Abstract
Our case involved a 1-year-old female with multiple admissions for chest infections. Given her family history and high clinical suspicion, a diagnosis of Griscelli syndrome and hemophagocytic lymphohistiocytosis was made. Her work-up included a brain MRI, which revealed diffuse volume loss and corpus callosum hypogenesis associated with a diffuse simplified pattern of the sulci and gyri compatible with lissencephaly.
We describe hypogenesis of the corpus callosum and lissencephaly for the first time in this syndrome.
Keywords: Griscelli, Hemophagocytic lymphohistiocytosis, Lissencephaly, Corpus callosum, Hypogenesis
Introduction
Griscelli syndrome is characterized by hypopigmentation of the skin, silver-gray hair, and severe immunodeficiency. It is inherited in an autosomal recessive pattern. The syndrome was first described by Claude Griscelli, a French pediatrician and immunology researcher, in 1978 [1].
Approximately 150 cases have been reported in the literature so far as per the Genetic and Rare Diseases Information Center [2].
Three main variations of Griscelli syndrome have been identified. Type 1 is associated with mutations in the Myosin VA gene, while the second type is related to mutations in RAS oncogene family (RAB27A). Both types are seen in chromosomal region 15q12. Type 3 Griscelli syndrome is caused by mutations in melanophilin [2].
The deficiency of melanocytes in the aforementioned genes may lead to the agglomeration of melanosomes near the microtubule organizing center and total loss of transfer to keratinocytes. It is thought that RAB27A-melanophilin-Myosin VA forms a tripartite complex facilitating intracellular melanosome transport [3].
Neurological symptoms such as hypotonia, seizures and developmental delay are the initial presentation of patients with type 1 Griscelli syndrome.
Patients with type 2 usually have concurrent hemophagocytic lymphohistiocytosis, which causes uncontrolled proliferation of lymphocytes and macrophages, leading to severe immunodeficiency. This condition is manifested clinically by high-grade fever, hepatosplenomegaly, lymphadenopathy and jaundice. Type 3 is clinically indolent.
All three variants present with hypopigmentation and light hair color [2].
Griscelli syndrome may be described as Chediak-Higashi-like syndrome, as both present in a similar fashion. They are differentiated by chromosomal analysis and lack of giant granules in Griscelli syndrome hair samples.
Treatment regimens are specifically dependent on the variant. Type 1 is tailored to the patient's symptoms and clinical presentation. Type 2 is usually fatal and requires bone marrow transplant, while type 3 does not require any treatment.
Case description
This case involved a 12-month-old female with a history of prior admissions for chest infections. Her first admission was at 3 months of age, while her second admission was at 6 months. Her family history was positive for consanguinity. She had silver-gray hair and fair skin, which was similar to her 8-year-old sister, who is known to have Griscelli syndrome with hemophagocytic lymphohistiocytosis.
Given her family history and clinical presentation, a hair sample was obtained and examined under a microscope. It showed small and irregular pigmentation, consistent with Griscelli syndrome. Chromosomal analysis confirmed these findings and demonstrated that mutations in chromosome region 15q21.3 affecting the gene RAB27A. Hence, the diagnosis of type 2 Griscelli syndrome with associated hemophagocytic lymphohistiocytosis was established.
The patient was admitted to the pediatric hematology oncology service at our hospital due to pyrexia, pancytopenia, hepatosplenomegaly and respiratory distress.
Her treatment regimen included chemotherapy, empirical antibiotics, blood transfusions and bone marrow transplant.
Suspicion of central nervous system involvement related to hemophagocytic lymphohistiocytosis and failure to thrive prompted the clinical team to request brain magnetic resonance imaging (MRI).
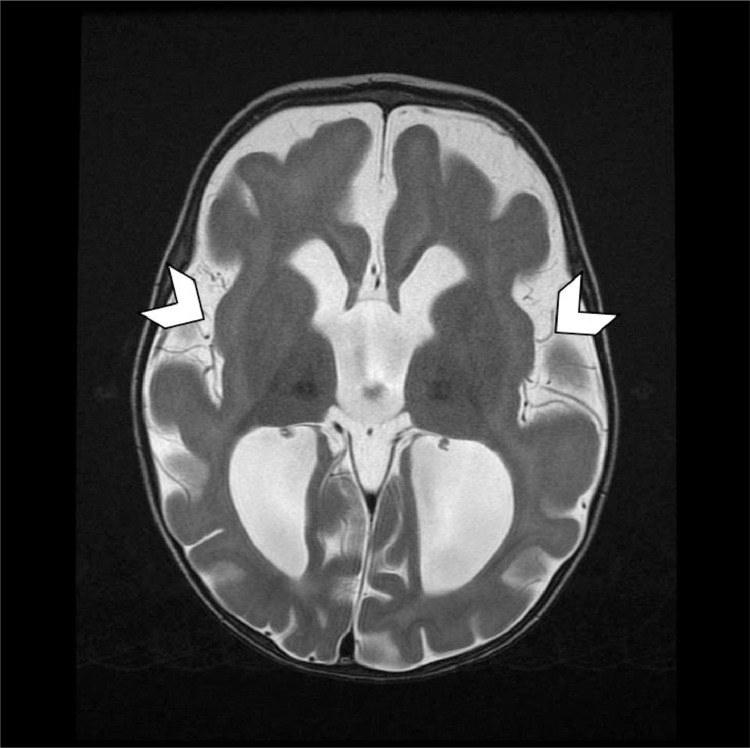
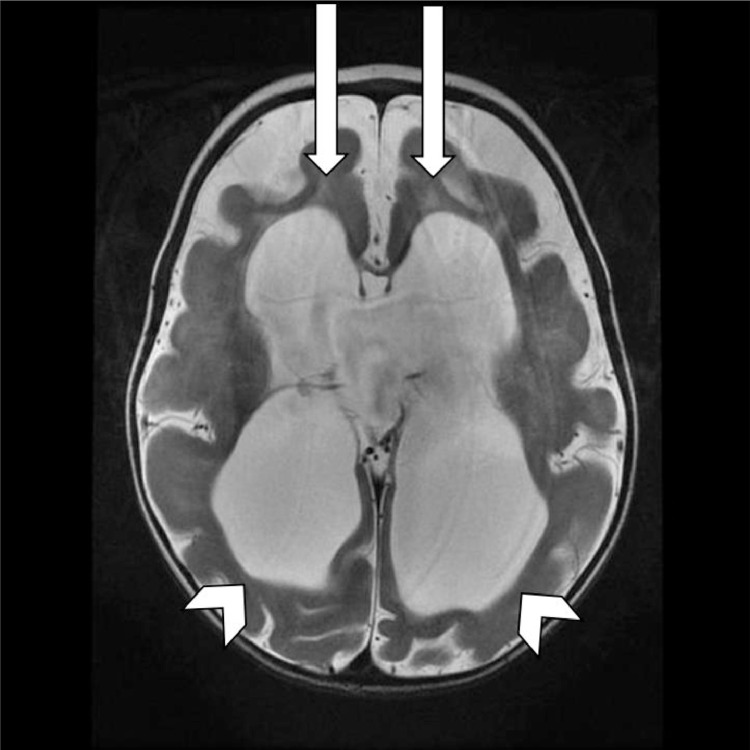
The MRI revealed diffuse prominence of the ventricles and extra-axial CSF (Cerebrospinal fluid) spaces related to diffuse volume loss, under opercularization of the sylvian fissures and cortical thickening (Fig. 3).
Fig. 3.
Axial T2 weighted image demonstrates under opercularization of the sylvian fissures (arrow heads).
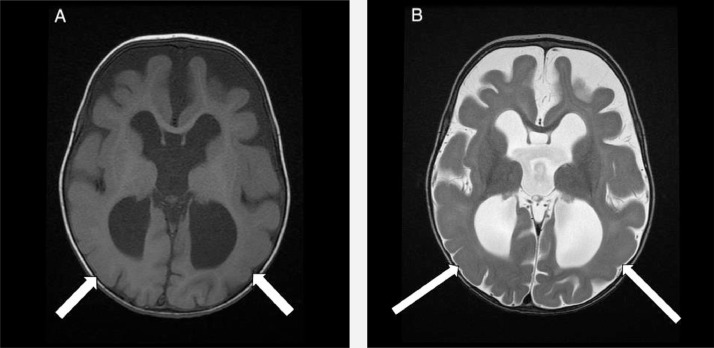
A simplified pattern of the sulci and gyri compatible with lissencephaly was also observed (Fig. 2).
Fig. 2.
Axial T1 (A) and Axial T2 (B) weighted images demonstrate diffuse simplified pattern of the sulci and gyri compatible with lissencephaly (white arrows).
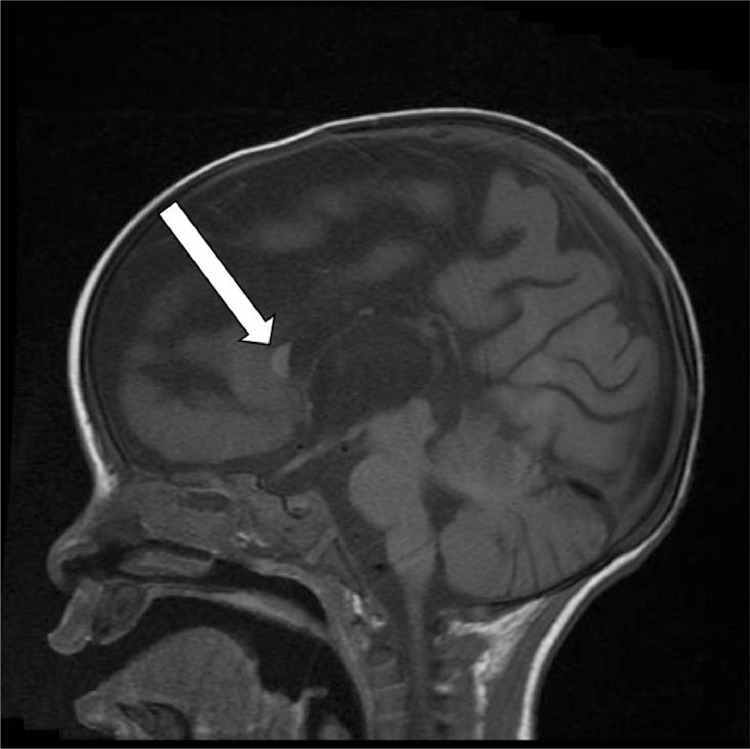
Dysplastic formation of the genu with hypogenesis of the remaining corpus callosum was noted (Fig. 1).
Fig. 1.
Sagittal T1 weighted image demonstrates Dysplastic formation of the genu (white arrow) with hypogensis of the remaining corpus callosum on the initial scan.
No CNS findings of hemophagocytic lymphohistiocytosis were identified on this exam.
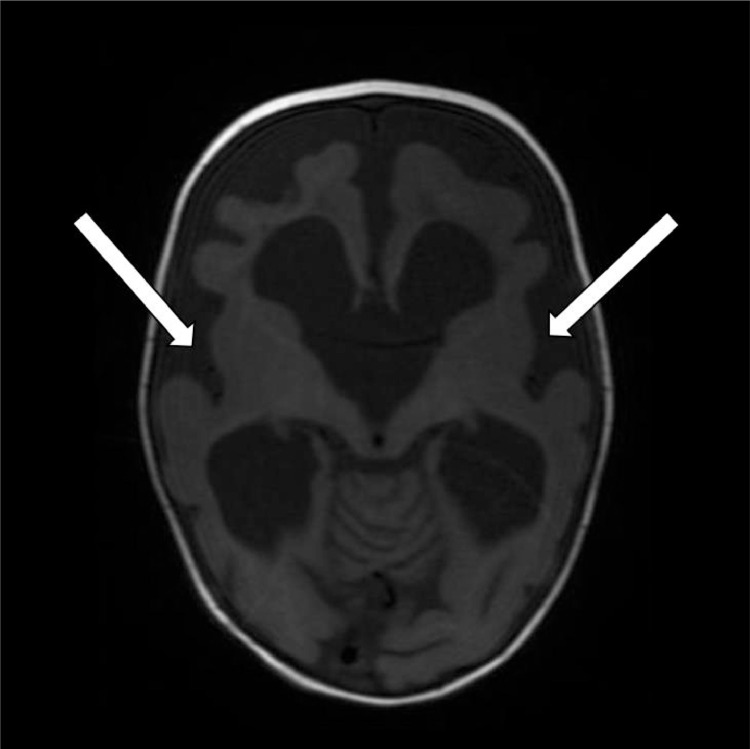
The patient remained in the hospital for four months due to her treatment regimen and chest infection. Within that time period, a follow-up MRI revealed marked interval progression of the diffuse parenchymal volume loss of the cerebrum and, to a lesser extent, the cerebellum (Fig. 4).
Fig. 4.
Axial T1 weighted image on follow up MRI demonstrates further progression of the diffuse parenchymal volume atrophy, progressive diffuse enlargement of the ventricular system and persistent enlargement of the cerebral sulci (white arrows).
The development of abnormal white matter T2 hyperintensities related to hemophagocytic lymphohistiocytosis was also noted (Fig. 5). Hypogenesis of the corpus callosum with diffuse simplified gyri and sulci was also redemonstrated.
Fig. 5.
Axial T2 weighted image on follow up MRI demonstrates development of bilateral frontoparietal confluent white matter hyperintensities (long arrows) and in the periventricular and subcortical regions (arrow heads).
She was later admitted to the pediatric intensive care unit in acute respiratory failure. This was due to an acute lung injury related to her bone marrow transplant and was worsened by her chronic lung disease secondary to unresolved disseminated pneumonia.
Her course was complicated by acute kidney injury and septic shock. The patient's condition unfortunately deteriorated, and she passed away.
Discussion
Griscelli syndrome has been described in the literature from clinical and genetic aspects; however, little is known about its radiological findings. In this case, we describe the neuroimaging findings of a patient with type 2 Griscelli syndrome as well as a review of the literature.
Given the high association of Griscelli syndrome with hemophagocytic lymphohistiocytosis (especially with type 2) [2], MRI is utilized to assess the neurological burden of the disease.
Findings in the literature can be categorized into nonspecific white matter changes, contrast enhancement and global volume loss [4]. High T2 signal lesions with enhancement have been described in the supra- and infratentorial brain with involvement of the basal ganglia, thalamus and corpus callosum [5,6]. These features could be attributed to hemophagocytic lymphohistiocytosis and may remain nonspecific.
In a case series described by Kalekar and Khadse in 2014, an Indian family of 9 siblings was clinically diagnosed with Griscelli syndrome and had brain MRIs as part of their work-up.
This majority of cases demonstrated dilated CSF spaces and cerebral and cerebellar atrophy.
Diffuse leptomeningitis, early hydrocephalus, nonenhancing frontal lesions, diffuse cerebral edema and tonsillar herniation were observed in one of the siblings. This was thought to be related to focal cerebritis and was followed up subsequently until it resolved.
One study demonstrated no abnormal findings, despite the patient presenting with seizures and developmental delay [7].
In addition to the aforementioned findings, involvement of the brain stem, cervical and thoracic spine have also been described to a lesser extent [8], [9], [10].
Brain computed tomography (CT) may be obtained on an emergency basis in the context of an acute insult.
CT findings include calcifications in the globus pallidus, parietal white matter and brachium pontis [11].
In our case, the first MRI did not show any features of hemophagocytic lymphohistiocytosis. It demonstrated diffuse prominence of the ventricles and extra-axial CSF spaces related to diffuse volume loss, under opercularization of the sylvian fissures and cortical thickening (Fig. 3). While these findings are recognized in patients with this rare syndrome, 2 unexpected findings were observed.
The corpus callosum was not completely formed with only partial formation of the genu, consistent with hypogenesis (Fig. 1). The sulci and gyri demonstrated a diffuse simplified pattern compatible with lissencephaly (Fig. 2).
A follow-up MRI of the brain was requested 3 months later. It revealed marked interval progression of the diffuse parenchymal volume loss (Fig. 4). Interval development of increased T2 signal seen in the frontoparietal region, subcortical and periventricular white matter was consistent with findings of hemophagocytic lymphohistiocytosis (Fig. 5).
Hypogenesis of the corpus callosum with diffuse simplified gyri and sulci were also demonstrated.
Lissencephaly and hypogenesis of the corpus callosum have not been described in the literature as imaging findings of this syndrome.
In the proper clinical context, such imaging findings may aid in the diagnosis of Griscelli syndrome. We hope that the findings will lead to further imaging classification/diagnosis for this rare entity.
References
- 1.Griscelli C, Prunieras M. Pigment dilution and immunodeficiency: a new syndrome. Int J Dermatol [Internet] 1978;17(10):788–791. doi: 10.1111/j.1365-4362.1978.tb05980.x. Available from: https://www.ncbi.nlm.nih.gov/m/pubmed/730432/ [DOI] [PubMed] [Google Scholar]
- 2.(NCATS) NC for ATS. Griscelli syndrome [Internet]. Available from: https://rarediseases.info.nih.gov/diseases/10913/griscelli-syndrome. Last updated: 11/1/2018
- 3.Van Gele M, Dynoodt P, Lambert J. Griscelli syndrome: a model system to study vesicular trafficking. Pigment Cell Melanoma Res [Internet] 2009;22(3):268–282. doi: 10.1111/j.1755-148X.2009.00558.x. https://pubmed.ncbi.nlm.nih.gov/19243575/ Available from: [DOI] [PubMed] [Google Scholar]
- 4.Meena KR, Kumar P, Anita A, Paul P. Griscelli Syndrome Type 2 With Hemophagocytic Lymphohistocytosis a Rare and Lethal Disorder. Int J Clin Pediatr. 2013;2(1) https://theijcp.org/index.php/ijcp/article/view/100/82 [Internet]. 2013; Available from: [Google Scholar]
- 5.Mishra K, Singla S, Sharma S, Saxena R, Batra VV. Griscelli syndrome type 2: a novel mutation in RAB27A gene with different clinical features in 2 siblings: a diagnostic conundrum. Korean J Pediatr [Internet] 2014;57(2):91–95. doi: 10.3345/kjp.2014.57.2.91. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3965801/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meschede IP, Santos TO, Izidoro-Toledo TC, Gurgel-Gianetti J, Espreafico EM. Griscelli syndrome-type 2 in twin siblings: case report and update on RAB27A human mutations and gene structure [Internet] Brazilian Journal of Medical and Biological Research. scielo. 2008;41:839–848. doi: 10.1590/s0100-879x2008001000002. https://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-879X2008001000002 Available from: [DOI] [PubMed] [Google Scholar]
- 7.Kalekar T, Khadse G. Mri imaging in griscelli syndrome. Eur J Pharm Med Res [Internet] 2014;1(1):272–277. https://www.ejpmr.com/home/abstract_id/2176 Available from: [Google Scholar]
- 8.Bindu PS, Mahadevan A, Taly AB, Chickabasaviah YT, Bharath RD, Nagappa M. Neuroimaging findings in Griscelli syndrome type 2 with primary neurological presentation. J Pediatr Neuroradiol [Internet] 2014;3:81–86. https://content.iospress.com/articles/journal-of-pediatric-neuroradiology/pnr091 Available from: [Google Scholar]
- 9.Rajadhyax M, Neti G, Crow Y, Tyagi A. Neurological presentation of Griscelli syndrome: obstructive hydrocephalus without haematological abnormalities or organomegaly. Brain Dev [Internet] 2007;29(4):247–250. doi: 10.1016/j.braindev.2006.09.007. https://pubmed.ncbi.nlm.nih.gov/17085000/ Available from: [DOI] [PubMed] [Google Scholar]
- 10.Panigrahi I, Suthar R, Rawat A, Behera B. Seizure as the presenting manifestation in Griscelli syndrome type 2. Pediatr Neurol [Internet] 2015;52(5):535–538. doi: 10.1016/j.pediatrneurol.2015.01.010. https://pubmed.ncbi.nlm.nih.gov/25801174/ Available from: [DOI] [PubMed] [Google Scholar]
- 11.Sarper N, Akansel G, Aydoğan M, Gedikbaşi D, Babaoğlu K, Gökalp AS. Neuroimaging abnormalities in Griscelli's disease. Pediatr Radiol [Internet] 2002;32(12):875–878. doi: 10.1007/s00247-002-0752-1. https://pubmed.ncbi.nlm.nih.gov/12447595/ Available from: [DOI] [PubMed] [Google Scholar]