Abstract
Objective
The purpose of our study was to assess organ function in 102 patients with severe COVID-19 infections, using retrospective clinical analysis.
Material and methods
A retrospective analysis was conducted on 102 patients with severe COVID-19 infections. The patients were divided into a survival group (n = 73) and a non-survival group (n = 29) according to their prognosis. The age, sex, underlying diseases, clinical laboratory data within 48 h (routine blood tests, ALT, AST, TBIL, ALB, BUN, CR, D-Dimer, PT, APTT, FIB, F VIII:C, CK-MB, CK, and LDH), and ventilation status were collected. The organ functions of these severe COVID-19 patients were assessed by comparing the differences between the two groups.
Results
AST, BUN, CR, CK-MB, LDH, and CK in the non-survival group were higher than those in the survival group, and the differences were statistically significant (P < 0.05). D-Dimer, PT, FIB, and F VIII:C in the non-survival group were higher than the values observed in the survival group, and the differences were statistically significant (P < 0.05). PLT, AST, BUN, CR, D-Dimer, PT, FIB, F VIII:C, CK-MB, CK, and LDH predicted the area under the ROC curve (AUC) of the COVID19 endpoint events and were 0.721, 0.854, 0.867, 0.757, 0.699, 0.679, 0.715, 0.811, 0.935, and 0.802, respectively.
Conclusion
The results showed that there were different degrees of damage to the liver, kidneys, blood coagulation, and heart function in the non-survival group. In addition, PLT, AST, BUN, CR, D-Dimer, PT, FIB, F VIII:C, CK-MB, CK, and LDH had value in evaluating disease prognosis.
Keywords: COVID-19, Pneumonia, Organ function, Prognosis
Abstract
Objetivo
Nuestro estudio tiene como objetivo evaluar la función del órgano en 102 pacientes con infección grave COVID-19 mediante análisis clínicos retrospectivos.
Materiales y métodos
Análisis retrospectivo de 102 pacientes con infección grave COVID-19. Los pacientes se dividieron en grupo de supervivencia (n = 73) y grupo de no supervivencia (n = 29) según la pre-fase. Edad, género, enfermedades subyacentes, datos de laboratorio clínico dentro de las 48 h (prueba de sangre de rutina, ALT, AST, TBIL, ALB, BUN, CR, dímero D, PT, APTT, FIB, F VIII: C, CK-MB, CK y LDH), y el estado de ventilación. Al comparar las diferencias entre los 2 grupos, se evaluó la función orgánica de estos pacientes graves con COVID-19.
Resultados
AST, BUN, CR, CK-MB, LDH y CK fueron todos más altos que el grupo de supervivencia en el grupo no sobreviviente, con una diferencia estadísticamente significativa (p < 0,05). Dímero D, PT, FIB y F VIII: C fueron mayores que el grupo de supervivencia en el grupo de no supervivencia, y la diferencia fue estadísticamente significativa (p < 0,05). PLT, AST, BUN, CR, dímero D, PT, FIB, F VIII: C, CK-MB, CK y LDH predijeron el área de curva inferior ROC (AUC) del evento final COVID-19, a 0,721, 0,854, 0,867, 0,757, 0,699, 0,679, 0,715, 0,811, 0,935 y 0,802, respectivamente.
Conclusión
Los resultados mostraron que el grupo de no supervivencia tenía diferentes grados de daño al hígado, riñón, coagulación y función cardíaca. Además, PLT, AST, BUN, CR, dímero D, PT, FIB, F VIII:C, CK-MB, CK y LDH tienen valor en la evaluación del pronóstico de la enfermedad.
Palabras clave: COVID-19, Neumonía, Función del órgano, Pronóstico
Introduction
In December 2019, pneumonia cases appeared in Wuhan, Hubei Province, China. Then the disease spread throughout China, and then to the entire world. The spread of Corona Virus Disease-2019 (COVID-19) has resulted in a large-scale pandemic, which already has had an irreparable impact on China and the world. Under the research of global experts, it was discovered that this infectious pneumonia is caused by a novel coronavirus infection. The disease caused by the novel coronavirus was officially named “COVID-19” by the World Health Organization (WHO) on February 11, 2020.
Coronavirus (CoV) is a single-stranded positive-stranded RNA virus. The International Committee on Taxonomy of Viruses (ICTV) divides CoVs into four types: Alphacoronavirus (αCoV), Betacoronavirus (βCoV), Deltacoronavirus (δCoV), and Gammacoronavirus (γCoV). Human coronavirus (HCoV) infection mainly refers to α or βCoV.1 The novel coronavirus (formerly known as 2019-nCoV) belongs to the βCoV type. At first, it was thought that the infection was not a result of human-to-human transmission. Later, it was found that the virus had clear human-to-human transmission, high infectivity, and severe pathogenicity.2, 3
The main clinical manifestations of COVID-19 are fever, cough, and fatigue. Patients with severe infections can experience acute respiratory distress syndrome (ARDS), and even multiple organ failure (MODS).3, 4 The purpose of this study was to assess organ function in infected patients who did not survive compared to patients who did survive in a cohort of severe COVID-19 patients. A clinical retrospective study was used that included collection of blood indices from 102 cases.
Material and methods
Clinical data
Clinical data were collected retrospectively from severe COVID-19 patients admitted to Huangshi Hospital of TCM (Infectious Disease Hospital) and E Dong Medical Group Huangshi Central Hospital in Hubei Province from February 2020, to March 2020. The patients were divided into a survival group and a non-survival group according to their prognosis.
The inclusion criteria were as follows: (1) The diagnosis of COVID-19 infection matched the novel coronavirus's diagnosis and treatment program established in China. (2) The patient met the criteria for the COVID-19 diagnosis and treatment program (7th edition) for severe cases. These criteria included respiratory distress (RR ≥ 30 times/min), oxygen saturation less than 93% in the resting state, and an oxygen partial pressure in arterial blood (PaO2)/oxygen concentration (FiO2) that was less than 300 mm Hg (1 mg Hg = 0.133 kPa). Also, the PaO2/FiO2 was corrected according to the formula, PaO2/FiO2 × [atmospheric pressure (mm Hg)/760]. Lastly, pulmonary imaging showed that the lesions progressed to more than 50% in 24–48 h.3 (3) The patients’ clinical data were complete.
Research methods
The patients with COVID-19 infections were retrospectively analyzed and divided into a survival group and a non-survival group according to their prognosis. Age, sex, and underlying disease data were collected. Routine blood tests were performed on the blood samples by using blood analyzer Abbott CD-3700; Blood biochemistry parameters[glutamic pyruvic transaminase (ALT), glutamic oxaloacetic transaminase (AST), total bilirubin (TBIL), albumin (ALB), urea nitrogen (BUN), creatinine (CR),creatine kinase isoenzyme (CK-MB), creatine kinase (CK), and lactate dehydrogenase (LDH)] were determined using Toshiba TBA-120FR automated biochemistry analyzer; Coagulation functions[D-Dimer, prothrombin time (PT), partially activated prothrombin time (APTT), fibrinogen (FIB), coagulation VIII factor activity (F VIII:C)]was detected by Sysmex CA50 Biomerieux mini-vidas automatic fluorescence immunoanalyzer. All the operations were done by specially assigned personnel and in strict accordance with the instructions regarding the use of the reagents. All the blood test indices were collected within 48 h Statistical. In all the blood test indices, one patient lacked F VIII:C test result, so We used imputation to get the complete data.
Statistical methods
SPSS, version 22.0, data analysis software was used to analyze the data. The Shapiro–Wilk test was used to assess the normality of the measured data. The data with normal distributions were expressed by X ± S, and the t-test was used for comparison between groups. The data with non-normal distribution were assessed using medians, and the quartile spacing was reflected by M (QR). The rank-sum test was used for inter-group comparisons. The area under the ROC curve (AUC) was calculated by drawing the working characteristic curve for the subjects. The differences were determined to be statistically significant at P < 0.05.
Results
Comparison of general data between the two groups
One hundred and two patients with severe COVID-19 infections were enrolled in the study. The patients included 59 males and 43 females, aged 32–90 years old, with an average age of 65.21 ± 14.27 years. The data obtained from all patients were tested for normality. The age and days of hospitalization for the two groups showed normal distributions, and the test for homogeneity of variance demonstrated that the variance was homogeneous. The average age of patients in the survival group and the non-survival group were 62.22 ± 13.61 and 72.60 ± 13.15 years old, respectively. The difference was statistically significant (P < 0.05). There was no significant difference in gender between the two groups, and the days of hospitalization for the two groups were 13.53 ± 8.31 and 18.54 ± 1.16 days, respectively, which also showed no significant differences between the two groups (P > 0.05). The 7-day and 28-day mortality rates of severe COVID-19 patients are respectively 6.86% and 24.51% as seen in Table 1 .
Table 1.
Baseline data and ventilation for novel coronavirus pneumonia in two groups.
| Group | Survival group (n = 73) | Non-survival group (n = 29) | P |
|---|---|---|---|
| Sex | |||
| Female | 34 | 20 | P = 0.404 |
| Male | 39 | 19 | |
| Age (year) | 72.60 ± 13.15 | 62.22 ± 13.61 | P = 0.015 |
| Length of stay (days) | 13.53 ± 8.31 | 18.54 ± 1.16 | P = 0.052 |
| 7-Day mortality | – | 7 (6.86%) | – |
| 28-Day mortality | – | 25 (24.51%) | – |
| Ventilation | |||
| Non-invasive ventilation | 23 | 4 | P = 0.01 |
| Invasive ventilationa | 4 | 25 | |
| Noneb, c | 46 | 0 | |
Indicates that the number of non-invasive and invasive ventilation patients in the survival group and the non-survival group is statistically different, P = 0.00.
Indicates that the number of patients without ventilation and the number of non-invasive ventilation patients in the survival group and the non-survival group is statistically different, P = 0.00.
Indicates that the number of patients without ventilation and the number of invasive ventilation patients in the survival group and the non-survival group is statistically different, P = 0.00.
Ventilation in the two groups
According to the ventilation statistics of the 102 patients in this study, it was observed that of the 73 patients in the survival group, 4 patients needed invasive ventilation, and 23 patients were treated with non-invasive ventilation. Of the patients in the non-survival group, 29 received auxiliary ventilation, including 4 patients who received non-invasive ventilation, and 25 patients who received invasive ventilation. The difference between these two groups was statistically significant (P < 0.05), as seen in Table 1.
Comparison of routine blood tests between the two groups
In this study, the average values for the lymphocyte ratio in the non-survival group and the survival group were 0.76 ± 0.47 and 0.76 ± 0.35, respectively. These values were below the reported normal lower limit value of 1.1 × 10^9/L and were significantly different from the normal lower limit when assessed using single sample t-tests (P < 0.05). However, there was no significant difference in the lymphocyte ratios between the two patient groups (P > 0.05). There also was no significant difference in the leukocyte counts between the two patient groups (P > 0.05). The platelet count in the survival group was significantly higher compared to the non-survival group (163.53 ± 59.20 vs. 126.80 ± 53.50, respectively, P < 0.05), as seen in Table 2 .
Table 2.
Blood index of 102 patients with severe COVID-19.
| Index | Variable | Survival group (n = 73) |
Non-survival group (n = 29) |
P value |
|---|---|---|---|---|
| Blood routine | WBC (×10^9/L) | 4.92 [2.83] | 5.70 [0.49] | 0.226 |
| LYM (×10^9/L) | 0.66 [0.49] | 0.64 [0.44] | 0.694 | |
| PLT (×10^12/L) | 153 [66] | 113 [58] | 0.013 | |
| Liver function | ALT (U/L) | 26.0 [21.0] | 31.0 [33.0] | 0.169 |
| AST (U/L) | 32.0 [13.0] | 51.0 [50.0] | 0.000 | |
| ALB (U/L) | 37.55 ± 4.56 | 35.11 ± 6.05 | 0.115 | |
| TBIL (μmol/L) | 10.3 [5.7] | 12.2 [14.0] | 0.090 | |
| Renal function | BUN (μmol/L) | 4.2 [2.0] | 7.0 [4.4] | 0.000 |
| CR (μmol/L) | 58.4 [31.8] | 86.4 [25.5] | 0.004 | |
| Coagulation function | D-Dmer (μg/L) | 0.24 [0.29] | 0.57 [3.25] | 0.025 |
| PT (s) | 11.6 [1.2] | 12.4 [2.6] | 0.044 | |
| APTT (s) | 40.2 [7.1] | 40.7 [8.5] | 0.511 | |
| FIB (g/L) | 5.13 [1.63] | 4.22 [2.59] | 0.065 | |
| FVIII:C (%) | 2.50 [0.00] | 3.17 [29.8] | 0.003 | |
| Cardiac function | CK-MB (U/L) | 1.8 [1.8] | 7.2 [11.7] | 0.000 |
| CK (U/L) | 68 [74] | 327 [773] | 0.000 | |
| LDH (U/L) | 352.1 ± 140.91 | 785.47 ± 274.40 | 0.047 |
Comparison of blood indices of organ function in the two groups
Liver function index
The measurement data from the patients were tested for normality. The ALB of the two groups conformed to a normal distribution, and the test for homogeneity of variance showed that the variance was homogeneous. Therefore, a t-test was used to compare the differences in ALB between the survival group and the non-survival group. The analysis showed that there was no significant difference between the two groups. The ALT, AST, and TBIL measurements from the two groups did not conform to normal distributions. Therefore, the rank-sum test was used to compare the differences of ALT, AST, and TBIL between the two patient groups. The results showed that there was a significant difference in AST between the two groups (P < 0.05), but there was no significant difference in ALT or TBIL between the two groups (P > 0.05).
Renal function index
The test for normality revealed that the data for BUN and CR from the two groups did not show normal distributions. The rank-sum test was used to compare the differences in BUN and CR between the survival group and the non-survival group, and the differences were statistically significant (P < 0.05).
Coagulation function index
When the data from the two patient groups were tested for normality for D-Dimer, PT, APTT, FIB, and F VIII:C, we observed that the data did not conform to normal distributions. Thus, the rank-sum test was used to compare the differences of D-Dimer, PT, APTT, FIB, and F VIII:C between the survival group and the non-survival group. The results showed that there were significant differences in D-Dimer, PT, F VIII:C between the two groups (P < 0.05), but there was no significant difference in APTT or FIB (P > 0.05), as shown in Table 2.
Cardiac function index
The data for cardiac function from the patients were tested for normal distribution. The LDH data from the two groups conformed to a normal distribution, and the test of variance showed that the variance was uneven. A t-test was used to compare the difference of LDH between the survival group and the non-survival group, and the difference between the two groups was statistically significant (P < 0.05). However, the CK-MB and CK data from the two groups did not show a normal distribution. Therefore, the differences in CK-MB and CK between the survival group and the non-survival group were compared using the rank-sum test, which showed that there were significant differences in CK-MB and CK between the two groups (P < 0.05). In addition, patients in both groups had underlying cardiovascular disease. There were 15 such cases in the non-survival group and 33 cases in the survival group. The chi-square test applied to these data indicated that the differences were not significant (P > 0.05).
ROC curve analysis of blood indices to evaluate the prognosis of COVID-19
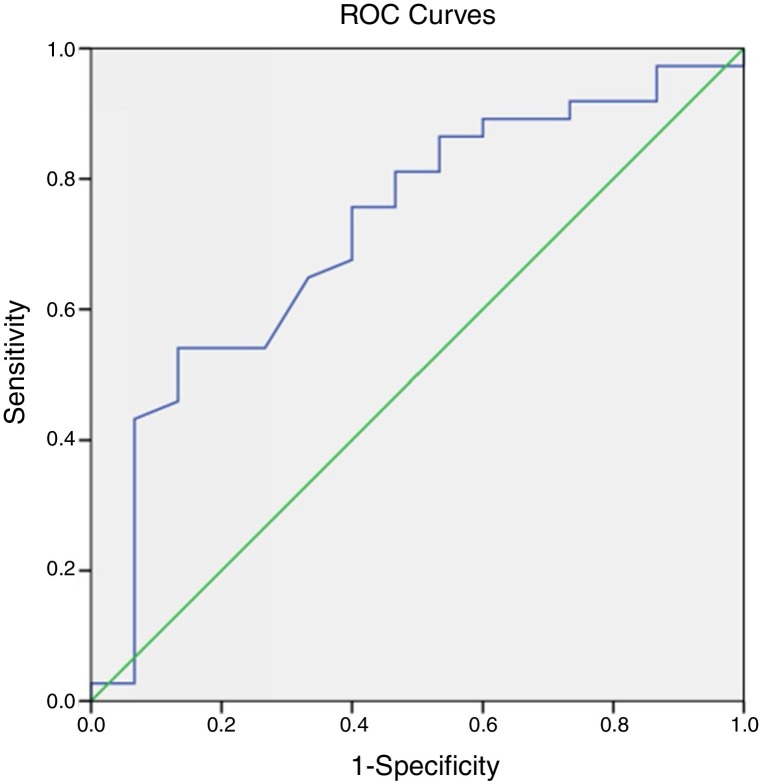
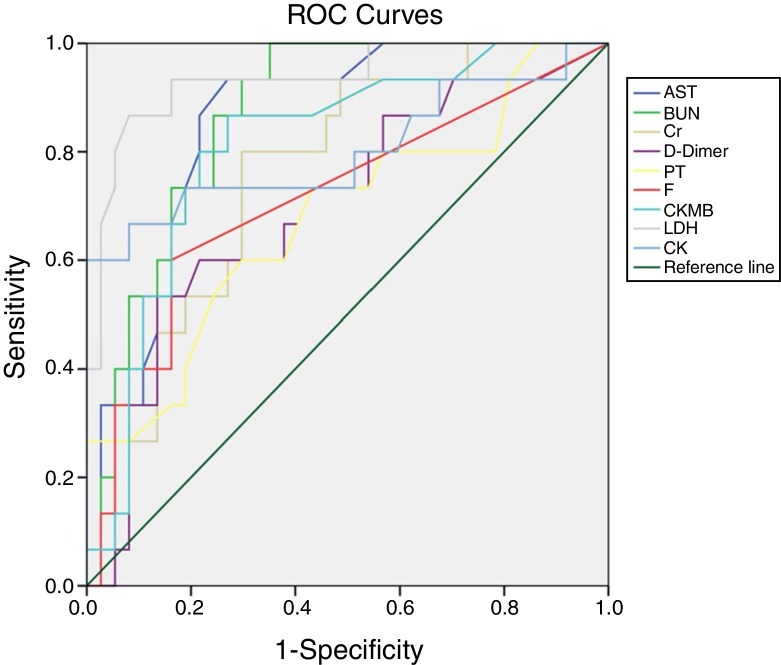
The prognostic evaluation of PLT in the survival group and the non-survival group was analyzed using ROC curves. The area under the curve was 0.721, indicating that PLT had some value in predicting the prognosis, as shown in Fig. 1 . The prognostic values of AST, BUN, CR, D-Dimer, PT, FIB, F VIII:C, CK-MB, CK, and LDH in both groups also were analyzed using the ROC curve analysis. The areas under the curve were 0.854, 0.867, 0.757, 0.699, 0.679, 0.715, 0.811, 0.935, and 0.802, respectively. These results demonstrated that these organ function blood indices had prognostic value in COVID-19 infections, as shown in Fig. 2 .
Fig. 1.
ROC curve analysis of PLT in evaluating the prognosis of COVID-19 patients.
Fig. 2.
ROC curve analysis of the blood indices of organ function to evaluate the prognosis of severe COVID-19.
Discussion
The novel coronavirus is a member of the β coronavirus type, with humans as the host. Since the first human coronavirus (HCoV) was isolated from the nasal secretions of patients with the common cold in 1965, six different strains of CoV are known to infect humans. These CoV strains include severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and the common cold viruses in individuals with strong immunity (such as 229E, OC43, NL63, and HKU1).1 This novel coronavirus, COVID-19, has more than 85% homology with the previously detected bat coronavirus (a SARS-related coronavirus species, that is, SARS coronavirus). However, there are clear differences in the genetic characteristics of COVID-19 compared with SARS-CoV and MERS-CoV.5
Through observation of the current global epidemic situation, we can demonstrate the infectivity and pathogenicity of COVID-19. To the treatment of COVID-19, the main treatment is antiviral therapy, such as interferon, lopinavir, ribavirin and so on. More than that to patients with severe COVID-19 infections, oxygen therapy and actively treatment of complications are indispensable. Of course, we should also inform patients to rest in bed and enhance nutrition,3 and for the complications, recent clinical work has shown that COVID-19 infections not only cause pneumonia but also cause damage to other organs such as the heart, liver, and kidneys, as well as the blood and immune systems.6
In this paper, 102 patients with severe COVID-19 infections were divided into two groups according to their prognosis, namely a non-survival group and a survival group. The blood indices for different organ functions were compared between these two groups.
There was a difference in ventilation between the two groups. Most patients in the survival group received noninvasive ventilation, while those in the non-survival group needed more invasive ventilation, and all patients in the non-survival group received Oxygen therapy. This result also suggests that there was a higher demand for auxiliary ventilation in patients with severe COVID19.
The decrease in the lymphocyte ratio is a clinical characteristic of CoV infections,7 which was similar to the observed immune injury of SARS in 2003. The results observed with SARS infections primarily showed decreases in T lymphocytes, CD4+, and CD8+T lymphocytes.8 The results of our study also showed that the lymphocyte ratios from both the non-survival group and the survival group were lower than the normal value. However, there was no significant difference in the lymphocyte ratio between the two groups. There were differences in platelets between the survival group and the non-survival group, and the possible reasons were as follows. First, the novel coronavirus directly affected the hematopoietic system, resulting in hematopoietic inhibition.9 Second, it is possible that infection led to the activation of blood coagulation and increased platelet consumption in the non-survival group.10 Thrombocytopenia is often associated with the prognosis of patients with pneumonia.
The causes of organ function damage due to CoV infection are as follows. (1) The novel coronavirus entered the body by binding to angiotensin-converting enzyme 2 (ACE-2) on the surface of alveolar epithelial cells, thus causing damage to the lungs of patients with CoV infection.11 When acute lung injury occurred, organ ischemia and hypoxia resulted in organ damage and organ dysfunction. (2) In previous studies of coronavirus, it has been reported that the critical threat of coronavirus infection to the human body was that the virus had considerable vasotropism and vascular endothelial injury was an important basis for the pathological changes in organs.12 (3) The immune cell state seen in COVID-19 infections was highly activated, which was mainly characterized by increased Th17 cells and the high cytotoxicity of CD8 cells, indicating that the patients had severe immune injuries, which affected organ function.13, 14 (4) In previous studies, CoV caused direct damage to the lungs and intestines,14 but the current pathological results from liver biopsies showed mild steatosis and slight abnormalities in the lobules and portal vein, which might be related to either viral damage or drug damage. A small amount of monocyte inflammatory infiltration has been seen in the heart tissue, and no other substantial damage has been reported.15 Therefore, COVID-19 infection might cause direct damage to various organs.
In our study, CK-MB, LDH, CK, AST, BUN, and CR were higher in the non-survival group compared to the survival group. However, due to the large number of middle-aged and elderly patients infected with COVID-19, the results concerning cardiac function may be affected by other underlying diseases. Inflammation, infection, and other factors lead to excessive activation of blood coagulation.16 In this study, the D-Dimer, PT, FIB, and F VIII:C in the non-survival group were higher than in the survival group, and the differences were statistically significant. The blood of the patients with COVID19 appeared to be in a hypercoagulable state, which resulted in the consumption of coagulation factors, and likely promoted the development of disseminated intravascular coagulation (DIC), resulting in the COVID19 endpoint events.17
In this study we think of patient's hospitalization as the survival time because of the time when COVID-19 patients infected is not clear. As described in the results, the 7-day and 28-day mortality rates of severe COVID-19 patients are respectively 6.86% and 24.51%. Therefore, the mortality rate of severe COVID-19 patients is relatively high.
The ROC curve area for PLT in this study was 0.721, which also suggested that thrombocytopenia indicates a poor prognosis in patients infected with COVID-19.
The prognostic values of AST, BUN, CR, D-Dimer, PT, FIB, F VIII:C, CK-MB, CK, and LDH were analyzed by ROC curve analysis, and the areas under the curve were 0.854, 0.867, 0.757, 0.699, 0.679, 0.715, 0.811, 0.935, and 0.802, respectively. The results indicated that these blood indices had prognostic value in COVID-19 infected patients. The indicators were significantly increased in covid-19 patients, Which indicated a higher risk of death. So we should actively prevent and treat complications and give organ function support.
Conclusion
The new virus outbreak is having a severe impact on the global economy, medical treatment, and public health. Therefore, attention should be paid to potential multiple organ damage during the treatment of patients who are critically ill with COVID-19. Early analysis of blood indicators and the protection of organ function can improve the survival rate of critically ill patients.
Financial support
This study was supported by the National Natural Science Foundation of Jiangsu Province (No. BK20170147), and the Nanjing Medical Science and Technique Development Foundation (No. YKK16181).
Conflict of interest
The authors declare that there is no conflict of interests.
Acknowledgements
All authors participated in the research design and took responsibility for the decision to submit for publication. We thank you for all patients and their families involved in the study.
References
- 1.Su S., Wong G., Shi W., Liu J., Lai Alexander C.K., Zhou J. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singhal Tanu. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novel coronavirus pneumonia diagnosis and treatment plan (Trial Seventh Edition). J Cardiopulmon Pulmon Dis, 2020;39:103–7.
- 4.Zhou Fei., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen Tuan M., Zhang Y., Paolo P.P. Virus against virus: a potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res. 2020;30:189–190. doi: 10.1038/s41422-020-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tianbing W., Du Z., Fengxue Z., Zhaolong C., Youzhong A., Gao Y. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395:e52. doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qu R., Ling Y., Zhang Y.-H.-Z., Wei L.-Y., Chen X., Li X.-M. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020 doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin L., Lianfeng L., Cao W., Taisheng L. Hypothesis for potential pathogenesis of SARS-CoV-2 infection – a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markus E., Gravemann U., Wiebke H., Frank T., Stefan R., Müller Thomas H. Inactivation of three emerging viruses – severe acute respiratory syndrome coronavirus, Crimean-Congo haemorrhagic fever virus and Nipah virus – in platelet concentrates by ultraviolet C light and in plasma by methylene blue plus visible light. Vox Sang. 2020 doi: 10.1111/vox.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rommel Marcel G.E., Christian M., Regina E., Harald S., Modlich U. Endothelial-platelet interactions in influenza-induced pneumonia: a potential therapeutic target. Anat Histol Embryol. 2019 doi: 10.1111/ahe.12521. [DOI] [PubMed] [Google Scholar]
- 11.Markus H., Hannah K.-W., Simon S., Nadine K., Tanja H., Sandra E. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiangyang L., Jingmin Z., Ning L., Bencheng Z., Ling L., Yi Y. Electron microscopic observation of multiple organ damage caused by SARS related coronavirus infection. Infect Inflamm Repair. 2003:145–148. [Google Scholar]
- 13.Mehta P., McAuley Daniel F., Brown M., Sanchez S., Tattersall Rachel S., Manson Jessica J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.To K.F., Tong Joanna H.M., Chan Paul K.S., Au Florence W.L., Chim Stephen S.C., Chan K.C.A. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J Pathol. 2004;202:157–163. doi: 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z., Shi L., Yijin W., Jiyuan Z., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H., Wang Y., Yupeng Z., Xu F., Jiepeng C., Duan L. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 2020;32:101500. doi: 10.1016/j.redox.2020.101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han H., Yang L., Liu R., Liu F., Wu K.-L., Li J. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]