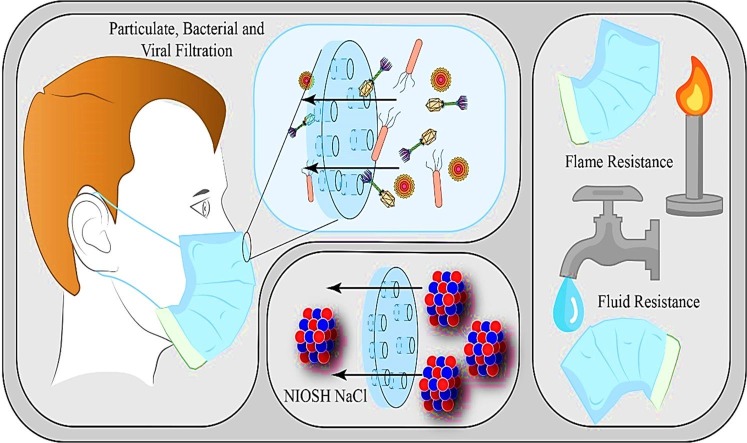
Graphical abstract
Keywords: Facemasks, Respirators, SARS-cov-2, Corona Virus Legislations, Testing methods, Viral Filtration Efficiency (VFE), Bacterial Filtration Efficiency (BFE), Particulate filtration efficiency (PFE), NIOSH NaCl method
Abstract
The COVID-19 outbreak has resulted in a shortage of personal protective equipment (PPE) throughout the world. This shortage has resulted in an increase in production of PPE to meet the demand, and as a result, several substandard equipment has entered the market. With face masks and respirators now beginning to see widespread use throughout the world, the standards and test with which they are required to undertake have become points of interest. The filtration efficiency of the masks is a key testing element that examines its ability to filter particles, bacteria and viruses; this examines the penetration efficiency percentage of each with lower results being preferable. Masks are also subjected to NaCl testing method, which allows a range of particle sizes to be examined and their penetration to be observed. The masks must also show considerable resistance to fluids and flames, to prevent the penetration of liquids and to be non-flammable. Various PPE testing protocols such as biological, chemical, fluid and flame resistances, protective ensemble, facepiece fit testing, NIOSH NaCl method and impact protection have been discussed. In addition, various tests involving bacterial and viral filtration efficiencies are also discussed. Differential pressure is examined to ascertain the comfort, airflow and breathability of the masks, whilst fit testing is examined to ensure a correct fit of the mask.
1. Introduction
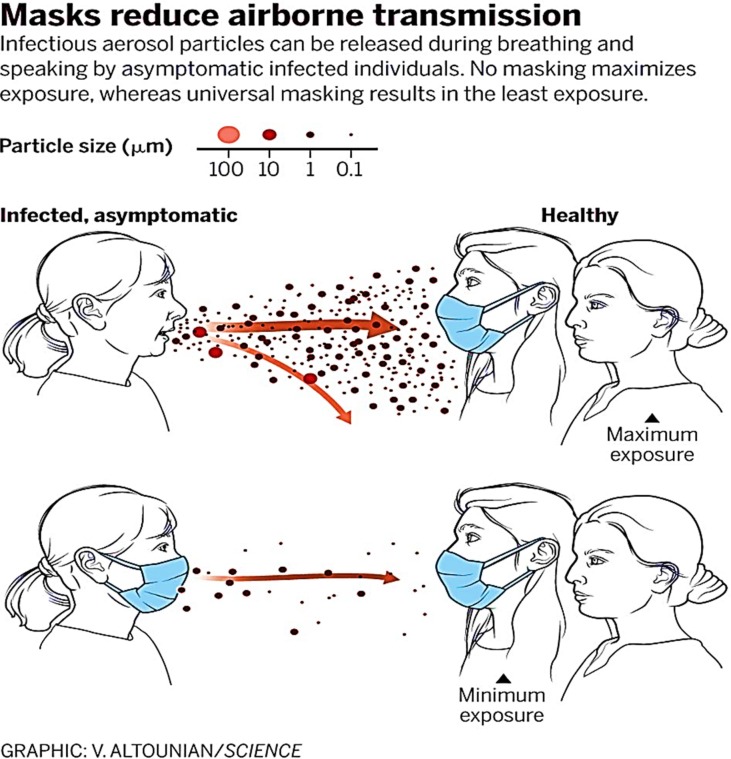
The Covid-19 pandemic has gripped the world’s attention for the year 2020. The SARS-cov-2 virus that is believed to have originated in Wuhan, China in December of 2019 (Zheng et al., 2020) has spread over 200 countries affecting over 7 million people and killing 400 thousand people as of the 8th of June 2020 (Coronavirus Update (Live), 2020). On the 30th of January, the World Health Organization (WHO) declared a global health emergency (Velavan and Meyer, 2020), and on the 19th of March, they advised the use of face masks or mouth coverings to prevent the spread of the disease (Infection Prevention and Control, 2020). Droplets exhaled from infected individuals’ respiratory tracts via coughing, breathing, sneezing and speaking are seen to be the main form of transmission (Fig. 1 ) (Chatterjee et al., 2020). When the droplets are emitted, they transfer the virus by three contact methods (WHO, 2020). Droplets that are inhaled directly are referred to as airborne, droplets that land on an individual’s hand or body and are then transferred to the face are contact and droplets that land on a surface and are then transferred are referred to as fomites. The use of face masks and respirators have now become recommend in several European countries such as Ireland (Gov.ie) but are not enacted into law. However, other countries such as Germany have made them compulsory on public transport and in shops (BBC) while Spain has made them compulsory outside as well (BBC World, 2020).
Fig. 1.
Mask prevention of transmission of the airborne virus (Chatterjee et al., 2020).
The pandemic has resulted in an increase in demand for PPE all over the world with six times the amount of surgical mask and three times that of respirators being required (Vijayakumar, 2020). This rise in demand has also seen cases of unsuitable PPE being sold that are unfit for use as they do not meet standards or legislation (rte news, 2020, Rawlinson, 2020). Strict testing and requirements have been set in place previously to ensure that these face masks and respirators meet certain standards. Filtration efficiency examines the masks ability to filter four different filtrates; particulates, bacterial, viral and NaCl (Rengasamy et al., 2017). The face masks and respirators are tested for fluid (Borkow et al., 2010) and flame (Rengasamy et al., 2018) resistance to ensure the materials are not susceptible to penetration, but liquids and can withstand acceptable heath levels. The fit of the face masks and respirators is also tested to ensure a suitable seal is obtained and that they are comfortable (OSHA).
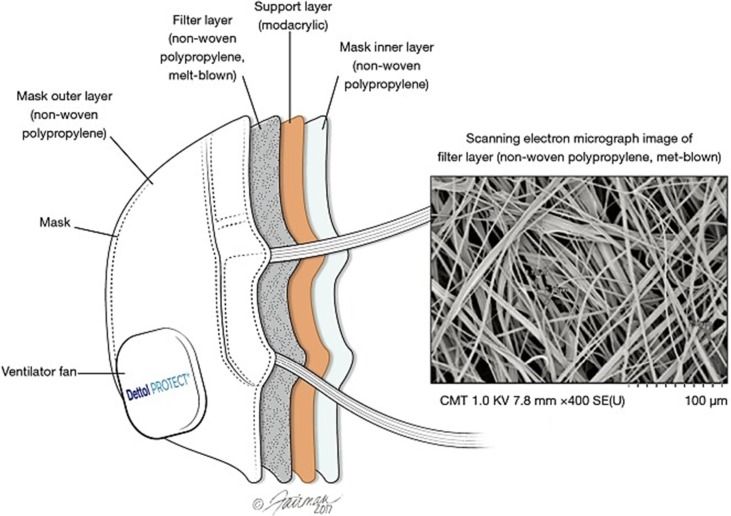
Nonwoven materials are the main material used in the filtration of aerosols (Scott, 2005). These can be made from man-made or natural fibres (paper not included) whereby a web or a coat of the materials are secured together. Melt blown fibre are produced by a hot polymer being blown out of a thin tube, these being deposited onto a collector to cool and form a web of material. N95 masks produced by 3 M are made up of multiple materials. Straps are composed of thermoplastic elastomer; nose clips are made from aluminium, nose foam form polyurethane, the filter from polypropylene and the shell and cover web are made from polyester (Solutions, 2020) N95 masks consist of multiple layers; the layers that are accountable for filtering efficiency are “polypropylene layers with an embedded electrostatic charge” (Juang and Tsai, 2020) (Fig. 2 ) (Steve Zhou et al., 2018). Surgical mask is comprised of three layers; the layers are made from non-woven fabric that have a material that is melt-blown between them (CEBM); these can be made from multiple materials (Table 1 ) (The Relationship of Fabric Properties and Bacterial Filtration).
Fig. 2.
N95 mask layers (Steve Zhou et al., 2018).
Table 1.
Different mask types and materials used in construction (Hall, 2014).
| Mask type | Materials |
|---|---|
| Tie-on Surgical Face Mask | 3-ply, pleated rayon outer web with polypropylene inner web |
| Classical surgical Mask, Blue | 3-ply, pleated cellulose polypropylene, polyester |
| Sofloop Extra Protection Mask | 3-ply, pleated blended cellulosic fibers with polypropylene and polyester, ethylene methyl acrylate strip |
| Aseptex Fluid Resistant | Molded rayon and polypropylene blend with acrylic binder |
| Surgine II Cone Mask | Molded polypropylene and polyester with cellulose fibers |
| Surgical Grade Con Style Mask | Molded polypropylene |
Disposable masks can be made from a variety of polymers such as polyethylene, polycarbonate and polyester (Fadare and Okoffo, 2020). These have three different layers with the outer layer being a waterproof nonwoven fibre, a melt-blown middle layer that filters most of the particulates and a soft fibre inner layer. Mask using filters are “fibrous”; they use layers of unwoven fibres to trap particulates. The size of the particulate they can trap depends on the thickness of the fibres, the openings left between the fibres and how many layers are present (McDiarmid et al., 2020).
2. Personal protective equipment (PPE) testing methods
Different PPE tests are utilized to guarantee the security and the safety of the PPE manufacturers in possibly hazardous workplace environments. The variety and the necessity of PPE testing are depending on the probable health risk to the user and the equipment’s intended use. Among all the examples of the PPE, wearing the face masks and respirators in public areas and hazardous workplaces are considered as an effective solution, which can hinder the dissemination of an infectious disease by avoiding the infectious droplets exhalation and spreading as well as the inhalation of their subsequent. In the event of the recent worldwide spread of COVID-19, which is affecting the respiratory system, the request for face masks and respirators is significantly increased by the public (https://www.tuvsud.com/en, xxxx, Konda et al., 2020).
Some general categories of PPE testing and their examples (along with their corresponding American National Standard, ANSI, (ANSI-American National Standards Institute) are shown in Table 2 . (https://www.tuvsud.com/en) The procedure/surgical masks can provide certified respiratory protection if they are tested, designed, and government- certified as a respirator (3M Personal Safety Division, 2020). The PPE tests, which are used in the United States, should comply with the US occupational safety and health administration (OSHA), as well as the requirements of product-specific American National Standards. For those, which are used or sold in Europe, it is necessary to meet Europe’s PPE Directive and the EU’s forthcoming PPE Regulation (https://www.tuvsud.com/en).
Table 2.
Specific types of PPE testing.
| PPE test methods (Personal Protective Equipment (PPE) Testing) | Applications (Personal Protective Equipment (PPE) Testing) |
|---|---|
| Biological resistance testing | Including antimicrobial and antibacterial testing, as well as penetration testing |
| Chemical resistance testing | Including chemical penetration and permeation testing (ANSI 103-2010 Standard) (S. Type) |
| Protective ensemble testing | For example, testing for vapour, chemical, or liquid splash resistance of clothing ensembles, and full-body protection systems (ASTM F2704) (S. Type) |
| Impact protection testing | In the case of sports helmets and occupational headwear and body protection equipment. (ANSI/ISEA 107-2004) |
| Protective hand and footwear testing | Such as gloves (ANSI/ISEA 105 (U.S. Standard)), and shoes (ANSI Z41.1) used in dangerous workplaces |
| Fall arrest equipment testing | For positioning belts and body harnesses used in construction and maintenance, as fall protection devices. (ANSI/ISEA Z359.1) |
Generally, If the user expects the performance of both a particulate respirator and a mask, they should select the PPE type which is tested and certified as a particulate respirator by the national institute for occupational safety and health (NIOSH) (CDC), and produced according to the food and drug administration (FDA) for the procedure/surgical mask (3M Personal Safety Division, 2020).
3. Common tests for respirators and surgical masks
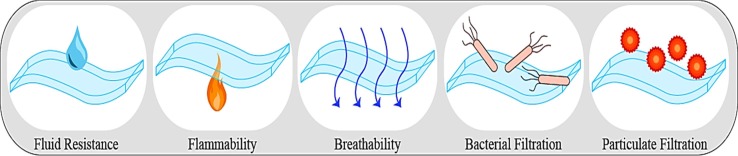
The ASTM standards are issued by the food and drug administration FDA, as the authorized standard in the US. There are several basic standard ASTM F2100-11 (2011), which specifies the performance necessities for the respirators and the face masks (3 Tips for Choosing the Right Face Mask). The ASTM F2100-11 specification explains the required properties and the testing protocols for the utilized material in the face masks production, which are used in the hospital, health care, and patient care. The face masks are classified based on their performance according to various testing such as flammability, fluid resistance, breathability, bacterial filtration efficiency etc. (Using and Spheres, 2005); which are illustrated in Fig. 3 . In the following sections, various testing methods are comprehensively explained.
Fig. 3.
Illustration of the common test to estimate the performance of face masks.
3.1. Filtration efficiency (FE)
There are different methods to measure the filtration efficiency in the 42 CFR Part 84 certification protocol, such as the particulate filtration efficiency (PFE), bacterial filtration efficiency (BFE), viral filtration efficiency (VFE), and NIOSH (Rengasamy et al., 2017, CDC, xxxx).
The PFE and BFE methods are associated with material efficiency, which is used as the barrier to protect the wearer against the aqueous viral aerosols. The filtration efficiency assessment is conducted according to the ASTM F2100-19E1 protocol utilizing the salt aerosol with a size of 100-nm (Using and Spheres, 2005).
In general, the filtration efficiency of the masks and respirators measure by Eq. (1), in which the Cu and Cd are the averages of particle concentrations per each upstream and the downstream test specimen. (Konda et al., 2020)
| (1) |
3.1.1. Particulate filtration efficiency (PFE)
The PFE method is led according to the American society of testing and materials (ASTM) F2299 protocol, and indicates the quality of the procedure/surgical masks; however, it cannot be considered as the indicator of respiratory protection performance (Velavan and Meyer, 2020). In other words, the PFE test procedures measure the quality of the masks for filtering the particles with different sizes (3M Personal Safety Division, 2020). According to the FDA guidance document, the PFE test can be conducted utilizing the 0.1-µm Polystyrene Latex particles. The use of latex spheres offers a precise test for estimating a submicron efficiency performance (C. for D. and R. Health, 2004). The Polystyrene Latex particles have been suspended in water, and the aerosols were created by means of a particle generator, which is adjustable and can provide the favorable of particles concentration. The particles can be counted utilizing a particle counter downstream. The concentration of the aerosol can be adjusted (from 10,000 to 15,000 particles) by passing through the drying chamber by means of HEPA filtered air, and after passing through the convex side of the test sample, it has been placed into a filter holder. According to the FDA protocols, the used particles are not charge neutralized (C. for D. and R. Health, 2004).
Besides, according to the ASTM 2299 protocol, the PFE testing has been carried out with a velocity between 1 cm/s to 25 cm/s utilizing the entire N95 FFRs, 90 cm2 surgical mask material, and surgical N95 FFRs. Furthermore, before PFE testing, the samples have been preconditioned at a relative humidity in the range of 30–50% at 21 ± 3 °C (ASTM International, 2010). The particulate filtering efficiency follows Eq. (2):
| (2) |
where Cu and Cd are the averages of upstream and the downstream counts, the PFE results are between 1 and 99.99%. The higher the percentage represents better mask filtration. For the PFE test, the used particle size can be in the size of 0.1 to 5.0 µm range. The size of the used particle (e.g. 0.6–1.0 µ) must be considered while the test results are being compared since the use of the particle with a larger size cause a misleading PFE assessment (What is the purpose of a medical-surgical face mask?). The minimum value of filtering efficiency for mask using non-oily (NaCl) particles is 30% (Standard and Yy, 2013).
3.1.2. Bacterial filtration efficiency (BFE)
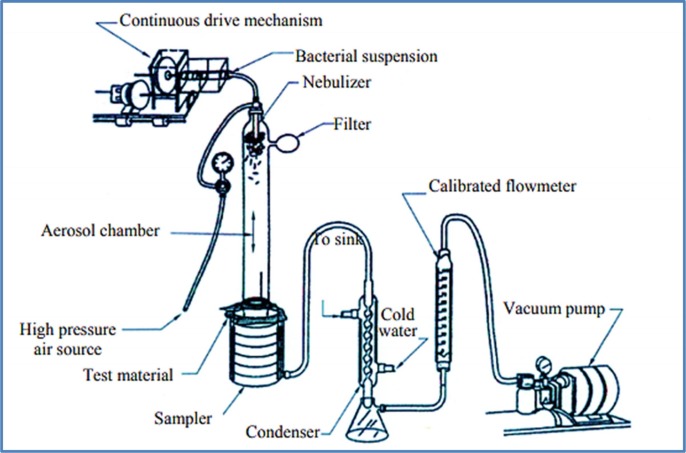
The BFE method is conducted according to the ASTM F2101 protocol that requires aqueous bacterial aerosol droplets (with the diameter size of 3 µm) (ASTM F2101 - 01), and measures the capability of the masks to stop the large particles from coughing, speech, and sneezing, with the size distribution from 0.6 µm up to thousands of microns expelling by the mask’s wearer (Anfinrud et al., 2020, Han et al., 2013, Yang et al., 2007). This method cannot be used to assess the filtration efficiency of the respirators, and it is not suitable to estimate the ability of the masks to protect the wearer from the external contaminants (https://www.clkmedicalsupply.com/masks-standard/, 2020, 3M Personal Safety Division, 2020). According to the ASTM F2101, the mask material sample is clamped between a six-stage cascade impactor and an aerosol chamber. The staphylococcus aureus aerosol is introduced into the chamber and drawn through the mask material utilizing a vacuum, which is attached to the cascade impactor (ASTM F2101 - 01). Before fixing the test sample, the air flow rate is adjusted at 28 L/min. The bacteria suspension is conducted to the nebulizer for 1 min, and the air pressure and cascade impactor run through the sampler for 2 min. The concentration of the bacteria suspension needs to be controlled; however, it can be maintained at (2200 ± 500) CFU per test, to avoid any adjustment during the test (ASTM F2101 - 01, xxxx, Standard and Yy, 2013). The measured mean value of the bacteria aerosol diameter should be in the range of 3.0 ± 0.3 μm, and the geometric standard deviation should not exceed the value of 1.5. (Standard and Yy, 2013) Fig. 4 displays the instrument of BFE test (ASTM F2101 - 01).
Fig. 4.
Graphical representation of the BFE test instrument (ASTM F2101 - 01).
The bacterial filtering efficiency of masks is given by Eq. (3):
| (3) |
where presents the average colony-forming units (the bacteria-containing aerosol) without the test filter, and is the average of colony-forming units with the test filters. The number of the particle units per each test is specified through the ASTM F2101 protocol. The BFE value varies between 1 and 99.9% depending upon the calculation’s methods and the test parameters (3M Personal Safety Division, 2020).
Furthermore, for a device, it is named a surgical or medical mask, if the minimum value of BFE rate is 95%. Also, the high protection and medium protection masks shall provide a minimum BFE rate of 98% and more than 99% (https://www.clkmedicalsupply.com/masks-standard).
3.1.3. Viral filtration efficiency (VFE)
The virions, as one of the smallest bioaerosol particles with a size diameter of 20–300 nm, can simply enter through the respiratory organ and result in various epidemic infections. The masks and respirators as protection equipment shall be tested in terms of virus filtration ability (Bałazy et al., 2006). The viral filtration efficiency (VFE) is one of the efficient methods, which has not been documented as a standard test protocol but has been modified by Nelson Laboratories (Medical Viral Penetration Test) according to the ASTM F2101 protocol. The resistance of the PPE equipment against the virus’s penetration can be determined through the viral penetration testing, which is a kind of pass and fail test, and shall be conducted based on the ASTM F1671 (ASTM International), AAMI PB70, which was confirmed by the FDA in 2004. The ANSI/AAMI PB70 and ASTM F1671 standard provide a classification protocol for PPEs such as isolation gowns, and surgical gowns utilize used in the health services, depending upon their liquid resistance ability This standard also specifies the test procedures for estimating the compliance of the PPEs with liquid barrier claims or liquid-borne microbial barrier claims (T.N.P.P.T. Laboratory, 2014, Li et al., 2019).
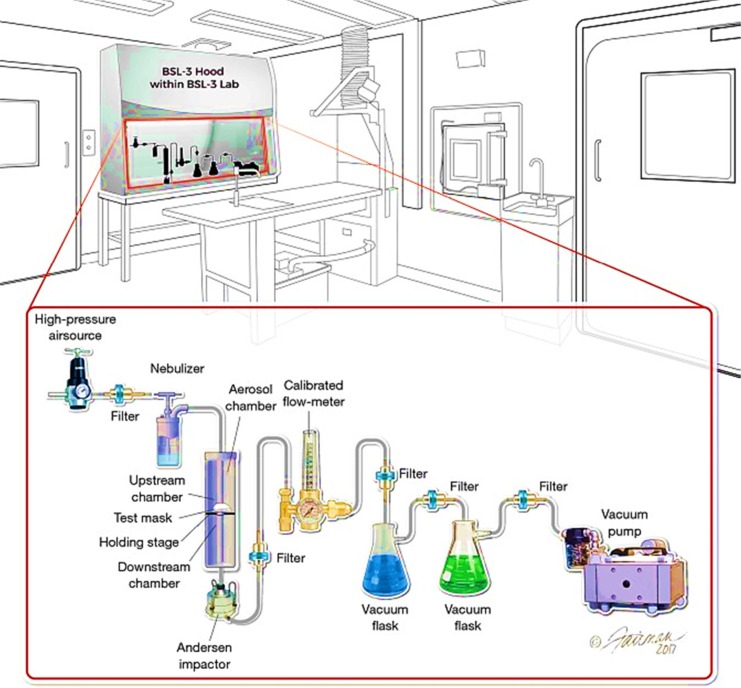
In addition to the VFE test requirement for the PPE manufacturers, this test has also been applied by the textile producers to guarantee the quality of the used materials for PPEs (Medical Viral Penetration Test). The virus filtration efficiency test can be performed through the aerosol filtration test device, which is shown in Fig. 5 (Steve Zhou et al., 2018). The test set up is assembled at Microbac per modified ASTM F2101-14 (ASTM F2101-14, 2014) and has been utilized to test the filtering ability of various masks against the penetration of influenza and rhinovirus so far. The samples are located between two chambers (upstream and downstream) (Steve Zhou et al., 2018).
Fig. 5.
The schematic illustration of the viral filtration test set up (Steve Zhou et al., 2018).
During the viral penetration test, the concentration of the segments should be considered both inside and outside of the samples (such as face respirators or the masks). The penetration value is presented as the fraction of the particles in a certain diameter, which can penetrate and pass from the barrier (Bałazy et al., 2006). The filtration ability of personal protective devices can be described through different calculation terms such as in Eqs. (1), (2), (3), which has been shown in the previous sections, or it can be defined by the term “assigned protection factor (APF)” (Current understanding on the Effectiveness) which is given by Eq. (4) as follows:
| (4) |
where the Cu and Cd follows a similar definition as previous equations, the APF value describes the capability of the masks and the respirators to decrease exposure determined under the conditions, which can replicate the workplace conditions most (Health and Safety Authority, 2010). Eq. (5) shows the relationship between the filtration efficiency percentage and the APF as follows:
| (5) |
The face masks and respirator performance can also be determined through Eq. (6), which defines the penetration efficiency (PE) of the devices as follows:
| (6) |
Furthermore, the penetration of a PPE device can be calculated through Eq. (7) as follows:
| (7) |
In which, C1, C2, present the aerosol concentration in front of the filter, behind the filter, respectively, and C0 stands for the aerosol concentration photometer reading for clean air (British Standard, 2008).
MacIntyre et al. (2015) studied the viral infections among the workers in hazardous places such as in a hospital. They summarized the efficiency of different masks (such as cloth masks and medical masks, etc.), which indicated that the medical masks are more efficient than the cloth masks since the penetration efficiency for cloth masks (97%) was much higher than that of medical masks (44%).
The particle concentrations and the size distributions of the tested samples can be estimated by exploiting the wide-range particle spectrometer (WPS), which is the combination of three various instruments, called the differential mobility diameter (DMA), the laser particle spectrometer (LPS), and the condensation particle counter (CPC). The presence of DMA and CPC provides the ability to count the particles with a diameter of 10–500 nm, while the LPS allows the particles of 350–10,000 nm. The DMA technique is the most effective method for assessing the aerosol particle size distribution with the size in the nanometer range, such as MS2 virions (Bałazy et al., 2006), SARS-CoV-2 (Santarpia et al., 2020), COVID-19 (Huang et al., 2020). Besides, based on the WPS manufacturer, fractional concentration of the particles can be calculated between 1 particle/cm3 to 10,000 particles/cm3 (T.W.P. Spectrometer).
3.1.4. NIOSH NaCl method
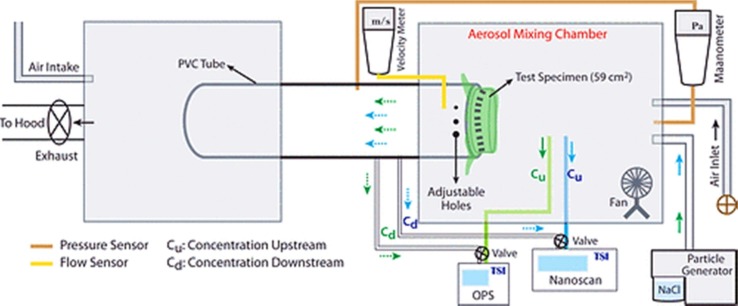
The face masks which are intended as respirator precertification might need valve leak DOP, NaCl, and inhalation/exhalation tests to fulfil the NIOSH standards (Respirator Precertification). The sodium chloride (NaCl) aerosol-based method is one of the most common testing methods for the face respirators meeting the requirements of the NIOSH protocol (42 FR Part 84 Respiratory Protective Devices, 2020, Rengasamy et al., 2018). There are various conditions and requirements for NaCl aerosol-based test set up, including a) Nontoxic polydisperse, uncharged aerosol particles (NaCl) which are used as the particle generator and provide the particles range sizes between 10 nm and 10 μm,(the average diameter of 300 nm) (Bałazy et al., 2006). b) The generation, mixing, and collection chambers, where the introduced aerosol mixed and passed through the mixing chamber in contact with the test specimen, and along with an air inlet, c) an airflow rate about 28 lpm (litres per minute), which is held on a tube linking the two chambers (Konda et al., 2020, 3M Personal Safety Division, 2020). The illustration of the experimental setup is illustrated in Fig. 6 . Where, the Cu value, and the Cd term, present aerosol, which is sampled before and after passing (upstream and downstream) through the specimen. (the), respectively (Konda et al., 2020).
Fig. 6.
Schematic illustration of filtering set up (Konda et al., 2020).
For instance, P2 or N95 face pieces, as one of the most common respirators can be certified through this method according to the NIOSH 42 CFR 84 protocol (CDC). The particulate respirators such as N95 protect the wearer only against the particles (not vapours or gases). Therefore, the airborne biological agents (e.g. bacteria or viruses) as particles, can be filtered using the disposable N95 face masks. Generally, the “p” term in the classification of the disposable high particulate is attributed to the size of the particle, which the face mask is intended to protect the wearer against that. The classified particulate filters are labelled as P1 (low-efficiency filters), P2 (medium efficiency filters) or P3 (high-efficiency filters) (Infection Prevention and Control Application, 2020). N95 respirators/masks and P2 respirators/masks are the same, and they are being applied to the similar conditions, however, there are slight differences in their testing and the certification performance between USA and Australia, which are shown in Table 3 (Infection Prevention and Control Application, 2020).
Table 3.
Difference between N95/P2 mask testing. (Produced by © Clinical Excellence Commission (CEC).)
| P2 Masks (Australian & New Zealand Standard) | N95 Masks (USA NIOSH Standard) | |
|---|---|---|
| Filter efficiency | At least 94% | At least 95% |
| Testing substance | Sodium Chloride Aerosol | Sodium Chloride Aerosol |
| Aerosol flow rate | 95 litres per minute | 85 litres per minute |
| Aerosol particle size | 0.3–0.6 µm | 0.3 µm |
The surgical masks cannot be certified through the NIOSH protocol. It has been reported that the penetration of the aerosol particles by N95 filtering devices cannot be greater than 5%, therefore according to Eqs. (6), (7), FE(%) shall be at least 95% (Bałazy et al., 2006).
Rengasamy et al. (2017) have compared the filtration efficiency of three models of surgical N95 FFRs, three models of SMs, and six models of NIOSH-approved non-FDA cleared N95 FFRs. They obtained lower efficiency through the NIOSH method, compared to the VFE, PFE, and BFE methods, which implicates that the VFE, PFE, and BFE, are not precise, documented testing protocols, and well-defined in comparison with the NIOSH NaCl method as a conservative test (https://www.clkmedicalsupply.com/masks-standard) (Table 4 ) (Rengasamy et al., 2017).
Table 4.
Filtration efficiencies for different models of face masks through the NIOSH NaCl, PFE, BFE, and VFE techniques (Rengasamy et al., 2017).
| Efficiency (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NIOSH NaCl |
PFE |
BFE |
VFE |
|||||||
| Type | Model | Sample Size | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| N95 FFR | A | 5 | 98.87 | 0.20 | 99.88 | 0.16 | 99.90 | 0.00 | 99.90 | 0.00 |
| N95 FFR | B | 5 | 99.66 | 0.03 | 99.99 | 0.02 | 99.90 | 0.00 | 99.90 | 0.00 |
| N95 FFR | C | 5 | 98.15 | 0.21 | 99.74 | 0.11 | 99.62 | 0.24 | 99.80 | 0.12 |
| N95 FFR | D | 5 | 99.32 | 0.13 | 99.93 | 0.07 | 99.90 | 0.00 | 99.90 | 0.00 |
| N95 FFR | E | 5 | 99.31 | 0.18 | 99.94 | 0.05 | 99.90 | 0.00 | 99.90 | 0.00 |
| N95 FFR | F | 5 | 99.33 | 0.07 | 99.86 | 0.29 | 99.90 | 0.00 | 99.90 | 0.00 |
| Surgical N95 | G | 5 | 98.93 | 0.20 | 99.97 | 0.02 | 99.80 | 0.12 | 99.88 | 0.04 |
| Surgical N95 | H | 5 | 99.68 | 0.24 | 99.98 | 0.03 | 99.86 | 0.09 | 99.88 | 0.04 |
| Surgical N95 | I | 5 | 98.27 | 0.37 | 99.84 | 0.05 | 99.90 | 0.00 | 99.90 | 0.00 |
| SM | Ja | 5 | 54.72 | 1.88 | 98.26 | 0.09 | 98.12 | 0.31 | 97.12 | 0.34 |
| SM | Ka | 5 | 88.40 | 1.48 | – | – | 99.80 | 0.10 | 99.88 | 0.04 |
| SM | La | 5 | 63.12 | 0.91 | 98.66 | 0.02 | 97.48 | 0.63 | 97.72 | 0.36 |
Significantly (p = <0.05) different from N95 FFRs and surgical N95 FFRs when tested by the NIOSH NaCl method. (SM: Surgical mask).
3.2. Fluid resistance
This test assesses the ability of the masks and respirators to lessen the squirted synthetic blood or any splashed or sprayed fluid that can penetrate the outer layer of the mask and transfer through the inner part by changing the pressure. The fluid resistance of the surgical masks and the surgical N95 is regulated according to the ASTM test technique F1862, “resistance to penetration by synthetic blood”, (ASTM F1862/F1862M), which is also utilized to measure the respirators’ fluid resistance (Borkow et al., 2010). According to the ASTM F 1862 protocol, the penetration resistance ability of the medical face mask is estimated using the high-velocity synthetic blood, which is in contact with the surface of the test sample (including a fixed volume in a specific time between 0 s and 2.5 s) (ISO, 2004). Several factors have a strong effect on the penetration and the wetting of the body fluids including, polarity, viscosity, and the surface tension, the structure, and the relative hydrophobicity or hydrophilicity of the face mask material (ISO, 2004).
The wetting characteristic of the blood can be simulated through adjusting the surface tension of synthetic blood, which should be lower than the surface tension range for body fluids, blood and also excluding saliva that is approximately between approximately 0,042 Nm−1 to 0,060 Nm−1 (Lentner, 1984). The surface tension for synthetic blood is in the range of 0,042 ± 0,002 Nm−1 (ISO, 2004). Fig. 7 displays the complete apparatus of the fluid resistance test, where the face mask sample is placed in the device by means of specimen-holding fixture and the synthetic blood is splashed on at the specific area of the face mask sample.
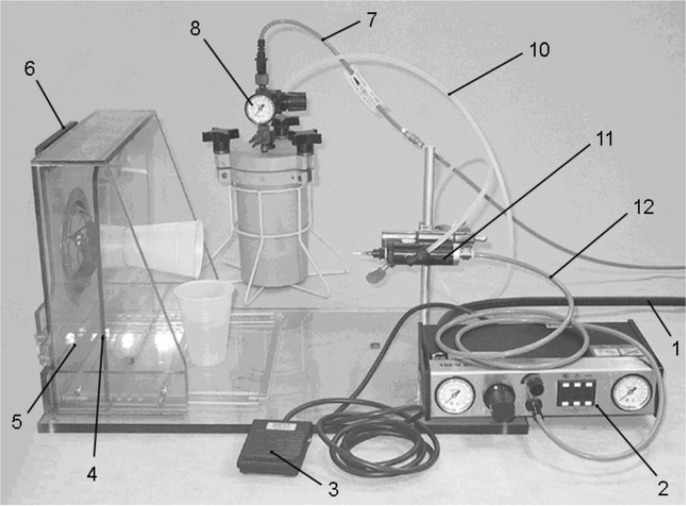
Fig. 7.
1: air line from supply to the controller, 2: EFD 1500 XL valve controller, 3: valve control switch, 4: targeting plate, 5: transparent plastic box, 6: hinged door with sample holding fixture, 7: air line from supply to the fluid reservoir, 8: fluid reservoir pressure gauge, 9: fluid reservoir (mount on bench top with base level to base of sample holding table), 10: fluid feed from the reservoir to the valve, 11: valve mounted on a ring stand mount, with canula, 12: air line from controller to valve (ISO, 2004). (Produced by https://www.iso.org/).
3.3. Flame resistance
The hospitals contain different sources of the heat, oxygen, and fuel, the ASTM F2100-11 standards requires a test regarding the flame resistance for all the medical masks. The used material for masks and respirators should not present any hazards for the users, and their flammability shall not be high. The flammability factor is determined based on the 16 CFR part 1610 for clothing textiles (U.S. CPSC, 2011). During the flammability test, the mask’s material should not be flamed or remain flamed after five seconds from burning. The flame spread test calculates the required time for the flame to reach the mask material in 5 in. distance (127 mm). “Class 1” represents the category of the material, which shows normal flame resistance, and they are suitable for the use in face masks and respirators. The tested material cannot be utilized after the flame resistance test (NEN-EN 149, 2009). Table 5 summarizes the characteristics of each class of materials that are categorized based on the 16 CFR part 1610 testing requirements (U.S. CPSC, 2011).
Table 5.
Classifications of face mask materials according to the flam resistance test (U.S. CPSC, 2011).
| Classification | Plain Surface | Raised Fiber Surface |
|---|---|---|
| Class 1 | Average burn time ≥ 3.5 s | Average burn time > 7.0 s OR Average burn time is 0–7 s with no base burns |
| Class 2 | N/A | Average burn time is 4–7 s with base burn |
| Class 3 | Average burn time < 3.5 s | Average burn time < 4.0 s with base burn |
3.4. Differential pressure (Delta-P)
The differential pressure test is an indicator of airflow resistance of the masks as well as their comfort and breathability (Lord, 1959). During this test, airflow passes through the mask in a controlled manner, and the various pressures are calculated for the inner and outer layers of the mask. The differential value is divided by the surface area (cm2) of the mask to estimate the breathability, where the higher Delta P values indicated a harder breath for the users. ΔP can be measured through Eq. (8), where PM represents the mean value of the differential pressure of the test sample, in Pa (Standard and Yy, 2013).
| (8) |
According to the ASTM F2100-11 protocol, the minimum value for Delta P should be less than 5.0 mm H2O/cm2 (or should not be greater than 49 Pa). The Delta P values less than 0.2 or more than 0.5 are not considered as standard values for the general surgical application (I. CLK Medical Supply). The standard requirements (ASTM F2100-11) for the performance of materials utilized in face masks are summarized in Table 6 (3 Tips for Choosing the Right Face Mask, xxxx).
Table 6.
The summary of ASTM F2100-11 requirements for face mask and respirators (3 Tips for Choosing the Right Face Mask, xxxx). (Produced by https://www.halyardhealth.com/).
| Test | Level 1 Barrier | Level 2 Barrier | Level 3 Barrier |
|---|---|---|---|
| ASTM F1862: (Fluid resistance) | 80 mmHg | 120 mmHg | 160 mmHg |
| MIL-M-36954 C: Delta p (Breathability) | <4 mm H2O | <5 mm H2O | <5 mm H2O |
| ASTM F2101: BFE (Filtration 3 μm) | ≥95% | ≥98% | ≥98% |
| ASTM F2299: PFE (Filtration 1 μm) | ≥95% @ 0.1 μ | ≥98% @ 0.1 μ | ≥98% @ 0.1 μ |
| 16 CFR Part 1610: (Flammability) | Class 1 | Class 1 | Class 1 |
4. Facepiece fit testing
The masks and respirator devices are worn by the wearer in high hazard environments. Thus, to increase the effectiveness of the devices, the wearers shall know how to use the devices correctly. The fit test aims to check and avoid any leakage existence before using the masks or respirators (Kiersma, 2014). The purpose of fit testing is to provide a perfect fit and appropriate seal for all tight facemasks and respirator facepieces, which can be done through two main methods. The first fit method is carried on utilizing either qualitative fit test methods or through the quantitative fit test techniques (Coffey et al., 2002), which are shown in the following section. The quantitative fit test, as astringent pass/fail test, is an effective method for full-face and half-face respirators that includes a laboratory test chamber or transferrable fit testing set up, which is utilized to measure the fit factor (FF) of the PPE (Health and Safety Authority, 2010).
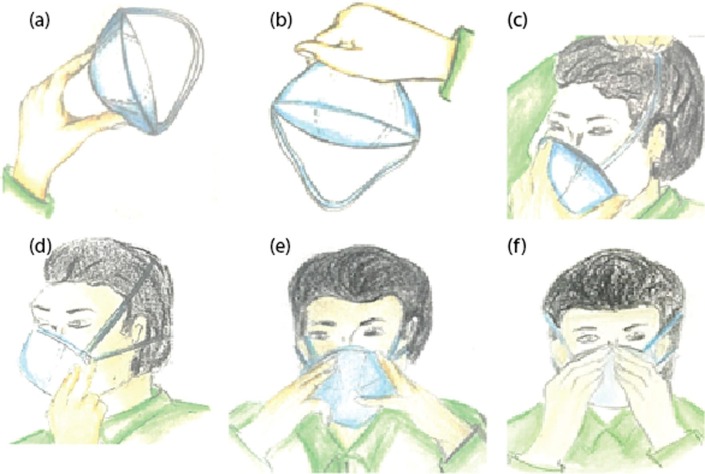
In the basis of the control of substances hazardous to health (COSHH) (Health and Safety Executive, 2016) protocols, there are different conditions in which the fit testing shall be repeated. These conditions are including a) the wearer has gained or lost weight, b) the wearer has experienced any dental work, c) the wearer has faced any imperfections such as scars, moles, etc., on his face, d) the wearer has changed the respiratory protective equipment (RPE) type, model, size, or material.(Tuberculosis) Fig. 8 illustrates the correct way to perform the fit check and to put on the FFP3 respirators through six steps.
Fig. 8.
Illustration of how to put on the well-fit of the FFP3 respirators (a-e), as well as their fit check (f).
4.1. Fit testing techniques
Facepieces fitting depends on its design features including (a) “negative pressure” or “positive pressure” method, which the respirator is functioning in, (b) The facepiece form and its ability to cover the face. In this case, for the respirators which operate in the “negative pressure” mode, the wearer should draw the air through a filter or chemical container into the facepiece, which makes a negative pressure inside the respirator compared to the pressure outside the facepiece, while the “positive pressure” respirator, pushes clean air into the facepiece by using a compressor or a fan, which produces a positive pressure inside the facepiece in comparison with the outside (Loeb, 2009).
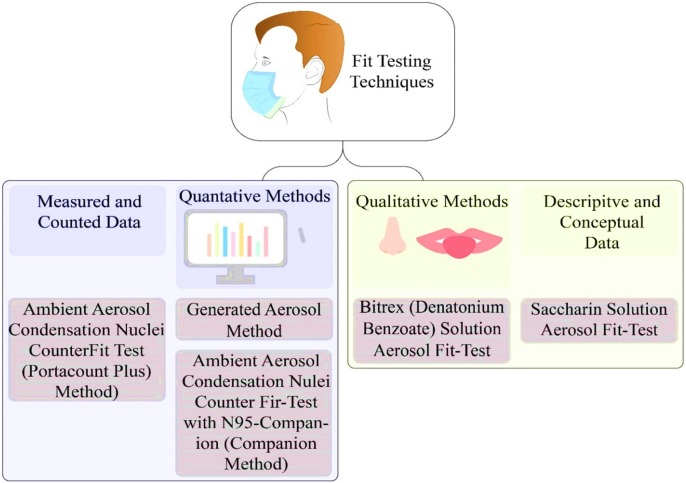
Besides, the design of the respirator affects the fit of the facepieces as fitting a half-facepiece respirators, which only cover the mouth and nose, is more difficult compared to that of full-facepiece respirators which also cover the eyes (Loeb, 2009) As was already mentioned, the fit-test for respiratory facepieces is mainly categorized into two groups, a) qualitative, and b) quantitative, that each can be done through different methods, which are summarized in Fig. 9 (Coffey et al., 2002, OSHA, xxxx).
Fig. 9.
Classification of the most common testing techniques.
For the qualitative test, a hood and a taste or odour solution are utilized to investigate the capability of the wearer to smell or taste the sample. Therefore, the qualitative method is a pass/fail test, which depends on the sensory detection of the wearer in terms of the taste and smell of the test agent, or the involuntary cough as a response to irritant smoke. Furthermore, a quantitative method utilizes an instrument to calculate the efficiency of the respirator numerically using electronic equipment, which calculates the air leakage into the face masks (Infection Prevention and Control Application, 2020). During the experiments through the fit testing methods, The fit factor can be obtained by using Eq. (9) as follows (Sietsema and Brosseau, 2016):
| (9) |
The overall fit factor can be calculated using the harmonic mean value of each exercise (Sietsema and Brosseau, 2016). Eq. (10) describes the calculation procedure of overall fit factors:
| (10) |
where “N” stands for the number of the exercises and the FFi, i = are the fit factor value for the individual exercise (Sietsema and Brosseau, 2016). If the N95 face mask is well fitted, it has the ability to filter out the small size particles less than 0.5 µm from the air (Infection Prevention and Control Application, 2020).
5. Conclusions
Face mask and respirators are to become and integral part of day to day life with the COVID pandemic. PPE undergoes analysis for a variety of tests to evaluate their performance and suitability for different environments. Face mask and respirators each have different standards to be met with respirators having the higher of the two. Particulate filtration, bacterial filtration viral filtration and NaCL method are evaluated to ascertain their efficiency in the filtration of each of the named bodies. Particulate filtration is measured using 0.1-µm latex spheres, and a percentage of how many are filtered through the maks from 1 to 99.99% with the higher the percentage, the better. Bacterial filtration efficiency measures the filtration of aerosol droplets of 3 µm in diameter as well ad well droplets formed from speaking coughing and sneezing down to the size of 0.6 µm, surgical or medical mask must obtain an efficiency of at least 95%. Viral efficiency examines the assigned protection factor; this evaluates the face mask and respirators ability to reduce exposure to a virus in workplace conditions. The NaCl method measures particles that are sized between 10 nm and 10 µm with N95 masks receiving a penetration of no more than 5%. The masks are further tested for their fluid and flame resistance, differential pressure and fit testing. Fluid resistance examines the penetration of and wetting of fluids on the mask, with bodily fluids, synthetic blood and saliva being measure. Flame resistance examines the flammability of materials used in masks, how much and how long the material will burn and how quickly the flame will spread. Differential pressure is used to measure the comfort and breathability of the masks and airflow resistance, the higher the differential pressure, the harder it is to breathe in the mask. Facepiece Fit Testing examines both qualitative and quantitative methods, with negative or positive pressure methods to study how well the mask or respirator fits on an individual face. By using all of these standards and their testing methods face masks and respirators can be approved for use in the prevention of the spread of COVID-19.
Acknowledgment
This work was supported by the “Presidents Bursary funding”, through the Institute of Technology Sligo.
References
- 3 Tips for Choosing the Right Face Mask|Halyard Health. https://www.halyardhealth.com/industry-news/2019/july/choosing-the-right-face-mask-3-things-to-know.aspx (accessed Jun. 03, 2020).
- 3M Personal Safety Division, 2020. Respirators and Surgical Masks: A Comparison, pp. 1–4.
- 42 CFR Part 84 Respiratory Protective Devices, 2020.
- Anfinrud P., Stadnytskyi V., Bax C.E., Bax A. Visualizing speech-generated oral fluid droplets with laser light scattering. New Engl. J. Med. 2020;382(21):2061–2063. doi: 10.1056/NEJMc2007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANSI-American National Standards Institute. https://www.ansi.org/.
- ASTM F1862/F1862M - 17 Standard Test Method for Resistance of Medical Face Masks to Penetration by Synthetic Blood (Horizontal Projection of Fixed Volume at a Known Velocity). https://www.astm.org/Standards/F1862.htm.
- ASTM F2101 - 01 Standard Test Method for Evaluating the Bacterial Filtration Efficiency (BFE) of Medical Face Mask Materials, Using a Biological Aerosol of Staphylococcus aureus. https://www.astm.org/DATABASE.CART/HISTORICAL/F2101-01.htm.
- ASTM F2101-14, 2014. Standard test method for evaluating the bacterial filtration efficiency (BFE) of medical face mask materials, using a biological aerosol of Staphylococcus aureus. American Society for Testing and Materials. https://wwwn.cdc.gov/PPEInfo/Standards/Info/ASTMF210114.
- ASTM International, 2010. ASTM F2299/F2299M - 03(2010)-Standard Test Method for Determining the Initial Efficiency of Materials Used in Medical Face Masks to Penetration by Particulates Using Latex Spheres. https://doi.org/10.1520/F2299_F2299M-03R10.
- ASTM International - Standard References for ASTM F1671/F1671M - 13. https://www.astm.org/DATABASE.CART/STD_REFERENCE/F1671.htm.
- Bałazy A., Toivola M., Adhikari A., Sivasubramani S.K., Reponen T., Grinshpun S.A. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am. J. Infect. Control. 2006;34(2):51–57. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- BBC World, 2020. Spain tightens mask rules for all older than five. Google Search. https://www.google.com/search?rlz=1C1GCEA_enIE905IE905&sxsrf=ALeKk01L1--jraSKCb7Tp5UV4Vwououp5A:1591877342300&q=BBC+World,+Spain+tightens+mask+rules+for+all+older+than+five.+2020.&spell=1&sa=X&ved=2ahUKEwi1z42c3fnpAhXHUxUIHTh4DDEQBSgAegQICxAn&biw=1366&bih.
- BBC. Coronavirus: Germans don compulsory masks as lockdown eases - BBC News. https://www.bbc.com/news/world-europe-52439926.
- Borkow G., Zhou S.S., Page T., Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkow G., Zhou S.S., Page T., Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Standard, 2008. Respiratory protective devices— Methods of test. Part 7: Determination of particle filter penetration. Bs En 13274-72008.
- C. for D. and R. Health, 2004. Guidance for Industry and FDA Staff: Surgical Masks - Premarket Notification [510 (k)] Submissions, p. 14.
- CDC - The National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/index.htm (accessed May 06, 2020).
- Chatterjee, P., et al., 2020. Healthcare workers & SARS-CoV-2 infection in India: a case-control investigation in the time of COVID-19, https://doi.org/10.4103/ijmr.IJMR_2234_20. [DOI] [PMC free article] [PubMed]
- Coffey C.C. Comparison of five methods for fit-testing N95 filtering-facepiece respirators. Appl. Occup. Environ. Hygiene. 2002;17(April):37–41. doi: 10.1080/1047322029010700. [DOI] [PubMed] [Google Scholar]
- Coronavirus Update (Live): 7,119,080 Cases and 406,655 Deaths from COVID-19 Virus Pandemic - Worldometer. 2020. - Google Search. https://www.google.com/search?q=Coronavirus+Update+(Live)%3A+7%2C119%2C080+Cases+and+406%2C655+Deaths+from+COVID-19+Virus+Pandemic+-+Worldometer.+2020.&rlz=1C1GCEA_enIE905IE905&oq=Coronavirus+Update+(Live)%3A+7%2C119%2C080+Cases+and+406%2C655+Deaths+from+.
- Current understanding on the Effectiveness of Face Masks and Respirators to Prevent the Spread of Respiratory Viruses, pp. 1–23.
- Fadare O.O., Okoffo E.D. Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140279. 140279–140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gov.ie - Guidance on safe use of face coverings. https://www.gov.ie/en/publication/aac74c-guidance-on-safe-use-of-face-coverings/.
- Hall, D., 2014. The Relationship of Fabric Properties and Bacterial Filtration Efficiency for Selected Surgical Face Masks, no. June.
- Han Z.Y., Weng W.G., Huang Q.Y. Characterizations of particle size distribution of the droplets exhaled by sneeze. J. R. Soc. Interface. 2013;10(88):20130560. doi: 10.1098/rsif.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health and Safety Authority, 2010. A Guide to Respiratory Protective Equipment [Online]. Available: http://www.hsa.ie/eng/Publications_and_Forms/Publications/Chemical_and_Hazardous_Substances/Respiratory Protective Equipment.pdf.
- Health and Safety Executive, “COSHH basics - COSHH,” 2002, 2016. https://www.hse.gov.uk/coshh/basics/index.htm.
- https://www.clkmedicalsupply.com/masks-standard/. https://www.clkmedicalsupply.com/masks-standard/ (accessed May 06, 2020).
- https://www.tuvsud.com/en. https://www.tuvsud.com/en (accessed May 05, 2020).
- Huang H. COVID-19: a call for physical scientists and engineers. ACS Nano. 2020 doi: 10.1021/acsnano.0c02618. [DOI] [PubMed] [Google Scholar]
- I. CLK Medical Supply, “Masks Standard”.
- Infection Prevention and Control Application of PPE During COVID-19 Version 2 June 2020 COVID-19 Infection Prevention and Control Utilisation Contents of PPE in Response to COVID-19, no. June, 2020.
- Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected, 2020. Google Search. https://www.google.com/search?q=Infection+prevention+and+control+during+health+care+when+novel+coronavirus+(nCoV)+infection+is+suspected.+2020.&rlz=1C1GCEA_enIE905IE905&oq=Infection+prevention+and+control+during+health+care+when+novel+coronavirus+(nCoV)+i.
- ISO, 2004. “SO 22609:2004 Clothing for protection against infectious agents — Medical face masks — Test method for resistance against penetration by synthetic blood (fixed volume, horizontally projected)”. https://www.iso.org/obp/ui/fr/#iso:std:iso:22609:ed-1:v1:en.
- Juang P.S.C., Tsai P. Emergency forum N95 respirator cleaning and reuse methods proposed by the inventor of the N95 mask material. J. Emerg. Med. 2020;58:817–820. doi: 10.1016/j.jemermed.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiersma, M.E., 2014. Occupational Safety and Health Administration. In: Encyclopedia of Toxicology: third ed., p. 642.
- Konda A., Prakash A., Moss G.A., Schmoldt M., Grant G.D., Guha S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 2020 doi: 10.1021/acsnano.0c03252. [DOI] [PubMed] [Google Scholar]
- Lentner, C. (Ed.), 1984. Geigy Scientific Tables, Volume 1 — Units of Measurement, Body Fluids, Composition of Blood, Hematology, Somatometric Data. Medical Education Division, Ciba-Geigy Corporation, West Caldwell, NJ, p. 413.
- Li M., Furlong J.L., Yorio P.L., Portnoff L. A new approach to measure the resistance of fabric to liquid and viral penetration. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0211827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. Surgical mask vs N95 respirator for preventing influenza among health care workers. JAMA. 2009;302(17):1865. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- Lord J. 35—The determination of the air permeability of fabrics. J. Text. Inst. Trans. 1959;50(10):T569–T582. doi: 10.1080/19447025908659937. [DOI] [Google Scholar]
- MacIntyre C.R. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5(4) doi: 10.1136/bmjopen-2014-006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDiarmid M., Harrison R., Nicas M. N95 respirators vs medical masks in outpatient settings. JAMA – J. Am. Med. Assoc. 2020;323(8):789. doi: 10.1001/jama.2019.20905. [DOI] [PubMed] [Google Scholar]
- Medical Viral Penetration Test|Nelson Labs. https://www.nelsonlabs.com/testing/viral-penetration-test/ (accessed May 14, 2020).
- NEN-EN 149, 2009. Respiratory protective devices - Filtering half masks to protect against particles - Requirements, testing, marking, Nen, vol. 1.
- OSHA, Fit Testing Procedures (Mandatory). - 1910.134 App A | Occupational Safety and Health Administration.
- Personal Protective Equipment (PPE) Testing|TÜV SÜD.” https://www.tuvsud.com/en/services/testing/personal-protective-equipment-testing (accessed Jul. 14, 2020).
- Rawlinson, K., 2020. Coronavirus PPE: all 400,000 gowns flown from Turkey for NHS fail UK standards.
- Rengasamy, S., et al., 2018. A comparison of total inward leakage measured using sodium chloride (NaCl) and corn oil aerosol methods for air-purifying respirators, 9624(May), https://doi.org/10.1080/15459624.2018.1479064. [DOI] [PMC free article] [PubMed]
- Rengasamy S., Shaffer R., Williams B., Smit S. A comparison of facemask and respirator filtration test methods. J. Occup. Environ. Hyg. 2017;14(2):92–103. doi: 10.1080/15459624.2016.1225157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy S., Niezgoda G., Shaffer R. Flammability of respirators and other head and facial personal protective equipment. J. Int. Soc. Respir. Prot. 2018;35(1):1–13. http://www.ncbi.nlm.nih.gov/pubmed/30364752 [PMC free article] [PubMed] [Google Scholar]
- Respirator Precertification (NIOSH) Tests - Nelson Labs. https://www.nelsonlabs.com/testing/respirator-pre-certification-tests-niosh/.
- rte news, 2020. Some new PPE ‘not fit for purpose’ - medical staff. Google Search. https://www.google.com/search?rlz=1C1GCEA_enIE905IE905&sxsrf=ALeKk01ABnyaJiVoMV7dO_-VxA7WMYdRvw:1591877533186&q=rte+news,+Some+new+PPE+%27not+fit+for+purpose%27+-+medical+staff.+2020.&spell=1&sa=X&ved=2ahUKEwj-u5D33fnpAhVlqHEKHQ2LAJAQBSgAegQICxAn&biw=1366&bih=576.
- S. Type, http://www.safeticorp.com/data/train_img_normal/Body_Protection.pdf, pp. 1–3.
- Santarpia, J.L., et al., 2020. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. medRxiv, p. 2020.03.23.20039446, https://doi.org/10.1101/2020.03.23.20039446.
- Scott, R.A., 2005. Textiles for Protection - Google Books.
- Sietsema M., Brosseau L.M. Comparison of two quantitative fit-test methods using N95 filtering facepiece respirators. J. Occup. Environ. Hyg. 2016;13(8):621–627. doi: 10.1080/15459624.2016.1159690. [DOI] [PubMed] [Google Scholar]
- Solutions, C., 2020. Scotch-Brite™ Purple Scour Pad.
- Standard, P.I. Yy, S., 2013. Pharmaceutical Industry Standard of the People’s Republic of China Surgical Mask.
- Steve Zhou S., Lukula S., Chiossone C., Nims R.W., Suchmann D.B., Ijaz M.K. Assessment of a respiratory face mask for capturing air pollutants and pathogens including human influenza and rhinoviruses. J. Thorac. Dis. 2018;10(3):2059–2069. doi: 10.21037/jtd.2018.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T.N.P.P.T. Laboratory, 2014. Considerations for Selecting Protective Clothing|NPPTL|NIOSH|CDC. https://www.cdc.gov/niosh/npptl/topics/protectiveclothing/#table3.
- T.W.P. Spectrometer, “Wide-Range Particle Spectrometer™”.
- (PDF) The Relationship of Fabric Properties and Bacterial Filtration Efficiency for Selected Surgical Face Masks. https://www.researchgate.net/publication/242297437_The_Relationship_of_Fabric_Properties_and_Bacterial_Filtration_Efficiency_for_Selected_Surgical_Face_Masks (accessed Jul. 06, 2020).
- U.S. CPSC, 2011. “United States Consumer Product Safety Commission,” no. October [Online]. Available: http://www.cpsc.gov/PageFiles/125782/diop.pdf.
- Using, P., Spheres, L., 2005. Standard Specification for Performance of Materials Used in Medical Face Masks 1, Test, vol. 11, no. 2018, pp. 19–21, https://doi.org/10.1520/F2100-11.2.
- Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop. Med. Int. Health. 2020;25(3):278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar V. Personal protection prior to preoperative assessment—little more an anaesthesiologist can do to prevent SARS-CoV-2 transmission and COVID-19 infection. Ain-Shams J. Anesthesiol. 2020;12(1) doi: 10.1186/s42077-020-00066-x. [DOI] [Google Scholar]
- “What is the purpose of a medical-surgical face mask?”.
- “What is the efficacy of standard face masks compared to respirator masks in preventing COVID-type respiratory illnesses in primary care staff? - CEBM.” https://www.cebm.net/covid-19/what-is-the-efficacy-of-standard-face-masks-compared-to-respirator-masks-in-preventing-covid-type-respiratory-illnesses-in-primary-care-staff/ (accessed Jul. 06, 2020).
- WHO, 2020. Advice on the use of masks in the context of COVID-19: interim guidance-2. Guía Interna la OMS, no. April, pp. 1–5, https://doi.org/10.1093/jiaa077.
- Yang S., Lee G.W.M., Chen C.M., Wu C.C., Yu K.P. The size and concentration of droplets generated by coughing in human subjects. J. Aerosol Med. Depos. Clear. Eff. Lung. 2007;20(4):484–494. doi: 10.1089/jam.2007.0610. [DOI] [PubMed] [Google Scholar]
- Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]