Abstract
Coronavirus disease 2019 (COVID-19) can result in deterioration of cardiac function, which is associated with high mortality. A simple point-of-care diagnostic test to screen for ventricular dysfunction would be clinically useful to guide management. We sought to review the clinical experience with an artificial intelligence electrocardiogram (AI ECG) to screen for ventricular dysfunction in patients with documented COVID-19. We examined all patients in the Mayo Clinic system who underwent clinically indicated electrocardiography and echocardiography within 2 weeks following a positive COVID-19 test and had permitted use of their data for research were included. Of the 27 patients who met the inclusion criteria, one had a history of normal ventricular function who developed COVID-19 myocarditis with rapid clinical decline. The initial AI ECG in this patient indicated normal ventricular function. Repeat AI ECG showed a probability of ejection fraction (EF) less than or equal to 40% of 90.2%, corroborated with an echocardiographic EF of 35%. One other patient had a pre-existing EF less than or equal to 40%, accurately detected by the algorithm before and after COVID-19 diagnosis, and another was found to have a low EF by AI ECG and echocardiography with the COVID-19 diagnosis. The area under the curve for detection of EF less than or equal to 40% was 0.95. This case series suggests that the AI ECG, previously shown to detect ventricular dysfunction in a large general population, may be useful as a screening tool for the detection of cardiac dysfunction in patients with COVID-19.
Cardiac injury is common among patients with coronavirus disease 2019 (COVID-19). The clinical presentation is variable, ranging from asymptomatic troponin elevation or chest pain with ST-segment elevation, to fulminant myocarditis with abrupt clinical deterioration due to the development of left ventricular (LV) dysfunction and even death.1, 2, 3, 4, 5 The ability to rapidly, safely, and noninvasively identify the presence of LV dysfunction may facilitate the management of patients with COVID-19. The most commonly performed test to assess LV function is transthoracic echocardiography, but this is effort intensive and requires significant expertise and prolonged exposure to health care staff during image acquisition.
We have previously shown that the application of artificial intelligence (AI) by means of a convolutional neural network to a standard 10-second, 12-lead electrocardiogram (ECG) identifies the presence of LV dysfunction (area under the receiver operator curve [AUC], 0.93) in a general population.6 We have also shown that AI ECG can be applied using smartphone-enabled electrodes and that it performs well among diverse ethnic, age, racial, and sex groups.7 , 8 On the basis of this work, on May 11, 2020, the US Food and Drug Administration issued an Emergency Use Authorization for the application of this algorithm in COVID-19 patients. Therefore, we sought to provide a preliminary report of the performance of the AI ECG for the identification of LV dysfunction specifically in COVID-19 patients.
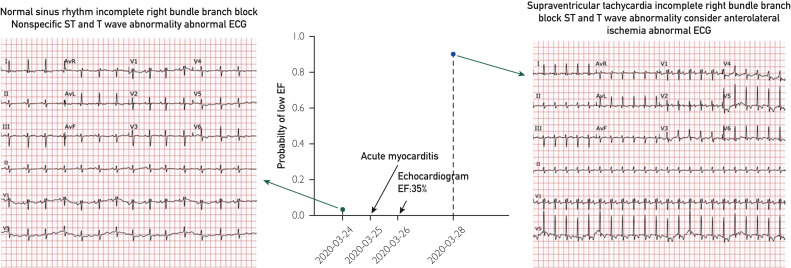
We identified 27 patients in the Mayo Clinic comprehensive electronic medical record with a positive COVID-19 test and a 12-lead ECG performed within 14 days of an echocardiogram (Table ). The mean age was 67±14 years, 67% were men, and 3 of 27 (11.1%) had depressed ventricular function and COVID-19, one presumed secondary to COVID-19 myocarditis (Figure ). This patient was a 77-year-old woman with transfusion-dependent myelodysplastic syndrome, chronic obstructive pulmonary disease requiring supplemental oxygen nocturnally, who presented with fevers and increased oxygen requirements, and fifth-generation troponin of 42 ng/L (peak during hospitalization 57 ng/L). She underwent cardiac magnetic resonance imaging 9 months before admission to assess for iron overload that showed ejection fraction (EF) at 61% (normal). The AI analysis of the admission 12-lead ECG indicated normal ventricular function (Figure). The patient was treated with intravenous immunoglobulin, methylprednisolone, and intravenous heparin for COVID-19 myocarditis, complicated by acute systolic heart failure. An echocardiogram on hospital day 2 showed an EF of 35%; a 12-lead ECG performed on hospital day 4 had a 90.2% probability of EF less than or equal to 40% with AI analysis. The patient developed progressive dyspnea and ultimately died from her illness. There were two other patients with LV dysfunction (EF≤40%), one before and after COVID-19 diagnosis, accurately detected by the AI ECG before and after COVID-19, and the other identified at the time of COVID-19 diagnosis. No other COVID-19 patient had EF less than or equal to 40%. The AI ECG test AUC was 0.95 for detection of an EF less than or equal to 40%.
Table.
Patient Characteristics
| Characteristic | Total cohort (n=27) | LVEF ≤40% (n=3) | LVEF >40% (n=24) |
|---|---|---|---|
| Age, years | 66.5±13.8 | 81.4±3.3 | 64.6±13.5 |
| Sex (M/F) | 18/9 | 1/2 | 17/7 |
| HTN, mm Hg | 12 | 2 | 10 |
| BMI, kg/mL2 | 28.9±4.2 | 24.8±3.8 | 29.4±4 |
| Ejection fraction, % | 57.1±13.5 | 25.6±11.1 | 61.1±7.1 |
| ARDS, n (%) | 15 (55.6) | 1 (33.3) | 14 (58.3) |
ARDS = acute respiratory distress syndrome; BMI = body mass index; F = female; HTN = hypertension; M = male.
Figure.
Electrocardiograms (ECGs) and artificial intelligence ECG results in a 77-year-old woman (patient #8) who developed left ventricular dysfunction due to coronavirus disease 2019 myocarditis. EF = ejection fraction.
We have previously shown that a neural network can be trained to identify subtle and nonspecific patterns in a standard ECG to identify the presence of occult cardiovascular disease including LV dysfunction, intermittent atrial fibrillation, and hypertrophic cardiomyopathy.9 , 10 The fact that the coronavirus spike protein binds to the angiotensin-converting enzyme 2 receptor, which is richly expressed in cardiac tissue, might explain the cardiotropic behavior of the virus. Given that the presence of cardiovascular disease is associated with a worse outcome and higher risk of death with COVID-19 infection, the ability to rapidly identify such individuals for specific therapeutic interventions or vaccine trials may be clinically important. Although the number of patients in this early series is small, the AUC of 0.95 to detect an EF less than or equal to 40% is consistent with the findings in a much larger general study that included 97,829 patients (AUC, 0.93), suggesting the performance of the network is generalizable to COVID-19 patients. This is an important observation because the data set used to train the network had no COVID-19–infected subjects. However, because this is a retrospective study, we lack routine rhythm surveillance, troponin measurements, or other clinical markers to identify the extent of cardiac involvement. Nonetheless, the ability of a 12-lead ECG to detect the presence of LV dysfunction may enhance clinical care while minimizing the risk of exposure associated with potentially unnecessary echocardiography in patients unlikely to have cardiac involvement. The fact that the algorithm worked in patients with ventricular dysfunction due to and independent of COVID-19 infection suggests that the AI ECG appears to detect ventricular dysfunction irrespective of causative disease, as per its design. Additionally, it suggests that COVID-19 infection does not impede AI ECG performance.
This early feasibility study, while requiring further validation, supports the Emergency Use Authorization issued by the US Food and Drug Administration and further study in this cohort.
Footnotes
Potential Competing Interests: Mayo Clinic has licensed the underlying technology to EKO, a maker of digital stethoscopes with embedded electrocardiogram electrodes. Mayo Clinic may receive financial benefit from the use of this technology, but at no point will Mayo Clinic benefit financially from its use for the care of patients at Mayo Clinic. Drs Friedman, Lopez-Jimenez, Kapa, and Attia may also receive financial benefit from this agreement. With the patent “WO2019070978A1”.
References
- 1.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with COVID-19 — a case series. N Engl J Med. 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attia Z.I., Kapa S., Lopez-Jimenez F. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019;25(1):70–74. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 7.Yasin O.Z., Attia Z., Dillon J.J. Noninvasive blood potassium measurement using signal-processed, single-lead ecg acquired from a handheld smartphone. J Electrocardiol. 2017;50(5):620–625. doi: 10.1016/j.jelectrocard.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noseworthy P.A., Attia Z.I., Brewer L.C. Assessing and mitigating bias in medical artificial intelligence: the effects of race and ethnicity on a deep learning model for ECG analysis. Circ Arrhythm Electrophysiol. 2020;13(3):e007988. doi: 10.1161/CIRCEP.119.007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attia Z.I., Noseworthy P.A., Lopez-Jimenez F. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394(10201):861–867. doi: 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]
- 10.Ko W.Y., Siontis K.C., Attia Z.I. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J Am Coll Cardiol. 2020;75(7):722–733. doi: 10.1016/j.jacc.2019.12.030. [DOI] [PubMed] [Google Scholar]