray S, Low DA, Raldow A, Steinberg ML, Lee P. Online Adaptive Radiation Therapy:Implementation of a New Process of Care. Cureus. 2017 Aug 27;9(8):e1618. doi:10.7759/cureus.1618. [DOI] [PMC free article] [PubMed]
- 19.Bertholet, G. Anastasi, D. Noble et al., Patterns of practice for adaptive and real-time radiation therapy (POP-ART RT) part II:Offline and online plan adaption for interfractional changes, Radiotherapy and Oncology, https://doi.org/10.1016/j.radonc.2020.06.017 [DOI] [PMC free article] [PubMed]
- 20.El-Bared N., Portelance L., Spieler B.O., Kwon D., Padgett K.R., Brown K.M. Dosimetric Benefits and Practical Pitfalls of Daily Online Adaptive MRI-Guided Stereotactic Radiation Therapy for Pancreatic Cancer. Pract Radiat Oncol. 2019 Jan;9(1):e46–e54. doi: 10.1016/j.prro.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Henke L., Kashani R., Yang D., Zhao T., Green O., Olsen L. Simulated Online Adaptive Magnetic Resonance-Guided Stereotactic Body Radiation Therapy for the Treatment of Oligometastatic Disease of the Abdomen and Central Thorax: Characterization of Potential Advantages. Int J Radiat Oncol Biol Phys. 2016 Dec 1;96(5):1078–1086. doi: 10.1016/j.ijrobp.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henke LE, Contreras JA, Green OL, Cai B, Kim H, Roach MC, et al,. Magnetic Resonance Image-Guided Radiotherapy (MRIgRT): A 4.5-Year Clinical Experience. Clin Oncol (R Coll Radiol). 2018 Nov;30(11):720-727. doi:0.1016/j.clon.2018.08.010. [DOI] [PMC free article] [PubMed]
- 23.Winkel D., Bol G.H., Kroon P.S., van Asselen B., Hackett S.S., Werensteijn-Honingh A.M. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019 Apr;2(18):54–59. doi: 10.1016/j.ctro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorino C., Guckemberger M., Schwarz M., van der Heide U.A., Heijmen B. Technology-driven research for radiotherapy innovation. Mol Oncol. 2020 Mar doi: 10.1002/1878-0261.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreier J., Genghi A., Laaksonen H., Morgas T., Haas B. Clinical evaluation of a full-image deep segmentation algorithm for the male pelvis on cone-beam CT and CT. Radiother Oncol. 2019 Dec;20(145):1–6. doi: 10.1016/j.radonc.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Purdy J.A. Intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 1996 Jul 1;35(4):845–846. doi: 10.1016/0360-3016(96)00223-4. [DOI] [PubMed] [Google Scholar]
- 27.Anastasi G., Bertholet J., Poulsen P., Roggen T., Garibaldi C., Tilly N. Patterns of practice for adaptive and real-time radiation therapy (POP-ART RT) part I: Intra-fraction breathing motion management. Radiother Oncol. 2020 Jun 23;S0167–8140(20):30343–30351. doi: 10.1016/j.radonc.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz D.L., Garden A.S., Shah S.J., Chronowski G., Sejpal S., Rosenthal D.I. Adaptive radiotherapy for head and neck cancer–dosimetric results from a prospective clinical trial. Radiother Oncol. 2013 Jan;106(1):80–84. doi: 10.1016/j.radonc.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Hoisak JD, Li N, Jiang C, Tian Z, Gautier Q, Zarepisheh M et al. Dosimetric benefit of adaptive re-planning in pancreatic cancer stereotactic body radiotherapy. Med Dosim. 2015 Winter;40(4):318-24. [DOI] [PubMed]
- 30.Verkooijen H.M., Kerkmeijer L.G.W., Fuller C.D., Huddart R., Faivre-Finn C., Verheij M. R-IDEAL: A Framework for Systematic Clinical Evaluation of Technical Innovations in Radiation Oncology. Front Oncol. 2017 Apr;3(7):59. doi: 10.3389/fonc.2017.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.