Climate change is challenging food production worldwide. We propose a strategy to accelerate the improvement of a nutritious resilient crop to meet the future food production demands in a changing climate.
Keywords: Chenopodium quinoa, drought tolerance, genome editing, molecular breeding, orphan crops, salt tolerance
Abstract
Crops tolerant to drought and salt stress may be developed by two approaches. First, major crops may be improved by introducing genes from tolerant plants. For example, many major crops have wild relatives that are more tolerant to drought and high salinity than the cultivated crops, and, once deciphered, the underlying resilience mechanisms could be genetically manipulated to produce crops with improved tolerance. Secondly, some minor (orphan) crops cultivated in marginal areas are already drought and salt tolerant. Improving the agronomic performance of these crops may be an effective way to increase crop and food diversity, and an alternative to engineering tolerance in major crops. Quinoa (Chenopodium quinoa Willd.), a nutritious minor crop that tolerates drought and salinity better than most other crops, is an ideal candidate for both of these approaches. Although quinoa has yet to reach its potential as a fully domesticated crop, breeding efforts to improve the plant have been limited. Molecular and genetic techniques combined with traditional breeding are likely to change this picture. Here we analyse protein-coding sequences in the quinoa genome that are orthologous to domestication genes in established crops. Mutating only a limited number of such genes by targeted mutagenesis appears to be a promising route for accelerating the improvement of quinoa and generating a nutritious high-yielding crop that can meet the future demand for food production in a changing climate.
Introduction
The challenge of sustainable food production in the future
Plant production is facing unprecedented challenges. In 2050, the human population will exceed 10 billion (FAO, 2017), and the demand for staple crops and livestock will have increased by 60% (Springmann et al., 2018). Agricultural growth relies on productivity gains through increased crop yields, but, following the yield increases achieved during the Green Revolution, the percentage increase in yield has tended to stagnate or decline over time (Lobell and Gourdji, 2012; Ray et al., 2012, 2013; Grassini et al., 2013). Climate change is predicted to drastically limit local plant production (Lobell and Gourdji, 2012). There is therefore an urgent need to develop crops that can tolerate abiotic stresses such as high temperatures, cold, frost, drought, soil salinization, and flooding. Drought and salt stress pose major challenges for agriculture because these adverse environmental factors prevent plants from realizing their full genetic potential. Non-optimal irrigation causes salinization of soils, and the shortage of high-quality irrigation water exacerbates problems caused by salinity. As a result, many of the arid regions that are presently cultivated may turn into marginal lands. To keep such lands productive, we will need resilient high-yielding crops that can replace current crops.
From a practical point of view, salt stress can be imposed more easily and precisely than drought stress in laboratory settings. Thus, most studies of drought tolerance have focused on salt stress, as plant responses to osmotic changes during both stress situations are closely related and the mechanisms overlap. Furthermore, as salinity imposes hyperosmotic stress on plants, salt-tolerant plants are also drought tolerant. However, genetically engineering salt-tolerant crops remains extremely challenging. As salt tolerance is a complex trait associated with multiple subtraits [e.g. ion homeostasis, osmotic balance, and reactive oxygen species (ROS) regulation], each having a complex genetic basis, manipulating a single or a limited number of genes has so far failed to yield salt-tolerant crops (Ismail and Horie, 2017).
The next sustainable Green Revolution should utilize a wider diversity of crops, so that food production can benefit from a broader set of species, each adapted for specific marginal conditions (Jacobsen et al., 2013, 2015). This approach would involve the focused breeding of divergent variants of the main crops cultivated today and, concurrently, the domestication of neglected species, with a focus on resilient plants. Resilient plants include plants with high nutritional value that are able to thrive in suboptimal environments. The output will be sustainable agricultural systems adapted to harsh environments.
Quinoa (Chenopodium quinoa) as a future major crop
Quinoa (Fig. 1A) was originally domesticated in the Andean region of South America as early as 7000 years ago, and is adapted to the harsh climatic conditions of the Andean area (Pearsall, 1992). Due to its high genetic diversity and its adaptation to extremely harsh conditions in the highlands of the Andes, quinoa can be grown on marginal soils and is resilient to frost, drought, and salinity, and to large temperature variations between day and night (Jacobsen et al., 2005, 2007; Ruiz et al., 2014). In addition, the seeds are rich in minerals and vitamins and have exceptional nutritional qualities. Compared with conventional grains, quinoa seeds lack gluten, have a superior ratio of proteins, lipids, and carbohydrates, and have a higher content of essential amino acids (Zurita-Silva et al., 2014; Filho et al., 2017; Pereira et al., 2019). However, grain consumption is limited by saponins that accumulate in the seed coat as a defence mechanism against pests and pathogens, and must be removed before consumption (Filho et al., 2017; Jarvis et al., 2017). ‘Sweet’ varieties with reduced amounts of saponins are available but may be more vulnerable to certain pests and herbivores (Singh and Kaur, 2018; McCartney et al., 2019).
Fig. 1.
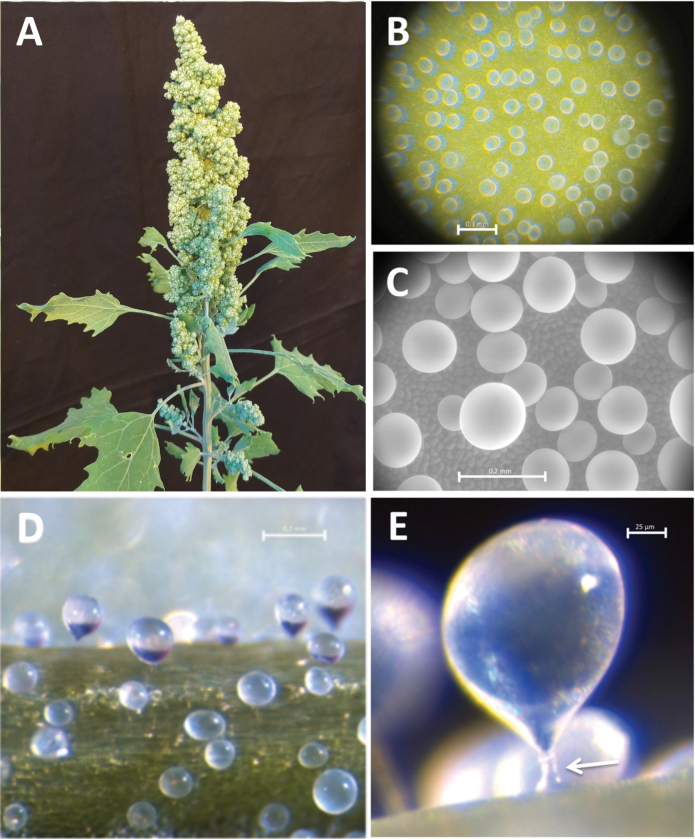
Quinoa is a salt-tolerant underutilized crop. (A) Panicle of quinoa (Chenopodium quinoa Willd. cv. Titicaca). (B–E) Leaves and stems of quinoa are covered with bladder cells, which are specialized trichomes into which salt is secreted. (B and C) Top view of a leaf. The surface is coated with numerous large bladders, visible under a (B) light microscope and (C) scanning electron microscope, where epidermal cells can be seen. (D) Side view of bladder cells. (E) Enlarged bladder complex consisting of an epidermal cell, stalk cell (marked by an arrowhead), and bladder cell. The salt concentration is expected to gradually increase from epidermal cells to bladder cell vacuoles.
Quinoa is traditionally cultivated in South America, where several cultivars have been developed, and a few varieties have been introduced in North America, Europe, China, and the Middle East (Bazile et al., 2016a, b; Murphy et al., 2016; Jacobsen, 2017; Katwal and Bazile, 2020). Although >16 000 accessions of the genus Chenopodium exist (FAO, 2010), access to genetic resources for quinoa has thus far been limited, greatly hindering genetic studies and molecular marker-assisted breeding efforts (Zurita-Silva et al., 2014; Peterson et al., 2015; Murphy et al., 2016, 2018). However, in 2017, two high-quality genome drafts were published based on inbred lines of a coastal Chilean quinoa accession (PI 614886) (Jarvis et al., 2017) and a Bolivian Real variety (Zou et al., 2017). These genome sequences provide insights into the basis for the exceptional nutritional value of quinoa and open up the possibility of targeted breeding of new quinoa varieties.
The molecular basis for salt/water stress tolerance of quinoa
Salt bladders (Fig. 1B–E), cell structures homologous to epidermal hair cells consisting of an epidermal cell, a stalk cell, and an epidermal bladder cell, occur in many halophytes (naturally evolved salt-tolerant plants), including quinoa, and could be critical for their salt tolerance by serving as salt dumps (Shabala et al., 2014). The direct involvement of the bladder complexes in salt tolerance of quinoa was first suggested by Kiani-Pouya et al. (2017). Gentle removal of bladders neither initiated wound metabolism nor affected the physiology and biochemistry of control-grown plants, but did have a pronounced effect on salt-grown plants, resulting in a salt-sensitive phenotype.
Bioinformatic analysis of the RNA profile of quinoa epidermal bladder cells showed a small number of differentially expressed genes and insignificant changes in the transcript level of most transporter genes under salt exposure (Zou et al., 2017; Böhm et al., 2018). The same transcriptome analysis suggested that high abscisic acid (ABA) levels are required to maintain the cellular response to osmotic stress within the bladder cell and that ABA transporters may be used to import ABA from the leaf, or that ABA is produced in bladder cells for export into other plant tissues. Because of the relatively small number of significant changes in transcript levels under salt stress for most transporter genes, one could suggest that bladder cells are ‘constitutively active’ in salt sequestration and that the transcript level responses of transporters only play a minor role under salt stress. Nevertheless, this transcriptome analysis enabled the identification of candidate genes likely to be involved in salt tolerance and suggested a model for how salt is transported into bladder cells (Böhm et al., 2018). However, many halophytes do not use glands or external bladder cells to regulate their tissue ion concentrations (Flowers and Colmer, 2008), and direct measurements of the ion composition of quinoa bladder cells are lacking; thus, it remains to be confirmed whether these bladder cells serve as salt dumps.
The identification of transporters differentially expressed in the bladder cell transcriptome and functional electrophysiological testing of key bladder cell transporters in Xenopus laevis oocytes revealed that loading of Na+ and Cl– into bladder cells is mediated by a set of tailored plasma- and vacuole membrane-based sodium-selective channel and Cl–-permeable transporters (Böhm et al., 2018). Two families of Na+ transport proteins are constitutively expressed in bladder cells and at high levels: HKT1-like Na+ transporters and NHX-like Na + transporters. HKT1-like transporters mediate Na+ or K+/Na+ transport across the plasma membrane and have previously been identified in genetic screens for salt-tolerant crops (Hauser and Horie, 2010), including salt-tolerant accessions of barley (Hordeum vulgare; Han et al., 2018). In quinoa, HKT1.2 is constitutively expressed in bladder cells and may be critical for Na+ loading. NHX-type Na+ transporters function as H+/Na+ antiporters transporting Na+ into the lumen of the vacuole of plants (Bassil and Blumwald, 2014). Epidermal bladder cells have high constitutive expression of two NHX1-like genes (Böhm et al., 2018). It is plausible that the products of these two genes sequester Na+ into the vacuole after it has been delivered into the cytoplasm. Apart from Na+ transporters, bladder cells exhibit high expression of a HAK-like K+ transporter, suggesting that these cells also take up K+ (Böhm et al., 2018).
In contrast to the bladder cell, nothing is known about the molecular nature and precise role of the stalk cell (Fig. 1E) which connects the epidermis cell with the bladder cell and serves as a transfer cell. No molecular picture of the transcellular ion transport of transfer cells exists so far either. To gain insight into the salt tolerance mechanism, it would be helpful to determine how stalk cells channel polar Na+, Cl–, and K+ as well as sugars and metabolites to supply the salt bladder with nutrients while compartmentalizing Na+ and Cl–.
Quinoa is also studied as a model organism to investigate water stress tolerance in plants that use the large volume of bladder cells as a water reservoir (Tester and Davenport, 2003). Hair cells probably contribute to the drought tolerance of cereal grasses by reducing water loss (Hameed et al., 2002; Saade et al., 2017). Likewise, bladder cells can be viewed as a kind of secondary epidermis that serves as a protective cover that reduces transpiration rates (Shabala and Mackay, 2011).
Quinoa has developed several other mechanisms that contribute to its high tolerance towards salt stress. In line with an increased K+ uptake under salt stress, quinoa can maintain high K+/Na+ ratios, which is a well-established indicator for salt tolerance (Maathuis and Amtmann, 1999; Shabala and Cuin, 2008; Hariadi et al., 2011). Na+ can also be compartmentalized in mesophyll vacuoles in old leaves and, when such leaves are shed, Na+ is also lost (Adolf et al., 2013; Bonales-Alatorre et al., 2013). The stomatal length was reduced in 114 quinoa accessions subjected to salt stress (Kiani-Pouya et al., 2019), suggesting that the design of the stomatal apparatus may also contribute to the water stress tolerance of quinoa (Hinojosa et al., 2019a; Kiani-Pouya et al., 2019).
Target traits for improvement
Efforts in quinoa breeding have primarily been carried out by academic institutions, and the lack of private investment has greatly hindered progress. Compared with cereals, quinoa has fairly low yields, one reason being the extreme conditions under which it is grown in the high Andes. However, yield stability varies even under favourable conditions, which can lead to large gaps between potential and realized yields. There is also a need for extensive processing for saponin removal. Still, quinoa remains popular due to its high market value, worldwide demand, and abiotic stress tolerance. Therefore, efforts to convert quinoa into a major crop must aim to increase yield, achieve yield stability, and reduce the saponin content of the seed (Rao and Shahid, 2012; Choukr-Allah et al., 2016; Ruiz et al., 2017; Gamboa et al., 2018; Präger et al., 2018). Because quinoa displays a natural resilience to adverse environmental factors, breeding goals for quinoa require crop improvements that optimize productivity with minimum inputs (Zurita-Silva et al., 2014; Yabe and Iwata, 2020).
We have previously proposed that domestication arises from changes in just a few domestication genes and that these events can be mimicked by mutagenesis of homologous genes in wild species (Palmgren et al., 2015; Østerberg et al., 2017). Strong support for this notion has come from the recent de novo domestication of wild tomato (Solanum pimpinellifolium) and groundcherry (Physalis pruinosa) (Lemmon et al., 2018; T. Li et al., 2018; Zsögön et al., 2018). The general applicability of these findings remains to be tested in a wider range of plant species. Recent reviews have stressed that the accelerated improvement of resilient crops holds a huge potential for agriculture (Bailey-Serres et al., 2019; Eshed and Lippman, 2019).
Quinoa competes well with other crops in the Bolivian Altiplano, but a key challenge in quinoa cultivation occurs under less adverse conditions where the yields are comparatively lower than those of widely grown cereals. Yield is a combination of many parameters including the number of seeds per plant, seed weight, and loss by seed shattering and pre-harvest sprouting (also called PHS). Beside parameters related to seed production, other factors, such as the number of plants per unit area, plant height, and variations in flowering time, also prevent quinoa from becoming a major food and feed source. In addition, most sweet quinoa varieties are extremely sensitive to mildew, resulting in large yield losses (Danielsen et al., 2000, 2003). Salt tolerance is likely to have an energetic cost for halophytes, as Na+ export diminishes the electrochemical gradient of H+ required for mineral uptake and turgor-driven processes in plants (Pedersen and Palmgren, 2017; Munns et al., 2020). As the expression of many salt tolerance genes in quinoa appears to be constitutive, energy loss may thus be a growth-limiting factor even when quinoa is grown in the absence of water stress. Thus, paradoxically, if quinoa is to compete with current crops on fertile soils, its resilience to environmental stress may become a barrier for its productivity.
Seed size
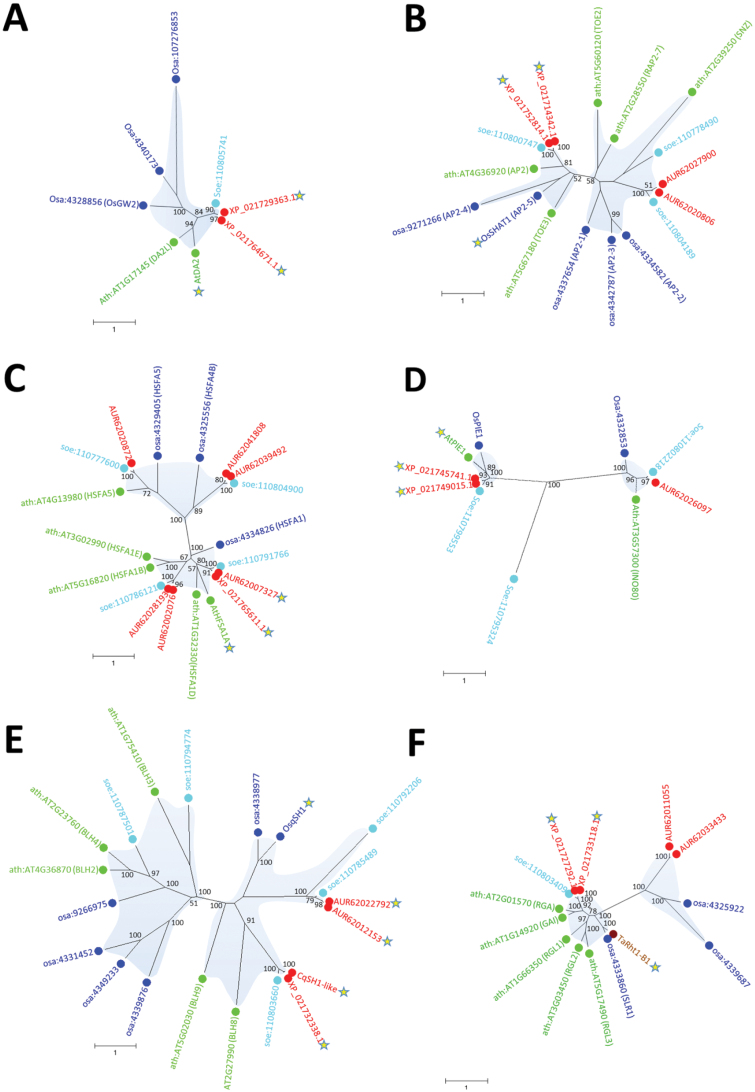
In rice, several genes have been associated with grain size control, including GRAIN WIDTH AND WEIGHT2 (GW2), encoding a RING-type E3 ubiquitin ligase (Song et al., 2007), and GRAIN INCOMPLETE FILLING1 (GIF1), encoding a cell wall invertase required for carbon partitioning during early grain filling (Wang et al., 2008). GW2, an orthologue of DA2 in Arabidopsis thaliana (Xia et al., 2013), is a negative regulator of cell division, and GW2 loss-of-function mutants show increased cell numbers, resulting in a wider spikelet hull (Song et al., 2007). This increase in spikelet size accelerates the grain milk filling rate and results in increased yields due to enhanced grain width and weight. Of the three orthologues in wheat (TaGW2-A1, -B1, and -D1), at least TaGW2-B1 and -D1 influence grain width and length (Zhang et al., 2018). GIF1 is responsible for the smaller grain sizes in wild rice (Oryza rufipogon) (Wang et al., 2008). Cumulative mutations in the GIF1 gene have resulted in larger grains in domesticated rice cultivars. In addition, overexpression of the domesticated variant of GIF1 under the control of its native promoter results in increased grain size (Wang et al., 2008). In addition to GIF1, several other negative regulators of grain size have been described, such as GRAIN SIZE3 (GS3) or Protein Phosphatase with Kelch-Like repeat domain1 (OsPPKL1) (Fan et al., 2006; Zhang et al., 2012; Gao et al., 2015). While we only identified one orthologue of GIF1 (AUR62006205) (Table 1; Fig. 2I) in the quinoa genome, two homologues of DA2 are present (AUR62041781 and AUR62037970) (Table 1; Fig. 2A). Loss-of-function mutations of the GIF1 orthologue in quinoa would therefore be an obvious starting point for increasing seed size.
Table 1.
Targets for accelerated domestication of quinoa
| Desired trait to modify | Genes involved in other species | Quinoa gene(s) | Subgenome | Gene chromosome coordinates (Phytozome v1.0) | % identity | Expression | Reference |
|---|---|---|---|---|---|---|---|
| Saponin biosynthesis | TSAR1 (Medicago truncatula) | AUR62017204 (TSARL1) | B | Chr16:68549573..68551812 | 32.00 | Seeds | Jarvis et al. (2017) |
| TSAR2 (Medicago truncatula) | AUR62017206 (TSARL2) | B | Chr16:68524854..68527010 | 30.86 | Roots | Jarvis et al. (2017) | |
| Seed size and number | DA2 (Arabidopsis)/GW2 (Oryza) | AUR62041781 | B | Chr17:39742130..39752168 | 56.69/45.16 | NA | This work |
| AUR62037970 | B | Chr05:34646253..34655250 | 56.66/45.57 | NA | This work | ||
| GIF1 (Oryza) | AUR62006205 | A | Chr15:3135695..3137782 | 60.21 | NA | This work | |
| GS3 (Oryza) | No close homologue | This work | |||||
| CKX5 (Arabidopsis)/Gn1a (Oryza) | AUR62034531 | B | Chr10: 7564646..7565207 | 68.67/43.49 | NA | This work | |
| AUR62014467 | B | Chr03: 74311653..74312220 | 68.67/44.01 | NA | This work | ||
| CKX3 (Arabidopsis)/Gn1a (Oryza) | AUR60229062 | A | Chr02: 37236856..37237243 | 38.09/43.30 | NA | This work | |
| AUR62033955 | NA | Chr00:184848685..184848904 | 35.82/41.65 | NA | This work | ||
| Seed shattering | SHP1/SHP2 (Arabidopsis) | AUR62035850 | A | Chr02:11045541..11052900 | 68.64/67.93 | NA | This work |
| AUR62027653 | B | Chr01:128347481..128357581 | 65.68/64.98 | NA | This work | ||
| SHAT1 (Oryza) | AUR62001901 | A | Chr07:69242892..69245843 | 55.86 | NA | This work | |
| AUR62003911 | A | Chr09:7603459..7606393 | 55.38 | NA | This work | ||
| SH4 (Oryza) | No close homologue | This work | |||||
| qSH1 (Oryza) | AUR62022792 | A | Chr04:3934578..3939232 | 39.41 | NA | This work | |
| AUR62012153 | B | Chr03:80004948..80009672 | 40.94 | NA | This work | ||
| AUR62022770 | A | Chr04:4527270..4527785 | 37.42 | NA | This work | ||
| AUR62029222 | n.a. | Chr00:42430804..42433133 | 36.69 | NA | This work | ||
| Height | Rht-B1 (Triticum aestivum) | AUR62039523 | B | Chr06:26006908..26013645 | 59.3 | NA | This work |
| AUR62014191 | A | Chr14:14625033..14626940 | 59.65 | NA | This work | ||
| Early flowering | FT1 (Beta vulgaris) | AUR62010060 (CqFT1A) | A | Chr15:4930835..4933952 | 81.71 | Flowers | Jarvis et al. (2017); Golicz et al. (2020) |
| AUR62013052 (CqFT1B) | B | Chr17:79266951..79277600 | 92.00 | Flowers | Jarvis et al. (2017); Golicz et al. (2020) | ||
| FT2 (Beta vulgaris) | AUR62000271 (CqFT2A) | A | Chr12:3192361..3196369 | 82.12 | Leaves | Jarvis et al. (2017); Golicz et al. (2020) | |
| AUR62006619 (CqFT2B) | B | Chr05:77596526..77601590 | 81.56 | Leaves | Jarvis et al. (2017); Golicz et al. (2020) | ||
| AUR62033889 | A | Chr15:31458414..31465667 | 63.79 | ND | Golicz et al. (2020) | ||
| TFL1 (Arabidopsis) | No close homologue | This work | |||||
| SOC1 (Arabidopsis) | AUR62004274 | B | Chr01:117180795..117186698 | 64.95 | NA | Golicz et al. (2020)/ This work | |
| AUR62033383 | B | Chr10:3492556..3498908 | 65.89 | NA | Golicz et al. (2020)/ This work | ||
| LFY (Arabidopsis) | AUR62043310 | NA | Chr00:74582790..74588853 | 64.01 | NA | Golicz et al. (2020) | |
| AUR62044212 | NA | Chr00:54562325..54568590 | 61.98 | NA | Golicz et al. (2020) | ||
| AUR62032216 | A | Chr08:14402581..14413925 | 60.53 | NA | Golicz et al. (2020) | ||
| ELF3 (Arabidopsis) | AUR62040202 | A | Chr04:10281102..10287617 | 38.16 | NA | Golicz et al. (2020) | |
| AUR62043053 | A | Chr04:11729489..11736003 | 38.31 | NA | Golicz et al. (2020) | ||
| AUR62009205 | B | Chr01:108898677..108906560 | 38.79 | NA | Golicz et al. (2020) | ||
| ELF4 (Arabidopsis) | AUR62012247 | B | Chr03:78738428..78738838 | 46.36 | NA | Golicz et al. (2020) | |
| AUR62022878 | A | Chr04:2907637..2908047 | 47.27 | NA | Golicz et al. (2020) | ||
| AUR62022877 | A | Chr04:2911065..2911460 | 44.23 | NA | Golicz et al. (2020) | ||
| AUR62012246 | B | Chr03:78752212..78752649 | 47.75 | NA | This work | ||
| PIE1 (Arabidopsis) | AUR62018509 | A | Chr07:85323308..85337723 | 60.55 | NA | This work | |
| AUR62020910 | B | Chr11:1213211..1228497 | 60.16 | NA | This work | ||
| Pre-harvest sprouting | MFT (Arabidopsis) | AUR62029959 | A | Chr08:39671124..39679767 | 73.41 | NA | This work |
| AUR62014698 | B | Chr01:29266367..29267601 | 49.13 | NA | This work | ||
| AUR62012495 | A | Chr02:4594321..4597301 | 61.21 | NA | This work | ||
| AUR62014699 | B | Chr01:29210009..29211182 | 60.47 | NA | This work | ||
| MKK3 (Hordeum vulgare) | AUR62015864 | B | Chr05: 956636..956737 | 62.03 | NA | This work | |
| AUR62026127 | A | Chr07: 82092195..82092329 | 59.96 | NA | This work | ||
| AUR62020359 | A | Chr12: 56190719..56190853 | 62.55 | This work | |||
| Heat stress | PIF4 (Arabidopsis) | No close homologueb | This work | ||||
| HSFA1 (Arabidopsis) | AUR62018674 | B | Chr16:76341712..76354887 | 52.89 | NA | This work | |
| AUR62007327 | A | Chr13:2302837..2307436 | 50.87 | NA | This work | ||
| DREB2A (Arabidopsis) | No close homologuec | This work |
NA, not available; ND none detected;
a 20 genes with E-scores <10–10.
b32 genes with E-scores <10–10.
c98 genes with E-scores <10–10.
Fig. 2.
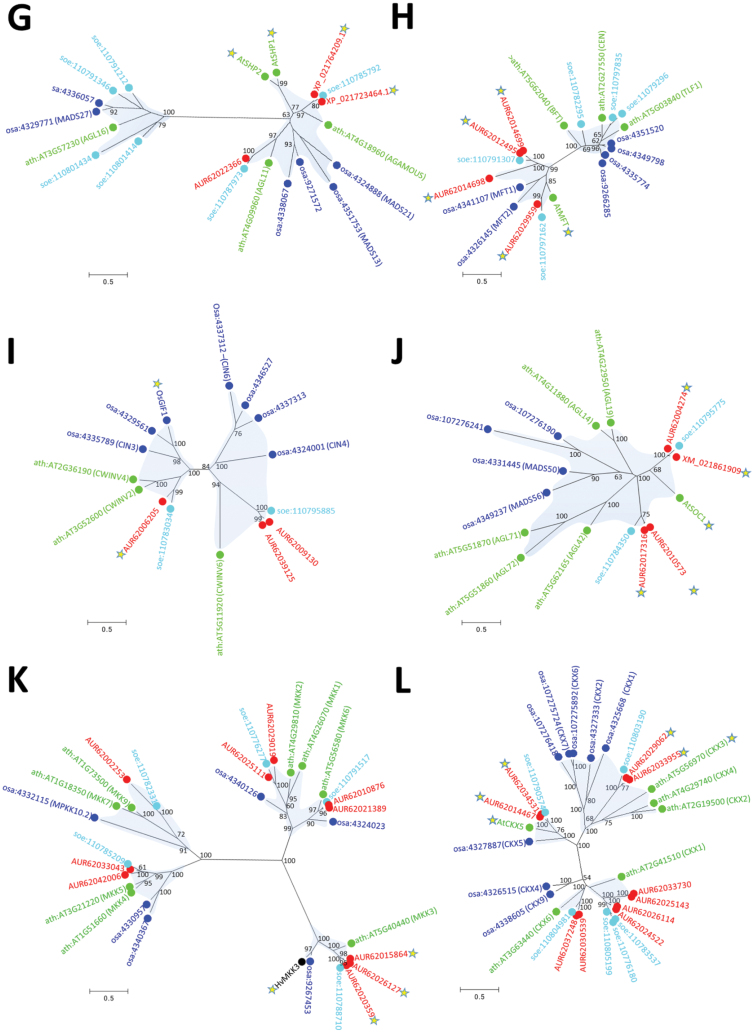
Phylogenetic tree of gene families in which members from rice (Oryza sativa), wheat (Triticum aestivum), and Arabidopsis thaliana control traits are suggested to be important for domestication of quinoa. Homologous genes in spinach (Spinachia oleracea), which is closely related to quinoa, are also shown. Species origins are highlighted by coloured text and circles: red, quinoa; blue, rice; green, Arabidopsis; turquoise, spinach; brown, wheat; black, barley (Hordeum vulgare). Domestication genes and their closest homologues in quinoa are marked by yellow stars. (A) OsGW2 controls seed size in rice. (B) OsSHAT1 controls seed shattering in rice. (C) AtHFSA1A controls heat stress in Arabidopsis. (D) OsPIE1 controls flowering time in rice. (E) OsqSH1 controls seed shattering in rice. (F) TaRht1-B1 controls plant height in wheat. (G) AtSHP1 controls seed dispersal in Arabidopsis. (H) AtMFT controls early sprouting in Arabidopsis. (I) OsGIF1 is involved in seed size in rice. (J) AtSOC1 controls flowering time in Arabidopsis. (K) HvMKK3 controls seed dormancy in barley. (L) Loss-of-function double mutation of AtCKX5 and AtCKX3 in Arabidopsis mimics the rice gn1a mutation related to increased grain numbers. (M) AtTFL1 is a time-of-flowering regulator in Arabidopsis and other species. For references, see main text. Accession numbers not given in the figure are as follows: AtDA2, Q93YV5; OsGIF1, Q6AVI1; OsGW2, B9F4Q9; AtMFT, Q6XFK7; AtHSFA1A, P41151; AtPIE1, Q7X9V2; OsqSH1, Q941S9; TaRHT1, Q9ST59; OsSHAT1, A0A0N7KJT8; AtSHP1, P29381; AtSHP2, P29385; AtSOC1, O64645; HvMKK3, A0A140JZ28; AtCKX5, Q67YU0; and AtCKX3, A0A1P8BER3. The basic local alignment search tool (BLAST) was used to search for genes in genomes annotated in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (https://www.genome.jp/tools/blast/), the KAUST Chenopodium database (https://www.cbrc.kaust.edu.sa/chenopodiumdb/), and the NCBI genome database (https://www.ncbi.nlm.nih.gov/genome/?term=quinoa). CqSH1-like (previously AUR62029222) was not correctly annotated and was corrected based on homology to the coding sequences of OsQSH1 and soe:110803660 guided by intron–exon splice sites in the quinoa genome sequence. The sequences were aligned using the multiple sequence comparison by the log-expectation (MUSCLE; Edgar, 2004) tool and subjected to maximum likelihood analysis by RAxML v. 8.2.12 (Stamatakis, 2014) assuming a Le and Gascuel (LG)+PROTGAMMA model (Le and Gascuel, 2008) and using the Extreme Science and Engineering Discovery Environment (XSEDE) at the CIPRES Science Gateway v. 3.3 (Miller et al., 2010). Bootstrap values from 1000 replicates are indicated on each node. Values <50 are not marked. Scale bars have numbers of amino acid substitutions per site indicated below.
Combining loci for increased grain number and seed size in the same genetic background would provide a strategy for tailor-made crop improvement. In rice, the combination of loss-of-function mutations in GRAIN NUMBER 1A (Gn1a) and GRAIN SIZE 3 (GS3) is responsible for the heavy panicle phenotype of elite hybrid rice (S. Wang et al., 2018). The null gn1a allele is the determinant factor for heavy panicles through increased grain number, while gs3 is associated with increased grain size and weight (S. Wang et al., 2018). In Arabidopsis, the rice gn1a mutation can be mimicked by deletion of two homologous genes: AtCKX5, the orthologue of rice Gn1a; and AtCKX3 (Bartrina et al., 2011). The quinoa genome encodes two close homologues of AtCKX5 (AUR6203453 and AUR62014467) and another two of AtCKX3 (AUR62029062 and AUR62033955) (Table 1; Fig. 2L), which could be potential targets for improving seed yield in quinoa. In contrast, no homologues of the rice GS3 gene could be identified.
Seed shattering
Through evolution, plants have acquired different mechanisms that allow them to release their seed upon maturation. This ability is crucial for survival of plant species in the wild, but would cause enormous losses in agricultural production systems. Thus, domesticated crop plants are characterized by an inactivation of the seed spreading mechanisms present in wild plants. In general, domestication has yielded crops with thicker cell walls around the abscission areas, resulting in an inability of the seeds or fruits to dehisce from the mother tissue (Dong and Wang, 2015; Ballester and Ferrándiz, 2017).
A number of transcription factors from heavily populated protein families are involved in seed shattering, acting in multicomponent systems where the activity of one type of transcription factor is controlled by transcription factors belonging to other protein families. In rice, one such multicomponent system is formed by qSH1, SH4, and SHAT1. The coordinated action of these transcription factors is necessary for abscission zone development, with SHAT1 being the main player, while SH4 positively regulates SHAT1 activity and qSH1 affects the expression of the other two transcription factors (Hofmann, 2012; Zhou et al., 2012). In Arabidopsis, the redundant MADS-box transcription factors SHATTERPROOF1 (SHP1) and SHP2 are required for dehiscent zone differentiation and seed dispersal (Liljegren et al., 2000). Two homologues of SHP1/2 exist in quinoa (AUR62035850 and AUR62027653) (Table 1; Fig. 2G). However, these genes are phylogenetically closer to Arabidopsis AGAMOUS (At4g18960), which controls flower architecture (Yanofsky et al., 1990), and might have functions unrelated to seed shattering. In contrast, there is no homologue of SH4, despite the presence of two homologous genes for both SHAT1 (AUR62001901 and AUR62003911) (Table 1; Fig. 2B) and qSH1 (AUR62022770 and AUR62029222) (Table 1; Fig. 2E).
Pre-harvest sprouting
An important challenge when growing quinoa as a crop in countries with rainy summers, such as those in northern Europe, is pre-harvest sprouting (Ceccato et al., 2011). Early rain spells during crop dry-down will lead to germination in the panicle, reducing marketable yields and grain quality. This yield constraint has been studied in other crops, and possible solutions may be expanded to quinoa. Modulating grain dormancy is an effective strategy for controlling pre-harvest sprouting and designing crops that are better adapted to regional climates and post-harvest applications. In rice, endosperm sugar accumulation caused by mutation of PHS8/ISA1 leads to pre-harvest sprouting (Du et al., 2018). In wheat domestication, independent mis-splicing mutations in TaPHS1 led to resistance to pre-harvest sprouting (Liu et al., 2015). TaPHS1 is a homologue of MOTHER OF FT AND TFL1 (MFT), which encodes a phosphatidylethanolamine-binding protein that regulates seed germination in Arabidopsis (Xi et al., 2010). Through phylogenetic analysis, we identified a close homologue of MFT (AUR62029959) in quinoa (Table 1; Fig. 2H), suggesting that pre-harvest sprouting might be a relatively easy trait to improve in this plant. Nevertheless, another three quinoa proteins are relatively close phylogenetically to MFT (AUR62014698, AUR62012495, and AUR62014699) (Table 1; Fig. 2H), which might complicate the task due to functional redundancy.
Mitogen-activated Protein Kinase Kinase 3 (MKK3) is the causal gene of the major grain dormancy quantitative trait loci (QTLs) Qsd2-AK (SD2) and PHS1 in barley and wheat, respectively (Nakamura et al., 2016; Torada et al., 2016). In rice, the MKKK62–MKK3–MAPK7/14 module controls seed dormancy via regulating OsMFT transcription (Mao et al., 2019). Exchange of the evolutionarily conserved amino acid N260 to T260 in MKK3 adapts barley to wet growth conditions in East Asia (Nakamura et al., 2016). Additionally, the semi-dominant ethylmethane sulfonate (EMS)-induced ERA8 allele of MKK3 (in which Glu365 is substituted with Lys) was shown to increase seed dormancy and thus pre-harvest sprouting tolerance in wheat (Martinez et al., 2020). The quinoa genome encodes three close homologues of MKK3 (AUR62015864, AUR62026127, and AUR62020359; Fig. 2K), and these are attractive targets for reducing pre-harvest sprouting.
Plant height
Lodging (bending over of the stems near ground level and stem breakage due to heavy panicles) is a common source of agricultural loss, due to the resulting difficulties in crop harvesting. This effect is more common with an increasing plant height. Thus, the so-called ‘Green Revolution’ genes in rice, barley, and wheat cause a decrease in plant height related to defects in the production or sensing of growth-controlling hormones (Hedden, 2003). REDUCED HEIGHT (Rht)-B1 and Rht-D1 in wheat and DWARF PLANT8 (Dwarf8) and Dwarf9 in maize (Zea mays) are orthologues of Arabidopsis GIBBERELIN INSENSITIVE (GAI) (Winkler and Freeling, 1994; Flintham et al., 1997; Peng et al., 1997, 1999; Fu et al., 2001; Lawit et al., 2010). Alteration of these genes results in defects in gibberellin sensing, and GAI expression in transgenic rice represses multiple gibberellin responses (Fu et al., 2001). In rice, the Green Revolution semi-dwarf (sd-1) phenotype is the result of a reduced content of active gibberellins caused by a defective biosynthetic enzyme (GA20ox2), in a similar manner to the sdw1/denso phenotype in barley (Peng et al., 1999; Monna, 2002; Spielmeyer et al., 2002; Jia et al., 2009).
Because plant hormones are multifunctional, gibberellin-related dwarfing mutations cause pleiotropic phenotypes, including a higher seed yield due to altered nutrient partitioning and increased number of panicles per area (Peng et al., 1997; Zhang et al., 2017). In South America, quinoa plants can grow up to 3 m high (Apaza et al., 2015), making lodging a potential problem. In addition, plant height in quinoa is affected by environmental factors, and some studies have identified a negative association between plant height and seed yield for several cultivars (Maliro et al., 2017). Therefore, genes affecting plant height should be a target of any attempt aimed at increasing quinoa yields. Two homologues of wheat Rht-B1/Rht-D1 are present in the quinoa genome (AUR62039523 and AUR62014191) (Table 1; Fig. 2F), and these genes are also homologues of Arabidopsis RGA1, which encodes a transcription factor involved in gibberellin signal transduction (Silverstone et al., 1998). In contrast, no clear homologue of the gene encoding GA20ox2 could be identified.
Flowering time
Production yields in quinoa are extremely sensitive to adverse weather conditions, generating a strong variation in flowering time amongst harvest seasons (Curti et al., 2016).
In Arabidopsis, flowering pathways are integrated by four main players: FLOWERING LOCUS C (FLC), SUPPRESSION OF CONSTANS OVEREXPRESSION1 (SOC1), FLOWERING LOCUS T (FT), and LEAFY (LFY) (van Dijk and Molenaar, 2017; Liu et al., 2020). Heterologous expression of the Arabidopsis FT gene in cassava (Manihot esculenta) improves flower development (Adeyemo et al., 2017), and overexpression of the rice homologues RFT1 and Hd3a results in extremely early flowering (Kojima et al., 2002; Pasriga et al., 2019). Likewise, overexpression of LFY homologues in different plants resulted in early flowering phenotypes (Blázquez et al., 1997; Tang et al., 2016; Liu et al., 2017). Heterologous expression of SOC1 orthologues from different plant species in Arabidopsis soc1 plants rescues the late flowering phenotype of this mutant and results in early flowering in wild-type Arabidopsis (Lee et al., 2004; Lei et al., 2013; Fudge et al., 2018; Liu et al., 2020). In turn, FLC is a MADS-box transcription factor that binds to the promoter of SOC1 and the first intron of FT, controlling their expression and repressing flowering (Helliwell et al., 2006; Searle et al., 2006). Consequently, null mutations in the FLC gene result in early flowering phenotypes (Michaels and Amasino, 1999). In addition, PHOTOPERIOD-INDEPENDENT EARLY FLOWERING1 (PIE1) activates FLC expression, and mutations in the PIE1 gene result in early flowering due to the elimination of FLC-mediated flowering repression (Noh and Amasino, 2003).
SOC1 and FLC are also important coordinators of cold responses and flowering time in Arabidopsis. SOC1 attenuates the expression of a number of cold-responsive genes by repressing the promoters of CRT/DRE-binding factors (CBFs) (Seo et al., 2009). In turn, CBFs activate FLC expression, repressing flowering. Although quinoa is quite resistant to cold temperatures (Jacobsen et al., 2005, 2007), low temperatures may result in delayed germination, and a reduction in growth and seed yield (Bertero et al., 2000; Jacobsen et al., 2005; Bois et al., 2006). Furthermore, flowering seems to be affected by the ability of the plant to reach the two-leaf stage, and temperature may affect the timing of this stage (Jacobsen et al., 2007). Therefore, homologues of SOC1 and FLC could be excellent targets for quinoa breeding.
Six homologues of Arabidopsis FT have been identified in quinoa (Table 1). Of these, only four are expressed at detectable levels (Golicz et al., 2020). In addition, several species, including Beta vulgaris, contain two orthologues of FT genes, FT1 and FT2. While BvFT2 promotes flowering, BvFT1 acts antagonistically, repressing flowering before vernalization (Dally et al., 2018). Of the four FT homologues expressed in quinoa, AUR62000271 and AUR62006619 are orthologues of BvFT2, making them the best targets to promote early flowering in quinoa through overexpression strategies. However, this could be a challenging task using current mutagenesis technologies.
The role of FT in flowering is mainly counteracted by the action of TERMINAL FLOWER 1 (TFL1), a close homologue belonging to the CENTRORADIALIS (CEN)-like subfamily of proteins. Indeed, FT and TFL1 have antagonistic roles in the regulation of flowering across different plant species (Seo et al., 2009; Pin et al., 2010; Wickland and Hanzawa, 2015; Wang et al., 2017; Kaneko-Suzuki et al., 2018; Lee et al., 2019; Wu et al., 2019). In Arabidopsis, rice, and soybean (Glycine max), TFL1 loss-of-function mutations cause early flowering and the generation of a terminal inflorescence (Shannon and Meeks-Wagner, 1991; Liu et al., 2010; Repinski et al., 2012; Kaneko-Suzuki et al., 2018). At least in Arabidopsis and rice, FT proteins activate the expression of flowering genes, while members of the TFL1 protein family are involved in the transcriptional repression of genes activated by FT (Kaneko-Suzuki et al., 2018; Lee et al., 2019). If a similar mechanism operates in quinoa, generating TFL1 loss-of-function mutations might be a simple alternative to FT mutagenesis to achieve early flowering phenotypes. However, true orthologues, or even close homologues, of TFL1 have yet to be identified in the quinoa genome.
While SOC1 has four homologues in quinoa (Table 1, Fig. 2J) (Golicz et al., 2020) and PIE1 has two homologues (Table 1; Fig. 2D), a high-throughput genomic analysis failed to identify an orthologue of FLC (Golicz et al., 2020). Despite the presence of a putative FLC orthologue (AUR62005643) in the quinoa genome, its similarity to several MADS-box genes that differ from FLC casts doubt about the identity of this gene as a true FLC orthologue. In addition to these central players, other Arabidopsis flowering genes are homologue rich in quinoa. These include LFY and several members of the EARLY FLOWERING (ELF) family, including ELF3 and ELF4, which have three orthologues each in quinoa (Table 1). Considering the number of protein families involved in flowering control and the presence of multiple orthologues in quinoa, any attempt to promote early flowering should incorporate multiplex genome editing.
Heat tolerance
The optimal temperature for quinoa germination is ~20 °C (González et al., 2017; Mamedi et al., 2017). Heat stress has profound effects on plant growth and development, affecting both vegetative and reproductive processes. At the subcellular level, heat stress rapidly inhibits photosynthesis by changing the internal structure of the chloroplasts, inactivating Rubisco, reducing the abundance of photosynthetic pigments, and damaging PSII (Allakhverdiev et al., 2008; Sharkey and Zhang, 2010; B. Li et al., 2018). Deleterious effects on reproductive development include inhibition of gametophyte development, reduced pollen germination and pollen tube growth, disturbances in pollen tube guidance and fertilization, and early embryo abortion (Sage et al., 2015; B. Li et al., 2018). This is specifically true for quinoa; temperatures of >35 °C during anthesis significantly reduce quinoa grain yield (Isobe et al., 2012; Lesjak and Calderini, 2017; Hinojosa et al., 2019b), largely as a result of a reduction in pollen viability (Hinojosa et al., 2019b). Furthermore, heat alters phytohormone production and signalling (Abdelrahman et al., 2017) and induces transcriptomic reprogramming and metabolomic changes. Heat stress also results in an increased accumulation of ROS (Zandalinas et al., 2018), thereby affecting protein and membrane stability and causing organelle malfunctioning. In this context, the peroxisome biogenesis genes PEX11C and FIS1A were proposed to be sensitive biochemical markers to screen for heat stress tolerance in quinoa (Hinojosa et al., 2019c).
Upon sensing an elevated ambient temperature, plants initiate signal transduction networks that regulate the expression of a series of genes, including those encoding HEAT SHOCK PROTEINS (HSPs) and ROS-scavenging enzymes, to increase their thermotolerance (B. Li et al., 2018). This signalling relies on rapid changes in cytosolic calcium, ROS, and nitric oxide (NO) levels that alter HSP activity via post-translational modification. HSPs then act as molecular chaperones, preventing protein denaturation and aggregation (Ohama et al., 2016). Cumulative evidence suggests that various signalling pathways are integrated to regulate the abundance and/or transcriptional activity of the basic helix–loop–helix transcription factor PHYTOCHROME INTERACTING FACTOR 4 (PIF4), which forms part of the central regulatory hub mediating the diurnal growth of plants under normal and high temperature conditions (B. Li et al., 2018). Also, HSFA1s, a family of HEAT SHOCK FACTOR (HSF) proteins, have emerged as master transcription factors affecting plant heat shock responses (Liu et al., 2011; Yoshida et al., 2011). HSFA1 activation stimulates the expression of a number of transcription factors that participate in a critical transcriptional regulatory cascade underlying the acquisition of thermotolerance in plants (Dickinson et al., 2018). In addition to HSFs, the ERF/AP2 family transcription factor DREB2A also functions in heat shock-mediated transcriptional regulatory networks (B. Li et al., 2018). Knocking out DREB2A expression resulted in a heat stress-sensitive phenotype in Arabidopsis, and plants overexpressing a constitutively active form of DREB2A showed enhanced thermotolerance (Sakuma et al., 2006).
No obvious orthologues of PFI4 or DREB2A are present in the quinoa genome. In contrast, two close homologues of HSFA1 exist (AUR62018674 and AUR62007327) (Table 1; Fig. 2C). As for the early flowering phenotypes, acquisition of thermotolerance by genetic engineering of HSFA1 would require changes in cis-regions to increase gene expression, and might be difficult to achieve with current mutation technologies. In addition, a glasshouse-based screen of 112 quinoa genotypes and their subsequent field evaluation showed substantial genetic variability in their heat stress tolerance (Hinojosa et al., 2019b), with a clear difference between sea-level and high-altitude varieties. Therefore, genome-wide association study (GWAS) analysis and/or genome sequencing of contrasting accessions may shed light on the molecular basis of differential heat tolerance in quinoa and suggest a strategy to incorporate this trait in high-yielding varieties.
Mildew tolerance
Downy mildew is a major cause of production loss in quinoa, with reductions of up to 99% in yield reported for susceptible cultivars (Danielsen et al., 2000, 2003). In quinoa, downy mildew is caused by Peronospora variabilis, and the resistance mechanisms to this disease are not yet understood. While it is generally assumed that bitter quinoa varieties with a high saponin content are less susceptible to microbial attack, there does not seem to be a correlation between downy mildew tolerance and saponin content in specific quinoa variants (Zurita-Silva et al., 2014). Further extensive research on the defence mechanisms of quinoa is needed to identify potential genetic targets for improved varieties, an approach that could be complemented with genetic assessments for resistance in planta.
Saponin content in seeds
Two beta helix–loop–helix transcription factors, AUR622017204 (TSARL1) and AUR62017206 (TSARL2), homologues of TSAR1 and TSAR2 in Medicago truncatula (Table 1), have been identified as controlling saponin biosynthesis in quinoa (Jarvis et al., 2017). Whereas TSARL2 is mainly expressed in roots, TSARL1 is expressed almost exclusively in seeds. Expression levels of TSARL1 are much lower in sweet quinoa varieties, most of which carry a single nucleotide polymorphism (SNP) in the last position of exon 3 of the TSARL1 gene. This SNP has been suggested to result in alternative splicing of the mRNA and generation of a premature stop codon. While not all sweet varieties of quinoa show this specific SNP, different mutations in the TSARL1 gene are present in all tested sweet varieties (Jarvis et al., 2017). In addition, sweet varieties have a thinner seed coat, which probably also contributes to their reduced saponin accumulation.
Methodological challenges for targeted breeding of quinoa
The advent of new breeding technologies, particularly CRISPR/Cas [clustered regularly interspaced palindromic repeats (CRISPR)/CRISPR-associated protein]-based systems, which allow the precise editing of several genes or alleles simultaneously, provides a promising platform for the targeted breeding of quinoa (Ma et al., 2015; Lowder et al., 2015; Qi et al., 2016; Čermák et al., 2017; Gao et al., 2017; Kim et al., 2017; W. Wang et al., 2018; Zhang et al., 2019; Zafar et al., 2020). However, transformation protocols are not well established in quinoa, complicating the delivery of the genome editing machinery. A full transformation procedure would require (i) delivery of transgenes into the cells; (ii) formation and selection of calli; and (iii) regeneration of full plants from callus tissue.
Agrobacterium tumefaciens has been used to transform quinoa cells in suspension cultures (Komari, 1990). This required the use of the highly virulent Agrobacterium strain A281. In addition, the binary plasmids used for the transformation had been modified to include a DNA fragment bearing an additional copy of the virB, virC, and virG virulence genes, generating a super-binary vector (Komari, 1990; Komari et al., 1996). While the efficiency of the transformation was suitable for delivery of a transgene into quinoa suspension cultures (10 positive calli out of 104 transformed), it might be too low for implementation of a genome editing strategy.
Another important drawback of the transformation approach was the size of the super-binary plasmid. Due to the instability of this plasmid in Escherichia coli, the amount of DNA that can be additionally included in such a plasmid through regular cloning strategies is limited. Nevertheless, two T-DNA vectors can be co-transformed into Agrobacterium (Komari et al., 1996). In this type of approach, one T-DNA plasmid would contain the selection marker and the required virulence genes, while the other would contain the DNA construct of interest. About 25% of the co-transformed Agrobacterium cells contain both plasmids. After co-cultivation with Agrobacterium, transformed quinoa cells would need to be plated in an appropriate medium for callus development and selection. Optimized conditions for callus formation in quinoa have recently been described (Telahigue and Toumi, 2017; Shahin, 2019).
The final challenge in quinoa transformation is the regeneration of quinoa plants. Somatic embryogenesis from callus has already been described in quinoa (Eisa et al., 2005), and it does not seem to require more than transfer of the callus to hormone-free Murashige and Skoog (MS) medium. Thus, while successful transformation followed by regeneration has not been reported in quinoa to date, all the necessary steps have been previously tested. Therefore, it should be technically possible to establish an Agrobacterium-mediated transformation protocol for quinoa based on tissue culture and regeneration of transformed plants from callus.
To improve the transformation efficiency of quinoa, booster genes can be used. Boosters, such as LEAFY COTYLEDON1 (Lotan et al., 1998), Lec1 (Lowe et al., 2003), LEAFY COTYLEDON2 (Stone et al., 2001), WUSCHEL (WUS) (Zuo et al., 2002), and BABY BOOM (BBM) (Boutilier et al., 2002), stimulate the production of embryo-like structures or somatic embryos on numerous explants and also enhance regeneration in both monocot and dicot plant species (Srinivasan et al., 2007; Deng et al., 2009). The co-overexpression of maize Bbm and Wus2 improves the transformation frequencies in sorghum (Sorghum bicolor) and sugarcane (Saccharum officinarum), which are recalcitrant to both biolistic and A. tumefaciens transformations (Lowe et al., 2016).
To circumvent the need for inefficient and time-consuming tissue culture in quinoa transformation, de novo induction of gene-edited meristems could be an alternative approach. In this approach, boosters and gene editing reagents are co-delivered to somatic cells, and the transferred somatic cells are subsequently induced to meristems that produce shoots with targeted DNA modifications and gene edits (Maher et al., 2020). Transgenic shoots in tomato, potato, and grapevine (Vitis vinifera) have been generated using the de novo induction of meristems (Maher et al., 2020). Collectively, a highly efficient transformation and genome editing system could be established in quinoa with the help of boosters and the de novo induction of meristems.
A central challenge for genetic engineering of quinoa plants is the fact that quinoa is an allotetraploid containing A and B genomes. In the worst-case scenario, all four copies of a gene of interest would need to be targeted. Recently, a protocol for CRISPR-mediated transformation of hexaploid wheat was developed (Zhang et al., 2019). In this work, ~10% of the transformed plants carried the desired mutation in all six copies in the genome, providing hope for the use of this technology in other polyploid species. Multiplex editing has also been successfully tested in other plants including maize, cotton (Gossypium hirsutum), barley, rice, and soybean (Lowder et al., 2015; Ma et al., 2015; Qi et al., 2016; Čermák et al., 2017; Gao et al., 2017; Kim et al., 2017; W. Wang et al., 2018).
Besides polyploidy, substantial genetic variation exists not only amongst quinoa cultivars, but also within local populations. Therefore, selection of the guide RNA sequence will need to be preceded by resequencing of the target gene in the individual genotype to be transformed. In potato, endogenous promoters have been used to greatly increase the efficiency of CRISPR-mediated genome editing (Liang et al., 2018; Johansen et al., 2019); similar strategies should be explored in quinoa. Moreover, engineering of wheat with the CRISPR/Cas9 system required codon optimization of the Cas9 sequence and the use of a maize promoter for expression (Zhang et al., 2019). Therefore, promoters, terminators, or other elements contained in common plasmids might need to be adapted to quinoa for efficient editing. However, with the publication of the quinoa reference genomes (Jarvis et al., 2017; Zou et al., 2017), and accumulating studies on the expression of different genes in this plant, selecting suitable DNA fragments for generating quinoa-optimized vectors should be achievable in the near future.
While genetically modifying quinoa using genome editing strategies seems to be feasible, such an approach would generate plants that might be subjected to strict GM regulation in some countries (Zhang et al., 2020). For instance, a recent ruling of the European Court (Case C-528/16) has declared that any plant product generated with the use of new genome editing technologies is subjected to GM regulation, regardless of whether or not a transgene is present. Nevertheless, genome editing techniques might become extremely valuable tools to accelerate the identification of relevant targets for other non-GM molecular breeding approaches.
As an alternative to genome editing, TILLING (Targeting Induced Local Lesions IN Genomes) methods may soon prove as effective and fast as gene editing technologies for the identification of induced genetic variants in any gene (Holme et al., 2019). Present quinoa TILLING libraries typically contain up to 3000 highly mutagenized individuals (Mestanza et al., 2018). However, the advent of advanced genetic screens now enables the establishment and screening of much larger libraries derived from fewer mutagenized individuals (Wendt et al., 2019). These enormous libraries can contain in excess of 300 000 individuals, which increases the probability of identifying a desired nucleotide change, and, once a desired mutant plant has been identified, it is less likely to have a non-perturbed phenotype due to a reduced mutation load per plant. Such large libraries are likely to represent a complete collection of loss-of-function versions of all genes in a genome and additionally provide instant access to numerous alternative functional alleles for every gene.
Conclusions and prospects
The publication of high-quality genome data for quinoa has opened up the possibility of using targeted genome editing for adapting this plant to cultivation conditions in new geographic areas, and improving its agronomic performance. Apart from an increase in seed size and seed numbers, factors such as flowering time, resistance to pathogens, and adaptation to heat stress are important traits to modify in this context. While novel genome-editing technologies, such as CRISPR, could provide an efficient strategy for accelerating the generation of new varieties of this allotetraploid plant, some countries require that such plants be regulated according to GM legislation, which precludes the use of new varieties for commercialization. As an alternative, high-end TILLING technologies could be used for directed molecular breeding of quinoa. The end result would consistently be a nutritious high-yielding crop that is already adapted to a changing climate.
Acknowledgements
This work was supported by grants to MP from the Carlsberg Foundation (RaisingQuinoa; project number CF18-1113), to MP and RL-M from the Novo Nordisk Foundation (NovoCrops; project number 2019OC53580), and to SS from ARC Discovery projects DP150101663 and DP170100430. We thank Jennifer Böhm for providing the images shown in Fig. 1B, D, and E, and Robert van Loo for sharing the quinoa genome annotation files and for fruitful discussions.
Author contributions
RL-M and MP wrote the first draft of the manuscript. AN performed the BLAST searches and carried out the phylogenetic analyses. All other authors proposed targets for the BLAST searches and/or contributed to the writing of the final version of the manuscript.
References
- Abdelrahman M, El-Sayed M, Jogaiah S, Burritt DJ, Tran LP. 2017. The ‘STAY-GREEN’ trait and phytohormone signaling networks in plants under heat stress. Plant Cell Reports 36, 1009–1025. [DOI] [PubMed] [Google Scholar]
- Adeyemo OS, Chavarriaga P, Tohme J, Fregene M, Davis SJ, Setter TL. 2017. Overexpression of Arabidopsis FLOWERING LOCUS T (FT) gene improves floral development in cassava (Manihot esculenta, Crantz). PLoS One 12, e0181460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolf VI, Jacobsen SE, Shabala S. 2013. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environmental and Experimental Botany 92, 43–54. [Google Scholar]
- Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P. 2008. Heat stress: an overview of molecular responses in photosynthesis. Photosynthesis Research 98, 541–550. [DOI] [PubMed] [Google Scholar]
- Apaza D, Carceres G, Estrada R, Pinedo R. 2015. Catalogue of commercial varieties of quinoa in Peru: a future planted thousands of years ago. Lima: FAO. [Google Scholar]
- Bailey-Serres J, Parker JE, Ainsworth EA, Oldroyd GED, Schroeder JI. 2019. Genetic strategies for improving crop yields. Nature 575, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester P, Ferrándiz C. 2017. Shattering fruits: variations on a dehiscent theme. Current Opinion in Plant Biology 35, 68–75. [DOI] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmülling T. 2011. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. The Plant Cell 23, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E, Blumwald E. 2014. The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Current Opinion in Plant Biology 22, 1–6. [DOI] [PubMed] [Google Scholar]
- Bazile D, Jacobsen S-E, Verniau A. 2016a. The global expansion of quinoa: trends and limits. Frontiers in Plant Science 7, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazile D, Pulvento C, Verniau A, et al. 2016b. Worldwide evaluations of quinoa: preliminary results from post international year of quinoa FAO projects in nine countries. Frontiers in Plant Science 7, 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero HD, King RW, Hall AJ. 2000. Photoperiod and temperature effects on the rate of leaf appearance in quinoa (Chenopodium quinoa). Australian Journal of Plant Physiology 27, 349–356. [Google Scholar]
- Blázquez MA, Soowal LN, Lee I, Weigel D. 1997. LEAFY expression and flower initiation in Arabidopsis. Development 124, 3835–3844. [DOI] [PubMed] [Google Scholar]
- Böhm J, Messerer M, Müller HM, et al. 2018. Understanding the molecular basis of salt sequestration in epidermal bladder cells of Chenopodium quinoa. Current Biology 28, 3075–3085. [DOI] [PubMed] [Google Scholar]
- Bois JF, Winkel T, Lhomme JP, Raffaillac JP, Rocheteau A. 2006. Response of some Andean cultivars of quinoa (Chenopodium quinoa Willd.) to temperature: effects on germination, phenology, growth and freezing. European Journal of Agronomy 25, 299–308. [Google Scholar]
- Bonales-Alatorre E, Pottosin I, Shabala L, Chen ZH, Zeng F, Jacobsen SE, Shabala S. 2013. Differential activity of plasma and vacuolar membrane transporters contributes to genotypic differences in salinity tolerance in a halophyte species, Chenopodium quinoa. International Journal of Molecular Sciences 14, 9267–9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, et al. 2002. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. The Plant Cell 14, 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccato DV, Bertero HD, Batlla D. 2011. Environmental control of dormancy in quinoa (Chenopodium quinoa) seeds: two potential genetic resources for pre-harvest sprouting tolerance. Seed Science Research 21, 133–141. [Google Scholar]
- Čermák T, Curtin SJ, Gil-Humanes J, et al. 2017. A multipurpose toolkit to enable advanced genome engineering in plants. The Plant Cell 29, 1196–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choukr-Allah R, Rao NK, Hirich A, Shahid M, Alshankiti A, Toderich K, Gill S, Butt KU. 2016. Quinoa for marginal environments: toward future food and nutritional security in MENA and Central Asia regions. Frontiers in Plant Science 7, 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti RN, de la Vega AJ, Andrade AJ, Bramardi SJ, Bertero HD. 2016. Adaptive responses of quinoa to diverse agro-ecological environments along an altitudinal gradient in North West Argentina. Field Crops Research 189, 10–18. [Google Scholar]
- Dally N, Eckel M, Batschauer A, Höft N, Jung C. 2018. Two CONSTANS-LIKE genes jointly control flowering time in beet. Scientific Reports 8, 16120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen S, Bonifacio A, Ames T. 2003. Diseases of quinoa (Chenopodium quinoa). Food Reviews International 19, 43–59. [Google Scholar]
- Danielsen S, Jacobsen S-E, Echegaray J, Ames T. 2000. Impact of downy mildew on the yield of quinoa. In: Scientist and farmer: partners in research for the 21st Century. Program Report 1999–2000. International Potato Center, 397–401. [Google Scholar]
- Deng W, Luo K, Li Z, Yang Y. 2009. A novel method for induction of plant regeneration via somatic embryogenesis. Plant Science 177, 43–48. [Google Scholar]
- Dickinson PJ, Kumar M, Martinho C, et al. 2018. Chloroplast signaling gates thermotolerance in Arabidopsis. Cell Reports 22, 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Wang YZ. 2015. Seed shattering: from models to crops. Frontiers in Plant Science 6, 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Xu F, Fang J, et al. 2018. Endosperm sugar accumulation caused by mutation of PHS8/ISA1 leads to pre-harvest sprouting in rice. The Plant Journal 95, 545–556. [DOI] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisa S, Koyro HW, Kogel KH, Imani J. 2005. Induction of somatic embryogenesis in cultured cells of Chenopodium qunioa. Plant Cell, Tissue and Organ Culture 81, 243–246. [Google Scholar]
- Eshed Y, Lippman ZB. 2019. Revolutions in agriculture chart a course for targeted breeding of old and new crops. Science 366, 705. [DOI] [PubMed] [Google Scholar]
- Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, Li X, Zhang Q. 2006. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theoretical and Applied Genetics 112, 1164–1171. [DOI] [PubMed] [Google Scholar]
- FAO . 2010. The second report on the state of the World’s plant genetic resources for food and agriculture. Rome: FAO. [Google Scholar]
- FAO . 2017. The future of food and agriculture: trends and challenges. Rome: FAO; . [Google Scholar]
- Filho AM, Pirozi MR, Borges JT, Pinheiro Sant’Ana HM, Chaves JB, Coimbra JS. 2017. Quinoa: nutritional, functional, and antinutritional aspects. Critical Reviews in Food Science and Nutrition 57, 1618–1630. [DOI] [PubMed] [Google Scholar]
- Flintham JE, Börner A, Worland AJ, Gale MD. 1997. Optimizing wheat grain yield: effects of Rht (gibberellin-insensitive) dwarfing genes. Journal of Agricultural Science 128, 11–25. [Google Scholar]
- Flowers TJ, Colmer TD. 2008. Salinity tolerance in halophytes. New Phytologist 179, 945–963. [DOI] [PubMed] [Google Scholar]
- Fu X, Sudhakar D, Peng J, Richards DE, Christou P, Harberd NP. 2001. Expression of Arabidopsis GAI in transgenic rice represses multiple gibberellin responses. The Plant Cell 13, 1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JB, Lee RH, Laurie RE, Mysore KS, Wen J, Weller JL, Macknight RC. 2018. Medicago truncatula SOC1 genes are up-regulated by environmental cues that promote flowering. Frontiers in Plant Science 9, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa C, Van den Broeck G, Maertens M. 2018. Smallholders’ preferences for improved quinoa varieties in the Peruvian Andes. Sustainability 10, 3735. [Google Scholar]
- Gao W, Long L, Tian X, Xu F, Liu J, Singh PK, Botella JR, Song C. 2017. Genome editing in cotton with the CRISPR/Cas9 system. Frontiers in Plant Science 8, 1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhang X, Lan H, Huang J, Wang J, Zhang H. 2015. The additive effects of GS3 and qGL3 on rice grain length regulation revealed by genetic and transcriptome comparisons. BMC Plant Biology 15, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golicz AA, Steinfort U, Arya H, Singh MB, Bhalla PL. 2020. Analysis of the quinoa genome reveals conservation and divergence of the flowering pathways. Functional & Integrative Genomics 20, 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JA, Buedo SE, Bruno M, Prado FE. 2017. Quantifying cardinal temperatures in quinoa (Chenopodium quinoa) cultivars. Lilloa 54, 179–194. [Google Scholar]
- Grassini P, Eskridge KM, Cassman KG. 2013. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nature Communications 4, 2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed M, Mansoor U, Ashraf M, Rao A-U-R. 2002. Variation in leaf anatomy in wheat germplasm from varying drought-hit habitats. International Journal of Agriculture & Biology 4, 12–16. [Google Scholar]
- Han Y, Yin S, Huang L, Wu X, Zeng J, Liu X, Qiu L, Munns R, Chen ZH, Zhang G. 2018. A sodium transporter HvHKT1;1 confers salt tolerance in barley via regulating tissue and cell ion homeostasis. Plant & Cell Physiology 59, 1976–1989. [DOI] [PubMed] [Google Scholar]
- Hariadi Y, Marandon K, Tian Y, Jacobsen SE, Shabala S. 2011. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. Journal of Experimental Botany 62, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser F, Horie T. 2010. A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant, Cell & Environment 33, 552–565. [DOI] [PubMed] [Google Scholar]
- Hedden P. 2003. The genes of the green revolution. Trends in Genetics 19, 5–9. [DOI] [PubMed] [Google Scholar]
- Helliwell CA, Wood CC, Robertson M, James Peacock W, Dennis ES. 2006. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. The Plant Journal 46, 183–192. [DOI] [PubMed] [Google Scholar]
- Hinojosa L, Kumar N, Gill KS, Murphy KM. 2019a. Spectral reflectance indices and physiological parameters in quinoa under contrasting irrigation regimes. Crop Science 59, 1927–1944. [Google Scholar]
- Hinojosa L, Matanguihan JB, Murphy KM. 2019b. Effect of high temperature on pollen morphology, plant growth and seed yield in quinoa (Chenopodium quinoa Willd.). Journal of Agronomy and Crop Science 205, 33–45. [Google Scholar]
- Hinojosa L, Sanad MNME, Jarvis DE, Steel P, Murphy K, Smertenko A. 2019c. Impact of heat and drought stress on peroxisome proliferation in quinoa. The Plant Journal 99, 1144–1158. [DOI] [PubMed] [Google Scholar]
- Hofmann NR. 2012. SHAT1, a new player in seed shattering of rice. The Plant Cell 24, 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme IB, Gregersen PL, Brinch-Pedersen H. 2019. Induced genetic variation in crop plants by random or targeted mutagenesis: convergence and differences. Frontiers in Plant Science 10, 1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AM, Horie T. 2017. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annual Review of Plant Biology 68, 405–434. [DOI] [PubMed] [Google Scholar]
- Isobe K, Uziie K, Hitomi S, Furuya U. 2012. Agronomic studies on quinoa (Chenopodium quinoa Willd.) cultivation in Japan. Japanese Journal of Crop Science 81, 167–172. [Google Scholar]
- Jacobsen SE. 2017. The scope for adaptation of quinoa in northern latitudes of Europe. Journal of Agronomy and Crop Science 203, 603–613. [Google Scholar]
- Jacobsen SE, Monteros C, Christiansen JL, Bravo LA, Corcuera LJ, Mujica A. 2005. Plant responses of quinoa (Chenopodium quinoa Willd.) to frost at various phenological stages. European Journal of Agronomy 22, 131–139. [Google Scholar]
- Jacobsen SE, Monteros C, Corcuera LJ, Bravo LA, Christiansen JL, Mujica A. 2007. Frost resistance mechanisms in quinoa (Chenopodium quinoa Willd.). European Journal of Agronomy 26, 471–475. [Google Scholar]
- Jacobsen SE, Sørensen M, Pedersen SM, Weiner J. 2013. Feeding the world: genetically modified crops versus agricultural biodiversity. Agronomy for Sustainable Development 33, 651–662. [Google Scholar]
- Jacobsen SE, Sørensen M, Pedersen SM, Weiner J. 2015. Using our agrobiodiversity: plant-based solutions to feed the world. Agronomy for Sustainable Development 35, 1217–1235. [Google Scholar]
- Jarvis DE, Ho YS, Lightfoot DJ, et al. 2017. The genome of Chenopodium quinoa. Nature 542, 307–312. [DOI] [PubMed] [Google Scholar]
- Jia Q, Zhang J, Westcott S, Zhang XQ, Bellgard M, Lance R, Li C. 2009. GA-20 oxidase as a candidate for the semidwarf gene sdw1/denso in barley. Functional & Integrative Genomics 9, 255–262. [DOI] [PubMed] [Google Scholar]
- Johansen IE, Liu Y, Jørgensen B, Bennett EP, Andreasson E, Nielsen KL, Blennow A, Petersen BL. 2019. High efficacy full allelic CRISPR/Cas9 gene editing in tetraploid potato. Scientific Reports 9, 17715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko-Suzuki M, Kurihara-Ishikawa R, Okushita-Terakawa C, Kojima C, Nagano-Fujiwara M, Ohki I, Tsuji H, Shimamoto K, Taoka KI. 2018. TFL1-like proteins in rice antagonize rice FT-like protein in inflorescence development by competition for complex formation with 14-3-3 and FD. Plant & Cell Physiology 59, 458–468. [DOI] [PubMed] [Google Scholar]
- Katwal TB, Bazile D. 2020. First adaptation of quinoa in the Bhutanese mountain agriculture systems. PLoS One 15, e0219804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani-Pouya A, Rasouli F, Bazihizina N, Zhang H, Hedrich R, Shabala S. 2019. A large-scale screening of quinoa accessions reveals an important role of epidermal bladder cells and stomatal patterning in salinity tolerance. Environmental and Experimental Botany 168, 103885. [Google Scholar]
- Kiani-Pouya A, Roessner U, Jayasinghe NS, Lutz A, Rupasinghe T, Bazihizina N, Bohm J, Alharbi S, Hedrich R, Shabala S. 2017. Epidermal bladder cells confer salinity stress tolerance in the halophyte quinoa and Atriplex species. Plant, Cell & Environment 40, 1900–1915. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim ST, Ryu J, Kang BC, Kim JS, Kim SG. 2017. CRISPR/Cpf1-mediated DNA-free plant genome editing. Nature Communications 8, 14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M. 2002. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant & Cell Physiology 43, 1096–1105. [DOI] [PubMed] [Google Scholar]
- Komari T. 1990. Transformation of cultured cells of Chenopodium quinoa by binary vectors that carry a fragment of DNA from the virulence region of pTiBo542. Plant Cell Reports 9, 303–306. [DOI] [PubMed] [Google Scholar]
- Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T. 1996. Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. The Plant Journal 10, 165–174. [DOI] [PubMed] [Google Scholar]
- Lawit SJ, Wych HM, Xu D, Kundu S, Tomes DT. 2010. Maize DELLA proteins dwarf plant8 and dwarf plant9 as modulators of plant development. Plant & Cell Physiology 51, 1854–1868. [DOI] [PubMed] [Google Scholar]
- Le SQ, Gascuel O. 1993. An improved general amino acid replacement matrix. Molecular Biology and Evolution 25, 1307–1320. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim J, Han JJ, Han MJ, An G. 2004. Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in rice. The Plant Journal 38, 754–764. [DOI] [PubMed] [Google Scholar]
- Lee C, Kim SJ, Jin S, Susila H, Youn G, Nasim Z, Alavilli H, Chung KS, Yoo SJ, Ahn JH. 2019. Genetic interactions reveal the antagonistic roles of FT/TSF and TFL1 in the determination of inflorescence meristem identity in Arabidopsis. The Plant Journal 99, 452–464. [DOI] [PubMed] [Google Scholar]
- Lei HJ, Yuan HZ, Liu Y, Guo XW, Liao X, Liu LL, Wang Q, Li TH. 2013. Identification and characterization of FaSOC1, a homolog of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 from strawberry. Gene 531, 158–167. [DOI] [PubMed] [Google Scholar]
- Lemmon ZH, Reem NT, Dalrymple J, Soyk S, Swartwood KE, Rodriguez-Leal D, Van Eck J, Lippman ZB. 2018. Rapid improvement of domestication traits in an orphan crop by genome editing. Nature Plants 4, 766–770. [DOI] [PubMed] [Google Scholar]
- Lesjak J, Calderini DF. 2017. Increased night temperature negatively affects grain yield, biomass and grain number in Chilean quinoa. Frontiers in Plant Science 8, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Gao K, Ren H, Tang W. 2018. Molecular mechanisms governing plant responses to high temperatures. Journal of Integrative Plant Biology 60, 757–779. [DOI] [PubMed] [Google Scholar]
- Li T, Yang X, Yu Y, Si X, Zhai X, Zhang H, Dong W, Gao C, Xu C. 2018. Domestication of wild tomato is accelerated by genome editing. Nature Biotechnology 36, 1160–1163. [DOI] [PubMed] [Google Scholar]
- Liang Z, Chen K, Zhang Y, Liu J, Yin K, Qiu JL, Gao C. 2018. Genome editing of bread wheat using biolistic delivery of CRISPR/Cas9 in vitro transcripts or ribonucleoproteins. Nature Protocols 13, 413–430. [DOI] [PubMed] [Google Scholar]
- Liljegren SJ, Ditta GS, Eshed Y, Savidge B, Bowman JL, Yanofsky MF. 2000. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404, 766–770. [DOI] [PubMed] [Google Scholar]
- Liu B, Watanabe S, Uchiyama T, et al. 2010. The soybean stem growth habit gene Dt1 is an ortholog of Arabidopsis TERMINAL FLOWER1. Plant Physiology 153, 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HC, Liao HT, Charng YY. 2011. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant, Cell & Environment 34, 738–751. [DOI] [PubMed] [Google Scholar]
- Liu S, Sehgal SK, Lin M, Li J, Trick HN, Gill BS, Bai G. 2015. Independent mis-splicing mutations in TaPHS1 causing loss of preharvest sprouting (PHS) resistance during wheat domestication. New Phytologist 208, 928–935. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao Q, Meng N, Song H, Li C, Hu G, Wu J, Lin S, Zhang Z. 2017. Over-expression of EjLFY-1 leads to an early flowering habit in strawberry (Fragaria × ananassa) and its asexual progeny. Frontiers in Plant Science 8, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wu X, Cheng M, Xie Z, Xiong C, Zhang S, Wu J, Wang P. 2020. Identification and functional characterization of SOC1-like genes in Pyrus bretschneideri. Genomics 112, 1622–1632. [DOI] [PubMed] [Google Scholar]
- Lobell DB, Gourdji SM. 2012. The influence of climate change on global crop productivity. Plant Physiology 160, 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan T, Ohto M, Yee KM, West MA, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ. 1998. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93, 1195–1205. [DOI] [PubMed] [Google Scholar]
- Lowder LG, Zhang D, Baltes NJ, Paul JW 3rd, Tang X, Zheng X, Voytas DF, Hsieh TF, Zhang Y, Qi Y. 2015. A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation. Plant Physiology 169, 971–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe K, Hoerster G, Sun X, Rasco-Gaunt S, Lazerri P, Ellis S, Abbitt S, Glassman K, Gordon-Kamm B. 2003. Maize LEC1 improves transformation in both maize and wheat. In: Vasil IK, ed. Plant biotechnology 2002 and beyond. Dordrecht, The Netherlands: Kluwer Academic Publishers, 283–284. [Google Scholar]
- Lowe K, Wu E, Wang N, et al. 2016. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. The Plant Cell 28, 1998–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Zhang Q, Zhu Q, et al. 2015. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Molecular Plant 8, 1274–1284. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Amtmann A. 1999. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Annals of Botany 84, 123–133. [Google Scholar]
- Maher MF, Nasti RA, Vollbrecht M, Starker CG, Clark MD, Voytas DF. 2020. Plant gene editing through de novo induction of meristems. Nature Biotechnology 38, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliro MF, Guwela VF, Nyaika J, Murphy KM. 2017. Preliminary studies of the performance of quinoa (Chenopodium quinoa Willd.) genotypes under irrigated and rainfed conditions of Central Malawi. Frontiers in Plant Science 8, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamedi A, Tavakkol R, Oveisi M. 2017. Cardinal temperatures for seed germination of three quinoa (Chenopodium quinoa Willd.) cultivars. Iranian Journal of Field Crop Science 1, 89–100. [Google Scholar]
- Mao X, Zhang J, Liu W, et al. 2019. The MKKK62–MKK3–MAPK7/14 module negatively regulates seed dormancy in rice. Rice 12, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez SA, Shorinola O, Conselman S, See D, Skinner DZ, Uauy C, Steber CM. 2020. Exome sequencing of bulked segregants identified a novel TaMKK3-A allele linked to the wheat ERA8 ABA-hypersensitive germination phenotype. Theoretical and Applied Genetics 133, 719–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney NB, Ahumada MI, Muñoz MP, Rosales IM, Fierro AM, Chorbadjian RA. 2019. Effects of saponin-rich quinoa (Chenopodium quinoa Willd.) bran and bran extract in diets of adapted and non-adapted quinoa pests in laboratory bioassays. Ciencia e Investigacion Agraria 46, 125–136. [Google Scholar]
- Mestanza C, Riegel R, Vásquez SC, Veliz D, Cruz-Rosero N, Canchignia H, Silva H. 2018. Discovery of mutations in Chenopodium quinoa Willd. through EMS mutagenesis and mutation screening using pre-selection phenotypic data and next-generation sequencing. Journal of Agricultural Science 156, 1196–1204. [Google Scholar]
- Michaels SD, Amasino RM. 1999. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. The Plant Cell 11, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010. New Orleans: IEEE, 1–8. [Google Scholar]
- Monna L, Kitazawa N, Yoshino R, Suzuki J, Masuda H, Maehara Y, Tanji M, Sato M, Nasu S, Minobe Y. 2002. Positional cloning of rice semidwarfing gene, sd-1: rice ‘green revolution gene’ encodes a mutant enzyme involved in gibberellin synthesis. DNA Research 9, 11–17. [DOI] [PubMed] [Google Scholar]
- Munns R, Passioura JB, Colmer TD, Byrt CS. 2020. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytologist 225, 1091–1096. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Bazile D, Kellogg J, Rahmanian M. 2016. Development of a worldwide consortium on evolutionary participatory breeding in quinoa. Frontiers in Plant Science 7, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Matanguihan JB, Fuentes FF, Gómez-Pando LR, Jellen EN, Maughan PJ, Jarvis DE. 2018. Quinoa breeding and genomics. In: Plant breeding reviews, Vol. 42. Hoboken, NJ: Wiley, 257–320. [Google Scholar]
- Nakamura S, Pourkheirandish M, Morishige H, et al. 2016. Mitogen-activated protein kinase kinase 3 regulates seed dormancy in barley. Current Biology 26, 775–781. [DOI] [PubMed] [Google Scholar]
- Noh YS, Amasino RM. 2003. PIE1, an ISWI family gene, is required for FLC activation and floral repression in Arabidopsis. The Plant Cell 15, 1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohama N, Kusakabe K, Mizoi J, et al. 2016. The transcriptional cascade in the heat stress response of Arabidopsis is strictly regulated at the level of transcription factor expression. The Plant Cell 28, 181–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østerberg JT, Xiang W, Olsen LI, et al. 2017. Accelerating the domestication of new crops: feasibility and approaches. Trends in Plant Science 22, 373–384. [DOI] [PubMed] [Google Scholar]
- Palmgren MG, Edenbrandt AK, Vedel SE, et al. 2015. Are we ready for back-to-nature crop breeding? Trends in Plant Science 20, 155–164. [DOI] [PubMed] [Google Scholar]
- Pasriga R, Yoon J, Cho LH, An G. 2019. Overexpression of RICE FLOWERING LOCUS T 1 (RFT1) induces extremely early flowering in rice. Molecules and Cells 42, 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearsall DM. 1992. The origins of plant cultivation in South America. In: Cowan CW, Watson PJ, ed. The origins of agriculture. An international perspective. Washington: Smithsonian Institution Press, 173–205. [Google Scholar]
- Pedersen JT, Palmgren M. 2017. Why do plants lack sodium pumps and would they benefit from having one? Functional Plant Biology 44, 473–479. [DOI] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP. 1997. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes & Development 11, 3194–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Richards DE, Hartley NM, et al. 1999. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400, 256–261. [DOI] [PubMed] [Google Scholar]
- Pereira E, Encina-Zelada C, Barros L, Gonzales-Barron U, Cadavez V, Ferreira ICFR. 2019. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: a good alternative to nutritious food. Food Chemistry 280, 110–114. [DOI] [PubMed] [Google Scholar]
- Peterson A, Jacobsen SE, Bonifacio A, Murphy K. 2015. A crossing method for Quinoa. Sustainability 7, 3230–3243. [Google Scholar]
- Pin PA, Benlloch R, Bonnet D, Wremerth-Weich E, Kraft T, Gielen JJ, Nilsson O. 2010. An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science 330, 1397–1400. [DOI] [PubMed] [Google Scholar]
- Präger A, Munz S, Nkebiwe PM, Mast B, Graeff-Hönninger S. 2018. Yield and quality characteristics of different quinoa (Chenopodium quinoa willd.) cultivars grown under field conditions in Southwestern Germany. Agronomy 8, 197. [Google Scholar]
- Qi W, Zhu T, Tian Z, Li C, Zhang W, Song R. 2016. High-efficiency CRISPR/Cas9 multiplex gene editing using the glycine tRNA-processing system-based strategy in maize. BMC Biotechnology 16, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NK, Shahid M. 2012. Quinoa—a promising new crop for the Arabian peninsula. Journal of Agriculture and Environmental Sciences 12, 1350–1355. [Google Scholar]
- Ray DK, Mueller ND, West PC, Foley JA. 2013. Yield trends are insufficient to double global crop production by 2050. PLoS One 8, e66428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray DK, Ramankutty N, Mueller ND, West PC, Foley JA. 2012. Recent patterns of crop yield growth and stagnation. Nature Communications 3, 1293. [DOI] [PubMed] [Google Scholar]
- Repinski SL, Kwak M, Gepts P. 2012. The common bean growth habit gene PvTFL1y is a functional homolog of Arabidopsis TFL1. Theoretical and Applied Genetics 124, 1539–1547. [DOI] [PubMed] [Google Scholar]
- Ruiz KB, Biondi S, Oses R, et al. 2014. Quinoa biodiversity and sustainability for food security under climate change. Agronomy for Sustainable Development 34, 349–359. [Google Scholar]
- Ruiz KB, Khakimov B, Engelsen SB, Bak S, Biondi S, Jacobsen SE. 2017. Quinoa seed coats as an expanding and sustainable source of bioactive compounds: an investigation of genotypic diversity in saponin profiles. Industrial Crops and Products 104, 156–163. [Google Scholar]
- Saade S, Kutlu B, Draba V, Förster K, Schumann E, Tester M, Pillen K, Maurer A. 2017. A donor-specific QTL, exhibiting allelic variation for leaf sheath hairiness in a nested association mapping population, is located on barley chromosome 4H. PLoS One 12, e0189446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage TL, Bagha S, Lundsgaard-Nielsen V, Branch HA, Sultmanis S, Sage RF. 2015. The effect of high temperature stress on male and female reproduction in plants. Field Crops Research 182, 30–42. [Google Scholar]
- Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2006. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. The Plant Cell 18, 1292–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, Amasino RA, Coupland G. 2006. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes & Development 20, 898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo E, Lee H, Jeon J, Park H, Kim J, Noh YS, Lee I. 2009. Crosstalk between cold response and flowering in Arabidopsis is mediated through the flowering-time gene SOC1 and its upstream negative regulator FLC. The Plant Cell 21, 3185–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Bose J, Hedrich R. 2014. Salt bladders: do they matter? Trends in Plant Science 19, 687–691. [DOI] [PubMed] [Google Scholar]
- Shabala S, Cuin TA. 2008. Potassium transport and plant salt tolerance. Physiologia Plantarum 133, 651–669. [DOI] [PubMed] [Google Scholar]
- Shabala S, Mackay A. 2011. Ion transport in halophytes. Advances in Botanical Research 57, 151–199. [Google Scholar]
- Shahin H. 2019. Callus formation and production of secondary metabolites by seedling explants of Chenopodium quinoa. Egyptian Journal of Botany 59, 451–460. [Google Scholar]
- Shannon S, Meeks-Wagner DR. 1991. A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. The Plant Cell 3, 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Zhang R. 2010. High temperature effects on electron and proton circuits of photosynthesis. Journal of Integrative Plant Biology 52, 712–722. [DOI] [PubMed] [Google Scholar]
- Silverstone AL, Ciampaglio CN, Sun T. 1998. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. The Plant Cell 10, 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Kaur A. 2018. Control of insect pests in crop plants and stored food grains using plant saponins: a review. LWT - Food Science and Technology 87, 93–101. [Google Scholar]
- Song XJ, Huang W, Shi M, Zhu MZ, Lin HX. 2007. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nature Genetics 39, 623–630. [DOI] [PubMed] [Google Scholar]
- Spielmeyer W, Ellis MH, Chandler PM. 2002. Semidwarf (sd-1), ‘green revolution’ rice, contains a defective gibberellin 20-oxidase gene. Proceedings of the National Academy of Sciences, USA 99, 9043–9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springmann M, Clark M, Mason-D’Croz D, et al. 2018. Options for keeping the food system within environmental limits. Nature 562, 519–525. [DOI] [PubMed] [Google Scholar]
- Srinivasan C, Liu Z, Heidmann I, et al. 2007. Heterologous expression of the BABY BOOM AP2/ERF transcription factor enhances the regeneration capacity of tobacco (Nicotiana tabacum L.). Planta 225, 341–351. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ. 2001. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proceedings of the National Academy of Sciences, USA 98, 11806–11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Tao YB, Fu Q, Song Y, Niu L, Xu ZF. 2016. An ortholog of LEAFY in Jatropha curcas regulates flowering time and floral organ development. Scientific Reports 6, 37306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telahigue D, Toumi L. 2017. Influence of medium and growth regulators on callogenesis of quinoa (Chenopodium quinoa Willd.) and effect of hydrous stress induced by P.E.G 6000 on the callus. Horticultural Biotechnology Research 3, 1–9. [Google Scholar]
- Tester M, Davenport R. 2003. Na+ tolerance and Na+ transport in higher plants. Annals of Botany 91, 503–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torada A, Koike M, Ogawa T, Takenouchi Y, Tadamura K, Wu J, Matsumoto T, Kawaura K, Ogihara Y. 2016. A causal gene for seed dormancy on wheat chromosome 4A encodes a MAP kinase kinase. Current Biology 26, 782–787. [DOI] [PubMed] [Google Scholar]
- van Dijk ADJ, Molenaar J. 2017. Floral pathway integrator gene expression mediates gradual transmission of environmental and endogenous cues to flowering time. PeerJ 5, e3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Wang J, Zhu X, et al. 2008. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nature Genetics 40, 1370–1374. [DOI] [PubMed] [Google Scholar]
- Wang S, Ma B, Gao Q, et al. 2018. Dissecting the genetic basis of heavy panicle hybrid rice uncovered Gn1a and GS3 as key genes. Theoretical and Applied Genetics 131, 1391–1403. [DOI] [PubMed] [Google Scholar]
- Wang W, Pan Q, He F, Akhunova A, Chao S, Trick H, Akhunov E. 2018. Transgenerational CRISPR–Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. The CRISPR Journal 1, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yang R, Devisetty UK, Maloof JN, Zuo Y, Li J, Shen Y, Zhao J, Bao M, Ning G. 2017. The divergence of flowering time modulated by FT/TFL1 is independent to their interaction and binding activities. Frontiers in Plant Science 8, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt T, Olsen O, Knudsen S, Thomsen HC, Skadhauge B, Rasmussen MW, Carciofi M, Striebeck A. 2019. Method to screen for a mutant within a population of organisms by applying a pooling and splitting approach. International patent WO2018001884, issued 4 January 2018. [Google Scholar]
- Wickland DP, Hanzawa Y. 2015. The FLOWERING LOCUS T/TERMINAL FLOWER 1 gene family: functional evolution and molecular mechanisms. Molecular Plant 8, 983–997. [DOI] [PubMed] [Google Scholar]
- Winkler RG, Freeling M. 1994. Physiological genetics of the dominant gibberellin-nonresponsive maize dwarfs, Dwarf8 and Dwarf9. Planta 193, 341–348. [Google Scholar]
- Wu Q, Bai X, Zhao W, et al. 2019. Investigation into the underlying regulatory mechanisms shaping inflorescence architecture in Chenopodium quinoa. BMC Genomics 20, 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Liu C, Hou X, Yu H. 2010. MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis. The Plant Cell 22, 1733–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Li N, Dumenil J, Li J, Kamenski A, Bevan MW, Gao F, Li Y. 2013. The ubiquitin receptor DA1 interacts with the E3 ubiquitin ligase DA2 to regulate seed and organ size in Arabidopsis. The Plant Cell 25, 3347–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe S, Iwata H. 2020. Genomics-assisted breeding in minor and pseudo-cereals. Breeding Science 70, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM. 1990. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346, 35–39. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ohama N, Nakajima J, et al. 2011. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Molecular Genetics and Genomics 286, 321–332. [DOI] [PubMed] [Google Scholar]
- Zafar SA, Zaidi SS, Gaba Y, Singla-Pareek SL, Dhankher OP, Li X, Mansoor S, Pareek A. 2020. Engineering abiotic stress tolerance via CRISPR/Cas-mediated genome editing. Journal of Experimental Botany 71, 470–479. [DOI] [PubMed] [Google Scholar]
- Zandalinas SI, Mittler R, Balfagón D, Arbona V, Gómez-Cadenas A. 2018. Plant adaptations to the combination of drought and high temperatures. Physiologia Plantarum 162, 2–12. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang J, Huang J, et al. 2012. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proceedings of the National Academy of Sciences, USA 109, 21534–21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li D, Zhang D, et al. 2018. Analysis of the functions of TaGW2 homoeologs in wheat grain weight and protein content traits. The Plant Journal 94, 857–866. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pribil M, Palmgren M, Gao C. 2020. A CRISPR way for accelerating improvement of food crops. Nature Food 1, 200–205. [Google Scholar]
- Zhang Y, Yu C, Lin J, Liu J, Liu B, Wang J, Huang A, Li H, Zhao T. 2017. OsMPH1 regulates plant height and improves grain yield in rice. PLoS One 12, e0180825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hua L, Gupta A, Tricoli D, Edwards KJ, Yang B, Li W. 2019. Development of an Agrobacterium-delivered CRISPR/Cas9 system for wheat genome editing. Plant Biotechnology Journal 17, 1623–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lu D, Li C, et al. 2012. Genetic control of seed shattering in rice by the APETALA2 transcription factor shattering abortion1. The Plant Cell 24, 1034–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C, Chen A, Xiao L, et al. 2017. A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Research 27, 1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsögön A, Čermák T, Naves ER, Notini MM, Edel KH, Weinl S, Freschi L, Voytas DF, Kudla J, Peres LEP. 2018. De novo domestication of wild tomato using genome editing. Nature Biotechnology 36, 1211–1216. [DOI] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Frugis G, Chua NH. 2002. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. The Plant Journal 30, 349–359. [DOI] [PubMed] [Google Scholar]
- Zurita-Silva A, Fuentes F, Zamora P, Jacobsen SE, Schwember AR. 2014. Breeding quinoa (Chenopodium quinoa Willd.): potential and perspectives. Molecular Breeding 34, 13–30. [Google Scholar]