Dear Editor,
The COVID-19 pandemic has brought numerous challenges to the provision of care for patients with inflammatory bowel disease (IBD) including limited access to healthcare systems and drugs, concerns about hospital visits and treatments and concerns about safety of IBD therapies [1], [2], [3]. Adherence to therapies and follow up assessments are key to maintain sustained remission in IBD. We performed a systematic review to identify the frequency and reasons for the disruption of care for IBD patients and attempted to identify regional differences in patterns of adherence to IBD therapies.
We searched the databases using PubMed and Embase on 6 August 2020 using the keywords inflammatory bowel disease, ulcerative colitis, Crohn disease, Crohn's disease combined using the operator ‘AND’ to combine with Coronavirus, COVID-19, SARS-COV-2, nCOV, coronaviridae infection or coronavirus disease 2019. After removal of duplicates, the eligible titles and abstracts were screened to identify studies reporting failure to adhere to IBD therapies during the COVID-19 pandemic irrespective of publication type. Studies which reported the discontinuation or delay of IBD therapy due to proven COVID-19 infection were excluded. Studies were also excluded if they did not provide complete/relevant data required or if they reported single cases. Failure to adhere to IBD medications was defined as either discontinuation or delay in administration of medications. The reasons for the failure to adhere were identified where reported in the studies. Relevant data were extracted from the included studies for overall IBD medications as well as biologics. The guidelines adapted by different societies on the use of immunosuppressive agents and biologics for IBD during the COVID-19 pandemic had variations, from complete stoppage of biologics in China to continuation of all biologics in Europe. Hence the data was stratified according to the continent from where the study is reported and analysed to identify differences in the failure to adhere to IBD therapies.
We calculated the pooled rates of failure to adhere to biologics and overall IBD therapy for all the studies together as also the rates separately for each continent. The statistical analysis was performed using R version 4.0.1 and meta package. The data were extracted as frequencies and pooled prevalence was computed by random effect model with inverse variance approach. The prevalence was logit transformed for computing the summary. Continuity correction of 0.5 was applied for cells with zero frequencies and events were computed per 100 observations. Heterogeneity was estimated by I2 and p value of heterogeneity. The risk of bias analysis was done using Joanna Briggs appraisal checklist for prevalence studies which looks at the appropriateness of study sample and selection, description of setting and subjects, completeness of provided data and analysis, and the appropriateness of measuring the condition.
We identified 438 titles of which 139 duplicate articles were excluded. After a full text assessment of 38 papers (Fig. 1 , PRISMA flowchart), data from 16 studies was used for analysis. Supplementary Table 1 provides the details of the studies included in the analysis and also lists the reasons and definitions of failure to adhere to IBD therapies. Supplementary Table 2 provides the reasons of exclusion for the studies excluded from the meta-analysis. Supplementary Table 3 provides the results of the risk of bias analysis.
Fig. 1.
PRISMA flowchart showing study selection.
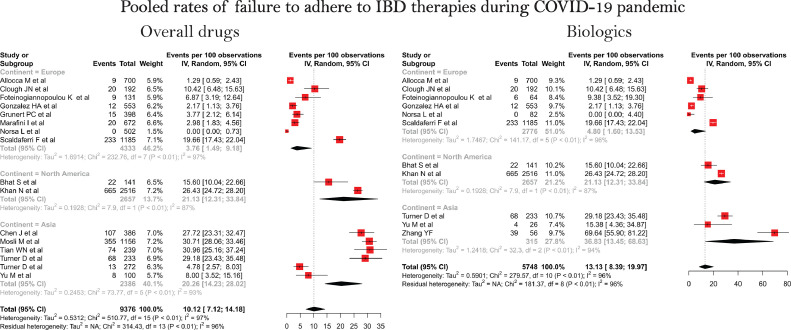
Sixteen studies provided the data of failure to adhere to overall IBD medications while eleven studies provided data vis-à-vis the use of biologics during the COVID-19 pandemic. Of the 16 studies included in the analysis eight were from Europe, six from Asia and two from North America. The pooled rate of failure to adhere to overall IBD medications was 10.12 (CI, 7.12–14.18, I2 = 97%) per 100 patients (Fig. 2 ). The corresponding pooled rates of failure to adhere to overall IBD medication were 3.76 (1.49–9.18, I2 = 97%) for European studies, 20.26 (14.23–28.02, I2 = 93%) for Asia and 21.13 (12.31–33.84, I2 = 87%) for North America suggesting geographic variation in the failure to adhere. The overall pooled rate of failure to adhere for biologics was 13.13 (8.39–19.97, I2 = 96%). Analysis based on the geography showed highest adherence in Europe (4.80, 1.60–13.53, I2 = 96%), intermediate in USA (21.13, 12.31–33.84, I2 = 87%) and lowest in Asia (36.83, 13.45–68.63, I2 = 94%). (Fig. 2).
Fig. 2.
Pooled rates of failure to adhere to IBD therapies during COVID-19 pandemic.
Our analysis identifies significant failure to adhere for IBD therapies including biologics during the COVID pandemic. This appears to be driven by concerns regarding safety amongst both the treating clinicians and patients [4]. The concerns amongst physicians are best exemplified by the data from China where at least one of the centers completely halted administration of biologics during this period [2,5]. Failure to adhere to IBD therapies may be associated with risk of disease flares and could necessitate hospital admissions and interventions including surgical procedures [3]. While the guidance from most professional societies suggested continuation of IBD therapies (except high dose steroids), our results show significant rates of failure to adhere to IBD therapies [6,7].
The analysis demonstrates significant heterogeneity which could be related to differences in the studies (geographical area), differences in definitions of adherence (stopping versus missing therapies), differences in measuring of adherence (from missing visits, telephonic survey, self-reporting) and differences in guidance by the local societies (eg. China recommendation to biologics). However, results also demonstrate that excellent adherence is possible even in wake of ongoing disruptions due to pandemic. The reasons for failure to adhere were a combination of factors related to social restriction (lockdowns, difficulty to access healthcare or therapies), patient related factors (fear of infection, choice, parental fear in pediatric studies) and physician related factors (fear of infection or adverse effects, local guidelines) [2,5,8]. Although our analysis is limited by lack of studies from Africa and South Asia, it demonstrates that adherence to therapies will remain a concern with the surge in COVID-19 in newer regions and with second surges. Use of certain innovative measures such as home monitoring for disease activity, home delivery of drugs and shifting to subcutaneous drugs where appropriate could help improve adherence [8]. It is apparent from the early data that sustained remission, avoiding disease activity and avoiding admissions may be the best adjuncts to public health measures in keeping IBD patients safe from the impact of the pandemic. To achieve this, there is a need for clear messaging from the clinicians and professional societies regarding the safety of most therapies, especially the biologics, during this pandemic.
Author contributions
Conception: VS, SS, AKS
Literature Search: AKS, VS
Screening: AKS, AJ,
Data Extraction: AKS, AJ, VS
RoB: AKS, AJ
Data Analysis: PKM
Initial Draft: AKS, PKM, AJ, VS
Manuscript revision for important intellectual content: VS and SS
Final Approval: All authors
Declaration of Competing Interest
None of the authors have any conflict of interest
Footnotes
Funding: No funding
Ethics: Since this work is a systematic review of already published data no ethical approval needed
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dld.2020.09.012.
Appendix. Supplementary materials
References
- 1.Danese S., Cecconi M., Spinelli A. Management of IBD during the COVID-19 outbreak: resetting clinical priorities. Nat Rev Gastroenterol Hepatol. 2020;17(5):253–255. doi: 10.1038/s41575-020-0294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian W.N., Huang Y.H., Dai C. The effect of the COVID-19 pandemic on the medical mode of patients with inflammatory bowel disease in China. Inflamm Bowel Dis. 2020 Jul 22 doi: 10.1093/ibd/izaa197. izaa197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y.F., Qiu Y., He J.S., Tan J.Y., Li X.Z., Zhu L.R., Chen Y., Liu Z.J., Iacucci M., Chen B.L., He Y., Ben-Horin S., Shen B., Zeng Z.R., Ghosh S., Chen M.H., Mao R. Impact of COVID-19 outbreak on the care of patients with inflammatory bowel disease: a comparison before and after the outbreak in South China. J Gastroenterol Hepatol. 2020 Aug 1 doi: 10.1111/jgh.15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Occhipinti V., Pastorelli L. Challenges in the care of IBD patients during the COVID-19 pandemic: report from a "Red Zone" area in Northern Italy. Inflamm Bowel Dis. 2020;26(6):793–796. doi: 10.1093/ibd/izaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An P., Ji M., Ren H. Prevention of COVID-19 in patients with inflammatory bowel disease in Wuhan, China. Lancet Gastroenterol Hepatol. 2020;5(6):525–527. doi: 10.1016/S2468-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin D.T., Feuerstein J.D., Wang A.Y., Cohen R.D. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology. 2020;159(1):350–357. doi: 10.1053/j.gastro.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin D.T., Abreu M.T., Rai V., Siegel C.A. International organization for the study of inflammatory bowel disease. Management of patients with crohn's disease and ulcerative colitis during the coronavirus disease-2019 pandemic: results of an international meeting. Gastroenterology. 2020;159(1):6–13. doi: 10.1053/j.gastro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marafini I., Salvatori S., Sena G., Calabrese E., Biancone L., Monteleone G. Low frequency of COVID-19 in inflammatory bowel diseases [published online ahead of print, 2020 Jun 13] Dig Liver Dis. 2020 doi: 10.1016/j.dld.2020.06.007. S1590-8658(20)30266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.