Abstract
Background
Hydroxychloroquine is generally considered safe in pregnancy for the treatment of rheumatic conditions, but studies have been too small to evaluate teratogenicity. Quantifying the risk of congenital malformations associated with early pregnancy exposure to hydroxychloroquine is important in both the context of its ongoing use for rheumatological disorders and its potential future use for coronavirus disease 2019 prophylaxis, for which a number of clinical trials are ongoing despite initial trials for coronavirus disease 2019 treatment having been negative.
Objective
The study objective was to evaluate the risk of major congenital malformations associated with exposure to hydroxychloroquine during the first trimester of pregnancy, the period of organogenesis.
Study Design
We performed a population-based cohort study nested in the Medicaid Analytic eXtract (MAX, 2000–2014) and IBM MarketScan Research Database (MarketScan, 2003–2015). The source cohort included 2045 hydroxychloroquine-exposed pregnancies and 3,198,589 pregnancies not exposed to hydroxychloroquine continuously enrolled in their respective insurance program for 3 months before the last menstrual period through at least 1 month after delivery; infants were enrolled for at least 3 months after birth. We compared the risk of congenital malformations in women using hydroxychloroquine during the first trimester of pregnancy with that of those not using hydroxychloroquine, restricting the cohort to women with rheumatic disorders and using propensity score matching to control for indication, demographics, medical comorbidities, and concomitant medications (1867 hydroxychloroquine-exposed pregnancies and 19,080 pregnancies not exposed to hydroxychloroquine). The outcomes considered included major congenital malformations diagnosed during the first 90 days after delivery and specific malformation types for which there were at least 5 exposed events: oral cleft, cardiac, respiratory, gastrointestinal, genital, urinary, musculoskeletal, and limb defects.
Results
Overall, 54.8 per 1000 infants exposed to hydroxychloroquine were born with a major congenital malformation versus 35.3 per 1000 unexposed infants, corresponding to an unadjusted relative risk of 1.51 (95% confidence interval, 1.27–1.81). Patient characteristics were balanced in the restricted, propensity score–matched cohort. The adjusted relative risk was 1.26 (95% confidence interval, 1.04–1.54); it was 1.33 (95% confidence interval, 1.08–1.65) for a daily dose of ≥400 mg and 0.95 (95% confidence interval, 0.60–1.50) for a daily dose of <400 mg. Among the different malformation groups considered, more substantial increases in the risk of oral clefts, respiratory anomalies, and urinary defects were observed, although estimates were imprecise. No pattern of malformation was identified.
Conclusion
Our findings suggest a small increase in the risk of malformations associated with first-trimester hydroxychloroquine use. For most patients with autoimmune rheumatic disorders, the benefits of treatment during pregnancy will likely outweigh this risk. If hydroxychloroquine were shown to be effective for coronavirus disease 2019 prophylaxis in ongoing trials, the risk of malformations would need to be balanced against such benefits.
Key words: coronavirus disease 2019, hydroxychloroquine, malformations, pregnancy, rheumatic disorders, systemic lupus erythematosus
Introduction
Hydroxychloroquine (HCQ) is an antimalarial drug widely used in the treatment of systemic lupus erythematosus (SLE) and other rheumatic disorders. It is generally considered to be safe for the treatment of autoimmune rheumatic conditions during pregnancy, and the continuation of HCQ during pregnancy is commonly recommended to improve disease management and pregnancy outcomes.1, 2, 3 However, studies have been too small to evaluate teratogenicity. Over the last several months, there has been heightened interest in HCQ because of it being a candidate drug for the treatment and/or prophylaxis of coronavirus disease 2019 (COVID-19).
AJOG at a Glance.
Why was this study conducted?
Although hydroxychloroquine (HCQ) is generally considered safe for the treatment of rheumatic conditions during pregnancy, most studies have been too small to evaluate teratogenicity.
Key findings
In this cohort study including 2045 HCQ-exposed pregnancies and 3,198,589 pregnancies not exposed to HCQ (1867 and 19,080, respectively, after restriction and matching), a 26% increase in the risk of major congenital malformations among HCQ-exposed patients was observed. The risk increase was seen with daily doses of ≥400 mg. No specific pattern of malformations was identified.
What does this add to what is known?
This is the third and by far the largest study to suggest a small increased risk. This signal warrants follow-up, given that HCQ is widely used for autoimmune rheumatic disorders in women of childbearing age. Should ongoing clinical trials indicate that there is a role for HCQ in coronavirus disease 2019 prophylaxis, the benefits of HCQ for this new indication would need to be weighed against the potential risk in pregnancy.
Between March 30, 2020, and June 15, 2020, HCQ was granted emergency use authorization by the Food and Drug Administration, allowing it to be used for COVID-19 outside the clinical trial setting, resulting in widespread use during that time window. At some hospitals, pregnant women with moderate COVID-19 have been treated with HCQ. Although several recent studies have failed to show a clear benefit of HCQ as a postexposure prophylaxis4, 5, 6 and the World Health Organization has discontinued the HCQ arm of the Solidarity Trial evaluating its efficacy for the treatment of patients who are hospitalized,7 numerous randomized controlled studies are still ongoing in particular to evaluate its effects for preexposure prophylaxis,8 including a trial in pregnant women.9
Most studies regarding the safety of HCQ when used for malaria and for rheumatic disorders, such as SLE, suggest no increase in the risk of common adverse obstetrical outcomes, such as spontaneous abortion, prematurity, and intrauterine growth restriction.10, 11, 12, 13 However, data regarding the risk of major congenital malformations associated with early pregnancy exposure are very limited, with the largest published cohort study including fewer than 200 exposed pregnancies (Supplemental Table 1).13 Quantification of the risk of congenital malformations associated with early pregnancy exposure to HCQ is therefore important in both the context of its ongoing use for rheumatological disorders and its potential future use for COVID-19, although its usefulness in this clinical context remains highly uncertain based on the results of initial trials. Given the limited data currently available, we evaluated the risk of major congenital malformations associated with HCQ using 2 large healthcare utilization databases.
Materials and Methods
Data sources and study cohorts
We conducted a cohort study of pregnancies nested in the Medicaid Analytic eXtract (MAX, 2000–2014), composed of all patients enrolled in Medicaid, and the IBM MarketScan Research Database (MarketScan, 2003–2015), composed of a nationally representative sample of patients with employer-provided health insurance. Both data sources included demographic and insurance enrollment information, medical visits and hospitalizations, diagnoses and procedures received as an in- or outpatient, and prescriptions filled on an outpatient basis. The development of the linked mother-infant pregnancy cohorts has been described previously.14 , 15 Briefly, we identified all completed pregnancies in women 12 to 55 years of age and linked these pregnancies to live-born infants by state, family identification number, and delivery and birth dates. Using a validated algorithm,16 we estimated the date of the last menstrual period on the basis of the delivery date and diagnostic codes indicative of preterm delivery. Mothers were required to be continuously insured from 3 months before the start of pregnancy to 1 month after delivery. Infants were required to be insured from birth to 3 months thereafter, unless they died sooner. These restrictions did not affect the age or race distribution in MAX but resulted in a decrease in the proportion of who become Medicaid eligible because of the occurrence of pregnancy and a corresponding increase in the proportion of who become eligible based on other criteria.17 Pregnancies with exposure to a known teratogenic medication (ie, warfarin, antineoplastic agents, lithium, isotretinoin, misoprostol, thalidomide) during the first trimester of pregnancy and pregnancies with chromosomal abnormalities were excluded.
Exposure
Women were considered exposed if they filled a prescription for HCQ during the first trimester of pregnancy (defined as the date of the last menstrual period to day 90 of pregnancy), the etiologically relevant exposure window for congenital malformations. To reduce the probability of exposure during early pregnancy from use of HCQ dispensed at an earlier time point, the reference group consisted of women without a prescription for HCQ for 3 months before the start of pregnancy to the end of the first trimester of pregnancy given HCQ’s long half-life.
Outcomes
The outcome of interest was major congenital malformations overall. In the secondary analyses, we also evaluated the specific malformation types for which we observed at least 5 exposed events across the 2 cohorts: oral cleft, cardiac, respiratory, gastrointestinal, genital, urinary, musculoskeletal, and limb defects. The presence of malformations was defined using validated algorithms based on inpatient or outpatient diagnoses and procedures, which have been shown to identify the outcomes with high specificity (ie, more than 1 date with the respective diagnostic codes recorded or 1 diagnostic code and a code for a procedure or surgery or infant death).18 Isolated congenital heart block was not included in the definition for cardiac malformations because its risk is increased in babies born to women with SLE.19 Supplemental Table 2 provides the details.
Covariates
Potential confounders and proxies for confounders considered included sociodemographic information (eg, state of residence, age, race and ethnicity [MAX only]), autoimmune rheumatic disorders (eg, rheumatoid arthritis, SLE, ankylosing spondylitis, psoriatic arthritis), other maternal conditions (eg, diabetes, hypertension, psychiatric conditions, renal disease, neurologic conditions, chronic respiratory conditions, anemia, infections), concomitant medication use (eg, systemic steroids, nonbiologic and biologic disease-modifying antirheumatic drugs [DMARDs], psychiatric medications, nonsteroidal antiinflammatory drugs [NSAIDs], suspected teratogens), and general markers of the burden of illness (eg, maternal comorbidity index, healthcare utilization measures) (Table ; Supplemental Tables 3 and 4).
Table.
Selected patient characteristics for HCQ-exposed pregnancies and pregnancies not exposed to HCQ
| Variable | Original source cohort |
Restricted matched cohorta |
||||||
|---|---|---|---|---|---|---|---|---|
| MarketScan (2003–2015) |
MAX (2000–2014) |
MarketScan (2003–2015) |
MAX (2000–2014) |
|||||
| HCQ exposed | Unexposed | HCQ exposed | Unexposed | HCQ exposed | Unexposed | HCQ exposed | Unexposed | |
| Number of pregnancies | 1359 | 1,317,520 | 686 | 1,881,069 | 1261 | 11,179 | 606 | 7901 |
| Age, mean (SD) | 33.0 (4.4) | 31.9 (4.6) | 27.8 (6.0) | 24.5 (5.9) | 33.1 (4.6) | 33.1 (4.4) | 27.9 (6.0) | 28.3 (5.9) |
| Autoimmune rheumatic disordersb | ||||||||
| Systemic lupus erythematosus | 759 (55.9) | 2188 (0.2) | 487 (71.0) | 2707 (0.1) | 675 (53.5) | 6065 (54.3) | 409 (67.5) | 5184 (65.6) |
| Rheumatoid arthritis | 408 (30.0) | 3028 (0.2) | 190 (27.7) | 3056 (0.2) | 392 (31.1) | 3515 (31.4) | 182 (30.0) | 2125 (26.9) |
| Ankylosing spondylitis | 14 (1.0) | 4654 (0.4) | <11 (0.7) | 2588 (0.1) | 14 (1.1) | 168 (1.5) | <11 (0.8) | 246 (3.1) |
| Psoriatic arthritis | 15 (1.1) | 404 (0.0) | <11 (0.2) | 206 (0.0) | 15 (1.2) | 127 (1.1) | <11 (0.2) | 42 (0.5) |
| Sicca syndrome | 134 (9.9) | 647 (0.0) | 60 (8.8) | 183 (0.0) | 122 (9.7) | 1113 (10.0) | 42 (6.9) | 625 (7.9) |
| Dermatomyositis | 17 (1.3) | 60 (0.0) | <11 (1.2) | 68 (0.0) | 13 (1.0) | 112 (1.0) | <11 (1.3) | 90 (1.1) |
| Other diffuse connective tissue disease | 221 (16.3) | 1000 (0.1) | 91 (13.3) | 423 (0.0) | 197 (15.6) | 1774 (15.9) | 73 (12.1) | 1075 (13.6) |
| Other autoimmune disease | 68 (5.0) | 1295 (0.1) | <11 (1.5) | 402 (0.0) | 67 (5.3) | 567 (5.1) | <11 (1.7) | 148 (1.9) |
| Sarcoidosis | 6 (0.4) | 275 (0.0) | <11 (1.3) | 455 (0.0) | 6 (0.5) | 50 (0.5) | <11 (1.5) | 121 (1.5) |
| Other maternal conditionsc | ||||||||
| Anemia | 93 (6.8) | 25,507 (1.9) | 79 (11.5) | 63,162 (3.4) | 84 (6.7) | 730 (6.5) | 71 (11.7) | 936 (11.9) |
| Diabetes | 34 (2.5) | 23,650 (1.8) | 33 (4.8) | 41,403 (2.2) | 34 (2.7) | 293 (2.6) | 30 (5.0) | 391 (4.9) |
| Hypertension | 93 (6.8) | 30,937 (2.4) | 74 (10.8) | 47,746 (2.5) | 85 (6.7) | 797 (7.1) | 65 (10.7) | 829 (10.5) |
| Neuropathic pain | 68 (5.0) | 27,054 (2.1) | 27 (3.9) | 27,145 (1.4) | 66 (5.2) | 577 (5.2) | 24 (4.0) | 351 (4.5) |
| Nonneuropathic pain | 450 (33.1) | 135,075 (10.3) | 316 (46.1) | 253,994 (13.5) | 421 (33.4) | 3773 (33.8) | 279 (46.0) | 3689 (46.7) |
| Serious infections | 43 (3.2) | 17,924 (1.4) | 59 (8.6) | 72,412 (3.9) | 38 (3.0) | 318 (2.8) | 52 (8.6) | 638 (8.1) |
| Renal disease | 58 (4.3) | 3379 (0.3) | 50 (7.3) | 6578 (0.4) | 45 (3.6) | 396 (3.5) | 35 (5.8) | 433 (5.5) |
| Concomitant medicationsc | ||||||||
| Systemic steroids | 565 (41.6) | 70,421 (5.3) | 383 (55.8) | 69,446 (3.7) | 485 (38.5) | 4262 (38.1) | 309 (51.0) | 4179 (52.9) |
| Biologic DMARDs | 64 (4.7) | 1303 (0.1) | 16 (2.3) | 459 (0.0) | 64 (5.1) | 575 (5.2) | 16 (2.6) | 227 (2.9) |
| Nonbiologic DMARDs | 150 (11.0) | 7535 (0.6) | 129 (18.8) | 4269 (0.2) | 117 (9.3) | 1021 (9.1) | 93 (15.4) | 872 (11.0) |
| Opioids | 296 (21.8) | 153,634 (11.7) | 311 (45.3) | 427,889 (22.8) | 272 (21.6) | 2402 (21.5) | 281 (46.4) | 3854 (48.8) |
| NSAIDs | 255 (18.8) | 65,980 (5.0) | 290 (42.3) | 317,721 (16.9) | 235 (18.6) | 2073 (18.5) | 254 (41.9) | 3361 (42.5) |
| Markers of burden of illnessd | ||||||||
| Maternal comorbidity index, mean (SD) | 3.0 (2.4) | 1.2 (1.5) | 3.4 (2.5) | 0.9 (1.4) | 2.9 (2.3) | 3.0 (2.4) | 3.3 (2.5) | 3.2 (2.6) |
| Number of distinct diagnoses, mean (SD) | 4.7 (3.9) | 2.1 (2.6) | 6.1 (4.7) | 2.8 (3.3) | 4.7 (3.9) | 4.8 (4.0) | 6.0 (4.7) | 6.2 (4.8) |
| Number of non-HCQ prescription drugs, mean (SD) | 3.4 (3.1) | 1.5 (2.2) | 4.9 (3.9) | 1.8 (2.5) | 3.3 (3.1) | 3.6 (3.5) | 4.7 (3.8) | 4.9 (4.0) |
| Number of outpatient visits, mean (SD) | 4.1 (4.4) | 2.1 (3.2) | 4.9 (5.9) | 2.1 (3.7) | 4.1 (4.4) | 4.4 (4.4) | 4.8 (4.9) | 5.0 (6.5) |
Data are presented as number (percentage), unless otherwise indicated. Cell size of <11 for the MAX cohort are suppressed in accord with the CMS cell size suppression policy.
CMS, Centers for Medicare and Medicaid Services; DMARD, disease-modifying antirheumatic drug; HCQ, hydroxychloroquine; MAX, Medicaid Analytic eXtract; NSAID, nonsteroidal antiinflammatory drug; SD, standard deviation.
Huybrechts et al. Hydroxychloroquine and birth defects. Am J Obstet Gynecol 2021.
Given the variable ratio matching, the counts for the unexposed group are weighted counts to demonstrate the balance in baseline covariates
Autoimmune rheumatic disorders were measured from 3 months before the start to the end of pregnancy
Maternal conditions and concomitant medication use were measured from 3 months before the start of pregnancy to the end of the first trimester of pregnancy
General markers of the burden of illness were measured during the 3 months before but not during pregnancy, as these measures may be affected by early detection of pregnancy complications.
Analyses
Baseline characteristics were compared between women exposed to HCQ and the reference group of unexposed women. Relative risks (RRs) with their 95% confidence intervals (CIs) were estimated using generalized linear models. As a first level of adjustment, the reference group was restricted to women with a recorded diagnosis of autoimmune rheumatic disorders commonly treated with HCQ (“restricted cohort”). In fully adjusted analyses, exposed and unexposed women in the restricted cohort were matched on the basis of their propensity score (PS), using a 1:200 variable ratio matching and a 0.01 caliper (“restricted matched cohort”). The PS, which reflects the probability of being treated with HCQ, was estimated using a logistic regression model, including all (>80) prespecified covariates. When evaluating the balance in baseline characteristics in the restricted matched cohort, the counts for the unexposed group were weighted to account for the variable ratio matching. We conducted analyses stratified by dose, using the highest daily dose dispensed during the first trimester of pregnancy (<400 mg and ≥400 mg daily) and duration of exposure (≤60 days and >60 days). In a sensitivity analysis, both the exposed and the reference groups were restricted to women with a recorded diagnosis of autoimmune rheumatic disorders. Estimates from both cohorts were combined using a meta-analytic approach with random effects.
For all analyses presented, results were described as similar or different from the reference group based on the magnitude of the point estimates, taking into account the precision of each estimate as reflected in the width of its 95% CI. We focused on estimating the magnitude of effects in preference to dichotomizing the results as statistically significant or not.20 The research was approved by the institutional review board of Brigham and Women’s Hospital, which waived the need for informed consent.
Results
The combined cohort included 2045 pregnancies exposed to HCQ during the first trimester of pregnancy (686 in MAX and 1359 in MarketScan) and 3,198,589 pregnancies not exposed to HCQ (1,881,069 in MAX and 1,317,520 in MarketScan). The mean daily dose of HCQ was 371 mg (standard deviation, 379 mg), and 61% of women used a daily dose of 400 mg. Among the exposed women, 25.9% were exposed for ≤30 days, 33.6% for 31 to 60 days, and 40.5% for >60 days during the first trimester of pregnancy.
Women exposed to HCQ tended to be older, had more comorbid conditions, took more concomitant medications (especially pain medications, steroids, NSAIDs, and DMARDs), and had greater healthcare utilization. After cohort restriction and adjustment through PS matching, all covariates—including treatment indications—were well balanced (Table; Supplemental Tables 3 and 4).
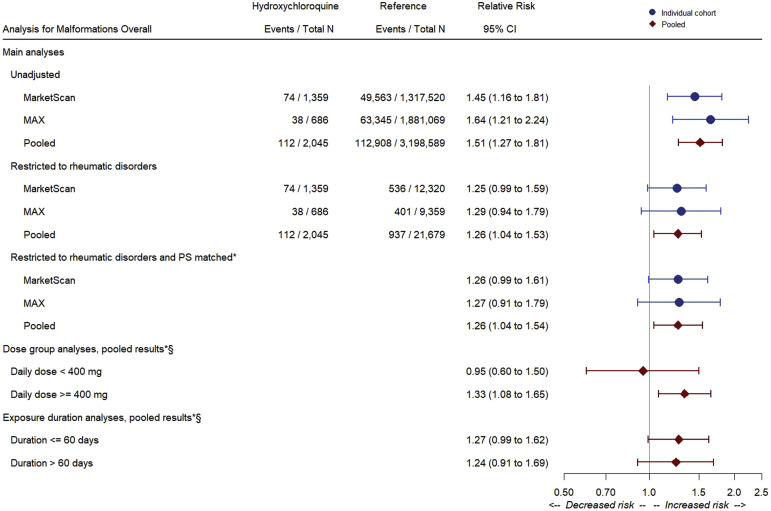
The pooled risk of any congenital malformation was 54.8 per 1000 HCQ-exposed infants (n=112 events) and 35.3 per 1000 infants not exposed to HCQ in the general population (n=112,908 events), corresponding to a pooled unadjusted RR of 1.51 (95% CI, 1.27–1.81). Restricting the reference group to women with rheumatic disorders resulted in an absolute risk of 44.1 per 1000 unexposed infants (506 of 11,468 events) and an RR of 1.26 (95% CI, 1.04–1.53). Adjusting for all potential confounding variables through PS matching did not result in further attenuation of the association (RR, 1.26; 95% CI, 1.04–1.54). Estimates were consistent between the 2 cohorts. The risk of malformations among the HCQ-exposed women was the same regardless of whether women had concomitant exposure to steroids. The adjusted RR was 1.33 (95% CI, 1.08–1.65) for a daily dose of ≥400 mg and 0.95 (95% CI, 0.60–1.50) for <400 mg. The risk was not affected by the duration of exposure (Figure 1 ), and results were similar when restricting both the exposed and the reference group to women with a recorded diagnosis of autoimmune rheumatic disorders (Supplemental Table 5).
Figure 1.
Relative risks of any congenital malformation: main and dose-stratified analyses
The asterisk symbol indicates that for the variable ratio matching PS analysis after restriction to deliveries with rheumatic disorders, the number of outcome events and the total number of deliveries in both the HCQ-exposed group and group not exposed to HCQ are not meaningful for absolute risk estimation; therefore, those counts are left blank in the Figure. The section symbol indicates that data are restricted to rheumatic disorders and PS-matched estimate.
CI, confidence interval; HCQ, hydroxychloroquine; MAX, Medicaid Analytic eXtract; PS, propensity score.
Huybrechts et al. Hydroxychloroquine and birth defects. Am J Obstet Gynecol 2021.
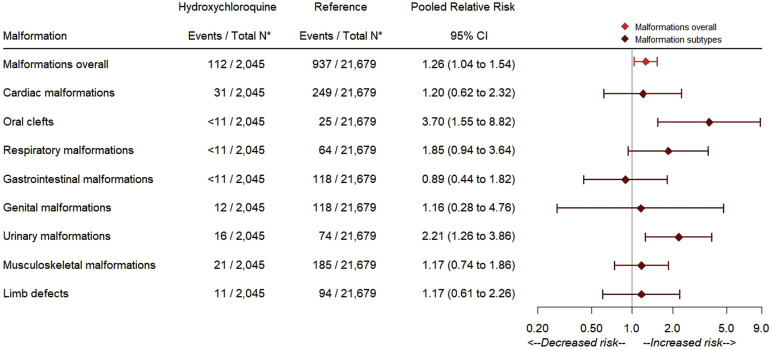
In the context of few events, risk estimates for the specific malformation types considered were relatively imprecise (Figure 2 ). The point estimates indicated an approximately 2- to 4-fold increase in the risks for oral clefts (RR, 3.70; 95% CI, 1.55–8.82), respiratory defects (RR, 1.85; 95% CI, 0.94–3.64), and urinary defects (RR, 2.21; 95% CI, 1.26–3.86), which were consistent between the 2 cohorts. None of the HCQ-exposed cases of oral clefts had concomitant exposure to steroids. The upper limit of the 95% CI for the pooled estimates suggested no more than a 2-fold increase in the risk of other specific malformation types with the exception of genital defects (upper limit 95% CI, 4.76). Among the 112 HCQ-exposed infants with malformations, 12 (10.7%) had more than 1 type of malformation recorded, with no specific pattern suggestive of a syndrome.
Figure 2.
Pooled adjusted relative risks in the PS-matched, restricted cohorts
The asterisk symbol indicates that for the variable ratio matching PS analysis after restriction to deliveries with rheumatic disorders, the number of outcome events and the total number of deliveries in both the HCQ-exposed group and the group not exposed to HCQ are not meaningful for absolute risk estimation; therefore, counts after restriction to deliveries with rheumatic disorders but before the PS matching are reported in the Figure.
HCQ, hydroxychloroquine; PS, propensity score.
Huybrechts et al. Hydroxychloroquine and birth defects. Am J Obstet Gynecol 2021.
Discussion
Principal findings
Using data from health plans that provide coverage for large populations of both commercially and publicly insured individuals in the United States, we identified a cohort of pregnant women with chronic autoimmune rheumatic diseases and assessed the relative prevalence of major congenital malformations in their newborns following exposure to HCQ during early pregnancy. Women who filled prescriptions for HCQ during the first trimester of pregnancy had a higher risk of malformations in their newborn than the general population. On the restriction to women with the indication (mainly SLE and rheumatoid arthritis), the RR attenuated but was still elevated. In utero–exposed newborns had an adjusted risk of major congenital malformations 26% higher than unexposed newborns overall and 33% higher for daily doses of ≥400 mg (although no increased risk was observed for lower doses based on the point estimate, reflecting the estimate most consistent with the data). A more substantial increase in the risk of oral clefts, respiratory anomalies, and urinary defects was observed, although CIs for specific malformations were wide. No pattern of malformation was identified.
Context
Previous studies evaluating the safety of HCQ in pregnancy included between 36 and 194 women and overall suggested no increased risk of pregnancy loss, prematurity, intrauterine growth retardation, preeclampsia, fetal distress, or induction of delivery compared with the reference groups.10 , 12 , 21, 22, 23, 24 As flares are associated with these and other complications and HCQ is effective at controlling them, drug use in pregnancy may improve pregnancy outcomes for women with rheumatic disorders23 and reduce the risk of congenital heart block in the neonate.25 Moreover, HCQ use reduces the dose of prednisone needed during pregnancy.12
However, most of these studies were too small to assess the risk of major malformations, and many based their conclusion on the statistical significance of underpowered comparisons.26 Given that HCQ crosses the placenta21 and inhibits cell division and DNA synthesis27 and that initial reports suggested an increased risk of chromosomal damage attributable to chloroquine,28 concerns regarding effects on rapidly dividing embryonic cells remain. Specific malformations reported among exposed newborns included cleft lip and palate (1 of 79)12 and pulmonary hypoplasia in a preterm infant (1 of 133).21 Moreover, 2 of the largest studies found a meaningful, although not statistically significant, increased risk of malformations overall. In 1 study, malformations were more common in the 194 HCQ-exposed patients (6.7%) than in the reference (2.3%) group (adjusted RR, 3.11; 95% CI, 0.99–9.77), with no clear pattern.13 In another study, the 114 HCQ-exposed patients had a prevalence of malformations of 7 of 97 (7.2%) and the reference group of 15 of 440 (3.4%) with a P value of .094,22 again with no clear pattern.
Research implications
For pregnant women with malaria or rheumatic disorders, the benefits of HCQ may still outweigh the potential risk,2 especially given that discontinuation of HCQ after conception would not necessarily prevent birth defects because the half-life is more than a month and would increase the risk of flares and their complications. Therefore, our findings of a potential small increase in the risk of malformations—although important for prescribers to be aware of—should not necessarily alter the treatment recommendation for a given woman with malaria or rheumatic disorders. For COVID-19, it will depend on whether the currently ongoing clinical trials demonstrate meaningful benefits of HCQ in reducing COVID-19 or its severity. Although initial trials using HCQ to treat COVID-19 have failed to demonstrate efficacy, trials regarding its use for preexposure prophylaxis have not yet been reported.
Strengths and limitations
In addition to several strengths (including a large and nationally representative population and a robust control for confounding through restriction and matching), our study is also subject to certain limitations, most of which would bias the results toward the null. First, we included only women with a live-born delivery because abortions and stillbirths are incompletely recorded in healthcare utilization data. This approach may have resulted in the exclusion of pregnancies with the outcome, as fetuses with malformations are more likely to experience fetal death or termination. Therefore, the incidence of major malformations reported in this study could underestimate the risk in pregnant women. If a higher proportion of women on HCQ had lethal malformations, more prenatal screening, or a higher propensity to terminate an affected pregnancy, this study would also underestimate the RR. However, differential terminations have been shown to be an unlikely source of selection bias.29 Second, the identification of major congenital malformations was based on the diagnosis and/or procedure codes recorded in claims. Misclassification would tend to bias RRs toward the null unless a higher proportion of malformation diagnoses were identified in women exposed to HCQ. Although others and we have shown a high positive predictive value for malformations,13 , 18 , 30 the potential for some misclassification remains. Third, information on HCQ exposure is obtained from the claims of filled prescriptions. As some women may fill prescriptions for medications but not use them, our study may misclassify unexposed pregnancies into the HCQ group, thus underestimating any potential effect; however, a large fraction of our cohort filled prescriptions for HCQ throughout the first trimester of pregnancy. Fourth, it is possible that some women in the reference group were taking immunomodulatory agents in lieu of HCQ. If these agents were teratogenic, we would be underestimating the effect of HCQ. However, their use is negligible during pregnancy, and on restriction and PS matching, our exposed and reference groups were balanced in the use of these medications. Fifth, disease flares in women with rheumatic disorders, such as SLE, have been associated with poor pregnancy outcomes, and HCQ use during pregnancy improves disease activity and reduces the antiphospholipid syndrome.12 , 23 Therefore, the reference group of women with the disease and without HCQ could have a higher risk, thus potentially underestimating the RR of flare-related adverse pregnancy outcomes, including fetal loss, fetal growth retardation, and prematurity. Alternatively, it is possible that there is a misclassification of the unexposed group with respect to the presence of underlying rheumatic disease or that women being treated with HCQ have more severe underlying disease than women without HCQ. Although neither rheumatic disorders nor flares have been associated with congenital malformations, it is conceivable that women with more severe disease receive higher doses of steroids and this may not be fully captured in our data. However, recent studies31 , 32 have refuted initial reports of strong associations between steroids and oral clefts.33 More directly, in this study, the absolute risk of malformations was the same among HCQ-exposed pregnancies with and without concomitant exposure to systemic steroids, and none of the cases of oral clefts in the HCQ-exposed pregnancies were exposed to steroids. Together, this suggests that steroid exposure is not a major threat to the validity of our analyses. Sixth, the MAX cohort included data through 2014—the most recent data available at the time of study conduct—and MarketScan included data through 2015, to avoid the use of International Classification of Diseases, 10th Revision–based algorithms for cohort creation and outcome identification that have not yet been validated. However, the biological association between HCQ exposure and malformations should not change over time. Finally, despite being the largest exposed cohort to date, the numbers were small for specific malformation groups, and CIs were wide; specific individual defects could not be examined. However, there is enough information to suggest that the magnitude of a potential risk of malformations would not be in the order of that associated with major teratogens.
Conclusions
In this study, there was no evidence of a large increase in the prevalence of major congenital malformations in the newborn from first-trimester maternal exposure to HCQ. However, it is the third study to suggest a moderate increased risk. For most patients with autoimmune rheumatic disorders, the benefits of treatment during pregnancy will likely outweigh this risk. If proven effective for COVID-19 prophylaxis in ongoing randomized trials, the benefits of HCQ would need to be weighed against the potential risk in pregnancy.
Footnotes
K.F.H. reports being an investigator on grants to Brigham and Women’s Hospital from Eli Lilly and Company and GlaxoSmithKline for unrelated studies. B.T.B. reports being an investigator on grants to Brigham and Women’s Hospital from Eli Lilly and Company, Baxalta, GlaxoSmithKline, and Pacira Pharmaceutical Inc for unrelated studies; received personal fees from Aetion Inc and from the Alosa Foundation outside the submitted work; and served on an expert panel for a postpartum hemorrhage quality improvement project that was conducted by the Association of Women’s Health, Obstetric, and Neonatal Nurses and funded by a grant from Merck for Mothers. S.C.K. reports being an investigator on grants to Brigham and Women’s Hospital from Pfizer, Bristol-Myers Squibb, AbbVie Inc, and Roche Holding AG for unrelated studies. R.J.D. reports being an investigator on grants to Brigham and Women’s Hospital from Bayer, Vertex, and Novartis for unrelated studies. S.H.-D. reports being an investigator on grants to her institution from Eli Lilly and Company and GlaxoSmithKline for unrelated studies; receiving personal fees from UCB outside the submitted work; and having served as an epidemiologist with the North American Antiepileptic Drug Pregnancy Registry, which is funded by multiple companies. The remaining authors report no conflict of interest.
The study was supported by internal funds of the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School. K.F.H., B.T.B., and S.H.-D. are supported by the National Institute of Mental Health (R01 MH116194), the National Institute on Drug Abuse (R01 DA044293 and R01 DA049822), and the National Institute of Child Health and Human Development (R01 HD097778).
Cite this article as: Huybrechts KF, Bateman BT, Zhu Y, et al. Hydroxychloroquine early in pregnancy and risk of birth defects. Am J Obstet Gynecol 2021;224:290.e1-22.
Supplementary Data
Supplementary Materials
Supplemental Table 1.
Summary of studies with information on HCQ and the risk of congenital malformations
| Author | Number of patients exposed to HCQ | Number of controls not exposed to HCQ | Outcome | Risk estimates | Comments |
|---|---|---|---|---|---|
| Levy et al, 19911 | 24 exposed pregnant women (27 pregnancies) Chloroquine=16 HCQ=8 |
No control | Congenital malformations | No congenital malformations were detected in 14 live births | Extremely limited sample to evaluate malformations. No control group |
| Buchanan et al, 19962 | 36 pregnancies | 53 unexposed pregnancies with systemic lupus erythematosus | Congenital malformations | No congenital malformations were detected in the HCQ-exposed group (1 Down syndrome); 1 case in control group (1 extra finger) | Extremely limited sample to estimate the RR of malformations |
| Parke et al, 19963 | 9 pregnancies | No control | Congenital malformations | No congenital malformations were detected in 9 live births | Extremely limited sample to evaluate malformations. No control group |
| Levy et al, 20014 | 10 pregnancies | 10 in the placebo group | Congenital malformations | No congenital malformations were detected in 19 live births | Extremely limited sample to evaluate malformations despite being a randomized controlled study |
| Costedoat-Chalumeau et al, 20035 | 133 pregnancies | 70 unexposed with similar disorders | Congenital malformations | 3 malformations were observed in the HCQ group (1 hypospadias, 1 craniostenosis, and 1 cardiac malformation, and 1 pulmonary hypoplasia in a preterm birth) vs 4 in the group not exposed to HCQ (1 hypospadias, 1 aplasia cutis of the scalp, 1 ulnar, and 1 severe cardiac malformation) | Modest sample to estimate the risk of malformations. Controlled study. Daily dose of 400–200 mg |
| Motta et al, 20056 | 40 pregnancies | No control | Congenital malformations | No congenital malformations were detected in 39 live births | Extremely limited sample to evaluate malformations. No control group |
| Clowse et al, 20067 | 56 pregnancies with continuous use of HCQ during pregnancy | 163 unexposed, with lupus | Congenital malformations | Miscarriage risk was 13% in the HCQ-exposed group and 4% in the group not exposed to HCQ and stillbirth 8% and 6%, respectively. Among the 47 live-born infants exposed to HCQ, 1 had cleft lip and palate. In the 145 not exposed to HCQ, 3 fetuses had fatal congenital anomalies and 1 had an abdominal hernia | Limited sample to estimate the RR of malformations. Controlled study |
| Viktil et al, 20128 | 58 pregnancies exposed between 3 months before delivery to delivery (34, first trimester of pregnancy) | Population reference | Congenital malformations and major congenital malformations | Exposed to HCQ, 4 of 58 (6.9%) Overall cohort, 5000 of 154,976 (3.2%) |
Limited sample to evaluate the risk of malformations. Reference group does not consider indication or other confounders |
| Diav-Citrin et al, 20139 | 114 pregnancies | 455 unexposed | Congenital malformations | 7 of 97 in exposed (7.2%) vs 15 of 440 (3.4%) in unexposed (malformations in the exposed: 1 spina bifida, 2 developmental dysplasia of the hip, 1 ventricular septal defect, 1 congenital hypothyroidism, 1 inguinal hernia, 1 congenital toxoplasmosis) | Modest sample to evaluate the RR of malformations; no control for indication. Daily dose of 200 to 400 mg |
| Cooper et al, 201410 | 194 pregnancies exposed during the first trimester | 171 women with similar indications treated before but not during, pregnancy | Congenital malformations; counts provided for specific malformations | RR, 3.11 (0.99–9.77). Malformations were more common in HCQ-exposed group (6.7%) than in the reference (2.3%) or other immunosuppressive medication (3%) group. Most common were genitourinary and cardiac but no clear pattern | Controlled study that adjusted for confounding (sociodemographic variables, chronic health diagnoses, medications used to treat chronic diseases, chronic immune-mediated diseases, geographic factors, and calendar year of pregnancy). Limited power to estimate the risk of specific congenital malformations |
| Gayed et al, 201411 | 149 pregnancies during pregnancy or breastfeeding | 139 unexposed with systemic lupus erythematosus | Congenital malformations | Exposed, 3 of 143 (2.1%) Unexposed 3 of 134 (2.2%) |
Modest sample to estimate the RR of malformations; no control for confounding other than indication. Exposure outside relevant period possible. Only abstract, no peer review |
| Koh et al, 201512 | 33 pregnancies exposed during pregnancy | No control group designed for risk estimates of congenital malformation | Goal not to assess congenital malformations, noted as remarks. Goal not to study treatments | No congenital malformations were detected in 33 HCQ-exposed pregnancies | Extremely limited sample to observe any malformation cases. No control group for malformations |
HCQ, hydroxychloroquine; RR, relation risk.
Huybrechts et al. Hydroxychloroquine and birth defects. Am J Obstet Gynecol 2021.
Supplemental Table 2.
Definition of congenital malformations
| Malformation group | Malformation subgroup | ICD-9 diagnosis code |
|---|---|---|
| Cardiovascular anomaliesa | Conotruncal defects | 745.0x, 745.1x, 745.2x |
| Single ventricle | 745.3x | |
| Ventricular septal defect | 745.4x | |
| Secundum atrial septal defect or patent foramen ovale | 745.5x and no preterm | |
| Atrioventricular septal defect | 745.6x | |
| Right-sided defects | 746.00, 746.01, 746.09, 746.1x, 746.2x, 746.83, 747.3x and no preterm, 746.02 and no preterm | |
| Left-sided defects | 747.1x, 747.2x, 746.3x, 746.5x, 746.7x, 746.81, 746.82, 746.84 | |
| Patent ductus arteriosus | 747.0x and no preterm | |
| Persistent pulmonary hypertension of the newborn | 416.0x or 747.83 and no preterm | |
| Anomalous pulmonary venous return | 747.4x | |
| Other cardiac malformation | 745.7x,745.8x, 746.8 (exclude if only 746.86), 746.85–746.87, 746.89 | |
| Cardiac malformation not otherwise specified | 745, 745.9, 746, 746.9x (exclude if only 746.99), 747 | |
| Central nervous system | Overall | 740.xx–742.xx |
| Microcephaly | 742.1x | |
| Hydrocephaly | 742.3x | |
| Reduction deformities of the brain | 742.2x | |
| Neural tube defects | 741.xx, 756.17, 740.0x, 740.2x, 742.0x | |
| Clubfoot | 754.50, 754.51, 754.59, 754.60, 754.62, 754.69, 754.70, 754.71, 754.79 | |
| Gastroschisis | 756.73 if coded after October 2009 | |
| 756.79 and ICD-9 procedure 54.71 if coded before October 2009 | ||
| Oral clefta | Cleft palate | 749.0x |
| Cleft lip | 749.1x | |
| Cleft palate with cleft lip | 749.2x | |
| Eye anomalies | 743.xx (exclude if only 743.6x and 743.8x) | |
| Ear anomalies | 744.xx (exclude if only 744.1x, 744.21, 744.29, and 744.4x–744.9x) | |
| Other vascular (noncardiac) anomalies | 747.6x–747.9x (exclude if only 747.83) | |
| Respiratory anomaliesa | 748.xx (exclude if only 748.1x) | |
| Gastrointestinal anomaliesa | 750.xx–751.xx (exclude if only 750.0x, 750.1x, 750.50, 751.0x) | |
| Genital anomaliesa | 752.xx (exclude if only 752.42, 752.52) (in addition, exclude 752.5x if preterm) | |
| Urinary anomalies | 753.xx (exclude if only 753.7x) | |
| Musculoskeletal anomaliesa | 754.xx and 756.xx (exclude if only 754.3x, 754.81, 754.82, 756.2x) | |
| Limb defectsa | 755.xx (exclude if only 755.65) | |
| Other anomalies | 757.xx; 759.xx (exclude if only 757.2–757.6, 759.81–759.83) |
In- and outpatient claims in the infant record between DoB and DoB + 90 days and/or in the maternal record between delivery and delivery + 30 days are considered.
-
•Greater than or equal to 2 dates with a code for a malformation within the group
-
○Exception: codes 747.3x, 746.02, 745.5x, and 747.0x require ≥2 dates with a code for a malformation of which at least 1 code is documented at ≥6 weeks after DoB.
-
○
-
•Greater than or equal to 1 date with a code for a malformation within the subgroup and cardiac procedure.
-
○Exception: code 746.02
-
○
-
•Greater than or equal to 1 date with a code for a malformation within the subgroup and infant died.
-
•If codes identified in the maternal record between LMP and LMP + 105 days and there are no codes in the infant record between DoB and DoB + 90 days (ie, only maternal codes between delivery and delivery + 30 days), the defect is considered a preexisting maternal defect.
-
•Any of the subgroups of cardiovascular anomalies is present.
-
•Greater than or equal to 2 dates with a code for any of the cardiac malformations (regardless of the subgroupb).
-
•Greater than or equal to 1 date with a code for any of the cardiac malformationsb and cardiac procedure.
-
•Greater than or equal to 1 date with a code for any of the cardiac malformationsb and infant died.
-
•If codes identified in the maternal record between LMP and LMP + 105 days and there are no codes in the infant record between DoB and DoB + 90 days (ie, only maternal codes between delivery and delivery + 30 days), the defect is considered a preexisting maternal defect.
-
•Greater than or equal to 2 dates with a code for the malformation group or subgroup
-
○Exception: for gastroschisis: if code 756.79 was used (before October 2009), requires ≥1 date with a code and ICD-9 procedure 54.71.
-
○
-
•Greater than or equal to 1 date with a code for the malformation group or subgroup and malformation-specific procedure.
-
•Greater than or equal to 1 date with a code for the malformation group of subgroup and infant died.
-
•If codes identified in the maternal record between LMP and LMP + 105 days and there are no codes in the infant record between DoB and DoB + 90 days (ie, only maternal codes between delivery and delivery + 30 days), the defect is considered a preexisting maternal defect.
-
•Any of the malformation groups or subgroups mentioned above is present.
DoB, date of birth; ICD-9, International Classification of Diseases, Ninth Revision; LMP, last menstrual period.
Huybrechts et al. Hydroxychloroquine and birth defects. Am J Obstet Gynecol 2021.
Specific malformation types that are considered individually
The following codes are not considered: 745.4x, 745.5x, 747.0x, 746.4x, 746.6x, 746.99, 747.3x if preterm, 746.02 if preterm, 747.5x, 416.0x if preterm, 747.83 if preterm, 746.08, 746.105.
Supplemental Table 3.
Patient characteristics for HCQ-exposed pregnancies and pregnancies not exposed to HCQ: MarketScan cohort (2003–2005)
| Variable | Original source cohort |
Restricted matched cohorta |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCQ exposed | Unexposed | Standardized difference | HCQ exposed | Unexposed | Standardized difference | |||||
| Total | 1359 | 1,317,520 | — | 1261 | 11,179 | — | ||||
| Age, mean (SD) | 33.0 | (4.4) | 31.9 | (4.6) | 0.234 | (33.1) | 4.4 | (33.1) | 4.6 | −0.016 |
| Region, n (%) | ||||||||||
| Northeast | 203 | (14.9) | 207,517 | (15.8) | −0.023 | 199 | (15.8) | 1754 | (15.7) | 0.003 |
| Midwest | 298 | (21.9) | 338,554 | (25.7) | −0.089 | 278 | (22.1) | 2548 | (22.8) | −0.018 |
| South | 608 | (44.7) | 520,269 | (39.5) | 0.106 | 553 | (43.9) | 4796 | (42.9) | 0.019 |
| West | 232 | (17.1) | 235,923 | (17.9) | −0.022 | 215 | (17.1) | 1949 | (17.4) | −0.010 |
| Unknown | 18 | (1.3) | 15,257 | (1.2) | 0.015 | 16 | (1.3) | 132 | (1.2) | 0.008 |
| Autoimmune rheumatic disordersb, n (%) | ||||||||||
| Systemic lupus erythematosus | 759 | (55.9) | 2188 | (0.2) | 1.581 | 675 | (53.5) | 6065 | (54.3) | −0.015 |
| Rheumatoid arthritis | 408 | (30.0) | 3028 | (0.2) | 0.914 | 392 | (31.1) | 3515 | (31.4) | −0.008 |
| Ankylosing spondylitis | 14 | (1.0) | 4654 | (0.4) | 0.082 | 14 | (1.1) | 168 | (1.5) | −0.035 |
| Psoriatic arthritis | 15 | (1.1) | 404 | (0.0) | 0.143 | 15 | (1.2) | 127 | (1.1) | 0.005 |
| Sicca syndrome | 134 | (9.9) | 647 | (0.0) | 0.464 | 122 | (9.7) | 1113 | (10.0) | −0.009 |
| Dermatomyositis | 17 | (1.3) | 60 | (0.0) | 0.158 | 13 | (1.0) | 112 | (1.0) | 0.003 |
| Other diffuse connective tissue disease | 221 | (16.3) | 1000 | (0.1) | 0.619 | 197 | (15.6) | 1774 | (15.9) | −0.007 |
| Other autoimmune disease | 68 | (5.0) | 1295 | (0.1) | 0.315 | 67 | (5.3) | 567 | (5.1) | 0.011 |
| Sarcoidosis | 6 | (0.4) | 275 | (0.0) | 0.087 | 6 | (0.5) | 50 | (0.5) | 0.004 |
| Other maternal conditionsc, n (%) | ||||||||||
| Attention deficit hyperactivity disorder | 13 | (1.0) | 6198 | (0.5) | 0.058 | 12 | (1.0) | 109 | (1.0) | −0.002 |
| Adjustment disorder | 4 | (0.3) | 3291 | (0.3) | 0.009 | 4 | (0.3) | 27 | (0.3) | 0.014 |
| Alcohol abuse or dependence | 2 | (0.2) | 1125 | (0.1) | 0.018 | 1 | (0.1) | 15 | (0.1) | −0.016 |
| Anxiety | 63 | (4.6) | 40,106 | (3.0) | 0.083 | 58 | (4.6) | 510 | (4.6) | 0.002 |
| Bipolar disorder | 5 | (0.4) | 4030 | (0.3) | 0.011 | 4 | (0.3) | 41 | (0.4) | −0.008 |
| Delirium | 1 | (0.1) | 638 | (0.1) | 0.010 | 1 | (0.1) | 10 | (0.1) | −0.003 |
| Depression | 76 | (5.6) | 51,324 | (3.9) | 0.080 | 72 | (5.7) | 645 | (5.8) | −0.003 |
| Drug abuse or dependence | 6 | (0.4) | 1850 | (0.1) | 0.056 | 6 | (0.5) | 46 | (0.4) | 0.010 |
| Other psychiatric disorders | 4 | (0.3) | 4017 | (0.3) | −0.002 | 4 | (0.3) | 40 | (0.4) | −0.008 |
| Personality disorder | 1 | (0.1) | 469 | (0.0) | 0.016 | 1 | (0.1) | 6 | (0.1) | 0.011 |
| Psychosis | 0 | (0.0) | 436 | (0.0) | −0.026 | 0 | (0.0) | 0 | (0.0) | — |
| Schizophrenia | 2 | (0.2) | 126 | (0.0) | 0.049 | 1 | (0.1) | 5 | (0.0) | 0.015 |
| Sleep disorder | 35 | (2.6) | 11,100 | (0.8) | 0.134 | 29 | (2.3) | 290 | (2.6) | −0.019 |
| Tobacco use | 10 | (0.7) | 9354 | (0.7) | 0.003 | 10 | (0.8) | 86 | (0.8) | 0.003 |
| Anemia | 93 | (6.8) | 25,507 | (1.9) | 0.241 | 84 | (6.7) | 730 | (6.5) | 0.005 |
| Asthma | 51 | (3.8) | 26,538 | (2.0) | 0.104 | 47 | (3.7) | 427 | (3.8) | −0.005 |
| Chronic obstructive pulmonary disease | 13 | (1.0) | 8896 | (0.7) | 0.031 | 11 | (0.9) | 116 | (1.0) | −0.017 |
| Chronic fatigue syndrome | 137 | (10.1) | 53,082 | (4.0) | 0.238 | 129 | (10.2) | 1187 | (10.6) | −0.013 |
| Diabetes | 34 | (2.5) | 23,650 | (1.8) | 0.049 | 34 | (2.7) | 293 | (2.6) | 0.005 |
| Obesity or overweight | 38 | (2.8) | 22,882 | (1.7) | 0.071 | 34 | (2.7) | 319 | (2.9) | −0.009 |
| Epilepsy or convulsions | 14 | (1.0) | 4025 | (0.3) | 0.089 | 14 | (1.1) | 124 | (1.1) | 0.000 |
| Fibromyalgia | 100 | (7.4) | 18,641 | (1.4) | 0.293 | 93 | (7.4) | 813 | (7.3) | 0.004 |
| Hypertension | 93 | (6.8) | 30,937 | (2.4) | 0.216 | 85 | (6.7) | 797 | (7.1) | −0.015 |
| Inflammatory myopathy | 1 | (0.1) | 102 | (0.0) | 0.033 | 1 | (0.1) | 7 | (0.1) | 0.005 |
| Inflammatory bowel disease | 20 | (1.5) | 14,652 | (1.1) | 0.032 | 20 | (1.6) | 170 | (1.5) | 0.005 |
| Irritable bowel syndrome | 16 | (1.2) | 5366 | (0.4) | 0.087 | 14 | (1.1) | 129 | (1.2) | −0.004 |
| Migraine or headache | 96 | (7.1) | 54,436 | (4.1) | 0.128 | 91 | (7.2) | 793 | (7.1) | 0.005 |
| Nausea and vomiting | 45 | (3.3) | 37,352 | (2.8) | 0.028 | 40 | (3.2) | 372 | (3.3) | −0.009 |
| Neuropathic pain | 68 | (5.0) | 27,054 | (2.1) | 0.160 | 66 | (5.2) | 577 | (5.2) | 0.003 |
| Nonneuropathic pain | 450 | (33.1) | 135,075 | (10.3) | 0.577 | 421 | (33.4) | 3773 | (33.8) | −0.008 |
| Other pain | 13 | (1.0) | 4290 | (0.3) | 0.079 | 12 | (1.0) | 108 | (1.0) | −0.001 |
| Infections | 43 | (3.2) | 17,924 | (1.4) | 0.121 | 38 | (3.0) | 318 | (2.8) | 0.010 |
| Renal disease | 58 | (4.3) | 3379 | (0.3) | 0.272 | 45 | (3.6) | 396 | (3.5) | 0.001 |
| Concomitant medicationsc, n (%) | ||||||||||
| Systemic steroids | 565 | (41.6) | 70,421 | (5.3) | 0.945 | 485 | (38.5) | 4262 | (38.1) | 0.007 |
| Biologic DMARDs | 64 | (4.7) | 1303 | (0.1) | 0.304 | 64 | (5.1) | 575 | (5.2) | −0.003 |
| Nonbiologic DMARDs | 150 | (11.0) | 7535 | (0.6) | 0.459 | 117 | (9.3) | 1021 | (9.1) | 0.005 |
| Anticonvulsants | 63 | (4.6) | 14,365 | (1.1) | 0.214 | 58 | (4.6) | 501 | (4.5) | 0.006 |
| Antidepressants | 220 | (16.2) | 99,633 | (7.6) | 0.269 | 187 | (14.8) | 1724 | (15.4) | −0.017 |
| Antipsychotics | 11 | (0.8) | 2986 | (0.2) | 0.081 | 8 | (0.6) | 67 | (0.6) | 0.004 |
| Anxiolytics | 3 | (0.2) | 2917 | (0.2) | 0.000 | 3 | (0.2) | 30 | (0.3) | −0.006 |
| Barbiturates | 40 | (2.9) | 14,607 | (1.1) | 0.130 | 34 | (2.7) | 317 | (2.8) | −0.008 |
| Benzodiazepines | 116 | (8.5) | 49,574 | (3.8) | 0.200 | 107 | (8.5) | 940 | (8.4) | 0.003 |
| Other hypnotics | 85 | (6.3) | 23,721 | (1.8) | 0.228 | 70 | (5.6) | 631 | (5.6) | −0.004 |
| Stimulants | 21 | (1.6) | 10,212 | (0.8) | 0.072 | 18 | (1.4) | 182 | (1.6) | −0.017 |
| Opioids | 296 | (21.8) | 153,634 | (11.7) | 0.274 | 272 | (21.6) | 2402 | (21.5) | 0.002 |
| Naloxone | 2 | (0.2) | 575 | (0.0) | 0.034 | 2 | (0.2) | 19 | (0.2) | −0.002 |
| Naltrexone | 0 | (0.0) | 97 | (0.0) | −0.012 | 0 | (0.0) | 0 | (0.0) | — |
| Buprenorphine | 2 | (0.2) | 623 | (0.1) | 0.032 | 2 | (0.2) | 19 | (0.2) | −0.002 |
| Methadone | 6 | (0.4) | 2902 | (0.2) | 0.039 | 6 | (0.5) | 46 | (0.4) | 0.010 |
| Acetaminophen | 45 | (3.3) | 16,027 | (1.2) | 0.141 | 38 | (3.0) | 352 | (3.2) | −0.008 |
| NSAIDs | 255 | (18.8) | 65,980 | (5.0) | 0.435 | 235 | (18.6) | 2073 | (18.5) | 0.002 |
| Antidiabetics | 58 | (4.3) | 32,993 | (2.5) | 0.098 | 55 | (4.4) | 436 | (3.9) | 0.023 |
| Antihypertensives | 147 | (10.8) | 40,106 | (3.0) | 0.310 | 127 | (10.1) | 1074 | (9.6) | 0.016 |
| Chloroquine | 1 | (0.1) | 243 | (0.0) | 0.026 | 1 | (0.1) | 6 | (0.1) | 0.010 |
| Insulin | 54 | (4.0) | 13,102 | (1.0) | 0.192 | 51 | (4.0) | 407 | (3.6) | 0.021 |
| Triptans | 38 | (2.8) | 17,138 | (1.3) | 0.106 | 37 | (2.9) | 302 | (2.7) | 0.014 |
| Suspected teratogensd | 219 | (16.1) | 100,774 | (7.7) | 0.264 | 199 | (15.8) | 1785 | (16.0) | −0.005 |
| Markers of burden of illnesse | ||||||||||
| Maternal comorbidity index, mean (SD) | 3.0 | (2.4) | 1.2 | (1.5) | 0.902 | 2.9 | (2.3) | 3.0 | (2.4) | −0.018 |
| Number of distinct diagnoses, mean (SD) | 4.7 | (3.9) | 2.1 | (2.6) | 0.776 | 4.7 | (3.9) | 4.8 | (4.0) | −0.042 |
| Number of non-HCQ prescription drugs, mean (SD) | 3.4 | (3.1) | 1.5 | (2.2) | 0.707 | 3.3 | (3.1) | 3.6 | (3.5) | −0.073 |
| Number of outpatient visits, mean (SD) | 4.1 | (4.4) | 2.1 | (3.2) | 0.536 | 4.1 | (4.4) | 4.4 | (4.4) | −0.055 |
| Number emergency department visits, mean (SD) | 0.1 | (0.4) | 0.1 | (0.3) | 0.107 | 0.1 | (0.4) | 0.1 | (0.4) | −0.002 |
| Hospitalization, n (%) | 19 | (1.4) | 9100 | (0.7) | 0.070 | 17 | (1.4) | 164 | (1.5) | −0.010 |
| Number of hospitalizations, mean (SD) | 0.0 | (0.1) | 0.0 | (0.1) | 0.069 | 0.0 | (0.1) | 0.0 | (0.1) | −0.014 |
| Number of d hospitalized, mean (SD) | 0.1 | (0.5) | 0.0 | (0.4) | 0.058 | 0.0 | (0.4) | 0.1 | (0.7) | −0.040 |
DMARD, disease-modifying antirheumatic drug; HCQ, hydroxychloroquine; NSAID, nonsteroidal antiinflammatory drug; SD, standard deviation.
Huybrechts et al. Hydroxychloroquine and birth defects. Am J Obstet Gynecol 2021.
Given the variable ratio matching, the counts for the unexposed group are weighted counts to demonstrate the balance in baseline covariates
Autoimmune rheumatic disorders were measured from 3 months before the start to the end of pregnancy
Maternal conditions and concomitant medication use were measured from 3 months before the start of pregnancy to the end of the first trimester
Women exposed to known teratogens have been excluded (ie, warfarin, antineoplastic agents, lithium, isotretinoin, misoprostol, thalidomide). Suspected teratogens considered include danazol, methimazole, propylthiouracil, aminoglycosides, trimethoprim, triamterene, sulfasalazine, spasmofen, cholestyramine, potassium iodide, tetracycline, and fluconazole
General markers of the burden of illness were measured during the 3 months before but not during pregnancy, as these measures may be affected by early detection of pregnancy complications
Supplemental Table 4.
Patient characteristics for HCQ-exposed and pregnancies not exposed to HCQ: MAX cohort (2000–2014)
| Variable | Original source cohort |
Restricted matched cohorta |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCQ exposed | Unexposed | Standardized difference | HCQ exposed | Unexposed | Standardized difference | |||||
| Total | 686 | 1,881,069 | 606 | 7901 | ||||||
| Age, mean (SD) | 27.8 | (6.0) | 24.5 | (5.9) | 0.568 | 27.9 | (6.0) | 28.3 | (5.9) | −0.068 |
| Race, n (%) | ||||||||||
| White | 219 | (31.9) | 763,706 | (40.6) | −0.181 | 209 | (34.5) | 2861 | (36.2) | −0.036 |
| Black or African American | 236 | (34.4) | 609,802 | (32.4) | 0.042 | 204 | (33.7) | 2599 | (32.9) | 0.016 |
| Hispanic or Latino | 109 | (15.9) | 271,736 | (14.5) | 0.040 | 97 | (16.0) | 1183 | (15.0) | 0.029 |
| Other or unknown | 122 | (17.8) | 235,825 | (12.5) | 0.147 | 96 | (15.8) | 1258 | (15.9) | −0.002 |
| Region, n (%) | ||||||||||
| Northeast | 171 | (24.9) | 330,760 | (17.6) | 0.180 | 146 | (24.1) | 1953 | (24.7) | −0.014 |
| Midwest | 200 | (29.2) | 592,663 | (31.5) | −0.051 | 180 | (29.7) | 2395 | (30.3) | −0.013 |
| South | 164 | (23.9) | 535,503 | (28.5) | −0.104 | 147 | (24.3) | 1926 | (24.4) | −0.003 |
| West | 151 | (22.0) | 422,143 | (22.4) | −0.010 | 133 | (22.0) | 1628 | (20.6) | 0.033 |
| Autoimmune rheumatic disordersb, n (%) | ||||||||||
| Systemic lupus erythematosus | 487 | (71.0) | 2707 | (0.1) | 2.200 | 409 | (67.5) | 5184 | (65.6) | 0.040 |
| Rheumatoid arthritis | 190 | (27.7) | 3056 | (0.2) | 0.867 | 182 | (30.0) | 2125 | (26.9) | 0.070 |
| Ankylosing spondylitis | <11 | (0.7) | 2588 | (0.1) | 0.090 | <11 | (0.8) | 246 | (3.1) | −0.165 |
| Psoriatic arthritis | <11 | (0.2) | 206 | (0.0) | 0.049 | <11 | (0.2) | 42 | (0.5) | −0.063 |
| Sicca syndrome | 60 | (8.8) | 183 | (0.0) | 0.437 | 42 | (6.9) | 625 | (7.9) | −0.038 |
| Dermatomyositis | <11 | (1.2) | 68 | (0.0) | 0.153 | <11 | (1.3) | 90 | (1.1) | 0.016 |
| Other diffuse connective tissue disease | 91 | (13.3) | 423 | (0.0) | 0.552 | 73 | (12.1) | 1075 | (13.6) | −0.047 |
| Other autoimmune disease | <11 | (1.5) | 402 | (0.0) | 0.168 | <11 | (1.7) | 148 | (1.9) | −0.017 |
| Sarcoidosis | <11 | (1.3) | 455 | (0.0) | 0.158 | <11 | (1.5) | 121 | (1.5) | −0.004 |
| Other maternal conditionsc, n (%) | ||||||||||
| Attention deficit hyperactivity disorder | <11 | (0.9) | 19,977 | (1.1) | −0.019 | <11 | (0.7) | 60 | (0.8) | −0.012 |
| Adjustment disorder | <11 | (0.7) | 10,427 | (0.6) | 0.022 | <11 | (0.7) | 53 | (0.7) | −0.001 |
| Alcohol abuse or dependence | <11 | (0.7) | 13,958 | (0.7) | −0.002 | <11 | (0.7) | 73 | (0.9) | −0.029 |
| Anxiety | 40 | (5.8) | 76,887 | (4.1) | 0.080 | 38 | (6.3) | 500 | (6.3) | −0.002 |
| Bipolar disorder | 11 | (1.6) | 25,851 | (1.4) | 0.019 | 11 | (1.8) | 172 | (2.2) | −0.026 |
| Delirium | <11 | (0.7) | 1701 | (0.1) | 0.100 | <11 | (0.7) | 33 | (0.4) | 0.034 |
| Depression | 75 | (10.9) | 120,954 | (6.4) | 0.160 | 66 | (10.9) | 873 | (11.1) | −0.005 |
| Drug abuse or dependence | 24 | (3.5) | 37,945 | (2.0) | 0.091 | 21 | (3.5) | 291 | (3.7) | −0.012 |
| Other psychiatric disorders | 14 | (2.0) | 22,868 | (1.2) | 0.065 | <11 | (1.7) | 104 | (1.3) | 0.027 |
| Personality disorder | <11 | (0.2) | 4345 | (0.2) | −0.020 | <11 | (0.2) | 17 | (0.2) | −0.011 |
| Psychosis | <11 | (0.4) | 4149 | (0.2) | 0.038 | <11 | (0.3) | 38 | (0.5) | −0.024 |
| Schizophrenia | <11 | (0.3) | 3133 | (0.2) | 0.026 | <11 | (0.3) | 32 | (0.4) | −0.012 |
| Sleep disorder | 18 | (2.6) | 14,869 | (0.8) | 0.142 | 18 | (3.0) | 211 | (2.7) | 0.018 |
| Tobacco use | 29 | (4.2) | 77,232 | (4.1) | 0.006 | 27 | (4.5) | 377 | (4.8) | −0.015 |
| Anemia | 79 | (11.5) | 63,162 | (3.4) | 0.315 | 71 | (11.7) | 936 | (11.9) | −0.004 |
| Asthma | 38 | (5.5) | 79,108 | (4.2) | 0.062 | 34 | (5.6) | 494 | (6.3) | −0.027 |
| Chronic obstructive pulmonary disease | 15 | (2.2) | 35,194 | (1.9) | 0.022 | 13 | (2.2) | 194 | (2.5) | −0.021 |
| Chronic fatigue syndrome | 63 | (9.2) | 60,757 | (3.2) | 0.249 | 57 | (9.4) | 764 | (9.7) | −0.009 |
| Diabetes | 33 | (4.8) | 41,403 | (2.2) | 0.142 | 30 | (5.0) | 391 | (4.9) | 0.000 |
| Obesity or overweight | 33 | (4.8) | 48,447 | (2.6) | 0.119 | 31 | (5.1) | 408 | (5.2) | −0.002 |
| Epilepsy or convulsions | 19 | (2.8) | 13,534 | (0.7) | 0.157 | 15 | (2.5) | 179 | (2.3) | 0.014 |
| Fibromyalgia | 73 | (10.6) | 19,343 | (1.0) | 0.419 | 62 | (10.2) | 906 | (11.5) | −0.040 |
| Hypertension | 74 | (10.8) | 47,746 | (2.5) | 0.335 | 65 | (10.7) | 829 | (10.5) | 0.007 |
| Inflammatory myopathy | <11 | (0.6) | 91 | (0.0) | 0.107 | <11 | (0.5) | 36 | (0.5) | 0.006 |
| Inflammatory bowel disease | 20 | (2.9) | 39,963 | (2.1) | 0.050 | 19 | (3.1) | 293 | (3.7) | −0.032 |
| Irritable bowel syndrome | <11 | (0.9) | 4722 | (0.3) | 0.083 | <11 | (0.8) | 76 | (1.0) | −0.015 |
| Migraine or headache | 98 | (14.3) | 141,233 | (7.5) | 0.219 | 86 | (14.2) | 1160 | (14.7) | −0.014 |
| Nausea and vomiting | 55 | (8.0) | 133,447 | (7.1) | 0.035 | 50 | (8.3) | 694 | (8.8) | −0.019 |
| Neuropathic pain | 27 | (3.9) | 27,145 | (1.4) | 0.154 | 24 | (4.0) | 351 | (4.5) | −0.024 |
| Nonneuropathic pain | 316 | (46.1) | 253,994 | (13.5) | 0.762 | 279 | (46.0) | 3689 | (46.7) | −0.013 |
| Other pain | 25 | (3.6) | 18,050 | (1.0) | 0.180 | 21 | (3.5) | 341 | (4.3) | −0.044 |
| Infections | 59 | (8.6) | 72,412 | (3.9) | 0.198 | 52 | (8.6) | 638 | (8.1) | 0.018 |
| Renal disease | 50 | (7.3) | 6578 | (0.4) | 0.368 | 35 | (5.8) | 433 | (5.5) | 0.013 |
| Concomitant medicationsc, n (%) | ||||||||||
| Systemic steroids | 383 | (55.8) | 69,446 | (3.7) | 1.387 | 309 | (51.0) | 4179 | (52.9) | −0.038 |
| Biologic DMARDs, n (%) | 16 | (2.3) | 459 | (0.0) | 0.215 | 16 | (2.6) | 227 | (2.9) | −0.014 |
| Nonbiologic DMARDs | 129 | (18.8) | 4269 | (0.2) | 0.667 | 93 | (15.4) | 872 | (11.0) | 0.128 |
| Anticonvulsants | 67 | (9.8) | 42,724 | (2.3) | 0.319 | 61 | (10.1) | 848 | (10.7) | −0.022 |
| Antidepressants | 161 | (23.5) | 174,161 | (9.3) | 0.391 | 140 | (23.1) | 1960 | (24.8) | −0.040 |
| Antipsychotics | <11 | (1.3) | 27,612 | (1.5) | −0.013 | <11 | (1.3) | 129 | (1.6) | −0.026 |
| Anxiolytics | <11 | (1.5) | 8696 | (0.5) | 0.102 | <11 | (1.3) | 143 | (1.8) | −0.040 |
| Barbiturates | 30 | (4.4) | 22,716 | (1.2) | 0.193 | 25 | (4.1) | 393 | (5.0) | −0.040 |
| Benzodiazepines | 49 | (7.1) | 64,984 | (3.5) | 0.165 | 46 | (7.6) | 617 | (7.8) | −0.008 |
| Other hypnotics | 65 | (9.5) | 68,885 | (3.7) | 0.236 | 55 | (9.1) | 820 | (10.4) | −0.044 |
| Stimulants | <11 | (1.3) | 15,280 | (0.8) | 0.049 | <11 | (1.3) | 117 | (1.5) | −0.014 |
| Opioids | 311 | (45.3) | 427,889 | (22.8) | 0.491 | 281 | (46.4) | 3854 | (48.8) | −0.048 |
| Naloxone | <11 | (0.4) | 4270 | (0.2) | 0.037 | <11 | (0.5) | 34 | (0.4) | 0.010 |
| Naltrexone | <11 | (0.2) | 237 | (0.0) | 0.047 | <11 | (0.0) | <11 | (0.0) | −0.010 |
| Buprenorphine | <11 | (0.4) | 4646 | (0.3) | 0.033 | <11 | (0.5) | 33 | (0.4) | 0.012 |
| Methadone | <11 | (0.3) | 6084 | (0.3) | −0.006 | <11 | (0.3) | 53 | (0.7) | −0.049 |
| Acetaminophen | 74 | (10.8) | 78,658 | (4.2) | 0.253 | 63 | (10.4) | 909 | (11.5) | −0.035 |
| NSAIDs | 290 | (42.3) | 317,721 | (16.9) | 0.579 | 254 | (41.9) | 3361 | (42.5) | −0.013 |
| Antidiabetics | 12 | (1.8) | 17,424 | (0.9) | 0.072 | <11 | (1.3) | 153 | (1.9) | −0.049 |
| Antihypertensives | 140 | (20.4) | 56,587 | (3.0) | 0.562 | 113 | (18.7) | 1548 | (19.6) | −0.024 |
| Chloroquine | <11 | (0.2) | 20 | (0.0) | 0.053 | <11 | (0.2) | 13 | (0.2) | 0.000 |
| Insulin | 17 | (2.5) | 16,250 | (0.9) | 0.126 | 14 | (2.3) | 181 | (2.3) | 0.001 |
| Triptans | 20 | (2.9) | 21,062 | (1.1) | 0.128 | 18 | (3.0) | 231 | (2.9) | 0.003 |
| Suspected teratogensd | 152 | (22.2) | 218,185 | (11.6) | 0.285 | 134 | (22.1) | 1918 | (24.3) | −0.051 |
| Markers of burden of illnesse | ||||||||||
| Maternal comorbidity index, mean (SD) | 3.4 | (2.5) | 0.9 | (1.4) | 1.203 | 3.3 | (2.5) | 3.2 | (2.6) | 0.016 |
| Number of distinct diagnoses, mean (SD) | 6.1 | (4.7) | 2.8 | (3.3) | 0.815 | 6.0 | (4.7) | 6.2 | (4.8) | −0.037 |
| Number of non-HCQ prescription drugs, mean (SD) | 4.9 | (3.9) | 1.8 | (2.5) | 0.959 | 4.7 | (3.8) | 4.9 | (4.0) | −0.053 |
| Number of outpatient visits, mean (SD) | 4.9 | (5.9) | 2.1 | (3.7) | 0.580 | 4.8 | (4.9) | 5.0 | (6.5) | −0.050 |
| Number of emergency department visits, mean (SD) | 0.5 | (1.1) | 0.3 | (0.9) | 0.195 | 0.5 | (1.0) | 0.6 | (1.3) | −0.040 |
| Hospitalization, n (%) | 51 | (7.4) | 67,486 | (3.6) | 0.169 | 42 | (6.9) | 525 | (6.7) | 0.011 |
| Number of hospitalizations, mean (SD) | 0.1 | (0.3) | 0.0 | (0.2) | 0.168 | 0.1 | (0.3) | 0.1 | (0.3) | 0.007 |
| Number of d hospitalized, mean (SD) | 0.4 | (2.5) | 0.1 | (1.2) | 0.128 | 0.4 | (2.4) | 0.3 | (1.7) | 0.025 |
Cell size of <11 for the MAX cohort are suppressed in accord with the CMS cell size suppression policy.
CMS, Centers for Medicare and Medicaid Services; DMARD, disease-modifying antirheumatic drug; HCQ, hydroxychloroquine; MAX, Medicaid Analytic eXtract; NSAID, nonsteroidal antiinflammatory drug; SD, standard deviation.
Huybrechts et al. Hydroxychloroquine and birth defects. Am J Obstet Gynecol 2021.
Given the variable ratio matching, the counts for the unexposed group are weighted counts to demonstrate the balance in baseline covariates
Autoimmune rheumatic disorders were measured from 3 months before the start to the end of pregnancy
Maternal conditions and concomitant medication use were measured from 3 months before the start of pregnancy to the end of the first trimester
Women exposed to known teratogens have been excluded (ie, warfarin, antineoplastic agents, lithium, isotretinoin, misoprostol, thalidomide). Suspected teratogens considered include danazol, methimazole, propylthiouracil, aminoglycosides, trimethoprim, triamterene, sulfasalazine, spasmofen, cholestyramine, potassium iodide, tetracycline, and fluconazole
General markers of the burden of illness were measured during the 3 months before but not during pregnancy, as these measures may be affected by early detection of pregnancy complications.
Supplemental Table 5.
Sensitivity analyses: adjusted RRs restricting the cohort to women with a recorded diagnosis of autoimmune rheumatic disorders
| Outcome | Fully adjusted pooled RR (95% CI) |
|
|---|---|---|
| Restricting the reference group to women with recorded diagnosis of autoimmune rheumatic disorders (original estimate) | Restricting both the HCQ and reference groups to women with recorded diagnosis of autoimmune rheumatic disorders (sensitivity analysis) | |
| Malformations overall | 1.26 (1.04–1.54) | 1.27 (1.03–1.57) |
| Cardiac malformations | 1.20 (0.62–2.32) | 1.36 (0.68–2.72) |
| Oral clefts | 3.70 (1.55–8.82) | 3.37 (1.32–8.56) |
| Respiratory malformations | 1.85 (0.94–3.64) | 1.63 (0.63–4.26) |
| Gastrointestinal malformations | 0.89 (0.44–1.82) | 0.86 (0.35–2.14) |
| Genital malformations | 1.16 (0.28–4.76) | 1.01 (0.26–3.90) |
| Urinary malformations | 2.21 (1.26–3.86) | 2.26 (1.27–4.02) |
| Musculoskeletal malformations | 1.17 (0.74–1.86) | 1.09 (0.65–1.83) |
| Limb defects | 1.17 (0.61–2.26) | 1.18 (0.60–2.35) |
CI, confidence interval; HCQ, hydroxychloroquine; RR, relative risk.
Huybrechts et al. Hydroxychloroquine and birth defects. Am J Obstet Gynecol 2021.
References
- 1.Sammaritano L.R., Bermas B.L., Chakravarty E.E. 2020 American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken) 2020;72:461–488. doi: 10.1002/acr.24130. [DOI] [PubMed] [Google Scholar]
- 2.Flint J., Panchal S., Hurrell A. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford) 2016;55:1693–1697. doi: 10.1093/rheumatology/kev404. [DOI] [PubMed] [Google Scholar]
- 3.Bermas B.L., Kim S.C., Huybrechts K. Trends in use of hydroxychloroquine during pregnancy in systemic lupus erythematosus patients from 2001 to 2015. Lupus. 2018;27:1012–1017. doi: 10.1177/0961203317749046. [DOI] [PubMed] [Google Scholar]
- 4.Geleris J., Sun Y., Platt J. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg E.S., Dufort E.M., Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulware D.R., Pullen M.F., Bangdiwala A.S. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Q&A: hydroxychloroquine and COVID-19. 2020. https://www.who.int/news-room/q-a-detail/q-a-hydroxychloroquine-and-covid-19 Available at:
- 8.Rome B.N., Avorn J. Drug evaluation during the Covid-19 pandemic. N Engl J Med. 2020;382:2282–2284. doi: 10.1056/NEJMp2009457. [DOI] [PubMed] [Google Scholar]
- 9.González R., García-Otero L., Pons-Duran C. Hydroxychloroquine efficacy and safety in preventing SARS-CoV-2 infection and COVID-19 disease severity during pregnancy (COVID-Preg): a structured summary of a study protocol for a randomised placebo controlled trial. Trials. 2020;21:607. doi: 10.1186/s13063-020-04557-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchanan N.M., Toubi E., Khamashta M.A., Lima F., Kerslake S., Hughes G.R. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann Rheum Dis. 1996;55:486–488. doi: 10.1136/ard.55.7.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costedoat-Chalumeau N., Amoura Z., Duhaut P. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty-three cases compared with a control group. Arthritis Rheum. 2003;48:3207–3211. doi: 10.1002/art.11304. [DOI] [PubMed] [Google Scholar]
- 12.Clowse M.E., Magder L., Witter F., Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006;54:3640–3647. doi: 10.1002/art.22159. [DOI] [PubMed] [Google Scholar]
- 13.Cooper W.O., Cheetham T.C., Li D.K. Brief report: risk of adverse fetal outcomes associated with immunosuppressive medications for chronic immune-mediated diseases in pregnancy. Arthritis Rheumatol. 2014;66:444–450. doi: 10.1002/art.38262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmsten K., Huybrechts K.F., Mogun H. Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald S.C., Cohen J.M., Panchaud A., McElrath T.F., Huybrechts K.F., Hernández-Díaz S. Identifying pregnancies in insurance claims data: methods and application to retinoid teratogenic surveillance. Pharmacoepidemiol Drug Saf. 2019;28:1211–1221. doi: 10.1002/pds.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margulis A.V., Setoguchi S., Mittleman M.A., Glynn R.J., Dormuth C.R., Hernández-Díaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf. 2013;22:16–24. doi: 10.1002/pds.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patorno E., Huybrechts K.F., Bateman B.T. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376:2245–2254. doi: 10.1056/NEJMoa1612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He M., Huybrechts K.F., Dejene S.Z. Validation of algorithms to identify adverse perinatal outcomes in the Medicaid Analytic eXtract database. Pharmacoepidemiol Drug Saf. 2020;29:419–426. doi: 10.1002/pds.4967. [DOI] [PubMed] [Google Scholar]
- 19.Friedman D., Duncanson L.J., Glickstein J., Buyon J. A review of congenital heart block. Images Paediatr Cardiol. 2003;5:36–48. [PMC free article] [PubMed] [Google Scholar]
- 20.Wasserstein R.L., Lazar N.A. The ASA statement on p-values: context, process, and purpose. Am Stat. 2016;70:129–133. [Google Scholar]
- 21.Costedoat-Chalumeau N., Amoura Z., Aymard G. Evidence of transplacental passage of hydroxychloroquine in humans. Arthritis Rheum. 2002;46:1123–1124. doi: 10.1002/art.10150. [DOI] [PubMed] [Google Scholar]
- 22.Diav-Citrin O., Blyakhman S., Shechtman S., Ornoy A. Pregnancy outcome following in utero exposure to hydroxychloroquine: a prospective comparative observational study. Reprod Toxicol. 2013;39:58–62. doi: 10.1016/j.reprotox.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Leroux M., Desveaux C., Parcevaux M. Impact of hydroxychloroquine on preterm delivery and intrauterine growth restriction in pregnant women with systemic lupus erythematosus: a descriptive cohort study. Lupus. 2015;24:1384–1391. doi: 10.1177/0961203315591027. [DOI] [PubMed] [Google Scholar]
- 24.Motta M., Tincani A., Faden D. Follow-up of infants exposed to hydroxychloroquine given to mothers during pregnancy and lactation. J Perinatol. 2005;25:86–89. doi: 10.1038/sj.jp.7211208. [DOI] [PubMed] [Google Scholar]
- 25.Izmirly P.M., Costedoat-Chalumeau N., Pisoni C.N. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012;126:76–82. doi: 10.1161/CIRCULATIONAHA.111.089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amrhein V., Greenland S., McShane B. Scientists rise up against statistical significance. Nature. 2019;567:305–307. doi: 10.1038/d41586-019-00857-9. [DOI] [PubMed] [Google Scholar]
- 27.Gabourel J.D. Effects of hydroxychloroquine on the growth of mammalian cells in vitro. J Pharmacol Exp Ther. 1963;141:122–130. [PubMed] [Google Scholar]
- 28.Neill W.A., Panayi G.S., Duthie J.J., Prescott R.J. Action of chloroquine phosphate in rheumatoid arthritis. II. Chromosome damaging effect. Ann Rheum Dis. 1973;32:547–550. doi: 10.1136/ard.32.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinke D., Rich-Edwards J.W., Williams P.L. Quantification of selection bias in studies of risk factors for birth defects among livebirths. Paediatr Perinat Epidemiol. 2020 doi: 10.1111/ppe.12650. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmsten K., Huybrechts K.F., Kowal M.K., Mogun H., Hernández-Díaz S. Validity of maternal and infant outcomes within nationwide Medicaid data. Pharmacoepidemiol Drug Saf. 2014;23:646–655. doi: 10.1002/pds.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hviid A., Mølgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ. 2011;183:796–804. doi: 10.1503/cmaj.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bay Bjørn A.M., Ehrenstein V., Hundborg H.H., Nohr E.A., Sørensen H.T., Nørgaard M. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. Am J Ther. 2014;21:73–80. doi: 10.1097/MJT.0b013e3182491e02. [DOI] [PubMed] [Google Scholar]
- 33.Carmichael S.L., Shaw G.M. Maternal corticosteroid use and risk of selected congenital anomalies. Am J Med Genet. 1999;86:242–244. doi: 10.1002/(sici)1096-8628(19990917)86:3<242::aid-ajmg9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
Supplemental References
- 1.Levy M., Buskila D., Gladman D.D., Urowitz M.B., Koren G. Pregnancy outcome following first trimester exposure to chloroquine. Am J Perinatol. 1991;8:174–178. doi: 10.1055/s-2007-999371. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan N.M., Toubi E., Khamashta M.A., Lima F., Kerslake S., Hughes G.R. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann Rheum Dis. 1996;55:486–488. doi: 10.1136/ard.55.7.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parke A., West B. Hydroxychloroquine in pregnant patients with systemic lupus erythematosus. J Rheumatol. 1996;23:1715–1718. [PubMed] [Google Scholar]
- 4.Levy R.A., Vilela V.S., Cataldo M.J. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus. 2001;10:401–404. doi: 10.1191/096120301678646137. [DOI] [PubMed] [Google Scholar]
- 5.Costedoat-Chalumeau N., Amoura Z., Duhaut P. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty-three cases compared with a control group. Arthritis Rheum. 2003;48:3207–3211. doi: 10.1002/art.11304. [DOI] [PubMed] [Google Scholar]
- 6.Motta M., Tincani A., Faden D. Follow-up of infants exposed to hydroxychloroquine given to mothers during pregnancy and lactation. J Perinatol. 2005;25:86–89. doi: 10.1038/sj.jp.7211208. [DOI] [PubMed] [Google Scholar]
- 7.Clowse M.E., Magder L., Witter F., Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006;54:3640–3647. doi: 10.1002/art.22159. [DOI] [PubMed] [Google Scholar]
- 8.Viktil K.K., Engeland A., Furu K. Outcomes after anti-rheumatic drug use before and during pregnancy: a cohort study among 150,000 pregnant women and expectant fathers. Scand J Rheumatol. 2012;41:196–201. doi: 10.3109/03009742.2011.626442. [DOI] [PubMed] [Google Scholar]
- 9.Diav-Citrin O., Blyakhman S., Shechtman S., Ornoy A. Pregnancy outcome following in utero exposure to hydroxychloroquine: a prospective comparative observational study. Reprod Toxicol. 2013;39:58–62. doi: 10.1016/j.reprotox.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Cooper W.O., Cheetham T.C., Li D.K. Brief report: risk of adverse fetal outcomes associated with immunosuppressive medications for chronic immune-mediated diseases in pregnancy. Arthritis Rheumatol. 2014;66:444–450. doi: 10.1002/art.38262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gayed M., Khamashta M., Culliford D. O58. Longterm outcomes of children born to mothers with SLE exposed to hydroxychloroquine in pregnancy. Rheumatology. 2014;53(Suppl 1):i55. [Google Scholar]
- 12.Koh J.H., Ko H.S., Kwok S.K., Ju J.H., Park S.H. Hydroxychloroquine and pregnancy on lupus flares in Korean patients with systemic lupus erythematosus. Lupus. 2015;24:210–217. doi: 10.1177/0961203314555352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.