Abstract
Background
Extensile lateral approach had been recognized as the gold standard technique for displaced intra-articular calcaneus fractures (DIACFs) while sinus tarsi approach had been increasingly valued by surgeons and comparative clinical outcome was shown in both techniques. Appropriate decisions could be made by the clinicians with the help of cost-utility analysis (CUA) about optimal healthcare for type II/III calcaneus fracture.
Method
A single-center, retrospective study was conducted in which basic characteristics, clinical outcomes, and health care costs of 109 patients had been obtained and analyzed. Changes in health-related quality of life (HRQoL) scores, validated by EuroQol five-dimensional-three levels (EQ-5D-3L), were used to enumerate quality-adjusted life years (QALYs). Cost-effectiveness was determined by the incremental cost per QALY.
Results
One hundred nine patients were enrolled in our study including 62 in the ELA group and 47 in the STA group. There were no significant differences between these two groups in mean total cost, laboratory, and radiographic evaluation expense, surgery, anesthesia, and antibiotic expense. The expense of internal fixation materials ($3289.0 ± 543.9) versus ($2630.6 ± 763.7) and analgesia ($145.8 ± 85.6) versus ($102.9 ± 62.7) in ELA group were significantly higher than in the STA group (P < .001, P = .008, respectively). Visual Analogue Scale (VAS) scores showed significant difference at postoperative 3 and 5 days (P < .001). American Orthopaedic Foot and Ankle Society (AOFAS) ankle-hindfoot scores and the Bohlers’ and Gissane angle showed no significant differences between the two groups before and after the operation. The cost-effectiveness ratios of ELA and STA were $8766.8 ± 2835.2/QALY and $7914.9 ± 1822.0/QALY respectively, and incremental cost-effectiveness ratio (ICERs) of ELA over STA was $32110.00/QALY, but both showed no significant difference.
Conclusion
Both ELA and STA techniques are effective operative procedures for the patients with calcaneus fracture. Moreover, STA seems to be more reasonable for its merits including less postoperative pain, and less expense of analgesia as well as internal fixation materials.
Level of evidence
5
Keywords: Cost-utility analysis, Calcaneus fracture, Extensile lateral approach, Sinus tarsi approach
Background
Calcaneus fracture is the most commonly happened one in tarsal bone, accounting for 2% of all fractures [1–3] and 75% of which are displaced intra-articular calcaneus fractures (DIACFs) [4, 5]. According to the classification system introduced by Sanders in 1993, calcaneus fracture is classified into four types based on the number and location of articular fragments seen on coronal and axial computed tomography (CT) [6, 7]. Nonoperative and operative treatments are the two traditional managements of calcaneus fractures. However, patients with conservative treatment may develop posttraumatic arthritis, chronic heel deformity, and malalignment of mechanical axis of the limb, which may seriously affect the quality of life [8, 9]. Surgical treatment is therefore necessary, and open reduction and internal fixation (ORIF) via extensile lateral approach (ELA) have been considered as the gold standard in the management of type II/III calcaneus fracture for its clear visualized for reduction and favorable outcomes in long-term [10–13]. However, some articles report multiple complications of ELA including hematoma, wound slough, dehiscence, and infection, and the occurrence rate is approaching 33% [14–18]. Many minimally invasive approaches including closed reduction, percutaneous screws fixation, and modified lateral approach (minimal invasive sinus tarsi approach) have been introduced in order to reduce the incidence of surgical complications [19–23]. Many investigators and surgeons have advocated using sinus tarsi approach (STA) for its lower reported wound complications rate, lower visual analog pain scale levels, comparative clinical outcomes, and increased overall satisfaction [24–26], and there has been a trend toward this technique in recent times. However, the optimal surgical approach for accurate reduction of DIACFs still remains controversial.
Owing to persistent pain, stiffness, and gait abnormalities, DIACFs may result in permanent disability and reduction of quality of life [5, 27–29]. Additionally, DIACFs are more frequently happened in the most economically active population such as adults and adolescents, which undoubtedly lay high socio-economic costs for its difficulties to return to work [30, 31]. Therefore, with growing pressure to reduce costs while high-quality care is still delivered, cost-effective research in calcaneus fracture is becoming increasingly important to discuss, which may help clinicians make appropriate surgical strategies and reach optimal health care [32].
Cost-utility analysis (CUA), which belongs to effectiveness analysis, evaluates the outcome in units of utilities standing for quantity and quality of life [33, 34]. Alterations in health-related quality of life (HRQoL) scores validated by EuroQol five-dimension three-levels (EQ-5D-3L) were applied to enumerate quality-adjusted life years (QALYs), which present the result of CUA. In addition, incorporated with cost information, an incremental cost-effectiveness ratio (ICER) can be used to enumerate between two techniques and display a cost per QALY value: lower figure represents more cost-effective strategy [35, 36].
To the best of our knowledge, there had not been any cost-effectiveness research comparing the two approaches of calcaneus fractures conducted in China. Therefore, the purpose of this study was to assess the cost and cost-effectiveness of two techniques for treatment of Sanders type II/III in calcaneus fractures, and thus, it may help us make optimal clinical decisions.
Method
General information
We conducted a retrospective review of 109 patients (62 in the ELA group and 47 in the STA group) who had been diagnosed as type II/III calcaneus fracture and had undergone ORIF surgery via ELA or STA from January 2013 to October 2018. Basic demographic and clinical characteristic of the patients were obtained including age, gender, type of fracture, anesthesia, the length of hospital stay, and follow-up time.
Inclusion and exclusion criteria
Participants were enrolled if they met the following eligibility criteria: (1) DIACFs classified as Sanders type II/III, (2) closed and fresh fractures, (3) fractures that underwent procedures via ELA or STA, and (4) follow-up period greater than 1 year. The exclusion criteria were as follows: (1) fractures classified as Sanders I or IV; (2) malunion, nonunion, open, and bilateral fractures; (3) associated or multiple trauma; and (4) severe systemic diseases and commodity.
Clinical assessment
The pain was measured using Visual Analogue Scale (VAS) with the score ranged from 0 to 10 points indicating no pain to worst pain. The VAS scores were collected at pre-operation and postoperative 3 days, 5 days, and 7 days. Functional outcome was measured by American Orthopaedic Foot and Ankle Society (AOFAS) ankle-hindfoot scores at pre-operation, at postoperative 3 months, and at final follow-up with the scores ranging from 0 to 100 points of pain, function, and alignment. Radiographic including Bohler’s angle and Gissane angle was measured on fluoroscopic images at pre-operation, at postoperative 3 months, and at final follow-up time. The specific radiographic views including the anteroposterior (AP), lateral, axial projections, and CT scans were collected preoperatively. Based on the anatomic characteristic of calcaneus fractures shown in preoperative radiographs and CT, surgeons could attain the information of the type, level, locations of the fractures and measure height, width, and Bohler’s and Gissane angle of the calcaneus.
Cost and cost-utility analysis
. The medical and financial records of patients including the expense of total costs, laboratory and radiographic evaluation, surgery, anesthesia, analgesia, internal fixation materials, and antibiotic were obtained from our hospital’s medical and financial information center. However, indirect costs including miss time from work or decreased productivity, rehabilitation, further consultation, and transportation were not enumerated because each patients’ situation and the employment status varied. All costs were converted into US dollars ($) at their value during January 2020.
Clinical outcome was monitored by recording health-related quality of life (HRQoL) scores before and after operation with a score ranging from − 0.11 to 1.0, and 1.0 indicates full health. Validation of HRQoL scores were converted by EQ-5D-3L including five dimensions: mobility, self-care, activities of daily life, pain, and anxiety/depression and each depicted by three levels from no, mild, or moderate to severe problems. Thus, this depictive system includes 243 combinations, or health states. Combining HRQoL index and time were used to estimate QALYs, which are enumerated for the area under the curve by trapezoidal method.
The CUA was conducted from the healthcare perspective and was presented by the cost-effectiveness ratio (CER) and incremental cost-utility ratio (ICER). An annual discount of 3% was adjusted to QALYs and mean total costs.
Different discount rates (0% and 5%) were used to mean total costs and QALYs for sensitivity analysis.
Surgical technique
The decisions including the surgical procedures, type of the incision—ELA or STA, and type of the implants were made by senior surgeons based on the anatomic characteristic of the fractures and their preference. There were three experienced surgeons included in total and were coded as A, B, and C in this study. They all learned from the same professor and trained in the same place, and therefore, the surgeons can perform the procedures with equivalent effectiveness and low complication rate.
Extensile lateral approach
Patients with a tourniquet at the thigh were under epidural, local, general, or subarachnoid block anesthesia in the lateral position. The incision of ELA, which originated perpendicularly from 5 cm over lateral malleolus or the midpoint between the fibula and Achilles tendon and stopped at the base of 5th metatarsal, was performed by experienced surgeons followed the way which was depicted by Benirshcke and Sangeorzan. With the flap held by several 2.0-mm Kirschner wires, this incision allowed the visualization of the lateral wall of calcaneus and subtalar joint and anatomic reduction was achieved directly under the guidance of C-arm fluoroscopy. Consequently, the length, height, and width of the calcaneus were restored (Fig. 1).
Fig.1.
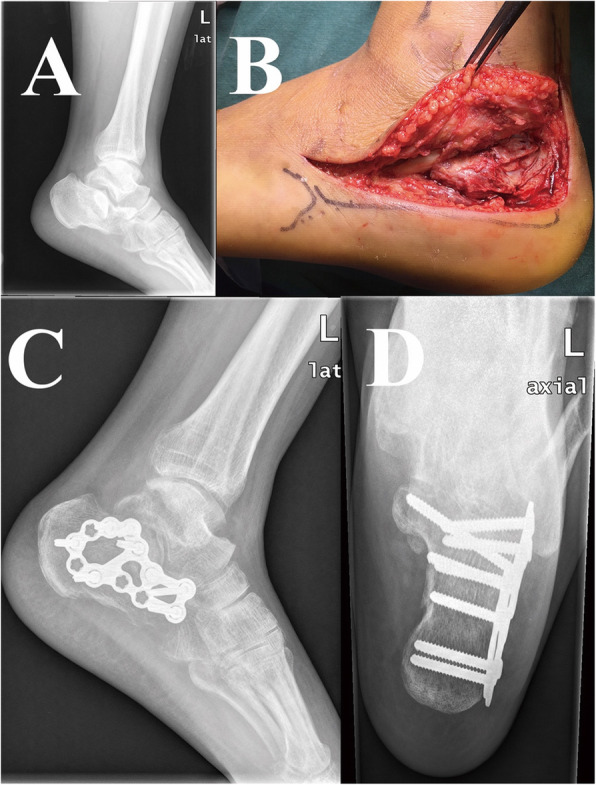
a The preoperative lateral image demonstrates collapse of the Bohler’s angle and posterior facet. b Intraoperative gross image of surgery via the extensile lateral approach (ELA). c The postoperative lateral image shows restoration of the Bohler’s angle and posterior facet by plate and screws. d The axial image shows restoration of the width and alignment of calcaneus
Sinus tarsi approach
Patients with a tourniquet at the thigh were under epidural, local, general, or subarachnoid block anesthesia in the lateral position. The incision was performed along a line from the tip of the lateral malleolus to the base of the fourth metatarsal, and its length was approximately 3–5 cm. This incision allowed the visualization of the posterior facet, anterior process, and even the calcaneocuboid joint with the calcaneofibular ligament dissected and the extensor brevis muscle elevated. Two 2.5-mm Schanz screw were inserted percutaneously into the calcaneal tuberosity and talus from medial to lateral, and tibia distraction device (Johnson & Johnson, USA) was applied to correct the deformity and restore the length, height, and width of the calcaneus with the guidance of C-arm fluoroscopy (Fig. 2).
Fig. 2.
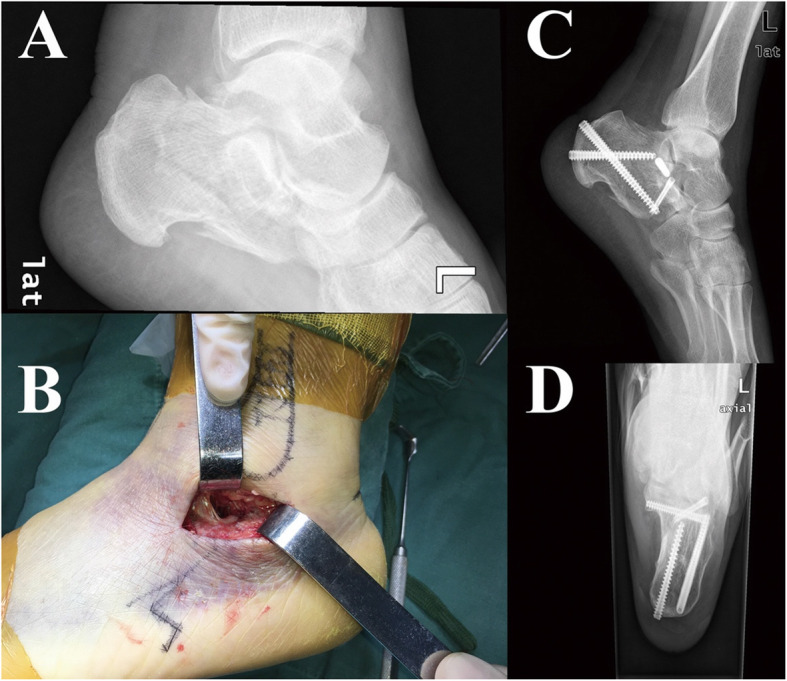
a The preoperative lateral image shows fracture line involving the subtalar joint. b Intraoperative gross image of surgery via the sinus tarsi approach (STA). c The postoperative lateral image shows restoration of Bohler’s angle, and fracture line involving subtalar joint was reduced by screws. d The axial image shows the normal alignment of calcaneus
Statistical analysis
The Kolmogorov-Smirnov test was applied to examine the normality of all variables. The between-group differences were compared by Student’s t test for normal distribution variables (mean ± standard deviation) or a Mann-Whitney U test for abnormal distribution variables (median (25% quartile–75% quartile)) or a chi-square test for categorical variables (n (%)). The differences in the longitudinal changes of the Bohler’s, Gissane angle and VAS scores between ELA and STA group were compared by applying non-parametric test. All analyses were performed using SPSS 20.0 software (SPSS, IL, USA). Differences were considered to be statistically significant when P value was less than .05.
Results
Patient information
A total of 109 patients were enrolled in our research, including 62 in ELA group (45.40 ± 12.91 years old, 82.26% male) and 47 in STA group (49.92 ± 14.98 years old, 80.85% male). In the ELA group, 36 were classified as Sanders type II and 26 were Sanders type III whereas in the STA group 28 were classified as Sanders type II and 19 were Sanders type III. A sum of 52 in the ELA group underwent spinal anesthesia and 10 underwent general anesthesia whereas among the patients in the STA group, 40 underwent spinal anesthesia and 7 were under general anesthesia. The length of hospital stay and follow-up time were comparative between the ELA group and STA group. There were no significant differences in age, gender, fracture type, antibiotics, anesthesia, the length of hospital stay, and the follow-up time between these groups (Table 1).
Table 1.
Patient information
| Parameters | ELA (N = 62) | STA (N = 47) | p value |
|---|---|---|---|
| Age (years) | 45.40 ± 12.91 | 49.92 ± 14.98 | .095 |
| Gender | .522 | ||
| Male | 51 | 38 | |
| Female | 11 | 9 | |
| Fracture type | .516 | ||
| Sanders II | 36 | 28 | |
| Sanders III | 26 | 19 | |
| Anesthesia | .860 | ||
| Spinal anesthesia | 52 | 40 | |
| General anesthesia | 10 | 7 | |
| The length of hospital stay | 10.9 ± 3.7 | 9.4 ± 3.6 | .060 |
| Follow-up time (month) | 30.5 ± 9.7 | 28.4 ± 13.5 | .150 |
Data are means ± SD and numbers of subjects. Significant difference (P < .05)
ELA Extensile lateral approach, STA Sinus tarsi approach
Clinical outcome
All the clinical outcomes including VAS scores, AOFAS scores, the Bohler’s angle, and the Gissane angle are presented in Table 2. Results showed that VAS score was significantly different at postoperative 3 and 5 days between the two groups (P < .001, P = .002, respectively). However, there was no significant difference in VAS scores before operation and at the final follow-up time between these two groups (P = .151, P = .693, respectively). AOFAS scores improved greatly 3 months after surgery, from 50.0 ± 8.0 to 80.3 ± 6.3 in the ELA group and from 51.2 ± 7.9 to 81.4 ± 5.6 in the STA group, but there showed no significant difference between two groups at these time points (P = .413, P = .325, respectively). Similarly, the Bohler’s angle and the Gissane angle were significantly improved 3 months after surgery, from 13.6° ± 7.3 to 30.2° ± 7.6 (P < .001) and from 102.8° ± 14.2 to 130.0° ± 9.2 (P < .001) in the ELA group whereas in the STA group, the Bohler’s angle changed from 16.0° ±7.4 to 28.2° ± 7.1 (P < .001) and the Gissane angle changed from 102.1° ± 12.5 to 126.0° ± 7.5 (P < .001). There showed no significant difference in the Bohler’s and the Gissane angle before operation (P = .086, P = .0530) and at postoperative 3 months (P = .920, P = .255). Moreover, there were no significant differences among AOFAS scores, the Bohler’s angle, and the Gissane angle at final follow-up between these two groups (P = .113, P = .065, P = .139, respectively).
Table 2.
Clinical outcome
| Parameters | ELA (N = 62) | STA (N = 47) | p value |
|---|---|---|---|
| VAS scores | |||
| Preoperative | 6.9 ± 1.1 | 6.6 ± 1.0 | .151 |
| Postoperative 3 days | 4.6 ± 0.9 | 3.0 ± 0.8 | < .001 |
| Postoperative 5 days | 2.3 ± 0.7 | 1.8 ± 0.5 | .002 |
| Postoperative 7 days | 1.6 ± 0.5 | 1.5 ± 0.5 | .693 |
| AOFAS | |||
| Preoperative | 50.0 ± 8.0 | 51.2 ± 7.9 | .413 |
| Postoperative 3 months | 80.3 ± 6.3 | 81.4 ± 5.6 | .325 |
| Final follow-up | 83.7 ± 4.6 | 85.0 ± 4.0 | .113 |
| Bohler’s angle (degrees) | |||
| Preoperation | 13.6 ± 7.3 | 16.0 ± 7.4 | .086 |
| Postoperative 3 months | 30.2 ± 7.6 | 28.2 ± 7.1 | .053 |
| Final follow-up | 29.9 ± 6.6 | 28.4 ± 6.3 | .065 |
| Gissane angle (degrees) | |||
| Pre-operation | 102.8 ± 14.2 | 102.0 ± 12.5 | .920 |
| Postoperative 3 months | 130.0 ± 9.2 | 127.7 ± 8.2 | .255 |
| Final follow-up | 126.0 ± 7.5 | 128.2 ± 7.8 | .139 |
| Preoperative EQ-5D score | 0.63 (0.57–0.68) | 0.59 (0.52–0.68) | .246 |
| Postoperative EQ-5D | 0.92 (0.89–0.95) | 0.93 (0.88–0.95) | .755 |
| Gained QALY | 0.77 (0.73–0.80) | 0.75 (0.69–0.79) | .250 |
EQ-5D EuroQol five-dimensional, VAS Visual Analogue Scale, AOFAS American Orthopaedic Foot and Ankle Society, QALY Quality-adjusted life-year
Health care costs
Table 3 summarizes the itemized mean costs of ELA and STA groups. There were no significantly statistical differences between these two groups in the total costs and expense of laboratory and radiographic evaluation, surgery, antibiotics, and anesthesia. However, the expense of internal fixation materials and analgesia exists significant difference between the two groups (P < .001, P = .008, respectively).
Table 3.
Health care cost
| Parameters | ELA (N = 62) | STA (N = 47) | p value |
|---|---|---|---|
| Laboratory expense | $143.1 ± 42.9 | $160.8 ± 109.5 | .605 |
| Radiography | $78.1 ± 21.8 | $80.8 ± 15.1 | .217 |
| CT | $46.3 ± 21.8 | $44.5 ± 21.7 | .506 |
| Surgery | $396.3 ± 130.5 | $442.9 ± 202.7 | .376 |
| Anesthesia | $146.3 ± 67.5 | $142.0 ± 70.1 | .778 |
| Analgesia | $145.8 ± 85.6 | $102.9 ± 62.7 | .008 |
| Internal fixation materials | $3289.0 ± 543.9 | $2630.6 ± 763.7 | < .001 |
| Antibiotic drugs | $85.0 ± 44.6 | $76.5 ± 54.9 | .104 |
| Total cost | $6481.0 ± 1504.4 | $5838.8 ± 1276.2 | .200 |
Values represent the mean cost per patient in January 2020 US dollars. Significant difference (P < .05)
CT Computed tomography
Cost-utility analysis
Changes in HRQoL scores validated by EQ-5D-3L in the two groups are presented as median (25% quartile, 75% quartile) in Table 2. The average baseline HRQoL scores of the ELA and STA group were similar (median 0.63; range, 0.57–0.68, and median 0.59; range, 0.52–0.68, respectively, P = .246). The average HRQoL scores at 1 year (0.92 in the ELA group and 0.93 in STA group) showed no significant difference (P = .755). The mean gained QALYs of the ELA and STA groups are 0.77 (range, 0.73–0.80) and 0.75 (range, 0.69–0.79), respectively. However, no significantly statistical difference exists in gained QALYs (P = .250).
The consequences of CUA are described on Table 4. The CERs of ELA and STA groups were $8766.8 ± 2835.2/QALY and $7914.9 ± 1822.0/QALY (P = .245), respectively. The difference between the mean total costs of the ELA and STA groups represents the incremental cost: $642.2 (ELA over STA). The mean gained QALYs and ICERs of ELA over STA were 0.02 and $32,110.00/QALY. With 3% and 5% annual discounts, the ICERs of ELA were $32,108.40/QALY and $32,126.05/QALY in the STA group, respectively (Table 5).
Table 4.
Cost-effectiveness ratios (CERs)
| Parameters | ELA (N = 62) | STA (N = 47) | p value |
|---|---|---|---|
| Cost | $6481.0 ± 1504.4 | $5838.8 ± 1276.2 | .200 |
| Utility (QALY) | 0.77 (0.73–0.80) | 0.75 (0.69–0.79) | .755 |
| CERs | $8766.8 ± 2835.2 | $7914.9 ± 1822.0 | .245 |
QALY Quality-adjusted life-year
Table 5.
Incremental cost-effectiveness ratio (ICER)
| Parameters | Normal | 3% Discount | 5% Discount |
|---|---|---|---|
| ΔCost | 642.20 | 623.51 | 605.36 |
| ΔQALY | 0.02 | 1.942 × 10−2 | 1.94 × 10−2 |
| ICER (Δcost/ΔQALY) | 32,110.00 | 32,106.59 | 31,794.26 |
ICER Incremental cost-effectiveness ratio
Discussion
Up to now, the optimal treatment of displaced intra-articular calcaneus fractures remains controversial even though the equipment and technologies have been developing rapidly. The ELA can provide good visualization of the fracture site, but several studies have reported that the postoperative wound complication rate including wound edge necrosis, dehiscence, hematoma, or infection is relatively high. The STA can minimize soft tissue damage and reduce the risk of postoperative complication while allowing comparative fracture reduction. Previous studies comparing the therapeutic efficacy and clinical outcomes of ORIF via ELA or STA have showed no significant differences [37–40]. From our results, the clinical outcomes including the Bohler’s and Gissane angle, VAS scores, and AOFAS scores were in accord with previous studies. Nevertheless, to the best of our knowledge, no studies comparing the direct costs and effectiveness of two techniques in China were conducted. Therefore, we have conducted this cost-utility analysis and provide another perspective for surgeons to make an optimally clinical decision in economic perspective.
In recent decades, surgery for treating calcaneus fractures has showed that it can bring great cost-effectiveness compared with nonoperative treatments. For both surgeons and patients, they have high requirements of satisfaction on clinical outcomes and cost-effectiveness due to the advancement of medicine, and therefore, costs are a crucial factor during making clinical decision. Cost-analysis is attached great significance of making clinical decision and is frequently used to evaluate which intervention can offer figure through comparing the cost and health impact of interventions [41]. Thus, surgeons are supposed to make full assessments of costs and take benefits into consideration when making clinical strategy to manage specific patients [42].
An article published in 2017 has used pooled data of western country to compare the cost and benefits in patients with calcaneus fractures classified as Sanders type II/III, which were managed with surgeries via extensile lateral approach or sinus tarsi approach or non-operative treatments [32]. It demonstrated that ORIF via STA is the least expensive option for treating Sanders type II/III concerning total costs, probability of working the same job, and duration out of work after ORIF. However, previous study did not conduct systematic retrospective review and report itemized details of the costs, which may hamper the ability to draw firm conclusions about cost-effectiveness by limited data. Moreover, long-term results after newer or refine ORIF techniques are unknown. In our study, we collected and checked the medical bills from single information center of all patients in order to conduct a cost-analysis.
In this retrospective view, only direct health costs were collected, and for the indirect health costs including miss time from work or decreased productivity, rehabilitation, further consultation, and transportation were not calculated. CUA can be performed even without indirect costs [43–45]. According to the results, we found no statistical differences in total costs, laboratory and radiographic evaluation expense, surgical expenses, antibiotic drugs expense, anesthesia expense, and the length of hospital stay. Our study found significant differences in the analgesia expenses and internal fixation materials costs. The costs of patients in ELA group were higher than the cost of patients in STA group ($145.8 ± 85.6 versus $102.9 ± 62.7, P = .080). Patients underwent surgical treatment via ELA which have caused much more severe injury to soft tissue and blood vessels comparing with STA. Owing to the larger wound caused by ELA, patients’ complaints of pain in the ELA group were more obvious. According to the pain measurement and VAS scores, there did exist significant difference in scores at 3 days and 5 days after surgery. Therefore, clinical strategies were made including using more effective analgesic drugs or extending the time of applying analgesic drugs, which resulted in the statistical difference in the expense of analgesia between these two groups. However, after postoperative treatments and caring, there showed no significant difference in VAS scores at 7 days after surgery.
Concerning the surgeon opinion for both techniques, surgeon A could master ELA and STA techniques proficiently while surgeon B prefers performing ELA technique and surgeon C prefers performing STA technique. Additionally, patients’ specific condition and subjective wishes are supposed to be taken into consideration, and therefore, there was no significant difference among surgeon rating for both surgical techniques to reconstruct the height, width, and Bohler’s and Gissane angle of the calcaneus, therefore reaching the anatomical reduction. In this study, the brand of materials used in the ELA group consists of Smith & nephew (29/62), Acumend (10/62), and Double Medical Technology Inc., China (23/62), while in STA group consists of Acumed (31/47) and Carefix, China (16/47). Normally, the option of choosing which type of plates mainly depends on patients’ condition and surgeon’s preferences. However, healthcare system varies in different countries and it can be assumed that there is some bias in choosing internal fixation materials based on patient income and insurance type. When conditions permit, surgeons provide patients with suggestions on therapeutic scheme patients and patients can choose imported or domestic internal fixation materials according to their financial status and wishes. Currently, domestic internal fixation materials have a price advantage and can reach similarly anatomical reduction with low complication rate compared with imported ones. In our study, the patients in the ELA group underwent ORIF with plates and five to eight 3.5-mm screws while the patients in the STA group underwent ORIF with three to four 6.5-mm cannulated screws solely. In the past 20 years, the technique of STA has been introduced in China and there were no comparable implants to match it, and therefore, this technique has not been popularized. Currently, as new generation of implants emerges, more and more surgeons tend to perform minimal invasive incisions and the cost of these implants are comparable to plate system in extensile lateral technique. Whether plate system or screws are applied via two different approaches, there were no definite indications for it. Based on our experience, normally, procedures via ELA do not apply with screws solely and procedures via STA do not apply with large plates. We tend to apply screws for patients with intact calcaneal wall and simple posterior calcaneal articular surface collapse. For those patients with factures of calcaneal anterior process or impingement of fracture fragments, we tend to apply plate systems to reach anatomic reduction. The selection of implants is based on the specific condition of each patient, and we control other parameters including patients’ information and fracture type, and that is the way we justified the cost of the patients.
As for the life of various types of implants, normally, the implants will be removed if situations including pain and infections happen. In our countries, a majority of patients choose to have their implants removed as long as the fracture site reaches clinical healing standard or 24 months after the surgery. However, it may not be possible to track and analyze the cost of removal internal fixations for it may not be performed in the same hospital.
In our study, no severe complications occurred and all the patients at final follow-up observations showed an excellent therapeutic effect after surgery according to direct measurement of clinical outcomes. The results showed that there were no significant differences between the ELA and STA groups among different surgeons on the clinical efficacy indicators. Changes in pre- and postoperative VAS and AOFAS scores are both the most direct measurement and rapid evidence of surgical outcomes. Altogether, the application of surgical incision on ELA or STA among surgeons A, B, and C depends on their own experience and preference. According to the clinical indicators, both incisions have comparative curative effects and equivalent precision ratio on patients with type II/III calcaneus fracture because there were no significant differences. They can alleviate patients’ pain and enable them to take their own responsibilities in the society.
The outcome of CUA was presented with gained QALYs, which were enumerated through multiplying the length of hospital stay by HRQoL weight (i.e., utility score) scaled from − 0.11 to 1.00 [46]. Direct elicitation methods, generic preference-based measures, and condition-specific measures were used to evaluate HRQoL. The EQ-5D-3L, regarded as the primary valuation study derived utility scores, are prevalently applied preference-based measures [47–49]. In this study, CER and ICERs were the outcomes of economic benefits, which could be comprehended that ICERs indicate how much extra health benefits via ELA can bring compared with via STA, and how much additional expense it will cost.
Consequently, we found no statistically significant differences in overall cost per patient and HRQoL between the two groups, but generally, surgery via ELA incurred much costs than via STA. Similarly, the incremental cost-utility ratio also showed there were no cost-utility benefits comparing the two groups.
Limitation
This study has its own limitations. Firstly, the chief limitation of study is that it is a retrospective cost-utility analysis and randomized clinical trials (RCTs) are insufficient. Therefore, there are inherent defect existing in the study. Secondly, all the data are collected from a sing center, which leads to the fact that the sample size is not adequate and it may result in single center analysis bias. Thirdly, indirect costs including rehabilitation, home care, and further consultation have not been collected as patients may go back to local hospital and produce fees that could not be tracked. Fourthly, only patients classified as Sanders type II/III were focused solely on in the study and future RCTs combined with CUA could emphasize on other types of calcaneus fractures such as Sanders I/IV, malunion, and nonunion. Lastly, there did exist some level of recall bias in patients’ accomplished questionnaires and limitation of all CUA in lacking of uniform methodology to track the preoperative outcome.
Conclusion
Both ELA and STA techniques are effective surgical procedures for the patients with calcaneus fracture. Moreover, STA seems to be more reasonable for its merits including less postoperative pain, fewer expenses of analgesia, and internal fixation materials.
Acknowledgements
Zihua Li, Xinbo Wu, and Haichao Zhou are co-first authors.
Abbreviations
- ELA
Extensile lateral approach
- STA
Sinus tarsi approach
- DIACFs
Displaced intra-articular calcaneus fractures
- CUA
Cost-utility analysis
- ORIF
Open reduction and internal fixation
- ICERs
Incremental cost-effectiveness ratio
- HRQoL
Health-related quality of life
- VAS
Visual analogue scale
- EQ-5D
EuroQol five-dimensional
- AOFAS
American orthopaedic foot and ankle society
- QALY
Quality-adjusted life-year
Authors’ contributions
LZH, WXB, and ZHC conceived and designed the study. XSC, XFJ, and HH measured and recorded the data. LZH and ZHC wrote the paper. WXB and YYF reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
1. National Natural Science Foundation of China (no. 31800782)
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This retrospective study was approved and consented to participate by the Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zihua Li, Xinbo Wu, and Haichao Zhou are co-first authors
References
- 1.Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691–697. doi: 10.1016/j.injury.2006.04.130. [DOI] [PubMed] [Google Scholar]
- 2.Zwipp H, Rammelt S, Barthel S. Fracture of the calcaneus. Der Unfallchirurg. 2005;108(9):737–747. doi: 10.1007/s00113-005-1000-6. [DOI] [PubMed] [Google Scholar]
- 3.Schepers T, van Lieshout EM, van Ginhoven TM, Heetveld MJ, Patka P. Current concepts in the treatment of intra-articular calcaneal fractures: results of a nationwide survey. Int Orthop. 2008;32(5):711–715. doi: 10.1007/s00264-007-0385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin D, Parsons N, Shaw E, et al. Operative versus non-operative treatment for closed, displaced, intra-articular fractures of the calcaneus: randomised controlled trial. BMJ. 2014;349(undefined):g4483. doi: 10.1136/bmj.g4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ocguder A, Gok H, Heycan C, Tecimel O, Tonuk E, Bozkurt M. Effects of custom-made insole on gait pattern of patients with unilateral displaced intra-articular calcaneal fracture: evaluation with computerized gait analysis. Acta Orthop Traumatol Turc. 2012;46(1):1–7. doi: 10.3944/aott.2012.2401. [DOI] [PubMed] [Google Scholar]
- 6.Sanders R, Fortin P, DiPasquale T, Walling A. Operative treatment in 120 displaced intraarticular calcaneal fractures. Results using a prognostic computed tomography scan classification. Clin Orthop Relat Res. 1993;(290):87–95. [PubMed]
- 7.Sanders R. Displaced intra-articular fractures of the calcaneus. J Bone Joint Surg Am. 2000;82(2):225–250. doi: 10.2106/00004623-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Clare MP, Lee WE, Sanders RW. Intermediate to long-term results of a treatment protocol for calcaneal fracture malunions. J Bone Joint Surg. 2005;87(5):963–973. doi: 10.2106/00004623-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Crosby LA, Fitzgibbons T. Intraarticular calcaneal fractures. Results of closed treatment. Clin Orthop Relat Res. 1993;(290):47–54. [PubMed]
- 10.Cottom JM, Baker JS. Restoring the anatomy of calcaneal fractures: a simple technique with radiographic review. Foot Ankle Spec. 2017;10(3):235–239. doi: 10.1177/1938640016679700. [DOI] [PubMed] [Google Scholar]
- 11.Makki D, Alnajjar HM, Walkay S, Ramkumar U, Watson AJ, Allen PW. Osteosynthesis of displaced intra-articular fractures of the calcaneum: a long-term review of 47 cases. J B Joint Surg Br Vol. 2010;92(5):693–700. doi: 10.1302/0301-620X.92B5.23542. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim T, Rowsell M, Rennie W, Brown AR, Taylor GJ, Gregg PJ. Displaced intra-articular calcaneal fractures: 15-year follow-up of a randomised controlled trial of conservative versus operative treatment. Injury. 2007;38(7):848–855. doi: 10.1016/j.injury.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Tennent TD, Calder PR, Salisbury RD, Allen PW, Eastwood DM. The operative management of displaced intra-articular fractures of the calcaneum: a two-centre study using a defined protocol. Injury. 2001;32(6):491–496. doi: 10.1016/S0020-1383(01)00024-9. [DOI] [PubMed] [Google Scholar]
- 14.Benirschke SK, Kramer PA. Wound healing complications in closed and open calcaneal fractures. J Orthop Trauma. 2004;18(1):1–6. doi: 10.1097/00005131-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Al-Mudhaffar M, Prasad CV, Mofidi A. Wound complications following operative fixation of calcaneal fractures. Injury. 2000;31(6):461–464. doi: 10.1016/s0020-1383(00)00026-7. [DOI] [PubMed] [Google Scholar]
- 16.Jw F. Early wound complications of operative treatment of calcaneus fractures : analysis of 190 fractures. J Orthop Trauma. 1994;13. [DOI] [PubMed]
- 17.Kline AJ, Anderson RB, Davis WH, Jones CP, Cohen BE. Minimally invasive technique versus an extensile lateral approach for intra-articular calcaneal fractures. Foot Ankle Int. 2013;34(6):773–780. doi: 10.1177/1071100713477607. [DOI] [PubMed] [Google Scholar]
- 18.Herscovici D Jr, Widmaier J, Scaduto JM, Sanders RW, Walling A. Operative treatment of calcaneal fractures in elderly patients. J Bone Joint Surg Am. 2005;87(6):1260–4. 10.2106/JBJS.D.01765. [DOI] [PubMed]
- 19.Mostafa MF, El-Adl G, Hassanin EY, Abdellatif MS. Surgical treatment of displaced intra-articular calcaneal fracture using a single small lateral approach. Strateg Trauma Limb Reconstr. 2010;5(2):87–95. doi: 10.1007/s11751-010-0082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Femino JE, Vaseenon T, Levin DA, Yian EH. Modification of the sinus tarsi approach for open reduction and plate fixation of intra-articular calcaneus fractures: the limits of proximal extension based upon the vascular anatomy of the lateral calcaneal artery. Iowa Orthop J. 2010;30(undefined):161–167. [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Ghalambor N, Nihal A, Trepman E. The modified Palmer lateral approach for calcaneal fractures: wound healing and postoperative computed tomographic evaluation of fracture reduction. Foot Ankle Int. 2003;24(10):744–753. doi: 10.1177/107110070302401003. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, Yanling S, Wei C, Qi Z, Wu Z, Zhang Y. Displaced intra-articular calcaneal fractures treated in a minimally invasive fashion. 2014;96(4):302–9. [DOI] [PubMed]
- 23.Xia S, Lu Y, Wang H, Wu Z, Wang Z. Open reduction and internal fixation with conventional plate via L-shaped lateral approach versus internal fixation with percutaneous plate via a sinus tarsi approach for calcaneal fractures - a randomized controlled trial. Int J Surg. 2014;12(5):475–480. doi: 10.1016/j.ijsu.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Kline AJ, Anderson RB, Davis WH, Jones CP, Cohen BE. Minimally invasive technique versus an extensile lateral approach for intra-articular calcaneal fractures. Foot Ankle Int. 2013;34(6):773–780. doi: 10.1177/1071100713477607. [DOI] [PubMed] [Google Scholar]
- 25.Yeo JH, Cho HJ, Lee KB. Comparison of two surgical approaches for displaced intra-articular calcaneal fractures: sinus tarsi versus extensile lateral approach. BMC Musculoskelet Disord. 2015;16(undefined):63. doi: 10.1186/s12891-015-0519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schepers T. The sinus tarsi approach in displaced intra-articular calcaneal fractures: a systematic review. Int Orthop. 2011;35(5):697–703. doi: 10.1007/s00264-011-1223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozkurt M, Kentel BB, Yavuzer G, Ocguder A, Heycan C, Tonuk E. Functional evaluation of intraarticular severely comminuted fractures of the calcaneus with gait analysis. J Foot Ankle Surg. 2004;43(6):374–379. doi: 10.1053/j.jfas.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Anja H, Konstantinidis L, Baur H, et al. Do changes in dynamic plantar pressure distribution, strength capacity and postural control after intra-articular calcaneal fracture correlate with clinical and radiological outcome? 2011;42(10):1135–43. [DOI] [PubMed]
- 29.Besch L, Radke B, Mueller M, et al. Dynamic and functional gait analysis of severely displaced intra-articular calcaneus fractures treated with a hinged external fixator or internal stabilization. J Foot Ankle Surg. 2008;47(1):19–25. doi: 10.1053/j.jfas.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Kuschnaroff CME, Muniz AMDS, Souza JBD, et al. Biomechanical evaluation of intra articular calcaneal fracture and clinical radiographic correlation. 2004;12(2):105–12.
- 31.Albin SR, Cornwall MW, McPoil TG, Van Boerum DH, Morgan JM. Plantar pressure and gait symmetry in individuals with fractures versus tendon injuries to the hindfoot. J Am Podiatr Med Assoc. 2015;105(6):469–477. doi: 10.7547/14-073.1. [DOI] [PubMed] [Google Scholar]
- 32.Clement RC, Lang PJ, Pettett BJ, Overman RA, Ostrum RF, Tennant JN. Sanders II/III calcaneus fractures in laborers: a cost-effectiveness analysis and call for effectiveness research. J Orthop Trauma. 2017;31(6):299–304. doi: 10.1097/BOT.0000000000000813. [DOI] [PubMed] [Google Scholar]
- 33.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 34.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on cost-effectiveness in health and medicine. JAMA. 1996;276(14):1172–1177. doi: 10.1001/jama.1996.03540140060028. [DOI] [PubMed] [Google Scholar]
- 35.Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ. 2005;14(5):487–496. doi: 10.1002/hec.944. [DOI] [PubMed] [Google Scholar]
- 36.Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ. 2004;13(12):1203–1210. doi: 10.1002/hec.901. [DOI] [PubMed] [Google Scholar]
- 37.Jiang N, Lin QR, Diao XC, Wu L, Yu B. Surgical versus nonsurgical treatment of displaced intra-articular calcaneal fracture: a meta-analysis of current evidence base. Int Orthop. 2012;36(8):1615–1622. doi: 10.1007/s00264-012-1563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang F, Tian H, Li S, et al. Meta-analysis of two surgical approaches for calcaneal fractures: sinus tarsi versus extensile lateral approach. ANZ J Surg. 2017;87(3):126–131. doi: 10.1111/ans.13869. [DOI] [PubMed] [Google Scholar]
- 39.Wei N, Yuwen P, Liu W, et al. Operative versus nonoperative treatment of displaced intra-articular calcaneal fractures: a meta-analysis of current evidence base. Medicine. 2017;96(49):e9027. doi: 10.1097/MD.0000000000009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo X, Li Q, He S, He S. Operative versus nonoperative treatment for displaced intra-articular calcaneal fractures: a meta-analysis of randomized controlled trials. J Foot Ankle Surg. 2016;55(4):821–828. doi: 10.1053/j.jfas.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 41.Grosse SD, Teutsch SM, Haddix AC. Lessons from cost-effectiveness research for United States public health policy. Ann Rev Public Health. 2007;28(undefined):365–391. doi: 10.1146/annurev.publhealth.28.021406.144046. [DOI] [PubMed] [Google Scholar]
- 42.Teutsch SM, Murray JF. Dissecting cost-effectiveness analysis for preventive interventions: a guide for decision makers. Am J Manag Care. 1999;5(3):301–305. [PubMed] [Google Scholar]
- 43.Bae EY. Guidelines for economic evaluation of pharmaceuticals in Korea. J Prev Med Public Health. 2008;41(2):80–83. doi: 10.3961/jpmph.2008.41.2.80. [DOI] [PubMed] [Google Scholar]
- 44.Glennie JL, Torrance GW, Baladi JF, et al. The revised Canadian Guidelines for the Economic Evaluation of Pharmaceuticals. Pharmacoeconomics. 1999;15(5):459–468. doi: 10.2165/00019053-199915050-00004. [DOI] [PubMed] [Google Scholar]
- 45.Torrance GW, Blaker D, Detsky A, et al. Canadian guidelines for economic evaluation of pharmaceuticals. Canadian collaborative workshop for pharmacoeconomics. Pharmacoeconomics. 1996;9(6):535–559. doi: 10.2165/00019053-199609060-00008. [DOI] [PubMed] [Google Scholar]
- 46.Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96(undefined):5–21. doi: 10.1093/bmb/ldq033. [DOI] [PubMed] [Google Scholar]
- 47.Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res. 2014;14(2):221–233. doi: 10.1586/14737167.2014.894462. [DOI] [PubMed] [Google Scholar]
- 48.Xie F, Gaebel K, Perampaladas K, Doble B, Pullenayegum E. Comparing EQ-5D valuation studies: a systematic review and methodological reporting checklist. Med Dec Making. 2014;34(1):8–20. doi: 10.1177/0272989X13480852. [DOI] [PubMed] [Google Scholar]
- 49.Payakachat N, Ali MM, Tilford JM. Can the EQ-5D detect meaningful change? A systematic review. PharmacoEconomics. 2015;33(11):1137–1154. doi: 10.1007/s40273-015-0295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


