Abstract
Chemical reactions on indoor surfaces play an important role in air quality in indoor environments, where humans spend 90% of their time. We focus on the challenges of understanding the complex chemistry that takes place on indoor surfaces and identify crucial steps necessary to gain a molecular-level understanding of environmental indoor surface chemistry: (1) elucidate key surface reaction mechanisms and kinetics important to indoor air chemistry, (2) define a range of relevant and representative surfaces to probe, and (3) define the drivers of surface reactivity, particularly with respect to the surface composition, light, and temperature. Within the drivers of surface composition are the roles of adsorbed/absorbed water associated with indoor surfaces and the prevalence, inhomogeneity, and properties of secondary organic films that can impact surface reactivity. By combining laboratory studies, field measurements, and modeling we can gain insights into the molecular processes necessary to further our understanding of the indoor environment.
Keywords: indoor chemistry, indoor surfaces, volatile and semi-volatile organic compounds, partitioning, surface chemistry, adsorption, photochemistry, acid-base chemistry, indoor air quality
Graphical Abstract
The Bigger Picture
Humans spend ∼90% of their time indoors. However, understanding the chemistry that occurs on indoor surfaces and its impact on air quality is still in its nascent stages due to the complexity of indoor surfaces. High surface-to-volume ratios indoors increase gas-surface collisions, but molecular mechanisms for surface reactions are often poorly understood, despite their importance becoming increasingly clear. Equilibrium thermodynamics poorly explain indoor surface chemistry, with key kinetic effects observed. Drivers of surface reactivity include relative humidity, temperature, light, and surface pH. Highlighted findings are the ubiquitous presence of aqueous and secondary organic films, their ability to act as reservoirs of contaminants, and impacts on gas and particle lifetimes. Indoor surface chemistry impacts multiple U.N. Sustainable Global Goals that point to the importance of further integration of laboratory, modeling, and real-world measurements to understand the air we breathe indoors.
Introduction: Surface Chemistry in Indoor Environments
Indoor air chemistry is a relatively new field of study that is rapidly growing in importance, as evidenced by the increasing number of publications and citations focused on this very important topic. 25 years ago there were only ∼20 papers with 70 citations on this topic, while by the end of 2019 there were 565 publications and roughly 20,000 citations.1 Understanding and predicting indoor air quality is essential for human health and sustainability, as people spend ∼90% or more of their lives indoors.2 Thus, the overall human exposure to gases and particulate matter (PM) is often greater in indoor environments than that found outdoors, yet, there remains a great deal about the chemistry of indoor environments that is not well understood from a detailed molecular perspective. This lack of scientific understanding is related to many unique sources,3 complex heterogeneous and multiphase chemical reactions,4 , 5 the high surface-to-volume ratio,6 , 7 and factors not present in outdoor environments (e.g., building materials, furnishings, ventilation, indoor lights, etc.) that all play a key role in indoor air chemistry and indoor air quality (Figure 1 ). Indoor surfaces are the interfaces between the built environment and indoor air, providing a source of emission, medium for multiphase reactions, and a sink for low volatility products, which affect indoor air quality. The chemistry that occurs on indoor surfaces is thought to be a key to this indoor air chemistry, as surfaces can adsorb and accumulate semi-volatile organic molecules and can also be reactive, facilitating the transformation of molecules once partitioned to the surface to less volatile forms leading to secondary organic films (SOFs). Earlier, studies were often focused on changes in the gas-phase composition with much less of a focus on the molecular view of interactions on the surface. Although past studies were groundbreaking from the perspective of beginning to understand the importance of indoor surface chemistry and its role in indoor air quality,8, 9, 10, 11, 12, 13 there still remains a dearth of knowledge about the fundamental chemical processes that occur on indoor surfaces. Furthermore, given recent events involving the spread of the corona virus disease 2019 (COVID-19),14 in part, potentially through surface-mediated processes, it is clear that knowledge of surfaces and surface chemistry indoors has the potential to improve our understanding of the adherence to and activity of biological organisms on surfaces and their role in the spread of infectious diseases.15
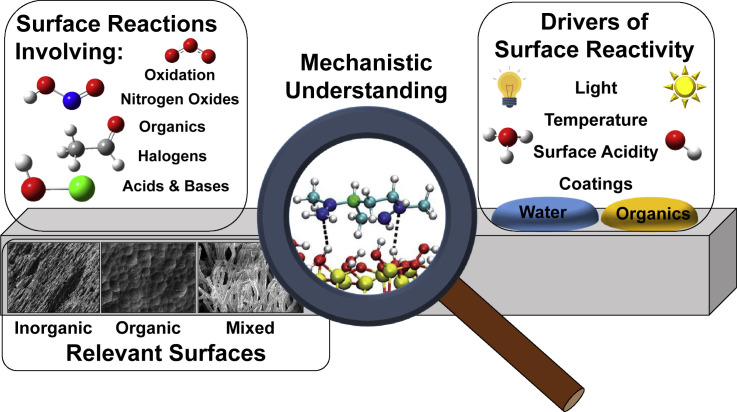
Figure 1.
The Complex Factors that Make Mechanistic Understanding of Indoor Surface Chemistry Challenging: Numerous Reactions, Complex Surfaces, and Different Drivers of Surface Reactivity
Image within magnifying glass reproduced from Fang et al.,5 Copyright 2019 Royal Society Chemistry.
Numerous aspects of indoor environments make the chemistry unique and need to be accounted for when establishing a framework for understanding chemistry occurring on indoor surfaces. For example, indoor environments have surface-to-volume ratios hundreds of times higher than outdoor environments (0.01 versus 3 m2/m3),6 , 7 , 16 which increases the relative importance of surface reactions for determining gas-phase concentrations and composition. As an example of the implications of these higher surface areas indoors, an ozone molecule indoors has a ∼40% chance of colliding with a surface before reacting versus ∼1% chance outdoors.17 From a slightly different perspective, a gas molecule will collide with an indoor surface ∼106 times before leaving via air-exchange, assuming it does not react. When combined with typical aerosol concentrations indoors,18 the importance of trace gases undergoing collisions with surfaces is far more important indoors than in the outdoor environment.
A second factor that is distinct for indoor environments is the higher concentrations of organic compounds (OCs) across a range of volatility.19 These high concentrations lead to different partitioning between gases, particles, and surfaces,20 with more volatile species partitioning to the condensed phase (i.e., surfaces) indoors than in the ambient atmosphere. In addition to the higher concentrations of OCs, including volatile organic compounds (VOCs) and semi-volatile organic compounds (SVOCs), the surfaces they are interacting with are complex and ever changing. Manuja et al.6 recently estimated the fraction of different surfaces within different residential rooms and highlighted the complex mixture of surfaces and variability room to room. This complexity is compounded by the fact that the surfaces inside buildings are constantly evolving. For example, Weschler3 documented considerable changes to indoor environments since the 1950s, including furnishings and building materials, which may have different chemical properties and reactivity. In the field of atmospheric chemistry, there are established land use models to estimate surface coverages,21 , 22 but the greater variability of indoor environments makes analogous efforts even more challenging.17 , 23 , 24
Lastly, the dynamic nature of air mixing and exchange with outdoor air is very important when considering the reaction rates and timescales for surface chemistry indoors. In a typical house, there are 0.5 air exchanges with the outdoor environment per hour, with exchange timescales ranging from 20 min to 10 h across building types.25 This means that equilibria are reestablished on a frequent basis compared with the outdoor air and slower gas-phase reactions that occur outdoors will not be competitive indoors (e.g., isoprene reaction with ozone).17 Accumulation of materials on surfaces—films of OCs and particle deposition—occurs on short and long timescales.26 Reactions that occur on these surfaces change as they age, evolve, and accumulate new materials.27, 28, 29 The time and length scales are smaller than those that have been studied for outdoor environments.
Indoor air chemistry is a rich field where the state-of-the-knowledge in the field has been summarized in several foundational reviews on the topic,3 , 30, 31, 32, 33, 34 as well as workshop reports,23 , 24 , 35 , 36 and other perspective-type articles.16 , 19 , 37 These foundational summaries and perspectives provide an important insight into many of the challenges of understanding indoor air chemistry, as well as opportunities and possible new directions. For example, a review on the role of interfaces in indoor air chemistry was published by Morrison in 2007.23 This critical review highlighted a number of short-, medium-, and long-term research priorities. Although, our knowledge of certain short-term priorities identified in that workshop, such as indoor ozone chemistry, have improved considerably in the intervening decade, significant gaps in our knowledge that were identified as long-term priorities, including key chemical mechanisms, still remain unresolved. Notable among these unresolved long-term priorities was the need for molecular-level understanding of phenomena such as hydrolysis, adsorption/desorption, aqueous thin-film chemistry (including film pH), SOFs, and secondary organic and inorganic aerosols.
To put the state-of-the science in perspective, the lack of knowledge of the mechanistic details and molecular descriptors of the chemistry that occurs on indoor surfaces is reminiscent of the state-of-the science in heterogeneous catalysis in the 1960s and heterogeneous atmospheric chemistry in the 1980s. Initially, these two fields were qualitative and empirical as quantitative information was nonexistent and only little, if any insights, was available on the molecular processes. This was due, in a large part, to the fact that there was little surface analysis incorporated into these earlier studies. Following the incorporation of the molecular-based studies, both of these fields—heterogeneous catalysis and heterogeneous atmospheric chemistry—were significantly advanced. Studies using different surface analytical techniques gave mechanistic information and important insights into the reactivity of different surfaces. Similarly, for indoor surface chemistry, surface analysis is needed to mechanistically understand the underlying chemical processes. As such, there is a clear need for further understanding of the fundamental chemistry of indoor surfaces from a physical chemistry perspective, using tools and techniques that provide insight into molecular processes that occur on relevant indoor surfaces.
To probe the physical and analytical chemistries occurring on indoor surfaces, one must start by defining key reactions. In many indoor studies, to date, multiphase chemistry has been defined as interactions of gas-phase species with a vaguely defined surface. This review will start by highlighting some of these key reactions before moving to discuss in greater detail the surfaces on which heterogeneous and multiphase chemistries can occur and the drivers of that surface reactivity.
Gas-Phase Reactants and Their Interactions with Surfaces
Surface chemistry in indoor environments is quite rich and includes oxidation chemistry, reactions of nitrogen oxides, surface adsorption and reactivity of OCs, halogen chemistry, and acid-base chemistry. Some of the questions and issues that are important to understand these reactions are now provided in more detail.
Surface Oxidation Chemistry of Organic Compounds in Indoor Environments
When evaluating the gas and multiphase chemistries occurring in any atmospheric system, the oxidants present and their reactions are crucial. While in the outdoor atmosphere the hydroxyl radical (OH) is abundant and commonly referred to as the detergent of the atmosphere, OH concentrations indoors are typically a factor of 10–20 lower than outdoors (∼ 4 × 105 molecules/cm3).38 Thus, much research to date has focused on a range of oxidants and their formation indoors.39 , 40 These oxidants are either directly harmful (e.g., ozone) or can lead to reactions forming oxidized species that may be harmful when inhaled. These oxidants lead to a range of volatile and non-volatile organic reaction products that are either unique indoors or present in higher concentrations indoors versus outdoors.41, 42, 43, 44
The most abundant oxidant indoors is ozone, which is primarily transported from outdoors to indoors, but can also form from indoor sources (e.g., printers).33 In the gas phase, ozone can react with many of the VOCs in indoor environments, but with smaller rate coefficients when compared with the equivalent reaction with the hydroxyl radical (OH).45 On surfaces, ozone decomposition is one of the most studied surface reactions and has been examined for a variety of surfaces and environmental conditions.8 , 40 , 46, 47, 48 For example, Morrison and Nazaroff monitored aldehyde emissions from exposure of carpet to ozone, observing C1–C13 aldehyde formation (60–800 μg m−2 hr−1),40 while Wisthaler and Weschler showed that ozone reacts with human skin oils.8 However, most of these studies have been conducted by monitoring loss rates of ozone to the surface, without probing the surface or explicitly describing how the ozone is interacting with the surface and whether decomposition to form molecular oxygen occurs as it does on solid surfaces (Equation 1a) or if organics are present with reactive sites (e.g., double bonds). These can lead to oxidized products as shown in Equation 1b, where (a) is adsorbed and (g) gas-phase products.
| (Equation 1a) |
| (Equation 1b) |
The detailed mechanism most likely involves the formation of reactive oxygen species (ROS), e.g., R3O2R4, on the surface before the formation of the final stable product, molecular oxygen, or oxidized organic compound.41 , 49 What, if any, chemistry do these ROS have on the surface? Can they react with co-adsorbed VOCs to lead to less volatile products? This will lead to SOF formation akin to SOA formation in the gas phase. Overall, the oxidant chemistry indoors is driven by a different set of species than the outdoor environment, but with equally important consequences, and may lead to unique chemistry with different reaction mechanisms and the formation of different products. Additionally, VOCs and SVOCs are present at higher concentrations in indoor environments than outdoors and their fate and reactivity are just beginning to be understood.50 , 51 Both VOCs43 , 44 and their lower volatility oxidized organic products41 are important to understand in terms of indoor exposures and health.30 , 32 , 52, 53, 54 Recent studies have focused on surface adsorption and oxidation chemistry of relevant OCs, including ozonolysis of an unsaturated triglyceride55 and squalene.56
Surface Chemistry of Nitrogen Oxides Including HONO Indoors
It is well known that nitrogen oxides, NO and NO2, are present in indoor air from combustion processes, including cooking, and outdoor transport.17 More recently, painted surfaces have been identified as a source of photochemically produced NO and NO2.57 NO2 is a precursor to nitrous acid (HONO), which was first observed indoors in the 1980s,10 but more recently, unexpectedly high levels have been observed in indoor environments (1– >20 ppb).58 The presence of HONO indoors is important as it decomposes readily to OH and NO,9 without needing the high-energy solar photons that initiate the reactions leading to OH production outdoors.59 The decomposition of HONO can occur with indoor light, as the absorption of this molecule starts just below 400 nm, with a peak maximum at 354 nm.60 As shown in Kowal et al.,61 there are many indoor light sources that can decompose HONO in the gas phase to form OH and NO. Due to its high reactivity, OH lifetimes are typically on the order of milliseconds, meaning that an OH molecule will have a mean free path of less than a meter before it reacts. Thus, since windows filter these higher energy wavelengths and OH does not live long enough to be transported indoors, any OH indoors must be generated through other processes. Additionally, OH production reactions can be spatially inhomogeneous because of the wide range of photon fluxes found indoors.62 Therefore, the formation of HONO must be understood in indoor environments. Furthermore, the formation of HONO and its dissociation is a topic of great interest, since much less is understood about HONO compared with NO and NO2.
A variety of surface reactions involving NO2, adsorbed nitrate, and photosensitizers have been proposed as mechanisms for the formation of HONO.9 , 63 , 64 This includes the NO2 hydrolysis reaction, which has been investigated with infrared spectroscopy:
| (Equation 2) |
In addition to its reactivity in the gas phase, HONO can also participate in heterogeneous chemistry. It was shown to react with nicotine adsorbed on model indoor surfaces (cellulose), forming tobacco-specific nitrosamines.65
Halogen Surface Chemistry
The last class of oxidants that has received attention recently has been the chlorine-containing gases. These are primarily produced indoors from cleaning product use, like bleach, which usually contains chlorine in the form of sodium hypochlorite (NaClO). When applied, the pH of this alkaline solution drops below the pKa (7.4) of HClO/ClO− and the equilibrium shifts to HOCl, which can lead to Cl2 formation (Equation 3).
| (Equation 3) |
Additional chlorinated species formed include ClNO2, Cl2O, NHCl2, and NCl3.66 After emission, these gases can also photolyze to form more halogen-containing oxidants (Cl and ClO), as well as OH, which are then taken up through reactions with indoor surfaces (Equation 4).
| (Equation 4) |
These HOCl-dominated formation pathways become far more important indoors than outdoors and have been shown to impact the overall indoor oxidant budgets.66 , 67 These halogen oxidants subsequently react with organic films68 and with VOCs to form aerosols that provide further surface area for indoor reactions.69 For example, HOCl can react with unsaturated molecules to form chlorohydrins (Equation 5).68
| (Equation 5) |
Acid-Base Surface Chemistry
Organic acids, ammonia, and nicotine are all important indoor species that can undergo an acid-base chemistry on surfaces. Many organic acids have been measured indoors, including water-soluble species such as lactic acid, pyruvic acid, and acetic acid.50 These organic acids can undergo interesting reactions on surfaces, such as pyruvic acids on oxide surfaces.70 Additionally, gases that partition into the aqueous phase on damp surfaces, such as glyoxal, can form different products (e.g., oxalic acid) than those that would form in the gas phase (e.g., formaldehyde).50 Another set of important organic gases that displays an acid-base behavior indoors is obtained from environmental tobacco smoke (ETS).71, 72, 73 For example, nicotine can exist in a protonated form, which has low volatility, but can partition to the gas phase in its free base (deprotonated form). Recent work has shown third-hand smoke from the re-emission of nicotine and other ETS compounds,71 indicating an important role for surface acidity, which is still highly uncertain. Ammonia concentrations are often a factor of 5–10 times higher indoors (∼30 ppb indoors versus 1–5 ppb outdoors), with concentrations reaching 100–1,000 ppb during certain activities.74 , 75 As such, ammonia can react with inorganic and organic acids to produce an aerosol mass and contributes to surface chemistry. These main reactions and equilibria indicate the importance of the acid-base chemistry in the indoor environment.
Indoor Relevant Surfaces for Laboratory Investigations
To gain a mechanistic insight into the reactions occurring at indoor surfaces, it is important to identify common surface materials for the community to focus on or use as standards for intercomparisons. Surface materials refers to the primary bulk materials, such as paints or carpet fibers, and excludes material that may accumulate on the substrate, such as organic films, salts, or grime. The broad array of indoor materials and its ever evolving nature3 make this challenging, but a few key surfaces have emerged during the recent expansion of indoor air chemistry research that are worth noting and that can serve as testbeds for many of the key scientific questions being studied, which are discussed below (Table 1 ). These surfaces can be grouped into inorganic, organic, and mixed inorganic/organic surfaces. To enable detailed molecular studies, model systems are listed that can facilitate a mechanistic understanding through their better-characterized chemical properties, as well as the ambient indoor surfaces they aim to represent, which are often more complex. As fundamental studies are conducted, the use of authentic indoor materials is also recommended to better understand the complexity of the system.
Table 1.
Recommended Indoor Surfaces for Surface Chemistry Studies, Including Model Systems and Their Molecular Formulas
| Material | Category | Model System | Chemical Formulas | Chemical Structure |
|---|---|---|---|---|
| Glass | inorganic | silicon dioxide | SiO2 | 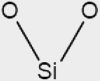 |
| Concrete | inorganic | quicklime (cement) limestone (aggregate) |
CaO CaCO3 |
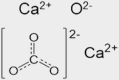 |
| Drywall | inorganic | Gypsum | CaSO4·2H2O | 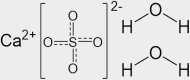 |
| Fabric | organic | polyethylene terephthalate (PET) (a.k.a. polyester) | [C10H8O4]n | 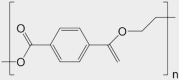 |
| Carpet | organic | nylon (e.g., nylon 6) | [NH(CH2)5CO]n |  |
| Wood/cotton | organic | cellulose | [C6H10O5]n | 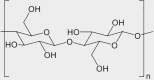 |
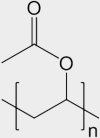
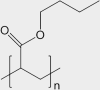
| Latex-painted drywall | mixed inorganic/organic |
synthetic rubber (e.g., co-polymer of vinyl acetate and butyl acrylate) | [CH3COOCH=CH2]n and [CH2CHCOO(CH2)3CH3]n |
|
Inorganic Surfaces
One of the most studied surfaces for coupled gas-phase and condensed-phase chemistry is glass. As most residential and commercial built environments contain windows, different forms of glass are ubiquitous in indoor settings. For detailed surface studies, silicon dioxide provides a model system that emulates many of the key properties of windows. As an example of its usage, Or et al.76 placed glass in different indoor locations, probed the composition and thickness of films, and deposited particles to learn about the coatings that form on glass. With its relatively well-defined structure, SiO2 represents an easily available surface for intercomparisons between studies of different indoor environments and controlled laboratory studies. A second inorganic surface, that is present in the built environment, is drywall, and the logical model system to study is the primary component of drywall, gypsum (CaSO4·2H2O). In contrast to glass, drywall is a less rigid surface, with greater surface roughness, which can both adsorb and absorb water. Thus, it represents a more heterogeneous interface in comparison with glass, providing a notable contrast between prevalent indoor surfaces. A third common, yet, less studied, inorganic surface we recommend for indoor air chemistry studies is related to concrete, which includes a mixture of calcium oxide (CaO) and calcium carbonate (CaCO3). While there are numerous other common inorganic surfaces that are important indoors, glass, drywall, and concrete provide a well-rounded set of systems for study and for intercomparisons between indoor chemistry studies.
Organic Surfaces
By surface area, organic materials are the most abundant indoors6 making it important to identify some of the most common surfaces for study, such as paints, fabrics, carpets, and wood. One of the challenges for identifying model systems is that each of these organic matrices has a vast array of compositions and properties, such that no universal model system exists in the way SiO2 can be used for glass. Thus, our recommendations are only examples of some prevalent materials. To represent fabric, we recommend a common polymer in many fabrics, polyethylene terephthalate (PET), also known as polyester. In addition to being used in many woven fibers, PET is also used in many common household plastics, including containers and indoor films, which broaden the appeal of studies of its surface chemistry. Carpets represent another common surface with a great deal of variety in chemical composition and physical properties.40 Different forms of nylon are among the most common polymers used in carpet (e.g., Nylon 6 and Nylon 66). The presence of nitrogen in the polymer (our only recommended surface with nitrogen) also has the potential to facilitate different chemistry in comparison with the PET. Lastly, wood is also common in many indoor environments (both bare and with a polymer coating), and the model system we recommend to replicate its properties is cellulose. This polymer of six-member rings provides a chemically unique material to compare with PET and its aromatic ring and Nylon with its nitrogen. Together, these chemically distinct surfaces provide a diverse set of systems that emulate much of the surface area in different indoor settings. Beyond the three materials that are the focus herein (nylon, PET, and cellulose), numerous other organic materials are present in indoor environments that could be investigated, including phenylurea-formaldehyde polymer (PUF) and polyvinyl chloride (PVC).
Mixed Inorganic/Organic Surfaces
Lastly, the most abundant surfaces in most indoor settings are paints, often painted drywall. As paint can have numerous formulations and unique properties, pinning down a single composition for a model system is difficult. We recommend a common synthetic rubber (i.e., latex) as a model system that consists of a co-polymer of vinyl acetate and butyl acrylate. Even using paint on different inorganic and organic surfaces listed above can lead to very different environments where reactions occur. The recent Manuja et al.6 study identified paint as having the largest surface area in many rooms in different U.S. residences (particularly the bedroom), making this a key surface to probe in detail. Our recommendation, as one of the most ubiquitous indoor surfaces in the industrialized world, is to use painted drywall as a model inorganic/organic system. While each of the surfaces listed above is important to study due to their prevalence indoors, it is also important to conduct studies considering some of the key factors and conditions that modify surface reactivity.
Drivers of Surface Reactivity
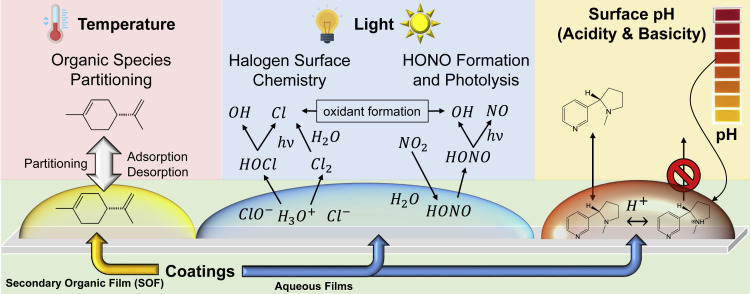
There are many drivers of surface reactivity (Figure 1), which impact the key reactions identified above (Figure 2 ). Besides the surface composition of the underlying material, the nature of the surface and its properties are also influenced by the presence of water and organic films.26 Light can drive photochemistry on surfaces, whether it is sunlight on windows or indoor lights, which have a variety of different spectral features.61 , 77 Temperatures play an important role in surface chemistry and there are temperature gradients and large temperature variations of surfaces throughout indoor spaces that will drive adsorption/desorption processes,20 as well as interfacial chemical reactions.18 These drivers are all discussed in some additional detail below.
Figure 2.
Schematic of Key Reactions Occurring on Indoor Surfaces Related to the Drivers of Surface Reactivity
Surface Composition and Properties
Surface composition, the underlying chemical nature of the surface, surface properties that include porosity, the presence of adsorbed water and water films, the presence of adsorbed OCs and organic films, and surface acidity will all play a role in surface reactions in indoor environments. Furthermore, these surfaces will be heterogeneous, containing regions with high organic content from the deposition of organic aerosol particles and the condensation of organic vapors, and regions with less organic deposition that are instead water rich, owing to the presence of adsorbed water and water films.76 , 78 Indoor surfaces are also dynamic entities and evolve over time.26 , 27 Given this complexity, heterogeneity, and temporal evolution, it is imperative to understand the range of chemical reactivities on surfaces in indoor environments if the underlying molecular processes are to be delineated.
Co-Adsorbed Water and Water Films Play an Important Role in Surface Reactivity
Depending on the nature of the surface chemistry, water on surfaces can enhance or inhibit surface reactivity toward the gas phase. For example, water-soluble gases can easily partition into water films,50 yet, a high relative humidity has also been shown to drive other organic molecules adsorbed onto surfaces back into the gas phase.79 Water-soluble organic species on and in indoor surfaces in the built environment, such as phthalates, may transfer into aqueous films.80 The pH-dependent multiphase chemistry of those species (e.g., hydrolysis) has been reported to be a source of harmful VOCs in the indoor atmosphere,81 , 82 but these processes are poorly quantified. Because of the partitioning of acidic gases and/or bases into these thin water films present on surfaces, and the deposition of aerosol particles, the question of surface acidity (or basicity) is important to address. Aqueous-phase chemistry depends on the solution pH in important ways and many multiphase aqueous chemical processes are pH dependent. For inorganic and organic acids, molecular and ionic speciation of acid/base conjugate pairs depend on pH, and for metals, such as iron, solubility and speciation are highly pH dependent. Therefore, understanding under what conditions these aqueous thin films are acidic or basic will be important in understanding the chemistry of the interfacial region, which includes the underlying surface. Recent progress in measuring acidity on surfaces, especially for aqueous aerosols deposited on to surfaces, has been achieved using acid-base equilibria coupled to micro-Raman spectroscopy.83 , 84 A related factor that could impact molecular interactions in the presence of thin films of water is that little is known about the interface, including surface tension and composition, as well as the ionic strength and/or dielectric properties of the water film. Additionally, recent studies have suggested that reaction kinetics are significantly accelerated within thin films and microdroplets. This has been attributed to partial solvation at the interface and fast diffusion within the thin film/microdroplet, as summarized in Wei et al.85 Other factors that can influence reactivity at the interface include molecular ordering and the protonation state of both organic and inorganic species. Measurements such as zeta potential to understand the surface charge and atomic force microscopy (AFM) for surface tension, are needed to unravel some of these complexities. AFM has been used to probe the surface tension of aqueous and organic environmental interfaces as a function of salt concentration and other important parameters.86 Extending many of these measurements to thin water films relevant to indoor environments will allow for a greater understanding of the role of acid-base chemistry indoors.
SOFs are thought to be ubiquitous on surfaces in indoor environments due to the many sources of VOCs and SVOCs that can adsorb and oxidize, leading to less volatile compounds as well as the deposition of organic particles onto indoor surfaces.34 It was recently shown that glass surfaces present in kitchen environments get coated with organic films with nearly 90% of the surface coated after a few months, whereas surfaces placed in other indoor areas had <10% area of the surface coated.76 The oxidation of organics on surfaces such as painted walls has been proposed to be an important source of gas-phase aldehydes, including nonanal.29 Other studies have shown that the oxidation of adsorbed terpenes, such as limonene, leads to the formation of secondary organic aerosols, SOAs, which can then deposit onto surfaces to form SOFs.87, 88, 89 Most recently, it was shown that adsorbed water can increase the amount of volatile gas-phase products of squalene thin-film oxidation, as well as changing the distribution of squalene oxidation products to yield more aldehyde and ketone products.90 Squalene films are models for skin and fabrics soiled with skin oil. It was also thought that surface reactions of other adsorbed OCs containing unsaturated carbon bonds will show a similar relative humidity dependence.
Light
In outdoor atmospheric chemistry, solar light is known to be a (the) major driver of the daytime chemistry. The smog prevalent in summertime urban environments is the result of photochemistry of atmospheric gases driven by sunlight and can lead to harmful consequences. Sunlight incident on glass windows, incandescent lights, and other light sources all have the potential to initiate chemistry on surfaces. Kowal et al.61 recently measured wavelength-dependent photon fluxes of a variety of indoor light sources, including halogen, incandescent, compact fluorescent lights, fluorescent tubes, and sunlight through windows. They also used these measured photon fluxes to predict radical production rates. Interestingly, they proposed that the photodissociation of nitrous acid, HONO, to produce OH + NO radicals, may be an important source of the gas-phase OH in the indoor environment from several of the measured light sources.
Given that light can also initiate reactions for surface-bound species, as well as within thin SOFs, it is anticipated that surface photochemistry can play a role in the chemistry of indoor environments. Photochemistry on glass windows with sunlight may be important in the oxidation of organics. Semiconductor photochemistry involving TiO2, which is present in paints and self-cleaning coatings, should impact the composition of indoor air. Indeed, such photosensitized chemistry has been observed in a proxy indoor environment: gas-phase NOx and HONO are released from nitrates deposited on surfaces coated with commercial indoor paints when these are illuminated with indoor light sources.57 The presence of photosensitizers within organic films may be important. Reactions that form nitrous acid have been shown to occur on semiconductor surfaces and in the presence of organic photosensitizers.77 Han et al. studied the photoenhanced uptake of NO2 by humic acid (HA) under dry and humidified conditions and found that the humic acid can photosensitize O2 to produce , which may be a reductant responsible for the photochemical uptake of NO2 on HA.77 Additionally, relative humidity and adsorbed H2O play a significant role in the generation of HONO through H+ transfer. Other photo-driven processes on painted surfaces release OCs into the air.91 Furthermore, recently, it was shown that indoor illumination of terpene and bleach can lead to particle formation.69 All of these represent interesting surface chemistry, yet, a quantitative understanding of the fundamental molecular processes involved has still not fully been achieved.
Temperature
Temperature variations in indoor environments can be quite extreme. This is particularly true in “hidden” spaces, such as attics and ducting, or on the high and low temperature coils for heating and cooling systems. An example of the impact of temperature is that the emission rates of phthalates (e.g., di(2-ethylhexyl) phthalate (DEHP)) can increase by a factor of 10 with a 10°C increase in temperature.92 Temperature will impact the lifetime of adsorbed species on the surface. For non-activated desorption processes, the desorption lifetime can be estimated from the expression:
| (Equation 6) |
where is the inverse of the period of vibration of the adsorbate-surface bond, is the heat of adsorption (the negative of the heat of desorption), k B is Boltzmann’s constant, and T is the temperature. At lower temperatures, the desorption lifetime of the adsorbed molecule will increase. For surface reactions, higher temperatures will enhance reactions by having the necessary energy to overcome reaction barriers. Thus, temperature can dictate the chemical lifetime of key molecular species on indoor surfaces and controls reaction rates.
Toward an Understanding of Molecular Processes on Indoor Surfaces: Future Directions
There are many challenges and research needs for developing a molecular understanding of the processes that take place on indoor surfaces relevant to indoor air chemistry and indoor air quality. Some future directions that, if further developed, will provide important steps toward understanding molecular processes on indoor surfaces are discussed and recommendations given.
Emerging Methods for Investigating Indoor Surfaces
Developing new measurement approaches for investigating the chemical composition and reaction mechanisms of indoor surfaces remains a frontier in indoor chemistry, as it lags behind the analysis of gases and particles indoors.37 Table 2 gives some recently utilized techniques for study of surfaces. Importantly, several of these methods include the direct analysis of surfaces. For example, Or et al.76 measured the inhomogenieties in surface morphology and composition across glass surfaces placed in different indoor environments for the first time using micro-spectroscopic probes of the surface. This detailed information gives insights into surface properties, including changes in surface area, the nature of organics that are present on the surface, and the surface coating coverage. Other methods target chemical characterization using mass spectrometry. Furthermore, the combination of micro-spectroscopy and mass spectrometry across these different experimental platforms, will provide new insights into chemical processes occurring on indoor surfaces.
Table 2.
Some Examples of Emerging Methods Used to Measure the Composition and Chemistry of Indoor Surfaces
| Method | Abbreviation | Target | Chemical Property Measured | Analyte Measurement | Select References |
|---|---|---|---|---|---|
| Indoor surface extractor | ISE | extractable organic films | Organic species (semi- to non-volatile) | GC-MS, LC-MS, high resolution MS, offline AMS | O'Brien et al.,93 |
| Direct analysis in real-time mass spectrometry | DART-MS | organic composition | organic species | ambient ionization mass spectrometry |
Zhou et al.,41,42,94 Schwartz-Narbonne et al.,68 |
| Sum frequency generation imaging | SFG imaging | surface order and structure | non-centrosymmetric vibrations | higher order vibrational spectroscopy |
Wang et al.95,96 |
| Atomic force microscopy with photothermal infrared spectroscopy | AFM-PTIR | thin-film composition | infrared absorption | photothermal expansion after absorption of infrared radiation | Or et al.,76,97 Bondy et al.98 |
| Acidity from Raman spectroscopy | Raman pH | Surface acidity | pH (through acid-conjugate base) | vibrational spectroscopy (Raman scattering) | Craig et al., 83 Rindelaub et al.84 |
Modeling Processes on Indoor Surfaces for Indoor Air Quality
Atmospheric chemistry modeling can be used to predict the chemical composition of the atmosphere. These models rely on understanding reaction mechanisms, kinetics, and thermodynamics of reaction processes. Similarly, for the indoor environment, indoor chemistry models are key to predicting and understanding indoor air quality38 and its impact on human health.35 As recently discussed in Shiraiwa et al.,99 modeling the different processes on indoor surfaces requires a range of computational and theoretical models that take into account the wide range of time and length scales. Integration of indoor chemistry models with laboratory experiments and field measurements can provide quantitative interpretation and mechanistic understanding by testing hypotheses. These models are also useful for extrapolation of such results under different conditions and to probe properties that are currently inaccessible by measurements.
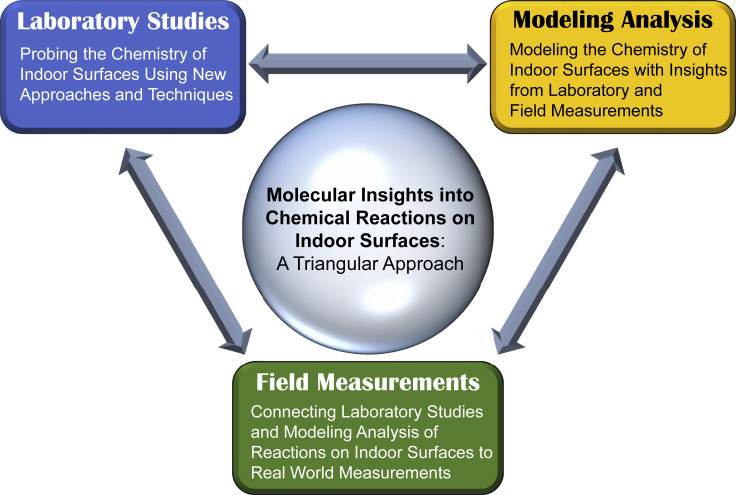
Integrated Field-Lab-Modeling Approach
Currently, the study of indoor environments includes three scientific approaches: (1) measurements within indoor environments, (2) laboratory studies of the chemistry of indoor environments, and (3) modeling analysis. As depicted in Figure 3 , only through the integration of field, laboratory, and modeling analysis can the importance of surface reactions in indoor air chemistry begin to be unraveled. The recent House Observations of Microbial and Environmental Chemistry (HOMEChem) experiment is an example of the kind of study that integrates field measurements in an indoor environment with the control of variables similar to a laboratory environment, with the goal of providing data for indoor air chemistry models.100 For example, from this combination of the state-of-the-art measurements with kinetic modeling, it was revealed that multiphase chemistry drives inorganic chlorinated and nitrogenated compounds in indoor air during bleach cleaning.67
Figure 3.
Conceptual Schematic of Recommended Approach to Indoor Surface Studies
Overall, as greater emphasis is placed on understanding the surface as an active participant in indoor chemistry, and not simply as an unknown entity that is a host to multiphase chemistry, the overall understanding of the chemistry of indoor environments and impacts on human health will improve. Central to improving the understanding of reactions occurring at surfaces are efforts to characterize the surfaces being probed, the use of standard surfaces across different indoor experiments to provide intercomparability and the understanding of the factors that drive surface chemistry. As outlined in this review article, there is a great deal of chemical complexity and thus many challenges in understanding surface chemistry indoors. These challenges give rise to opportunities for researchers ready to take on the chemical complexity of indoor surfaces and their reactivity, which is becoming increasingly clear can dictate indoor air quality and subsequently impact human health.
Acknowledgments
Dr. Paula Olsiewski is acknowledged for her leadership of the Chemistry of Indoor Environments program, which enabled this review. This work was supported by the Sloan Foundation’s Chemistry of Indoor Environments (CIE) program, specifically grant G-2018-10122 (Ault), which funded the “Molecular Insights into Chemical Reactions on Indoor Surfaces” workshop at the University of Michigan, May 7–8, 2018, organized by Professors Ault and Grassian, which inspired many of the ideas behind this work. The Sloan Surface Consortium for Chemistry of Indoor Environments (SURF-CIE) grew out of this workshop and has driven further efforts (Sloan grant: G-2019-12365). The MOdelling Consortium for Chemistry of Indoor Environments (MOCCIE) modeling effort (Sloan grant: G-2019-12306) has helped drive the exploration of the role of the surface in indoor air chemistry. Professor Doug Tobias is acknowledged for the molecular model representation of an indoor surface depicted through the magnifying glass in Figure 1. Nicole Olson is acknowledged for the electron microscopy images in Figure 1. Lightbulb and thermometer in Figure 2 are from FlatIcon. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. Mention of any commercial product or trade name does not constitute endorsement by the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health.
Author Contributions
A.P.A. and V.H.G. proposed the topic of the review, organized the workshop that was the genesis of the review, conducted the literature review, wrote the manuscript, and made the figures. N.C., D.B.C., H.D., D.J.D., D.K.F., J.L.J., V.F.M., G.C.M., R.E.O., M.S., M.E.V., J.R.W., and W.X. participated in the workshop, provided input on the manuscript, and contributed revisions and edits.
References
- 1.Clarivate Web of science search. 2020. https://clarivate.com/webofsciencegroup/solutions/web-of-science/
- 2.Klepeis N.E., Nelson W.C., Ott W.R., Robinson J.P., Tsang A.M., Switzer P., Behar J.V., Hern S.C., Engelmann W.H. The national human activity pattern survey (NHAPS): a resource for assessing exposure to environmental pollutants. J. Expo Anal Environ Epidemiol. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 3.Weschler C.J. Changes in indoor pollutants since the 1950s. Atmos. Environ. 2009;43:153–169. [Google Scholar]
- 4.Fang Y., Riahi S., McDonald A.T., Shrestha M., Tobias D.J., Grassian V.H. What is the driving force behind the adsorption of hydrophobic molecules on hydrophilic surfaces? J. Phys. Chem. Lett. 2019;10:468–473. doi: 10.1021/acs.jpclett.8b03484. [DOI] [PubMed] [Google Scholar]
- 5.Fang Y., Lakey P.S.J., Riahi S., McDonald A.T., Shrestha M., Tobias D.J., Shiraiwa M., Grassian V.H. A molecular picture of surface interactions of organic compounds on prevalent indoor surfaces: limonene adsorption on SiO2. Chem. Sci. 2019;10:2906–2914. doi: 10.1039/c8sc05560b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manuja A., Ritchie J., Buch K., Wu Y., Eichler C.M.A., Little J.C., Marr L.C. Total surface area in indoor environments. Environ. Sci.: Processes Impacts. 2019;21:1384–1392. doi: 10.1039/c9em00157c. [DOI] [PubMed] [Google Scholar]
- 7.Singer B.C., Hodgson A.T., Hotchi T., Ming K.Y., Sextro R.G., Wood E.E., Brown N.J. Sorption of organic gases in residential rooms. Atmos. Environ. 2007;41:3251–3265. [Google Scholar]
- 8.Wisthaler A., Weschler C.J. Reactions of ozone with human skin lipids: sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air. Proc. Natl. Acad. Sci. USA. 2010;107:6568–6575. doi: 10.1073/pnas.0904498106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finlayson-Pitts B.J., Wingen L.M., Sumner A.L., Syomin D., Ramazan K.A. The heterogeneous hydrolysis of NO2 in laboratory systems and in outdoor and indoor atmospheres: an integrated mechanism. Phys. Chem. Chem. Phys. 2003;5:223–242. [Google Scholar]
- 10.Pttts J.N., Wallington T.J., Biermann H.W., Winer A.M. Identification and measurement of nitrous acid in an indoor environment. Atmos. Environ. 1985;19:763–767. [Google Scholar]
- 11.Liu Q.T., Chen R., McCarry B.E., Diamond M.L., Bahavar B. Characterization of polar organic compounds in the organic film on indoor and outdoor glass windows. Environ. Sci. Technol. 2003;37:2340–2349. doi: 10.1021/es020848i. [DOI] [PubMed] [Google Scholar]
- 12.Shu S., Morrison G.C. Rate and reaction probability of the surface reaction between ozone and dihydromyrcenol measured in a bench scale reactor and a room-sized chamber. Atmos. Environ. 2012;47:421–427. [Google Scholar]
- 13.Ongwandee M., Morrison G.C., Guo X., Chusuei C.C. Adsorption of trimethylamine on zirconium silicate and polyethylene powder surfaces. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2007;310:62–67. [Google Scholar]
- 14.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 — navigating the uncharted. N. Engl. J. Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nazaroff W.W., Weschler C.J., Corsi R.L. Indoor air chemistry and physics. Atmos. Environ. 2003;37:5451–5453. [Google Scholar]
- 17.Weschler C.J., Carslaw N. Indoor chemistry. Environ. Sci. Technol. 2018;52:2419–2428. doi: 10.1021/acs.est.7b06387. [DOI] [PubMed] [Google Scholar]
- 18.Johnson A.M., Waring M.S., DeCarlo P.F. Real-time transformation of outdoor aerosol components upon transport indoors measured with aerosol mass spectrometry. Indoor Air. 2017;27:230–240. doi: 10.1111/ina.12299. [DOI] [PubMed] [Google Scholar]
- 19.Nazaroff W.W., Goldstein A.H. Indoor chemistry: research opportunities and challenges. Indoor Air. 2015;25:357–361. doi: 10.1111/ina.12219. [DOI] [PubMed] [Google Scholar]
- 20.Wang C., Collins D.B., Arata C., Goldstein A.H., Mattila J.M., Farmer D.K., Ampollini L., DeCarlo P.F., Novoselac A., Vance M.E., et al. Surface reservoirs dominate dynamic gas-surface partitioning of many indoor air constituents. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aay8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner B.L., Lambin E.F., Reenberg A. The emergence of land change science for global environmental change and sustainability. Proc. Natl. Acad. Sci. USA. 2007;104:20666–20671. doi: 10.1073/pnas.0704119104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novotny E.V., Bechle M.J., Millet D.B., Marshall J.D. National satellite-based land-use regression: NO2 in the United States: NO2 in the United States. Environ. Sci. Technol. 2011;45:4407–4414. doi: 10.1021/es103578x. [DOI] [PubMed] [Google Scholar]
- 23.Morrison G. Interfacial chemistry in indoor environments. Environ. Sci. Technol. 2008;42:3494–3499. doi: 10.1021/es087114b. [DOI] [PubMed] [Google Scholar]
- 24.Morrison G.C., Carslaw N., Waring M.S. A modeling enterprise for chemistry of indoor environments (CIE) Indoor Air. 2017;27:1033–1038. doi: 10.1111/ina.12407. [DOI] [PubMed] [Google Scholar]
- 25.Breen M.S., Burke J.M., Batterman S.A., Vette A.F., Godwin C., Croghan C.W., Schultz B.D., Long T.C. Modeling spatial and temporal variability of residential air exchange rates for the near-road exposures and effects of urban air pollutants study (Nexus) Int. J. Environ. Res. Public Health. 2014;11:11481–11504. doi: 10.3390/ijerph111111481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weschler C.J., Nazaroff W.W. Growth of organic films on indoor surfaces. Indoor Air. 2017;27:1101–1112. doi: 10.1111/ina.12396. [DOI] [PubMed] [Google Scholar]
- 27.Zhou S., Hwang B.C.H., Lakey P.S.J., Zuend A., Abbatt J.P.D., Shiraiwa M. Multiphase reactivity of polycyclic aromatic hydrocarbons is driven by phase separation and diffusion limitations. Proc. Natl. Acad. Sci. USA. 2019;116:11658–11663. doi: 10.1073/pnas.1902517116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darling E., Corsi R.L. Field-to-laboratory analysis of clay wall coatings as passive removal materials for ozone in buildings. Indoor Air. 2017;27:658–669. doi: 10.1111/ina.12345. [DOI] [PubMed] [Google Scholar]
- 29.Kruza M., Lewis A.C., Morrison G.C., Carslaw N. Impact of surface ozone interactions on indoor air chemistry: a modeling study. Indoor Air. 2017;27:1001–1011. doi: 10.1111/ina.12381. [DOI] [PubMed] [Google Scholar]
- 30.Śmiełowska M., Marć M., Zabiegała B. Indoor air quality in public utility environments-a review. Environ Sci Pollut Res Int. 2017;24:11166–11176. doi: 10.1007/s11356-017-8567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weschler C.J. Roles of the human occupant in indoor chemistry. Indoor Air. 2016;26:6–24. doi: 10.1111/ina.12185. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell C.S., Zhang J.J., Sigsgaard T., Jantunen M., Lioy P.J., Samson R., Karol M.H. Current state of the science: health effects and indoor environmental quality. Environ. Health Perspect. 2007;115:958–964. doi: 10.1289/ehp.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weschler C.J. Ozone in indoor environments: concentration and chemistry. Indoor Air Int. J. Indoor Air Qual. Clim. 2000;10:269–288. doi: 10.1034/j.1600-0668.2000.010004269.x. [DOI] [PubMed] [Google Scholar]
- 34.Abbatt J.P.D., Wang C. The atmospheric chemistry of indoor environments. Environ. Sci.: Processes Impacts. 2020;22:25–48. doi: 10.1039/c9em00386j. [DOI] [PubMed] [Google Scholar]
- 35.Wells J.R., Schoemaecker C., Carslaw N., Waring M.S., Ham J.E., Nelissen I., Wolkoff P. Reactive indoor air chemistry and health-a workshop summary. Int. J. Hyg. Environ. Health. 2017;220:1222–1229. doi: 10.1016/j.ijheh.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wargocki P., Lai A. Editorial - special issue on indoor pollutants, chemistry and health. Selected papers presented at indoor Air 2014 conference in Hong Kong. Build. Environ. 2015;93:1–2. [Google Scholar]
- 37.Farmer D.K. Analytical challenges and opportunities for indoor air chemistry field studies. Anal. Chem. 2019;91:3761–3767. doi: 10.1021/acs.analchem.9b00277. [DOI] [PubMed] [Google Scholar]
- 38.Carslaw N. A new detailed chemical model for indoor air pollution. Atmos. Environ. 2007;41:1164–1179. [Google Scholar]
- 39.Wang H., Morrison G.C. Ozone-initiated secondary emission rates of aldehydes from indoor surfaces in four homes. Environ. Sci. Technol. 2006;40:5263–5268. doi: 10.1021/es060080s. [DOI] [PubMed] [Google Scholar]
- 40.Morrison G.C., Nazaroff W.W. Ozone interactions with carpet: secondary emissions of aldehydes. Environ. Sci. Technol. 2002;36:2185–2192. doi: 10.1021/es0113089. [DOI] [PubMed] [Google Scholar]
- 41.Zhou S.M., Forbes M.W., Abbatt J.P.D. Kinetics and products from heterogeneous oxidation of squalene with ozone. Environ. Sci. Technol. 2016;50:11688–11697. doi: 10.1021/acs.est.6b03270. [DOI] [PubMed] [Google Scholar]
- 42.Zhou S.M., Forbes M.W., Katrib Y., Abbatt J.P.D. Rapid oxidation of skin oil by ozone. Environ. Sci. Technol. Lett. 2016;3:170–174. [Google Scholar]
- 43.Liu S., Thompson S.L., Stark H., Ziemann P.J., Jimenez J.L. Gas-phase carboxylic acids in a university classroom: abundance, variability, and sources. Environ. Sci. Technol. 2017;51:5454–5463. doi: 10.1021/acs.est.7b01358. [DOI] [PubMed] [Google Scholar]
- 44.Liu S., Li R., Wild R.J., Warneke C., de Gouw J.A., Brown S.S., Miller S.L., Luongo J.C., Jimenez J.L., Ziemann P.J. Contribution of human-related sources to indoor volatile organic compounds in a university classroom. Indoor Air. 2016;26:925–938. doi: 10.1111/ina.12272. [DOI] [PubMed] [Google Scholar]
- 45.Seinfeld J.H., Pandis S.N. John Wiley & Sons; 2016. Atmospheric Chemistry and Physics: from Air Pollution to Climate Change. [Google Scholar]
- 46.Rim D., Gall E.T., Maddalena R.L., Nazaroff W.W. Ozone reaction with interior building materials: influence of diurnal ozone variation, temperature and humidity. Atmos. Environ. 2016;125:15–23. [Google Scholar]
- 47.Wang H., Morrison G. Ozone-surface reactions in five homes: surface reaction probabilities, aldehyde yields, and trends. Indoor Air. 2010;20:224–234. doi: 10.1111/j.1600-0668.2010.00648.x. [DOI] [PubMed] [Google Scholar]
- 48.Sabersky R.H., Sinema D.A., Shair F.H. Concentrations, decay rates, and removal of ozone and their relation to establishing clean indoor air. Environ. Sci. Technol. 1973;7:347–353. [Google Scholar]
- 49.Shiraiwa M., Sosedova Y., Rouvière A., Yang H., Zhang Y., Abbatt J.P., Ammann M., Pöschl U. The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles. Nat. Chem. 2011;3:291–295. doi: 10.1038/nchem.988. [DOI] [PubMed] [Google Scholar]
- 50.Duncan S.M., Sexton K.G., Turpin B.J. Oxygenated VOCs, aqueous chemistry, and potential impacts on residential indoor air composition. Indoor Air. 2018;28:198–212. doi: 10.1111/ina.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price D.J., Day D.A., Pagonis D., Stark H., Algrim L.B., Handschy A.V., Liu S., Krechmer J.E., Miller S.L., Hunter J.F., et al. Budgets of organic carbon composition and oxidation in indoor air. Environ. Sci. Technol. 2019;53:13053–13063. doi: 10.1021/acs.est.9b04689. [DOI] [PubMed] [Google Scholar]
- 52.Tang X.C., Misztal P.K., Nazaroff W.W., Goldstein A.H. Volatile organic compound emissions from humans indoors. Environ. Sci. Technol. 2016;50:12686–12694. doi: 10.1021/acs.est.6b04415. [DOI] [PubMed] [Google Scholar]
- 53.Coleman B.K., Destaillats H., Hodgson A.T., Nazaroff W.W. Ozone consumption and volatile byproduct formation from surface reactions with aircraft cabin materials and clothing fabrics. Atmos. Environ. 2008;42:642–654. [Google Scholar]
- 54.Bernstein J.A., Alexis N., Bacchus H., Bernstein I.L., Fritz P., Horner E., Li N., Mason S., Nel A., Oullette J., et al. The health effects of non-industrial indoor air pollution. J. Allergy Clin. Immunol. 2008;121:585–591. doi: 10.1016/j.jaci.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Z., Zhou S., Abbatt J.P.D. Kinetics and condensed-phase products in multiphase ozonolysis of an unsaturated triglyceride. Environ. Sci. Technol. 2019;53:12467–12475. doi: 10.1021/acs.est.9b04460. [DOI] [PubMed] [Google Scholar]
- 56.Lakey P.S.J., Morrison G.C., Won Y., Parry K.M., von Domaros M., Tobias D.J., Rim D., Shiraiwa M. The impact of clothing on ozone and squalene ozonolysis products in indoor environments. Commun. Chem. 2019;2:56. [Google Scholar]
- 57.Schwartz-Narbonne H., Jones S.H., Donaldson D.J. Indoor lighting releases gas phase nitrogen oxides from indoor painted surfaces. Environ. Sci. Technol. Lett. 2019;6:92–97. [Google Scholar]
- 58.Gligorovski S. Nitrous acid (HONO): an emerging indoor pollutant. J. Photochem. Photobiol. A. 2016;314:1–5. [Google Scholar]
- 59.Finlayson-Pitts B.J., Pitts J.N. Academic Press; 2000. Chemistry of the Upper and Lower Atmosphere. [Google Scholar]
- 60.Stutz J., Kim E.S., Platt U., Bruno P., Perrino C., Febo A. UV-visible absorption cross sections of nitrous acid. J. Geophys. Res. 2000;105:14585–14592. [Google Scholar]
- 61.Kowal S.F., Allen S.R., Kahan T.F. Wavelength-resolved photon fluxes of indoor light sources: implications for HOx production. Environ. Sci. Technol. 2017;51:10423–10430. doi: 10.1021/acs.est.7b02015. [DOI] [PubMed] [Google Scholar]
- 62.Won Y., Waring M., Rim D. Understanding the spatial heterogeneity of indoor OH and HO2 due to photolysis of HONO using computational fluid dynamics simulation. Environ. Sci. Technol. 2019;53:14470–14478. doi: 10.1021/acs.est.9b06315. [DOI] [PubMed] [Google Scholar]
- 63.Rubasinghege G., Grassian V.H. Photochemistry of adsorbed nitrate on aluminum oxide particle surfaces. J. Phys. Chem. A. 2009;113:7818–7825. doi: 10.1021/jp902252s. [DOI] [PubMed] [Google Scholar]
- 64.Monge M.E., D'Anna B., George C. Nitrogen dioxide removal and nitrous acid formation on titanium oxide surfaces—an air quality remediation process? Phys. Chem. Chem. Phys. 2010;12:8991–8998. doi: 10.1039/b925785c. [DOI] [PubMed] [Google Scholar]
- 65.Sleiman M., Gundel L.A., Pankow J.F., Jacob P., Singer B.C., Destaillats H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc. Natl. Acad. Sci. USA. 2010;107:6576–6581. doi: 10.1073/pnas.0912820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong J.P.S., Carslaw N., Zhao R., Zhou S., Abbatt J.P.D. Observations and impacts of bleach washing on indoor chlorine chemistry. Indoor Air. 2017;27:1082–1090. doi: 10.1111/ina.12402. [DOI] [PubMed] [Google Scholar]
- 67.Mattila J.M., Lakey P.S.J., Shiraiwa M., Wang C., Abbatt J.P.D., Arata C., Goldstein A.H., Ampollini L., Katz E.F., DeCarlo P.F., et al. Multiphase chemistry controls inorganic chlorinated and nitrogenated compounds in indoor air during bleach cleaning. Environ. Sci. Technol. 2020;54:1730–1739. doi: 10.1021/acs.est.9b05767. [DOI] [PubMed] [Google Scholar]
- 68.Schwartz-Narbonne H., Wang C., Zhou S., Abbatt J.P.D., Faust J. Heterogeneous chlorination of squalene and oleic acid. Environ. Sci. Technol. 2019;53:1217–1224. doi: 10.1021/acs.est.8b04248. [DOI] [PubMed] [Google Scholar]
- 69.Wang C., Collins D.B., Abbatt J.P.D. Indoor illumination of terpenes and bleach emissions leads to particle formation and growth. Environ. Sci. Technol. 2019;53:11792–11800. doi: 10.1021/acs.est.9b04261. [DOI] [PubMed] [Google Scholar]
- 70.Alves M.R., Fang Y., Wall K.J., Vaida V., Grassian V.H. Chemistry and photochemistry of pyruvic acid adsorbed on oxide surfaces. J. Phys. Chem. A. 2019;123:7661–7671. doi: 10.1021/acs.jpca.9b06563. [DOI] [PubMed] [Google Scholar]
- 71.DeCarlo P.F., Avery A.M., Waring M.S. Thirdhand smoke uptake to aerosol particles in the indoor environment. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aap8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ongwandee M., Morrison G.C. Influence of ammonia and carbon dioxide on the sorption of a basic organic pollutant to carpet and latex-painted gypsum board. Environ. Sci. Technol. 2008;42:5415–5420. doi: 10.1021/es071935j. [DOI] [PubMed] [Google Scholar]
- 73.Ongwandee M., Sawanyapanich P. Influence of relative humidity and gaseous ammonia on the nicotine sorption to indoor materials. Indoor Air. 2012;22:54–63. doi: 10.1111/j.1600-0668.2011.00737.x. [DOI] [PubMed] [Google Scholar]
- 74.Ampollini L., Katz E.F., Bourne S., Tian Y., Novoselac A., Goldstein A.H., Lucic G., Waring M.S., DeCarlo P.F. Observations and contributions of real-time indoor ammonia concentrations during HOMEChem. Environ. Sci. Technol. 2019;53:8591–8598. doi: 10.1021/acs.est.9b02157. [DOI] [PubMed] [Google Scholar]
- 75.Brauer M., Koutrakis P., Keeler G.J., Spengler J.D. Indoor and outdoor concentrations of inorganic acidic aerosols and gases. J Air Waste Manage Assoc. 1991;41:171–181. doi: 10.1080/10473289.1991.10466834. [DOI] [PubMed] [Google Scholar]
- 76.Or V.W., Alves M.R., Wade M., Schwab S., Corsi R.L., Grassian V.H., Clear C. Crystal Clear? Microspectroscopic Imaging and Physicochemical Characterization of Indoor Depositions on Window Glass. Environ. Sci. Technol. Lett. 2018;5:514–519. [Google Scholar]
- 77.Stemmler K., Ammann M., Donders C., Kleffmann J., George C. Photosensitized reduction of nitrogen dioxide on humic acid as a source of nitrous acid. Nature. 2006;440:195–198. doi: 10.1038/nature04603. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz-Narbonne H., Donaldson D.J. Water uptake by indoor surface films. Sci Rep. 2019;9:11089. doi: 10.1038/s41598-019-47590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang M.J., Cziczo D.J., Grassian V.H. Interactions of water with mineral dust aerosol: water adsorption, hygroscopicity, cloud condensation, and ice nucleation. Chem. Rev. 2016;116:4205–4259. doi: 10.1021/acs.chemrev.5b00529. [DOI] [PubMed] [Google Scholar]
- 80.Jaeger R.J., Rubin R.J. Plasticizers from plastic devices extraction, metabolism, and accumulation by biological systems. Science. 1970;170:460–462. doi: 10.1126/science.170.3956.460. [DOI] [PubMed] [Google Scholar]
- 81.Sohoni P., Sumpter J.P. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- 82.Norbäck D., Wieslander G., Nordström K., Wålinder R. Asthma symptoms in relation to measured building dampness in upper concrete floor construction, and 2-ethyl-1-hexanol in indoor air. Int. J. Tuberc. Lung Dis. 2000;4:1016–1025. [PubMed] [Google Scholar]
- 83.Craig R.L., Nandy L., Axson J.L., Dutcher C.S., Ault A.P. Spectroscopic determination of aerosol pH from acid–base equilibria in inorganic, organic, and mixed systems. J. Phys. Chem. A. 2017;121:5690–5699. doi: 10.1021/acs.jpca.7b05261. [DOI] [PubMed] [Google Scholar]
- 84.Rindelaub J.D., Craig R.L., Nandy L., Bondy A.L., Dutcher C.S., Shepson P.B., Ault A.P. Direct measurement of pH in individual particles via Raman microspectroscopy and variation in acidity with relative humidity. J. Phys. Chem. A. 2016;120:911–917. doi: 10.1021/acs.jpca.5b12699. [DOI] [PubMed] [Google Scholar]
- 85.Wei Z., Li Y., Cooks R.G., Yan X. Accelerated reaction kinetics in microdroplets: overview and recent developments. Annu. Rev. Phys. Chem. 2020;71:31–51. doi: 10.1146/annurev-physchem-121319-110654. [DOI] [PubMed] [Google Scholar]
- 86.Lee H.D., Estillore A.D., Morris H.S., Ray K.K., Alejandro A., Grassian V.H., Tivanski A.V. Direct surface tension measurements of individual sub-micrometer particles using atomic force microscopy. J. Phys. Chem. A. 2017;121:8296–8305. doi: 10.1021/acs.jpca.7b04041. [DOI] [PubMed] [Google Scholar]
- 87.Waring M.S., Siegel J.A. Indoor secondary organic aerosol formation initiated from reactions between ozone and surface-sorbed d-limonene. Environ. Sci. Technol. 2013;47:6341–6348. doi: 10.1021/es400846d. [DOI] [PubMed] [Google Scholar]
- 88.Youssefi S., Waring M.S. Transient secondary organic aerosol formation from limonene ozonolysis in indoor environments: impacts of air exchange rates and initial concentration ratios. Environ. Sci. Technol. 2014;48:7899–7908. doi: 10.1021/es5009906. [DOI] [PubMed] [Google Scholar]
- 89.Destaillats H., Lunden M.M., Singer B.C., Coleman B.K., Hodgson A.T., Weschler C.J., Nazaroff W.W. Indoor secondary pollutants from household product emissions in the presence of ozone: a bench-scale chamber study. Environ. Sci. Technol. 2006;40:4421–4428. doi: 10.1021/es052198z. [DOI] [PubMed] [Google Scholar]
- 90.Arata C., Heine N., Wang N., Misztal P.K., Wargocki P., Bekö G., Williams J., Nazaroff W.W., Wilson K.R., Goldstein A.H. Heterogeneous ozonolysis of squalene: gas-phase products depend on water vapor concentration. Environ. Sci. Technol. 2019;53:14441–14448. doi: 10.1021/acs.est.9b05957. [DOI] [PubMed] [Google Scholar]
- 91.Gandolfo A., Marque S., Temime-Roussel B., Gemayel R., Wortham H., Truffier-Boutry D., Bartolomei V., Gligorovski S. Unexpectedly high levels of organic compounds released by indoor photocatalytic paints. Environ. Sci. Technol. 2018;52:11328–11337. doi: 10.1021/acs.est.8b03865. [DOI] [PubMed] [Google Scholar]
- 92.Liang Y., Xu Y. Emission of phthalates and phthalate alternatives from vinyl flooring and crib mattress covers: the influence of temperature. Environ. Sci. Technol. 2014;48:14228–14237. doi: 10.1021/es504801x. [DOI] [PubMed] [Google Scholar]
- 93.O'Brien R.E., Ridley K.J., Canagaratna M.R., Jayne J.T., Croteau P.L., Worsnop D.R., Budisulistiorini S.H., Surratt J.D., Follett C.L., Repeta D.J., Kroll J.H. Ultrasonic nebulization for the elemental analysis of microgram-level samples with offline aerosol mass spectrometry. Atmos. Meas. Tech. 2019;12:1659–1671. [Google Scholar]
- 94.Zhou S.M., Forbes M.W., Abbatt J.P.D. Application of direct analysis in real time-mass spectrometry (DART-MS) to the study of gas-surface heterogeneous reactions: focus on ozone and PAHs. Anal. Chem. 2015;87:4733–4740. doi: 10.1021/ac504722z. [DOI] [PubMed] [Google Scholar]
- 95.Wang H., Chen W., Wagner J.C., Xiong W. Local ordering of lattice self-assembled SDS@2β-CD materials and adsorbed water revealed by vibrational sum frequency generation microscope. J. Phys. Chem. B. 2019;123:6212–6221. doi: 10.1021/acs.jpcb.9b04928. [DOI] [PubMed] [Google Scholar]
- 96.Wang H., Gao T., Xiong W. Self-phase-stabilized heterodyne vibrational sum frequency generation microscopy. ACS Photonics. 2017;4:1839–1845. [Google Scholar]
- 97.Or V.W., Estillore A.D., Tivanski A.V., Grassian V.H. Lab on a tip: atomic force microscopy- photothermal infrared spectroscopy of atmospherically relevant organic/inorganic aerosol particles in the nanometer to micrometer size range. Analyst. 2018;143:2765–2774. doi: 10.1039/c8an00171e. [DOI] [PubMed] [Google Scholar]
- 98.Bondy A.L., Kirpes R.M., Merzel R.L., Pratt K.A., Banaszak Holl M.M., Ault A.P. Atomic force microscopy-infrared spectroscopy of individual atmospheric aerosol particles: subdiffraction limit vibrational spectroscopy and morphological analysis. Anal. Chem. 2017;89:8594–8598. doi: 10.1021/acs.analchem.7b02381. [DOI] [PubMed] [Google Scholar]
- 99.Shiraiwa M., Carslaw N., Tobias D.J., Waring M.S., Rim D., Morrison G., Lakey P.S.J., Kruza M., Von Domaros M., Cummings B.E., et al. Modelling consortium for chemistry of indoor environments (MOCCIE): integrating chemical processes from molecular to room scales. Environ. Sci.: Processes Impacts. 2019;21:1240–1254. doi: 10.1039/c9em00123a. [DOI] [PubMed] [Google Scholar]
- 100.Farmer D.K., Vance M.E., Abbatt J.P.D., Abeleira A., Alves M.R., Arata C., Boedicker E., Bourne S., Cardoso-Saldaña F., Corsi R., et al. Overview of HOMEChem: house observations of microbial and environmental chemistry. Environ. Sci.: Processes Impacts. 2019;21:1280–1300. doi: 10.1039/c9em00228f. [DOI] [PubMed] [Google Scholar]