Abstract
White cord syndrome is a rare condition involving sudden neurological deterioration following a decompressive cervical spinal surgery and characterized by the appearance of hyperintensity on T2-weighted magnetic resonance imaging. We present a report of a pediatric male patient who presented with the condition. This case shows that white cord syndrome can also be present in pediatric patients. We provide a brief review of the literature highlighting the main radiologic findings.
Keywords: White cord syndrome, Spine, Reperfusion injury
Introduction
White cord syndrome, also known as reperfusion injury of the spinal cord, is a rare complication of spinal decompression surgery and only occurs in chronically compressed cords. Neurological deficits are a rare complication of spinal decompression surgery and are usually the result of an expansive hematoma or iatrogenic injury. In the absence of any clear etiology, the cause of post-operative neurological deficits may be related to a reperfusion injury. White cord syndrome refers to sudden neurological deterioration following cervical decompression surgery and is characterized by the appearance of an increase in signal intensity on T2-weighted magnetic resonance imaging (MRI) — We report a case of white cord syndrome and review the imaging and clinical findings of this rare entity.
Case report
A 12-year-old male patient with a ventriculoperitoneal shunt and chronic nonprogressive encephalopathy, secondary to neonatal pneumococcal meningitis at 23 days old, evolves with spastic tetraparesis and epilepsy. No other history was elicited. On admission the patient presented with headache and dystonic posture of the upper limb. During examination the patient was alert and oriented. A non-contrast-enhanced computed tomography was performed revealing no signs of shunt failure. An MRI of the brain was subsequently performed revealing a cyst in the anterior portion of the cervical canal leading to cord compression (Fig. 1). Following this, an MRI of the cervical spine was acquired confirming the presence of the cyst in the intradural extramedullary space of the anterior portion of the cervical spinal canal, extending from C2 to C4 (Fig. 2). The cyst followed the signal intensity of the cerebral spinal fluid on T2WI and T1WI and was interpreted as an acquired arachnoid cyst (postmeningitis). The spinal cord below the level of the compression (C4) showed swelling and an increase in the signal intensity on T2WI, suggestive of edema or gliosis. These findings were consistent with compressive myelopathy. The patient was started on oral dexamethasone and ketorolac.
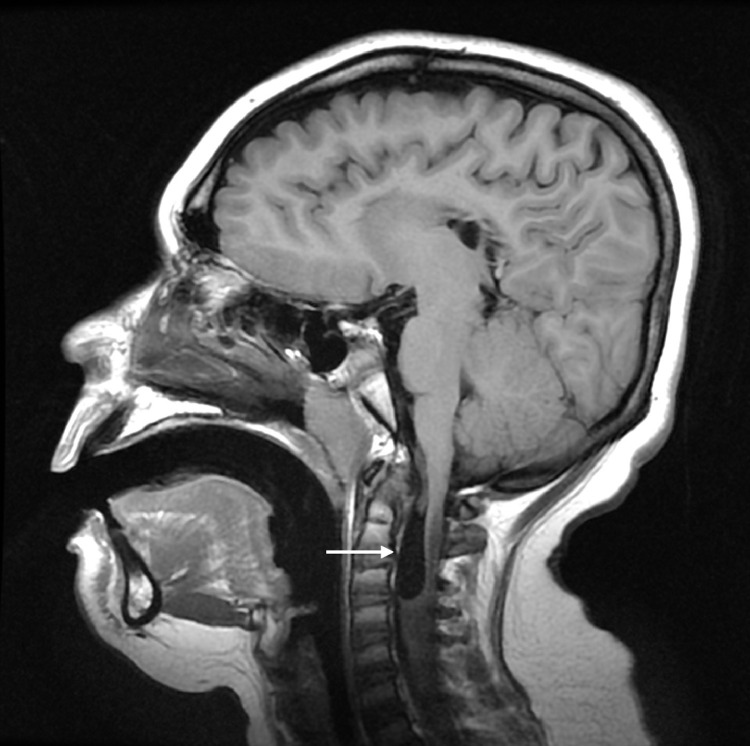
Fig. 1.
Brain MRI. Sagittal T1WI shows a cyst structure in the anterior portion of the cervical canal (white arrow).
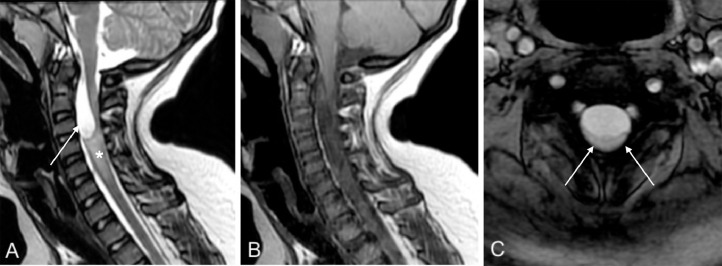
Fig. 2.
Cervical spine MRI. Sagittal T2WI (A) shows the presence of the cyst (–arrow) in the intradural extramedullary space of the cervical spinal canal, extending from C2 to C4 and causing cord compression. The cervical cord shows swelling and hyperintense signal on T2WI (asterisk). Postcontrast sagittal T1WI (B) shows no enhancement. Axial GRE (C) shows marked compression and increase in the signal intensity in the spinal cord (–arrows).
Based on the clinical and radiological findings posterior cervical decompressive surgery and arachnoid cyst fenestration were performed 4 days later. During the surgical procedure extensive fibrosis and arachnoid adhesions were detected in the intradural space of the spinal canal. This was in addition to the thick wall of the cyst, which supported our theory of the presence of an acquired arachnoid cyst. No complications were reported during the procedure. Immediately after the surgery the child presented with right arm monoplegia. Postsurgical MRI performed the same day of the intervention revealed a marked increase in the extension of the intramedullary and mainly central hyperintensity on T2WI (Fig. 3) with no contrast enhancement in postgadolinium T1WI. The differential diagnosis from the imaging included an iatrogenic cord injury and white cord syndrome. The patient continued on dexamethasone and ketorolac as well as starting on paracetamol.
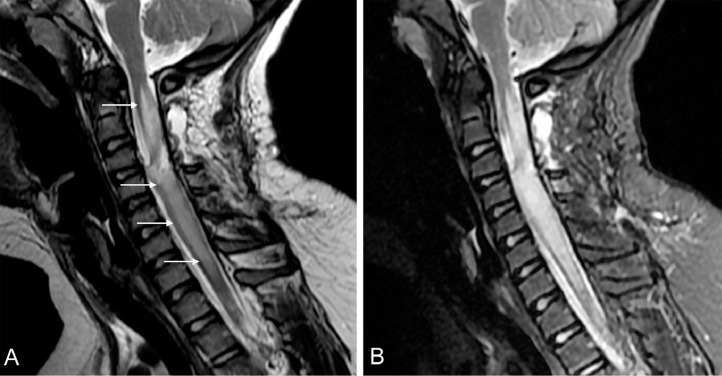
Fig. 3.
Postsurgical cervical MRI. Marked increase in the extension of the hyperintensity in the spinal cord (white arrows) show on sagittal T2WI (A) and STIR (B). The hyperintensity on T2 extend from C2 to C7-T1.
One month and 9 days later, the patient presented with facial flushing, profuse sweating, nausea, severe headaches, and tachycardia during inpatient rehabilitation. These were interpreted as symptoms of dysautonomia. The patient was referred and admitted to our institution, where an MRI was performed. The MRI revealed an increase in the extension of the hyperintensity on T2WI, now extending to the dorsal cord. They also revealed an enlargement of the cervical cord (Fig. 4). Intravenous dexamethasone was administered based on the diagnosis of white cord syndrome. The patient's clinical response to high-dose steroids was excellent. Right arm mobility gradually started to improve with rehabilitation therapy, while the steroids were gradually decreased. A follow-up MRI was performed 2 months after the surgery. The images revealed a marked decrease in the extension of the hyperintensity on T2WI as well as a decrease in the swelling of the cord (Fig. 5). The MRI showed a residual hyperintense area on T2WI in the cervical cord, suggestive of gliosis, as well as the reappearance of the cyst. However, the cyst was now smaller than the one that was originally observed. The patient suffered chronic neuralgic pain in the right arm as a sequel but demonstrated adequate mobility.
Fig. 4.
Spinal MRI performed 1 month and 9 days after the surgery. Sagittal T2 (A) shows swelling (asterisk) of the spinal cord and increase of the extension of the hyperintensity, extending to the dorsal cord (T5). Axial T2 (B) at the level of T3 shows hyperintensity in the dorsal cord (asterisk).
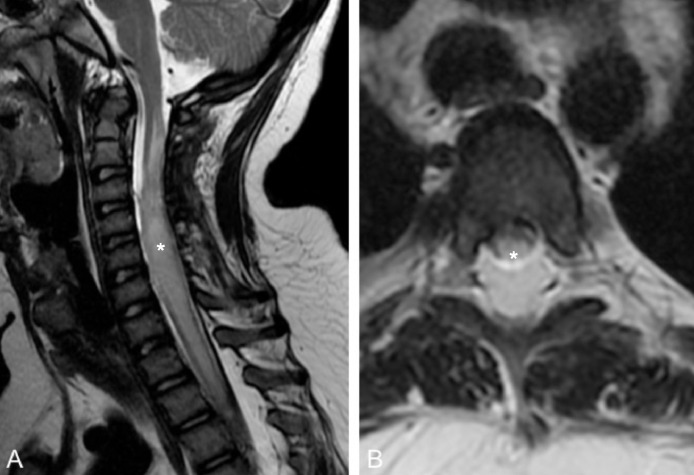
Fig. 5.
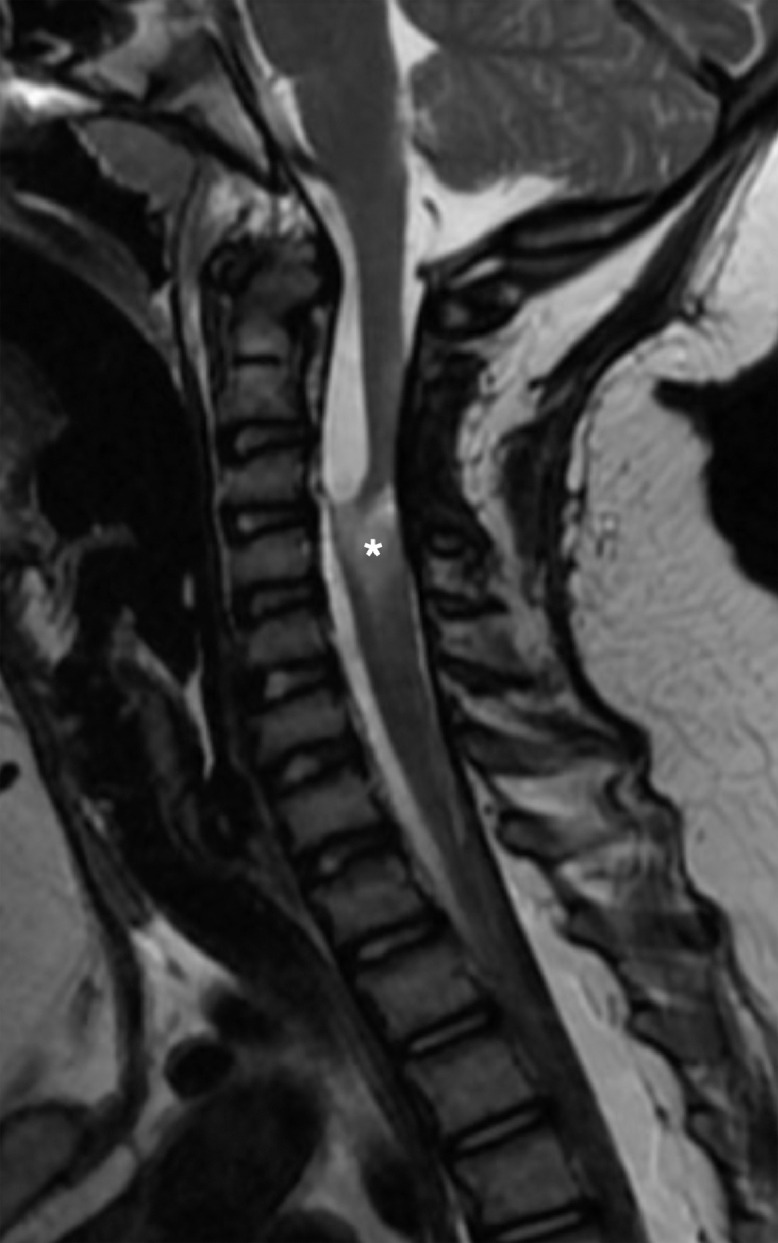
Follow up by MRI 2 months later. Sagittal T2WI shows a residual hyperintense lesion on in the cervical cord, compatible with myelomalacia (asterisk).
Discussion
White cord syndrome is a very rare condition. It is characterized by an ischemic injury in the spinal cord following spinal decompression surgery in the absence of an iatrogenic cord injury or perioperative complications [1]. This ischemic injury associated with edema of the cord is attributed to an injury caused by immediate reperfusion of the newly decompressed cord [2]. Clinically speaking, this condition is characterized by sudden neurological deterioration following spinal decompression surgery and occurs in a chronically compressed cord. A recent case report showed a late onset of symptoms, following 24 hours of surgery. This is in contrast to other reports, which describe the neurologic deficit appearing during surgery or immediately after [3]. From a radiological perspective, white cord syndrome is characterized by a new hyperintense lesion on T2WI in the previously compressed spinal cord. This hyperintensity is usually detected through an MRI scan following the decompressive surgery. Furthermore, it can also be characterized by an increase in the extension of the hyperintensity on T2WI in a previously compressed cord with compressive myelopathy. The term “white cord syndrome” was introduced by Chin et al [4], in reference to the appearance of the spinal cord on T2WI sagittal imaging. On T1WI the lesion shows a hypointense signal. None of the previous case reports demonstrated contrast enhancement following the administration of gadolinium. Swelling of the spinal cord can also occur, as is shown in our case. This is the sixth case report of white cord syndrome and, to the best of our knowledge, the first case occurring in a pediatric patient reported in the English literature. In this case the cause of the compression is an acquired arachnoid cyst. In the previous reports, the causes of the compression included disc herniation, cervical canal stenosis, and vertebral metastasis. Furthermore, the extension of the hyperintensity on T2WI was more marked than in previous reports, to the extent that there was even cord enlargement. There was also a worsening of the symptomatology 1 month after the surgery. This was demonstrated by a progression of the T2WI hyperintensity within the spinal cord on the MRI images, a finding that is both unusual and previously unreported. The cyst reported in the present case is secondary to arachnoid inflammation as a result of neonatal pneumococcal meningitis. All 6 cases of white cord syndrome (including the present case) were managed with high doses of steroids. The outcomes of the cases were different, with 4 cases presenting partial recovery and 2 presenting no recovery. Our case started to improve clinically 1 month and 10 days after the initial presentation. This presentation differs from the previous cases reporting partial recovery [1,2,4]. In these 3 cases, all of which involved adult patients, only a few days were needed in order to see an improvement in the symptoms. There are no risk factors associated with white cord syndrome. All reported cases presented in the presurgical MRI, with hyperintensity on T2WI in the spinal cord suggestive of myelomalacia. This may seem to suggest that patients with cervical myelopathy are more vulnerable to reperfusion injuries. However, the development of white cord syndrome following decompressive surgery on a spinal cord with compressive myelopathy is exceptionally rare. The etiology of white cord syndrome is unknown. Nevertheless, the most widely accepted theory is that it develops from a reperfusion injury on a chronically compressed cord [1,2]. In this sense, the chronically compressed cord becomes susceptible to ischemia due to occlusive thrombi and the presence of edema from an increase in vascular permeability and venous congestion [4]. As with cerebral reperfusion injuries in animals, the blood-spinal barrier is probably disrupted in the ischemic cord. This leads to an increase in vascular permeability, which in turn leads to the release of inflammatory mediators such as TNF-a. These inflammatory mediators then increase the levels of oxygen-derived free radicals, resulting in neuronal membrane damage [5], [6], [7]. The positive response to high doses of steroids reported in several cases (including the present case) would seem to support the previous hypothesis.
The main differential diagnosis of white cord syndrome is an iatrogenic injury and cord compression from a postsurgical hematoma. The surgical history in addition to the imaging follow up can help to discard an iatrogenic injury. A postsurgical hematoma is easily recognizable in the MRI scan.
Ischemia by hypoperfusion and any undiagnosed demyelinating disorder should be ruled out. Diffusion-weighted imaging sequence can be useful to perform the differential diagnosis with ischemia [8]. The clinical history and the study of the brain with MRI could help to rule out a demyelinating disease.
Conclusion
White cord syndrome is an extremely rare condition. We have described a case to occur in a pediatric patient. Furthermore, in our case the patient presents with a late clinical and radiological exacerbation. MRI is an essential tool for identifying this entity. This is because it allows some of the other common causes of sudden neurological deterioration as a complication of cervical spinal decompression surgery to be ruled out. In the absence of any other clear etiology, the diagnosis of white cord syndrome is based on the clinical history of the acute neurological deficit and the appearance of T2 hyperintense lesions on MRI of the spinal cord following decompressive cervical spinal surgery.
Patient Consent Statement
The adult responsible for the presented patient has signed the authorization for the use of the case for academic purposes.
Contributor Information
Francisco Sepulveda, Email: francisco.sepulvedah@redsalud.gov.cl.
Leandro Carballo, Email: lcarballo@fleni.org.ar.
Martin Carnevale, Email: mcarnevale@fleni.org.ar.
Paulina Yañez, Email: pyanez@fleni.org.ar.
References
- 1.Giammalva G, Maugeri R, Graziano F, Guli C, Giugno A, Basile L. White cord syndrome after non-contiguous double-level anterior cervical decompression and fusion (ACDF): a “no reflow phenomenon. Interdisc Neurosurg Adv Tech Case Managt. 2017;7:47–49. [Google Scholar]
- 2.Antwi P, Grant R, Kuzmik G, Abbed K. “White Cord Syndrome” of Acute Hemiparesis After Posterior Cervical Decompression and Fusion for Chronic Cervical Stenosis. World Neurosurg. 2018;113:33–36. doi: 10.1016/j.wneu.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Papaioannou I, Repantis T, Baikousis A, Korovessis P. Spinal cord series and cases: clinical management in spinal cord disorders. 2019;5:28. doi: 10.1038/s41394-019-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin K, Seale J. Cumming V,“White cord syndrome”of acute tetraplegia after an anterior cervical decompression and fusion for chronic spinal cord compression: a case report. Case Rep Orthop. 2013 doi: 10.1155/2013/697918. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Yoshioka H, Pak H, Chan P. Oxidative stress in ischemic brain damage: mechanism of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan W, Banks W, Kastin A. Blood-brain barrier permeability to ebiratide and TNF in acute spinal cord injury. Exp Neurol. 1997;146:367–373. doi: 10.1006/exnr.1997.6533. [DOI] [PubMed] [Google Scholar]
- 7.Shan L, Sai M, Qiu X, Zhou Y, Zhang Y, Zheng L. Hydroxysafflor Yellow A protects spinal cords from ischemia/reperfusion injury in rabbits. BMC Neurosci. 2010;11:98. doi: 10.1186/1471-2202-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurnher MM, Bammer R. Diffusion-weighted MR imaging (DWI) in spinal cord ischemia. Neuroradiology. 2006;48(11):795–801. doi: 10.1007/s00234-006-0130-z. [DOI] [PubMed] [Google Scholar]