Abstract
Rationale & Objective
Underlying kidney disease is an emerging risk factor for more severe coronavirus disease 2019 (COVID-19) illness. We examined the clinical courses of critically ill COVID-19 patients with and without pre-existing chronic kidney disease (CKD) and investigated the association between the degree of underlying kidney disease and in-hospital outcomes.
Study Design
Retrospective cohort study.
Settings & Participants
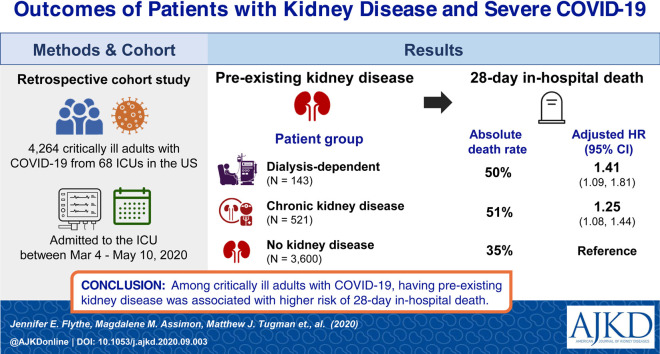
4,264 critically ill patients with COVID-19 (143 patients with pre-existing kidney failure receiving maintenance dialysis; 521 patients with pre-existing non-dialysis-dependent CKD; and 3,600 patients without pre-existing CKD) admitted to intensive care units (ICUs) at 68 hospitals across the United States.
Predictor(s)
Presence (vs absence) of pre-existing kidney disease.
Outcome(s)
In-hospital mortality (primary); respiratory failure, shock, ventricular arrhythmia/cardiac arrest, thromboembolic events, major bleeds, and acute liver injury (secondary).
Analytical Approach
We used standardized differences to compare patient characteristics (values > 0.10 indicate a meaningful difference between groups) and multivariable-adjusted Fine and Gray survival models to examine outcome associations.
Results
Dialysis patients had a shorter time from symptom onset to ICU admission compared to other groups (median of 4 [IQR, 2-9] days for maintenance dialysis patients; 7 [IQR, 3-10] days for non-dialysis-dependent CKD patients; and 7 [IQR, 4-10] days for patients without pre-existing CKD). More dialysis patients (25%) reported altered mental status than those with non-dialysis-dependent CKD (20%; standardized difference = 0.12) and those without pre-existing CKD (12%; standardized difference = 0.36). Half of dialysis and non-dialysis-dependent CKD patients died within 28 days of ICU admission versus 35% of patients without pre-existing CKD. Compared to patients without pre-existing CKD, dialysis patients had higher risk for 28-day in-hospital death (adjusted HR, 1.41 [95% CI, 1.09-1.81]), while patients with non-dialysis-dependent CKD had an intermediate risk (adjusted HR, 1.25 [95% CI, 1.08-1.44]).
Limitations
Potential residual confounding.
Conclusions
Findings highlight the high mortality of individuals with underlying kidney disease and severe COVID-19, underscoring the importance of identifying safe and effective COVID-19 therapies in this vulnerable population.
Index Words: Coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), critical illness, chronic kidney disease (CKD), end-stage kidney disease (ESKD), dialysis, in-hospital mortality, clinical course, intensive care unit (ICU), end-stage renal disease (ESRD), COVID-19 outcome, altered mental status, prognosis, severe COVID-19, glomerular filtration rate (GFR), renal function, clinical trajectory
Graphical abstract
Plain-Language Summary.
Individuals with underlying kidney disease may be particularly vulnerable to severe coronavirus disease 2019 (COVID-19) illness, marked by multisystem organ failure, thrombosis, and a heightened inflammatory response. Among 4,264 critically ill adults with COVID-19 admitted to 68 intensive care units across the United States, we found that both non-dialysis-dependent chronic kidney disease patients and maintenance dialysis patients had a 28-day in-hospital mortality rate of ∼50%. Patients with underlying kidney disease had higher in-hospital mortality than patients without pre-existing kidney disease, with patients receiving maintenance dialysis having the highest risk. As evidenced by differences in symptoms and clinical trajectories, patients with pre-existing kidney disease may have unique susceptibility to COVID-19–related complications, which warrants additional study and special consideration in the pursuit and development of targeted therapies.
Editorial, p. 175
Since its emergence in Wuhan, China, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has snowballed into a global pandemic, infecting more than 28 million people across the globe and killing more than 900,000 as of mid-September 2020.1 Emerging data suggests that individuals with underlying kidney dysfunction have worse COVID-19–related outcomes than those without kidney dysfunction.2, 3, 4, 5, 6, 7, 8 Similar outcome differences across patients with and without kidney dysfunction have been observed in other illness states (eg, general critical illness9 and influenza10) and may relate in part to innate immunity impairment, vascular dysfunction, and the heightened inflammatory state that are characteristic of advanced chronic kidney disease (CKD).11, 12, 13
As such, individuals with underlying CKD may be particularly vulnerable to COVID-19–related critical illness, marked by multi-system organ failure, thrombosis, and a heightened inflammatory response. COVID-19–related critical illness affects ∼10% of patients hospitalized with COVID-19 and has an exceedingly high mortality rate.14, 15, 16, 17 Data from the United States indicate that patients with critical COVID-19 illness complicated by acute kidney injury have worse outcomes than those without acute kidney injury.18, 19, 20, 21 Single-center and regional studies suggest that similarly poor outcomes occur among individuals with critical COVID-19 illness and pre-existing CKD, especially in patients with kidney failure receiving maintenance dialysis, but study sample sizes were limited and most lacked comparator populations.20, 21, 22, 23, 24, 25 Given the rapidly changing landscape of COVID-19 therapeutics and the potential for reduced kidney function to limit therapeutic options (eg, remdesivir), granular and broadly representative data characterizing the clinical courses of critically ill patients with COVID-19 and pre-existing CKD are needed to inform the management of this vulnerable population.
To address this knowledge gap, we used data from the Study of the Treatment and Outcomes in Critically Ill Patients With COVID-19 (STOP-COVID), a cohort study of more than 4,000 patients with COVID-19 admitted to intensive care units (ICUs) at 68 hospitals across the United States and examined the clinical courses of critically ill COVID-19 patients with and without pre-existing CKD. We also investigated the association between degree of underlying kidney disease and occurrence of in-hospital mortality and other outcomes (eg, respiratory failure, shock, and thromboembolic events).
Methods
Patient Population and Study Design
We used data from STOP-COVID, a multicenter cohort study that enrolled consecutive adults (aged ≥18 years) with laboratory-confirmed COVID-19 admitted to ICUs at 68 geographically diverse US hospitals (Item S1). Cohort compilation and initial results have been previously reported.26 The STOP-COVID parent study was approved by the institutional review boards at each participating site. This ancillary study was approved by the University of North Carolina at Chapel Hill Institutional Review Board (#20-1395). A waiver of informed consent was granted due to the anonymity of the STOP-COVID limited data set used for this project.
In this ancillary study, focused on pre-existing kidney disease, we included 4,264 critically ill COVID-19 patients with and without pre-existing CKD admitted to 68 ICUs in the United States between March 4, 2020 and May 10, 2020. Using a retrospective cohort study design, we followed patients forward in historical time from ICU admission to in-hospital death, hospital discharge, or June 6, 2020, the date of database locking for these analyses. All patients still hospitalized at the time of data analysis had at least 28 days of follow-up. We excluded patients without documented vital signs on ICU day 1 (n = 5).
Data Collection
Study personnel at each STOP-COVID site collected data by detailed medical chart review and used a standardized electronic case report form to enter data into a secure Research Electronic Data Capture (REDCap) database. Abstracted data included demographics, comorbid conditions, and home medications; symptoms and vital signs at ICU admission; longitudinal laboratory and physiologic parameters, therapeutic interventions, and acute organ injury and support during the first 14 days after ICU admission; and dates and contributing causes of in-hospital death. For individuals with pre-existing kidney failure receiving maintenance dialysis, we also collected data on dialysis modality, dialysis vintage, and vascular access type (for hemodialysis patients), all preceding hospital admission.
Exposures
The exposures of interest were the presence of pre-existing non-dialysis-dependent CKD and kidney failure requiring maintanence dialysis. We defined pre-existing non-dialysis-dependent CKD as a baseline estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 (based on either the Modification of Diet in Renal Disease [MDRD] Study27 or CKD Epidemiology Collaboration [CKD-EPI]28 equations) prior to hospitalization on at least 2 consecutive occasions at least 12 weeks apart or, in cases where pre-hospitalization eGFRs were unavailable, the presence of non-dialysis-dependent CKD in the medical chart problem list or past medical history. Individuals with prior kidney transplant were classified according to their baseline eGFR. We defined pre-existing kidney failure requiring maintanence dialysis as medical chart-documented maintenance dialysis therapy prior to hospital admission. We categorized patients without evidence of CKD or maintenance dialysis therapy as having no pre-existing CKD, and these individuals served as our referent group.
Outcomes
Primary outcomes were 14- and 28-day in-hospital mortality. Secondary outcomes included 14-day in-hospital respiratory failure, shock, ventricular arrhythmia or cardiac arrest, thromboembolic events (including ischemic stroke, pulmonary embolism, or deep vein thrombosis), major bleeds, and acute liver injury (Table S1).
Statistical Analysis
All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc). We described patient characteristics on ICU day 1, therapies administered, and laboratory parameters across exposure groups as count (percentage) for categorical variables and as median [interquartile range (IQR)] for continuous variables. We compared baseline covariate distributions using absolute standardized mean differences (ASMDs). An ASMD > 0.10 represents an imbalance (i.e., difference) between exposure groups.29 , 30 A larger ASMD is indicative of a larger between-group difference.
We assessed the association between the presence of pre-existing kidney disease (non-dialysis-dependent CKD and kidney failure requiring maintenance dialysis, separately) versus no pre-existing CKD and 14- and 28-day in-hospital mortality using Fine and Gray proportional subdistribution hazards models. Individuals were followed forward in historical time from ICU admission to the first occurrence of an outcome, censoring event (completion of 14 and 28 days of follow-up), or competing event (hospital discharge). Pre-specified subgroup analyses evaluated the association between vascular access type before hospital admission and dialysis vintage (separately) and mortality in hemodialysis patients, and baseline serum creatinine level (pre-hospital serum creatinine <1.2, 1.2-1.9, and ≥2.0 mg/dL) and mortality in patients with non-dialysis-dependent CKD.
We used similar methods to examine the association between the presence (vs absence) of pre-existing CKD and the occurrence of select secondary outcomes during the 14 days after ICU admission. We restricted these secondary analyses to individuals who were alive and free of the outcome of interest on ICU day 1. Follow-up began on ICU day 2, with both in-hospital death and hospital discharge treated as competing events. We adjusted associative models for demographics (model 1) and separately for demographics and comorbid conditions (model 2) when the number of outcome events was sufficient. Model 1 assesses the association of outcomes and underlying kidney disease overall, whereas model 2 assesses the association of outcomes and underlying kidney disease independent of comorbid conditions known to associate with COVID-19 outcomes.17 , 21 , 31, 32, 33, 34, 35 Using analogous methods, we performed separate sensitivity analyses excluding patients receiving therapeutic-level anticoagulation on ICU day 1 from models examining major bleeds and thromboembolic events, and excluding patients with a history of liver disease from models examining acute liver injury.
Results
Patient Characteristics
A total of 4,264 individuals with COVID-19 critical illness were included in the study: 143 (3%) with pre-existing kidney failure receiving maintenance dialysis, 521 (12%) with pre-existing non-dialysis-dependent CKD, and 3,600 (85%) without pre-existing CKD. Tables 1 and S2 to S4 display the demographic and clinical characteristics on ICU day 1 across study groups. Most (58%) patients in the study were cared for in ICUs located in the northeastern United States. Non-dialysis-dependent CKD patients were older than dialysis patients (median ages of 69 [IQR, 60-76] and 65 [IQR, 56-71] years, respectively; ASMD = 0.31) and patients without pre-existing CKD (median age of 61 [IQR, 51-70] years; ASMD = 0.55). Comorbid conditions including diabetes and cardiovascular conditions were more common in patients with pre-existing CKD (both maintenance dialysis and non-dialysis-dependent CKD patients) compared to those without CKD.
Table 1.
Characteristics of Critically Ill COVID-19 Patients on ICU Day 1
| Characteristica | Maintenance Dialysis Patients (n = 143) | Patients With Non-Dialysis-Dependent CKD (n = 521) | Patients Without Pre-existing CKD (n = 3,600) |
|---|---|---|---|
| Demographics | |||
| Age, y | 65 [56-71] | 69 [60-76] | 61 [51-70] |
| Male sex | 77 (54%) | 323 (62%) | 2,314 (64%) |
| Racea | |||
| White | 48 (34%) | 184 (35%) | 1,403 (39%) |
| Black | 71 (50%) | 232 (45%) | 963 (27%) |
| Other race | 5 (3%) | 34 (7%) | 309 (9%) |
| Unknown/not reported | 19 (13%) | 71 (14%) | 925 (26%) |
| Ethnicitya | |||
| Hispanic | 29 (20%) | 66 (13%) | 919 (26%) |
| Non-Hispanic | 107 (75%) | 411 (79%) | 2,218 (62%) |
| Unknown/not reported | 7 (5%) | 44 (8%) | 463 (13%) |
| BMI, kg/m2 | 28.3 [23.8-34.7] | 30.3 [26.3-36.3] | 30.4 [26.5-35.6] |
| US geographic region | |||
| Northeast | 78 (55%) | 241 (46%) | 2,175 (60%) |
| South | 27 (19%) | 65 (12%) | 388 (11%) |
| Midwest | 31 (22%) | 169 (32%) | 717 (20%) |
| West | 7 (5%) | 46 (9%) | 320 (9%) |
| Comorbid conditions | |||
| Diabetes | 97 (68%) | 329 (63%) | 1,337 (37%) |
| Hypertension | 125 (87%) | 451 (87%) | 2,036 (57%) |
| Coronary artery disease | 55 (38%) | 146 (28%) | 374 (10%) |
| Heart failure | 44 (31%) | 136 (26%) | 233 (6%) |
| Atrial fibrillation or flutter | 31 (22%) | 70 (13%) | 211 (6%) |
| Asthma or COPD | 21 (15%) | 118 (23%) | 617 (17%) |
| Chronic liver disease | 7 (5%) | 33 (6%) | 103 (3%) |
| Home medications | |||
| ACEi or ARB | 35 (24%) | 243 (47%) | 1,127 (31%) |
| β-Blocker | 98 (69%) | 264 (51%) | 768 (21%) |
| Other antihypertensive | 69 (48%) | 296 (57%) | 937 (26%) |
| Statin | 80 (56%) | 317 (61%) | 1,226 (34%) |
| Aspirin | 65 (45%) | 213 (41%) | 668 (19%) |
| Anticoagulant | 38 (27%) | 95 (18%) | 294 (8%) |
| NSAID | 3 (2%) | 26 (5%) | 322 (9%) |
| ICU admission source | |||
| Emergency department | 82 (57%) | 299 (57%) | 1,981 (55%) |
| Hospital ward | 47 (33%) | 165 (32%) | 1,114 (31%) |
| Transfer from another hospital | 12 (8%) | 50 (10%) | 487 (14%) |
| Other | 2 (1%) | 6 (1%) | 18 (1%) |
| Days from symptom onset to ICU admission | 4 [2-9] | 7 [3-10] | 7 [4-10] |
| Symptoms | |||
| Shortness of breath | 85 (59%) | 360 (69%) | 2,733 (76%) |
| Cough | 81 (57%) | 322 (62%) | 2,698 (75%) |
| Fever | 82 (57%) | 294 (56%) | 2,486 (69%) |
| Altered mental status | 36 (25%) | 106 (20%) | 418 (12%) |
| Myalgia or arthralgia | 15 (10%) | 94 (18%) | 815 (23%) |
| Headache | 4 (3%) | 27 (5%) | 343 (10%) |
| Nausea or vomiting | 27 (19%) | 60 (12%) | 573 (16%) |
| Diarrhea | 26 (18%) | 115 (22%) | 708 (20%) |
Note: Values are given as number (percent) for categorical variables and as median [interquartile range] for continuous variables. Absolute standardized mean differences comparing the patient groups with one another are presented in Tables S2 to S4. Variables with missing values are presented in Table S13.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; ICU, intensive care unit; NSAID, nonsteroidal anti-inflammatory drug.
Information on race and ethnicity were abstracted from the electronic health record of each patient. In the United States, people of Hispanic ethnicity are those of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin regardless of race.
Of the 143 maintenance dialysis patients, 128 (90%) received in-center hemodialysis, 9 (6%) received peritoneal dialysis, 2 (1%) received home hemodialysis, and 4 (3%) had an undocumented modality before hospital admission. Of the 128 hemodialysis patients with a known vascular access type, 82 (64%), 35 (27%), and 11 (9%) dialyzed via a fistula, catheter, and graft, respectively, prior to admission.
The median time from COVID-19–related symptom onset to ICU admission was 4 (IQR, 2-9) days among maintenance dialysis patients, 7 (IQR, 3-10) days among non-dialysis-dependent CKD patients, and 7 (IQR, 4-10) days among patients without pre-existing CKD. In general, dialysis patients reported COVID-19–related symptoms before ICU admission at a lower frequency than patients without CKD, with 1 exception: the percentage of dialysis patients reporting altered mental status was more than twice that of patients without CKD (25% vs 12%; ASMD = 0.36) and slightly more than that of patients with non-dialysis-dependent CKD (25% vs 20%; ASMD = 0.12). In addition, respiratory symptoms were less frequent in dialysis patients compared to the other groups.
Tables 2 and S2 to S4 display COVID-19 severity and laboratory findings on ICU day 1 across study groups. A modestly higher percentage of patients without pre-existing CKD (63%) required invasive mechanical ventilation on ICU day 1 compared to maintenance dialysis patients (56%; ASMD = 0.15). Median white blood cell counts, platelet counts, and fibrinogen concentrations on ICU day 1 were lower in dialysis patients compared to patients without pre-existing CKD, whereas median C-reactive protein, interleukin 6, ferritin, and troponin levels were higher in dialysis patients (all ASMDs > 0.10). Similar laboratory patterns were observed for platelet counts, fibrinogen concentrations, and troponin levels when comparing patients with non-dialysis-dependent CKD to patients without pre-existing CKD, but the differences were of lower magnitudes.
Table 2.
Illness Severity and Laboratory Findings of Critically Ill COVID-19 Patients on ICU Day 1
| Characteristic | Maintenance Dialysis Patients (n = 143) | Patients With Non-Dialysis-Dependent CKD (n = 521) | Patients Without Pre-existing CKD (n = 3,600) |
|---|---|---|---|
| Vital signs | |||
| Highest temperature, °C | 37.8 [37.1-38.5] | 37.7 [37.1-38.4] | 38.0 [37.2-38.9] |
| Fever: >38°C | 65 (45%) | 213 (41%) | 1,837 (51%) |
| Lowest systolic BP, mm Hg | 92 [81-108] | 96 [84-112] | 97 [85-110] |
| Highest heart rate, beats/min | 101 [88-119] | 101 [87-116] | 105 [92-121] |
| Highest respiratory rate, breaths/min | 29 [23-35] | 30 [26-36] | 32 [26-38] |
| Severity of illness markers | |||
| Invasive mechanical ventilation | 80 (56%) | 311 (60%) | 2,284 (63%) |
| PEEP, cm H2O | 10 [8-14] | 10 [10-15] | 12 [10-15] |
| Pao2:Fio2 ratio, mm Hg | 164 [101-267] | 125 [79-190] | 122 [83-191] |
| Noninvasive mechanical ventilation | 3 (2%) | 16 (3%) | 86 (2%) |
| High-flow nasal cannula or nonrebreather mask | 29 (20%) | 121 (23%) | 801 (22%) |
| AKI requiring dialysis | — | 27 (5%) | 55 (2%) |
| Renal SOFA scorea | |||
| 0 (creatinine < 1.2 mg/dL) | 0 (0%) | 62 (12%) | 2,347 (65%) |
| 1 (creatinine 1.2-1.9 mg/dL) | 0 (0%) | 168 (32%) | 844 (23%) |
| 2 (creatinine 2.0-3.4 mg/dL) | 0 (0%) | 143 (27%) | 255 (7%) |
| 3 (creatinine 3.5-4.9 mg/dL) | 0 (0%) | 68 (13%) | 71 (2%) |
| 4 (creatinine ≥ 5.0 mg/dL or KRT) | 143 (100%) | 80 (15%) | 83 (2%) |
| Liver SOFA scoreb | |||
| 0 (bilirubin < 1.2 mg/dL) | 132 (92%) | 482 (93%) | 3,253 (90%) |
| 1 (bilirubin 1.2-1.9 mg/dL) | 7 (5%) | 28 (5%) | 251 (7%) |
| ≥ 2 (bilirubin ≥ 2.0 mg/dL) | 4 (3%) | 11 (2%) | 96 (3%) |
| Coagulation SOFA scorec | |||
| 0 (platelet count ≥ 150 K/μL) | 90 (63%) | 408 (78%) | 2,989 (83%) |
| 1 (platelet count 100-149 K/μL) | 37 (26%) | 80 (15%) | 467 (13%) |
| ≥2 (platelet count < 100 K/μL) | 16 (11%) | 33 (6%) | 144 (4%) |
| Vasopressor or inotrope use | 72 (50%) | 219 (42%) | 1,482 (41%) |
| Shockd | 17 (12%) | 75 (14%) | 370 (10%) |
| Coinfections | |||
| Bacterial pneumonia | 24 (17%) | 85 (16%) | 489 (14%) |
| Bacteremia or endocarditis | 11 (8%) | 16 (3%) | 80 (2%) |
| Laboratory findings | |||
| WBC count, ×103/μL | 7.5 [5.7-10.4] | 8.2 [5.8-11.6] | 8.5 [6.1-11.9] |
| Lymphocyte count, ×103/μL | 9.7 [5.4-15.1] | 9.4 [5.7-14.9] | 10.0 [6.0-15.1] |
| Hemoglobin, g/dL | 10.3 [9.0-11.8] | 11.4 [9.5-12.9] | 12.8 [11.4-14.2] |
| Platelet count, ×103/μL | 167 [124-212] | 202 [155-258] | 218 [167-281] |
| Serum creatinine, mg/dL | 7.7 [5.6-10.1] | 2.2 [1.5-3.6] | 1.0 [0.8-1.4] |
| AST, U/L | 46 [30-72] | 48 [33-80] | 54 [36-85] |
| ALT, U/L | 23 [17-40] | 28 [18-47] | 38 [24-62] |
| Bilirubin, mg/dL | 0.6 [0.4-0.7] | 0.5 [0.3-0.8] | 0.6 [0.4-0.8] |
| Lactate, mmol/L | 1.6 [1.0-2.6] | 1.6 [1.0-2.4) | 1.6 [1.1-2.3] |
| CRP, mg/L | 170.0 [73.0-277.7] | 142.4 [84.8-217.5] | 151.0 [81.6-235.7] |
| IL-6, pg/mL | 121.4 [41.3-320.4] | 52.0 [19.0-175.3] | 56.0 [18.0-156.6] |
| Arterial pH | 7.37 [7.28-7.43] | 7.34 [7.26-7.40] | 7.38 [7.30-7.44] |
| Fibrinogen, mg/dL | 542 [369-619] | 588 [442-732] | 614 [491-764] |
| D-Dimer, ng/mL | 1,347 [609-2,623] | 1,385 [670-3,018] | 1,270 [652-3,459] |
| Ferritin, ng/mL | 3,406 [1,795-6,271] | 984 [450-1,981] | 947 [489-1,919] |
| Troponin T, ng/mL | 175 [95-364] | 51 [25-110] | 12 [4-40] |
| Troponin I, ng/mL | 140 [70-330] | 55 [20-170] | 30 [10-110] |
Note: Values are given as number (percent) for categorical variables and as median [interquartile range] for continuous variables. Absolute standardized mean differences comparing the patient groups to one another are presented in Tables S2 to S4. Variables with missing values are presented in Table S13.
Abbreviations: AKI, acute kidney injury; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BP, blood pressure; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; Fio2, fraction of inspired oxygen; ICU, intensive care unit; IL-6, interleukin 6; KRT, kidney replacement therapy; PEEP, positive end-expiratory pressure; SOFA, Sequential Organ Failure Assessment.
Renal component of the SOFA score was based on the serum creatinine level. Patients who did not have a serum creatinine drawn on ICU day 1 were classified as having renal SOFA score of 0, and patients on KRT were classified as having a renal SOFA score of 4.49
Liver component of the SOFA score. Patients who did not have a serum bilirubin drawn on ICU day 1 were classified as having a liver SOFA score of 0.49
Coagulation component of the SOFA score. Patients who did not have a platelet count drawn on ICU day 1 were classified as having a coagulation SOFA score of 0.49
Shock is defined as the requirement of 2 or more vasopressors or inotropes.
Targeted Therapies and Clinical Trajectories
Table 3 displays COVID-19–targeted therapies administered during the 14 days after ICU admission in each group (Table S5: corresponding ASMDs). Compared to maintenance dialysis patients, a higher percentage of patients without pre-existing CKD were mechanically ventilated (74% vs 80%; ASMD = 0.15). Prone positioning was used in a higher percentage of patients without pre-existing CKD (42%) compared to non-dialysis-dependent CKD (27%; ASMD = 0.30) and dialysis (24%; ASMD = 0.37) patients. Remdesivir was more commonly administered to patients without pre-existing CKD (7%) compared to non-dialysis-dependent CKD patients (2%; ASMD = 0.22). No maintenance dialysis patients received remdesivir. Patients without pre-existing CKD received tocilizumab (19%) more often than non-dialysis-dependent CKD (14%; ASMD = 0.12) and dialysis (9%; ASMD = 0.28) patients.
Table 3.
COVID-19 Targeted Therapies Administered During the First 14 Days After ICU Admission
| Therapy | Maintenance Dialysis Patients (n = 143) | Patients With Non-Dialysis-Dependent CKD (n = 521) | Patients Without Pre-existing CKD (n = 3,600) |
|---|---|---|---|
| Anti-infective agent | |||
| HCQ or CQa | 83 (58%) | 352 (68%) | 2,487 (69%) |
| Azithromycina | 63 (44%) | 274 (53%) | 1,959 (54%) |
| HCQ or CQ + azithromycina | 36 (25%) | 127 (24%) | 1,127 (31%) |
| Remdesivir | 0 (0%) | 13 (2%) | 262 (7%) |
| Ribavirin | 0 (0%) | 2 (0%) | 14 (0%) |
| Lopinavir/ritonavir | 5 (3%) | 15 (3%) | 154 (4%) |
| Anti-inflammatory agent | |||
| Any corticosteroidb | 52 (36%) | 204 (39%) | 1,357 (38%) |
| Dexamethasoneb | 3 (2%) | 11 (2%) | 137 (4%) |
| NSAID | 1 (1%) | 10 (2%) | 160 (4%) |
| Aspirin | 54 (38%) | 151 (29%) | 596 (17%) |
| Statin | 55 (38%) | 182 (35%) | 822 (23%) |
| Tocilizumab | 13 (9%) | 73 (14%) | 667 (19%) |
| Vitamin C | 10 (7%) | 53 (10%) | 371 (10%) |
| Respiratory and cardiac intervention | |||
| Invasive mechanical ventilation | 106 (74%) | 404 (78%) | 2,891 (80%) |
| Neuromuscular blockade | 27 (19%) | 163 (31%) | 1,410 (39%) |
| Inhaled epoprostenol | 1 (1%) | 29 (6%) | 185 (5%) |
| Inhaled nitric oxide | 4 (3%) | 19 (4%) | 132 (4%) |
| Prone positioning | 35 (24%) | 142 (27%) | 1,495 (42%) |
| ECMO | 1 (1%) | 3 (1%) | 157 (4%) |
| Vasopressor or inotrope | 106 (74%) | 366 (70%) | 2,502 (70%) |
| Mechanical cardiac supportc | 1 (1%) | 1 (0%) | 5 (0%) |
| Other | |||
| Therapeutic anticoagulationd | 64 (45%) | 232 (45%) | 1,656 (46%) |
| Convalescent serum | 4 (3%) | 10 (2%) | 132 (4%) |
Note: Values are given as number (percent) of patients. Absolute standardized mean differences comparing the patient groups to one another are presented in Table S5.
Abbreviations: CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; CQ, chloroquine; ECMO, extracorporeal membrane oxygenation; HCQ, hydroxychloroquine; ICU, intensive care unit; NSAID, nonsteroidal anti-inflammatory drug.
The anti-infective medication categories of HCQ or CQ, azithromycin, and HCQ or CQ + azithromycin are not mutually exclusive.
The anti-inflammatory agent categories of any corticosteroid and dexamethasone are not mutually exclusive.
Mechanical cardiac support included an intra-aortic balloon pump, Impella heart pump (ABIOMED), and left and right ventricular assist devices.
Therapeutic anticoagulation included continuous drips of heparin, argatroban, or bivalirudin; subcutaneous enoxaparin (1.5 mg/kg once per day), dalteparin (150-200 U/kg once per day or 100 U/kg twice per day, fondaparinux (≥5 mg per day); and oral anticoagulants (eg, warfarin, apixaban, rivaroxaban, edoxaban, and dabigatran).
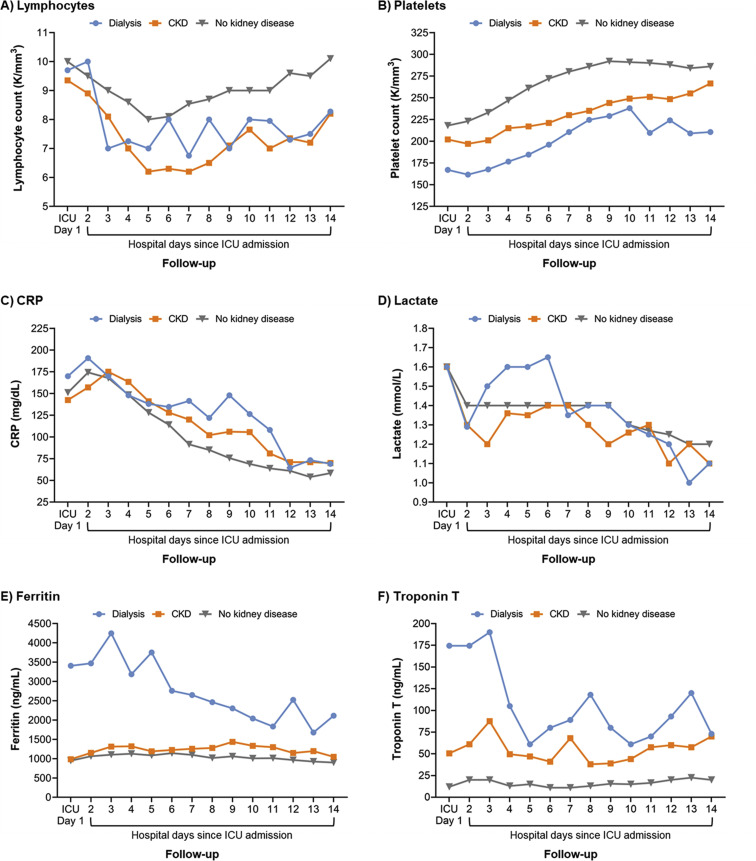
Figures 1 and S1 display laboratory parameter trajectories during the first 14 days after ICU admission across groups. In general, maintenance dialysis and non-dialysis-dependent CKD patients had lower platelet counts and higher C-reactive protein levels compared to patients without pre-existing CKD. Lactate levels on ICU day 1 were similar across groups, but elevated levels persisted longer in dialysis patients compared to the other groups. Longitudinally, ferritin and troponin levels were highest in dialysis patients and lowest in patients without pre-existing CKD.
Figure 1.
Trajectories of key laboratory values in the first 14 days after intensive care unit (ICU) admission. Median values are presented in the figure. Dialysis represents patients with pre-existing kidney failure receiving maintenance dialysis. CKD represents patients with pre-existing non-dialysis-dependent CKD. No kidney disease represents patients without pre-existing CKD. Figure S1 displays analogous figures for the laboratory values of creatinine, interleukin 6, fibrinogen, D-dimer, direct bilirubin, and troponin I. Abbreviations: CKD, chronic kidney disease; CRP, C-reactive protein.
In-Hospital Outcomes
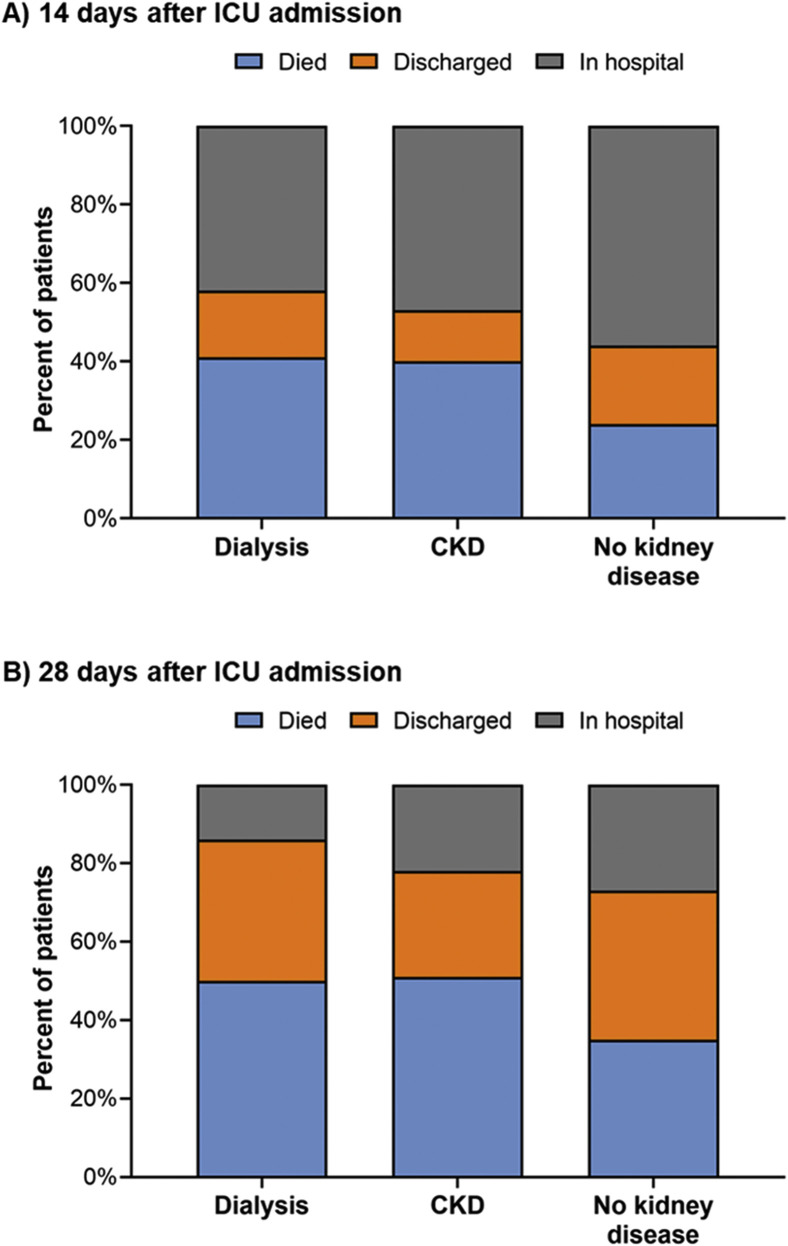
Figure 2 depicts patient disposition at 14 and 28 days after ICU admission. The leading contributing cause of death across all patient groups was respiratory failure (Fig S2). Compared to no pre-existing CKD, non-dialysis-dependent CKD and kidney failure requiring maintenance dialysis were associated with higher risks of 14- and 28-day in-hospital mortality (Table 4 ). In models examining the association between in-hospital mortality and pre-existing kidney disease status, independent of other comorbid conditions (ie, models adjusted for demographic and comorbid conditions), the associations were slightly attenuated but remained statistically significant (fully adjusted hazard ratios [HRs] for 28-day in-hospital mortality of 1.25 [95% CI, 1.08-1.44] and 1.41 [95% CI, 1.09-1.81] for non-dialysis-dependent CKD and maintenance dialysis-dependent kidney failure, respectively). Models evaluating 14-day in-hospital mortality produced similar results. Of the patients who died during the 28 days following ICU admission, median times from ICU admission to death were 8 (IQR, 5-11), 8 (IQR, 5-13), and 10 (IQR, 6-16) days for the maintenance dialysis, non-dialysis-dependent CKD, and no pre-existing CKD groups, respectively. Mortality rates across exposure groups were stable during the study period (Table S6).
Figure 2.
Patient disposition at 14 and 28 days after intensive care unit (ICU) admission. Dialysis represents patients with pre-existing kidney failure receiving maintenance dialysis. CKD represents patients with pre-existing non-dialysis-dependent CKD. No kidney disease represents patients without pre-existing CKD. Abbreviation: CKD, chronic kidney disease.
Table 4.
Association Between Pre-existing CKD and 14- and 28-Day In-Hospital Mortality Among Critically Ill COVID-19 Patients
| Patient Group | No. of Deaths | Unadjusted HR (95% CI) | Model 1 HR (95% CI)a | Model 2 HR (95% CI)b |
|---|---|---|---|---|
| 14-Day In-Hospital Mortality | ||||
| No pre-existing CKD | 876 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Non-dialysis-dependent CKD | 207 | 1.80 (1.55-2.09) | 1.44 (1.23-1.68) | 1.32 (1.13-1.55) |
| Maintenance dialysis | 59 | 1.89 (1.46-2.45) | 1.75 (1.35-2.28) | 1.56 (1.19-2.04) |
| 28-Day In-Hospital Mortality | ||||
| No pre-existing CKD | 1,261 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Non-dialysis-dependent CKD | 265 | 1.67 (1.47-1.91) | 1.35 (1.18-1.55) | 1.25 (1.08-1.44) |
| Maintenance dialysis | 72 | 1.67 (1.31-2.12) | 1.58 (1.24-2.02) | 1.41 (1.09-1.81) |
Note: Fine and Gray proportional subdistribution hazards models were used to estimate the association between the presence of pre-existing kidney disease (kidney failure receiving maintenance dialysis and non-dialysis-dependent CKD, separately) versus no pre-existing CKD and 14- and 28-day in-hospital mortality. Hospital discharge was treated as a competing event.
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; HR, hazard ratio, No., number.
Model 1 was adjusted for age, sex, race, and Hispanic ethnicity.
Model 2 was adjusted for model 1 covariates plus diabetes, hypertension, coronary artery disease, heart failure, and atrial fibrillation or flutter.
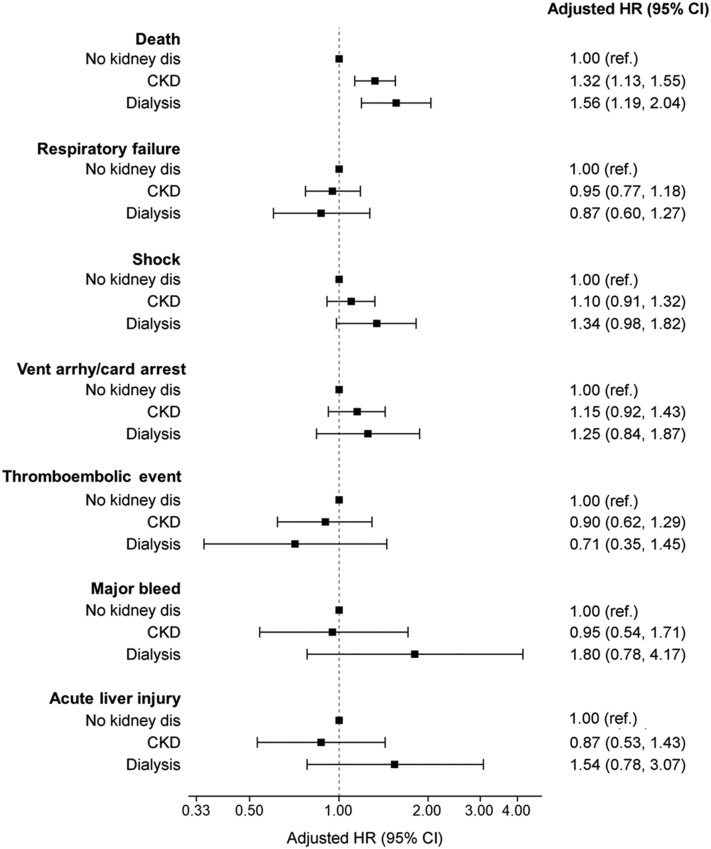
Figure 3 and Table S7 display secondary outcome associations across patient groups. Dialysis patients had nominally higher risks of shock, ventricular arrhythmia or cardiac arrest, major bleeding events, and acute liver injury during the 14 days after ICU admission, though these findings were not statistically significant. The occurrence of thromboembolic events was similar across patient groups. Sensitivity analyses excluding patients receiving therapeutic anticoagulation from major bleed and thromboembolic event models and excluding patients with a history of liver disease from acute liver injury models produced similar results (Tables S8 and S9).
Figure 3.
Association between pre-existing CKD and 14-day in-hospital outcomes among critically ill patients with COVID-19. Dialysis represents patients with pre-existing kidney failure receiving maintenance dialysis. CKD represents patients with pre-existing non-dialysis-dependent CKD. No kidney disease represents patients without pre-existing CKD. Fine and Gray proportional subdistribution hazards models were used to estimate the association between the presence of pre-existing CKD (kidney failure receiving maintenance dialysis and non-dialysis-dependent CKD, separately) versus no pre-existing CKD and 14-day in-hospital outcomes. In mortality analyses, hospital discharge was treated as a competing event. In analyses of other outcomes, both in-hospital death and hospital discharge were treated as competing events. Analyses assessing mortality, respiratory failure, shock, and ventricular arrhythmia or cardiac arrest were adjusted for age, sex, race, Hispanic ethnicity, diabetes, hypertension, coronary artery disease, heart failure, and atrial fibrillation or flutter. Analyses evaluating thrombotic events, major bleeding events, and acute liver injury were only adjusted for age, sex, race, and Hispanic ethnicity due to the low number of event counts. Abbreviations: CI, confidence interval; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; HR, hazard ratio; ref., referent; vent arrhyth/card arrest, ventricular arrhythmia or cardiac arrest.
Pre-existing Kidney Disease Subgroups
Tables S10 to S12 display results from all pre-existing kidney disease subgroup analyses. Of the 397 individuals with pre-existing non-dialysis-dependent CKD and known baseline serum creatinine levels, there was a nominally higher risk of in-hospital mortality with higher baseline serum creatinine concentrations, but results did not reach statistical significance. Among the 128 in-center hemodialysis patients, dialysis through a catheter (vs an arteriovenous access) was associated with higher 28-day in-hospital mortality (demographic-adjusted HR, 1.94 [95% CI, 1.09-3.44]).
Discussion
In this study of more than 4,200 critically ill adults admitted to 68 U.S. ICUs with COVID-19, we found that having pre-existing kidney disease was associated with higher in-hospital mortality rates, with the strength of this association varying by the degreee of baseline kidney dysfunction. Compared to no pre-existing CKD, the presence of pre-existing maintenance dialysis-dependent kidney failure was associated with the highest hazard of in-hospital death, while pre-existing non-dialysis-dependent CKD had an intermediate association. Our findings highlight the importance of identifying effective COVID-19 therapies that can be safely administered to patients with an underlying kidney dysfunction. Moreover, they underscore the urgency of proactive pre-hospital advance care planning conversations with this vulnerable population.
Our findings from a large geographically diverse sample of critically ill COVID-19 patients expand on the existing evidence base demonstrating higher in-hospital mortality among patients with underlying kidney disease and newly report detailed clinical trajectories and outcomes among non-dialysis-dependent CKD patients. The observed association between pre-existing kidney disease (dialysis and non-dialysis-dependent) and in-hospital mortality persisted in models adjusted for medical conditions known to associate with poorer COVID-19 outcomes, suggesting that underlying kidney disease confers risk for individuals with severe COVID-19 beyond that related to the comorbid disease burden characteristic of the disease state. Such findings may relate, in part, to uremia-induced innate immune system changes that hinder neutrophil, monocyte, and B- and T-cell function, thereby impairing bactericidal capacity and antimicrobial ability.11, 12, 13
We also found that maintenance dialysis patients receiving ICU-level care for COVID-19 had an in-hospital death rate of 50%, which is lower than rates reported in regional studies.36, 37, 38 Strikingly, the unadjusted death rate among non-dialysis-dependent CKD patients (51%) was equivalent to that of dialysis patients (50%) and notably higher than that of patients without underlying CKD (35%). These findings not only highlight the importance of discussing COVID-19 risks with dialysis and non-dialysis-dependent CKD patients, but also highlight the importance of engaging in advance care planning conversations in the ambulatory setting, prior to patients falling ill with COVID-19. These discussions are particularly germane for individuals with kidney disease because remdesivir, one of the few evidence-based COVID-19 therapeutic options currently available, is generally not recommended for adults with an eGFR < 30 mL/min/1.73 m2.39 However, the purported risks of remdesivir in the setting of kidney dysfunction stem from concerns related to accumulation of its carrier, sulfobutylether-β-cyclodextrin, may be overstated.39 Moreover, individuals with kidney dysfunction are being excluded from clinical trials of other potential therapeutic agents (eg, favipiravir [Clinicaltrials.gov identifier NCT0435854940]). Such exclusions may represent yet another example of “renalism” and deserve healthy skepticism by the nephrology community.41 Lack of safe and effective therapeutic options for patients with COVID-19 and reduced kidney function is a critical gap in our clinical repertoire.
Beyond their elevated in-hospital mortality risks, individuals with pre-existing CKD (dialysis and non-dialysis-dependent) had different symptoms prior to ICU admission than patients without pre-existing CKD. For example, altered mental status afflicted 25% of maintenance dialysis patients compared to just 12% of patients without pre-existing CKD. Dialysis patients have unique neurologic vulnerability related to vascular disease, and dialysis treatment–induced ischemia and osmolar shifts that may leave them susceptible to both the direct (ie, neuroinvasion) and indirect (ie, oxidative stress, hypoxia, and ischemia) neurologic effects of severe COVID-19.42 , 43 In addition, a lower percentage of patients with pre-existing CKD (dialysis and non-dialysis-dependent) reported respiratory symptoms (shortness of breath and cough) and fever compared to patients without pre-existing CKD. These findings are consistent with existing reports23 , 37 and highlight the necessity of vigilance for nontraditional COVID-19 symptoms such as altered mental status and gastrointestinal symptoms.
Finally, given the rapidly changing COVID-19 therapeutic landscape, the timing of our study cohort deserves consideration. We studied critically ill individuals who were admitted to ICUs between March 4 and May 10, 2020. This period was relatively early in the US COVID-19 pandemic when cases and mortality were surging in the Northeast, and there were few proven effective treatments (although remdesivir was showing potential). In the intervening months, there have been important therapeutic advances. Several randomized controlled trials have demonstrated that systemic steroid therapy reduces mortality among critically ill patients with COVID-19.44 Smaller studies have suggested benefit from prone positioning.45, 46, 47 In addition, on August 23, 2020, the US Food and Drug Administration issued an emergency use authorization for COVID-19 convalescent plasma for the treatment of hospitalized patients with COVID-19.48 We did not design our observational study to evaluate the effect of specific therapeutics on outcomes, but instead shine light on the critical importance of testing and identifying therapies that are safe and effective for individuals with a pre-existing kidney dysfunction. Beyond the imperative to include patients with kidney disease in trials of COVID-19 therapeutics, future studies should examine the efficacy and safety of evidence-based therapies in individuals with underlying CKD. Moreover, investigations of temporal and geographic trends of evidence-based therapies to determine whether proven therapies are achieving adequate clinical uptake among individuals with underlying kidney disease are warranted.
Our study has several strengths. First, we used data from a cohort of more than 4,200 critically ill individuals with COVID-19 who were admitted to 68 geographically diverse US ICUs, increasing the generalizability of our findings and expanding the evidence base about critically ill patients with COVID-19 and pre-existing CKD. Second, we performed detailed chart reviews using standardized data extraction tools to collect granular information on patients’ clinical courses. This obviated the need for reliance on administrative billing codes that may lead to misclassification and supported the study of detailed comparisons across study groups. Third, data were collected from critically ill patients consecutively admitted to each ICU, minimizing potential selection bias. Fourth, whereas some prior studies of maintenance dialysis patients hospitalized with COVID-19 had limited follow-up time, we followed up patients until the occurrence of hospital discharge, death, or 28 days.
We also acknowledge several study limitations. First, as with all observational studies, residual confounding may exist. However, to examine the association between underlying kidney disease and outcomes independent of coexistent medical conditions, we accounted for key demographic factors and comorbid conditions known to have strong associations with outcomes in individuals with COVID-19 in our multivariable models. Second, we defined pre-existing kidney disease based on the presence of prior eGFR assessments or documentation of CKD in the admitting hospital’s medical record. It is possible that some exposure misclassification may have occurred. Third, data for organ injury and organ support were captured during the first 14 days following ICU admission only. Events after the 14-day period may have been missed. However, it is reassuring that most of the observed events occurred early in ICU courses, suggesting that most events were likely captured. Fourth, data on inflammatory markers were not available for many patients (Table S12) and may not have been missing at random (ie, laboratory values were likely drawn more often in patients with more severe COVID-19). As such, it is possible that the observed trends in such markers may not generalize to individuals with less severe COVID-19. Related, it is possible that individuals with pre-existing CKD (dialysis and non-dialysis-dependent) may have been preferentially declined ICU admission or died before ICU admission, raising the possibility of potential selection bias in our cohort. However, such selection bias would likely bias our findings toward the null. Fifth, we did not have information for 14- and 28-day vital status for patients who were discharged from the hospital before these time points. Finally, limited numbers of some secondary outcomes (eg, major bleeding events and acute liver injury) in the pre-existing CKD groups (dialysis and non-dialysis-dependent) may have limited our ability to detect significant associations. Therefore, these findings should be considered hypothesis generating and fodder for future study.
In conclusion, in this multicenter, nationally representative cohort of US adults with COVID-19 critical illness, we found that both non-dialysis-dependent CKD patients and kidney failure patients receiving maintenance dialysis had a 28-day in-hospital mortality rate of ∼50% and patients with underlying kidney disease had higher in-hospital mortality than patients without pre-existing CKD, with maintenance dialysis patients having the highest risk in adjusted analyses. As evidenced by differences in symptoms and clinical trajectories, patients with pre-existing kidney disease may have unique vulnerability to COVID-19–related complications that warrant additional study and special consideration in the pursuit and development of targeted therapies.
Article Information
STOP-COVID Investigators
A full list of the STOP-COVID Investigators is provided in Item S2.
Authors’ Full Names and Academic Degrees
Jennifer E. Flythe, MD, MPH, Magdalene M. Assimon, PharmD, PhD, Matthew J. Tugman, BA, Emily H. Chang, MD, Shruti Gupta, MD, MPH, Jatan Shah, MD, Marie Anne Sosa, MD, Amanda DeMauro Renaghan, MD, Michal L. Melamed, MD, MHS, F. Perry Wilson, MD, Javier A. Neyra, MD, MSCS, Arash Rashidi, MD, Suzanne M. Boyle, MD, MSCE, Shuchi Anand, MD, MS, Marta Christov, MD, PhD, Leslie F. Thomas, MD, Daniel Edmonston, MD, and David E. Leaf, MD, MMSc.
Authors’ Contributions
Research idea and study design: JEF, MMA; data acquisition: all authors; data analysis/interpretation: all authors; statistical analysis: MMA; supervision or mentorship: JEF, DEL. JEF and MMA contributed equally to this work. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
Drs Flythe and Assimon are supported by R01 HL152034 from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH). Dr Flythe is supported by K23 DK109401 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH. Dr Wilson is supported by R01DK113191 and P30DK097310 from the NIDDK of the NIH. Dr Anand is supported by K23 DK101826 from the NIDDK of the NIH. Dr Christov is supported by the Westchester Community Foundation – Renal Clinic Fund. Dr Leaf is supported by R01DK125786 from the NIDDK of the NIH and R01HL144566 from the NHLBI of the NIH. The funders played no role in study design; data collection, analysis, or reporting; or the decision to submit for publication.
Financial Disclosure
In the last 3 years, Dr Flythe received speaking honoraria from American Renal Associates, American Society of Nephrology, Dialysis Clinic, Inc, National Kidney Foundation, and multiple universities; received investigator-initiated research funding from the Renal Research Institute, a subsidiary of Fresenius Medical Care, North America; is on the medical advisory board of NxStage Medical, Inc; and has received consulting fees from Fresenius Medical Care, North America and AstraZeneca. In the last 3 years, Dr Assimon received investigator-initiated research funding from the Renal Research Institute and honoraria from the International Society of Nephrology. In the last 3 years, Dr Chang received investigator-initiated funding from the Renal Research Institute. Dr Gupta is a scientific coordinator for GlaxoSmithKline’s ASCEND trial. In the last 3 years, Dr Anand received the Normon S. Coplon Applied Pragmatic Research Award sponsored by Satellite Health Care and has consulted for DURECT. The remaining authors declare that they have no relevant financial interests.
Acknowledgements
The authors thank the study site research teams who invested countless hours in electronic health record review and data entry.
Peer Review
Received July 24, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form September 15, 2020. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Footnotes
Complete author and article information provided before references.
Figure S1: Trajectories of other laboratory values in the first 14 days after ICU admission.
Figure S2: Contributing cause(s) of death.
Item S1: List of participating STOP-COVID sites.
Item S2: List of STOP-COVID Investigators.
Table S1: Outcome definitions.
Table S2: Patient characteristics on ICU day 1, dialysis patients vs non-dialysis-dependent CKD patients.
Table S3: Patient characteristics on ICU day 1, dialysis patients vs patients without pre-existing CKD.
Table S4: Patient characteristics on ICU day 1, non-dialysis-dependent CKD patients vs patients without pre-existing CKD.
Table S5: Standardized differences comparing therapies administered in the first 14 days after ICU admission.
Table S6: Percentage of 14- and 28-day in-hospital deaths that occurred earlier and later in the study period.
Table S7: Association between pre-existing kidney disease and 14-day in-hospital outcomes.
Table S8: Sensitivity analyses excluding patients on therapeutic anticoagulation on ICU day 1.
Table S9: Sensitivity analyses excluding patients with history of liver disease.
Table S10: Association between baseline serum creatinine and 14- and 28-day in-hospital mortality.
Table S11: Association between vascular access type and 14- and 28-day in-hospital mortality.
Table S12: Association between dialysis vintage and 14- and 28-day in-hospital mortality.
Table S13: ICU day 1 variables with missing data.
Contributor Information
STOP-COVID Investigators:
Carl P. Walther, Samaya J. Anumudu, Justin Arunthamakun, Kathleen F. Kopecky, Gregory P. Milligan, Peter A. McCullough, Thuy-Duyen Nguyen, Shahzad Shaefi, Megan L. Krajewski, Sidharth Shankar, Ameeka Pannu, Juan D. Valencia, Sushrut S. Waikar, Zoe A. Kibbelaar, Ambarish M. Athavale, Peter Hart, Shristi Upadhyay, Ishaan Vohra, Adam Green, Jean-Sebastien Rachoin, Christa A. Schorr, Lisa Shea, Daniel L. Edmonston, Christopher L. Mosher, Alexandre M. Shehata, Zaza Cohen, Valerie Allusson, Gabriela Bambrick-Santoyo, Noor ul aain Bhatti, Bijal Mehta, Aquino Williams, Samantha K. Brenner, Patricia Walters, Ronaldo C. Go, Keith M. Rose, Lili Chan, Kusum S. Mathews, Steven G. Coca, Deena R. Altman, Aparna Saha, Howard Soh, Huei Hsun Wen, Sonali Bose, Emily A. Leven, Jing G. Wang, Gohar Mosoyan, Girish N. Nadkarni, Pattharawin Pattharanitima, Emily J. Gallagher, Allon N. Friedman, John Guirguis, Rajat Kapoor, Christopher Meshberger, Katherine J. Kelly, Chirag R. Parikh, Brian T. Garibaldi, Celia P. Corona-Villalobos, Yumeng Wen, Steven Menez, Rubab F. Malik, Carmen Elena Cervantes, Samir C. Gautam, Mary C. Mallappallil, Jie Ouyang, Sabu John, Ernie Yap, Yohannes Melaku, Ibrahim Mohamed, Siddhartha Bajracharya, Isha Puri, Mariah Thaxton, Jyotsna Bhattacharya, John Wagner, Leon Boudourakis, H. Bryant Nguyen, Afshin Ahoubim, Kianoush Kashani, Shahrzad Tehranian, Leslie F. Thomas, Dheeraj Reddy Sirganagari, Pramod K. Guru, Yan Zhou, Paul A. Bergl, Jesus Rodriguez, Jatan A. Shah, Mrigank S. Gupta, Princy N. Kumar, Deepa G. Lazarous, Seble G. Kassaye, Michal L. Melamed, Tanya S. Johns, Ryan Mocerino, Kalyan Prudhvi, Denzel Zhu, Rebecca V. Levy, Yorg Azzi, Molly Fisher, Milagros Yunes, Kaltrina Sedaliu, Ladan Golestaneh, Maureen Brogan, Neelja Kumar, Michael Chang, Jyotsana Thakkar, Ritesh Raichoudhury, Akshay Athreya, Mohamed Farag, Edward J. Schenck, Soo Jung Cho, Maria Plataki, Sergio L. Alvarez-Mulett, Luis G. Gomez-Escobar, Di Pan, Stefi Lee, Jamuna Krishnan, William Whalen, David Charytan, Ashley Macina, Sobaata Chaudhry, Benjamin Wu, Frank Modersitzki, Anand Srivastava, Alexander S. Leidner, Carlos Martinez, Jacqueline M. Kruser, Richard G. Wunderink, Alexander J. Hodakowski, Juan Carlos Q. Velez, Eboni G. Price-Haywood, Luis A. Matute-Trochez, Anna E. Hasty, Muner M.B. Mohamed, Rupali S. Avasare, David Zonies, David E. Leaf, Shruti Gupta, Meghan E. Sise, Erik T. Newman, Samah Abu Omar, Kapil K. Pokharel, Shreyak Sharma, Harkarandeep Singh, Simon Correa, Tanveer Shaukat, Omer Kamal, Wei Wang, Heather Yang, Jeffery O. Boateng, Meghan Lee, Ian A. Strohbehn, Jiahua Li, Ariel L. Mueller, Roberta Redfern, Nicholas S. Cairl, Gabriel Naimy, Abeer Abu-Saif, Danyell Hall, Laura Bickley, Chris Rowan, Farah Madhani-Lovely, Vasil Peev, Jochen Reiser, John J. Byun, Andrew Vissing, Esha M. Kapania, Zoe Post, Nilam P. Patel, Joy-Marie Hermes, Anne K. Sutherland, Amee Patrawalla, Diana G. Finkel, Barbara A. Danek, Sowminya Arikapudi, Jeffrey M. Paer, Peter Cangialosi, Mark Liotta, Jared Radbel, Sonika Puri, Jag Sunderram, Matthew T. Scharf, Ayesha Ahmed, Ilya Berim, Jayanth S. Vatson, Shuchi Anand, Joseph E. Levitt, Pablo Garcia, Suzanne M. Boyle, Rui Song, Jingjing Zhang, Sang Hoon Woo, Xiaoying Deng, Goni Katz-Greenberg, Katharine Senter, Moh’d A. Sharshir, Vadym V. Rusnak, Muhammad Imran Ali, Anip Bansal, Amber S. Podoll, Michel Chonchol, Sunita Sharma, Ellen L. Burnham, Arash Rashidi, Rana Hejal, Eric Judd, Laura Latta, Ashita Tolwani, Timothy E. Albertson, Jason Y. Adams, Ronald Reagan, Steven Y. Chang, Rebecca M. Beutler, Santa Monica, Carl E. Schulze, Etienne Macedo, Harin Rhee, Kathleen D. Liu, Vasantha K. Jotwani, Jay L. Koyner, Alissa Kunczt, Chintan V. Shah, Vishal Jaikaransingh, Stephanie M. Toth-Manikowski, Min J. Joo, James P. Lash, Javier A. Neyra, Nourhan Chaaban, Rajany Dy, Alfredo Iardino, Elizabeth H. Au, Jill H. Sharma, Marie Anne Sosa, Sabrina Taldone, Gabriel Contreras, David De La Zerda, Hayley B. Gershengorn, Salim S. Hayek, Pennelope Blakely, Hanna Berlin, Tariq U. Azam, Husam Shadid, Michael Pan, Patrick O’ Hayer, Chelsea Meloche, Rafey Feroze, Rayan Kaakati, Danny Perry, Abbas Bitar, Elizabeth Anderson, Kishan J. Padalia, John P. Donnelly, Andrew J. Admon, Jennifer E. Flythe, Matthew J. Tugman, Emily H. Chang, Brent R. Brown, Amanda K. Leonberg-Yoo, Ryan C. Spiardi, Todd A. Miano, Meaghan S. Roche, Charles R. Vasquez, Amar D. Bansal, Natalie C. Ernecoff, Sanjana Kapoor, Siddharth Verma, Huiwen Chen, Csaba P. Kovesdy, Miklos Z. Molnar, Ambreen Azhar, S. Susan Hedayati, Mridula V. Nadamuni, Shani Shastri, Duwayne L. Willett, Samuel A.P. Short, Amanda D. Renaghan, Kyle B. Enfield, Pavan K. Bhatraju, A. Bilal Malik, Matthew W. Semler, Anitha Vijayan, Christina Mariyam Joy, Tingting Li, Seth Goldberg, Patricia F. Kao, Greg L. Schumaker, Nitender Goyal, Anthony J. Faugno, Greg L. Schumaker, Caroline M. Hsu, Asma Tariq, Leah Meyer, Ravi K. Kshirsagar, Daniel E. Weiner, Aju Jose, Marta Christov, Jennifer Griffiths, Sanjeev Gupta, Aromma Kapoor, Perry Wilson, Tanima Arora, and Ugochukwu Ugwuowo
Supplementary Material
Figures S1-S2; Items S1-S2; Tables S1-S13.
References
- 1.Johns Hopkins University Coronavirus Resource Center, 2020. https://coronavirus.jhu.edu/ Accessed September 13, 2020.
- 2.Williamson E.J., Walker A.J., Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry B.M., Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52(6):1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapp J, Lieberman-Cribbin W, Tuminello S, Taioli E. Male sex, severe obesity, older age, and chronic kidney disease are associated with COVID-19 severity and mortality in New York City [published online ahead of print August 2020]. Chest. 2020; 10.1016/j.chest.2020.08.2065. [DOI] [PMC free article] [PubMed]
- 6.Gansevoort R.T., Hilbrands L.B. CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol. 2020;26:1–2. doi: 10.1038/s41581-020-00349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried MW, Crawford JM, Mospan AR, et al. Patient characteristics and outcomes of 11,721 patients with COVID19 hospitalized across the United States [published online ahead of print August 2020]. Clin Infect Dis. 2020; 10.1093/cid/ciaa1268. [DOI] [PMC free article] [PubMed]
- 8.Intensive Care National Audit and Research Centre COVID-19 Report, 2020. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports2020 Accessed September 10, 2020.
- 9.Hutchison C.A., Crowe A.V., Stevens P.E., Harrison D.A., Lipkin G.W. Case mix, outcome and activity for patients admitted to intensive care units requiring chronic renal dialysis: a secondary analysis of the ICNARC Case Mix Programme Database. Crit Care. 2007;11(2):R50. doi: 10.1186/cc5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbertson D.T., Rothman K.J., Chertow G.M. Excess deaths attributable to influenza-like illness in the ESRD population. J Am Soc Nephrol. 2019;30(2):346–353. doi: 10.1681/ASN.2018060581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ando M., Shibuya A., Tsuchiya K., Akiba T., Nitta K. Reduced expression of Toll-like receptor 4 contributes to impaired cytokine response of monocytes in uremic patients. Kidney Int. 2006;70(2):358–362. doi: 10.1038/sj.ki.5001548. [DOI] [PubMed] [Google Scholar]
- 12.Girndt M., Sester M., Sester U., Kaul H., Köhler H. Defective expression of B7-2 (CD86) on monocytes of dialysis patients correlates to the uremia-associated immune defect. Kidney Int. 2001;59(4):1382–1389. doi: 10.1046/j.1523-1755.2001.0590041382.x. [DOI] [PubMed] [Google Scholar]
- 13.Syed-Ahmed M., Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26(1):8–15. doi: 10.1053/j.ackd.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 15.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer A.J., Morley E.J., Meyers K. Cohort of four thousand four hundred four persons under investigation for COVID-19 in a New York hospital and predictors of ICU care and ventilation. Ann Emerg Med. 2020;76(76):394–404. doi: 10.1016/j.annemergmed.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan L, Chaudhary K, Saha A, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2020 Sep 3;ASN.2020050615. 10.1681/ASN.2020050615. [published online ahead of print]. [DOI]
- 19.Gupta S, Coca S, Chan L, et al. Acute kidney injury requiring renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol. 2020; 10.1016/j.jcrc.2020.07.025. [DOI]
- 20.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan W.J., Liang W.H., Zhao Y. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J., Li J., Zhu G. Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Clin J Am Soc Nephrol. 2020;15(8):1139–1145. doi: 10.2215/CJN.04160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbett R.W., Blakey S., Nitsch D. Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol. 2020;31(8):1815–1823. doi: 10.1681/ASN.2020040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Renal Association COVID-19 Data, 2020. https://renal.org/covid-19/data/2020 Accessed September 10, 2020.
- 26.Gupta S., Hayek S.S., Wang W. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(180):1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey A.S., Coresh J., Greene T. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–1234. [Google Scholar]
- 30.Yang D., Dalton J. Paper 335-2012: A unified approach to measuring the effect size between two groups using SAS®. https://support.sas.com/resources/papers/proceedings12/335-2012.pdf Accessed September 10, 2020.
- 31.Moon A.M., Webb G.J., Aloman C. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73(3):705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan Y., Yang Y., Wang F. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1) doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Killerby M.E., Link-Gelles R., Haight S.C. Characteristics associated with hospitalization among patients with COVID-19 - metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):790–794. doi: 10.15585/mmwr.mm6925e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valeri A.M., Robbins-Juarez S.Y., Stevens J.S. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31(7):1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma Y., Diao B., Lv X. Epidemiological, clinical, and immunological features of a cluster of COVID-19 contracted hemodialysis patients. Kidney Int Rep. 2020;5(8):1333–1341. doi: 10.1016/j.ekir.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher M., Milagros Y., Mokrzycki M., Golestaneh L., Alahiri E., Coco M. Chronic hemodialysis patients hospitalized with COVID-19- short-term outcomes in Bronx, New York. Kidney360. 2020;1(8):755–762. doi: 10.34067/KID.0003672020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adamsick M.L., Gandhi R.G., Bidell M.R. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol. 2020;31(7):1384–1386. doi: 10.1681/ASN.2020050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Study of the use of favipiravir in hospitalized subjects with COVID-19, 2020. https://clinicaltrials.gov/ct2/show/NCT04358549 Accessed September 10, 2020.
- 41.Chertow G.M., Normand S.L., McNeil B.J. “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol. 2004;15(9):2462–2468. doi: 10.1097/01.ASN.0000135969.33773.0B. [DOI] [PubMed] [Google Scholar]
- 42.Varatharaj A., Thomas N., Ellul M.A. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nuzzo D., Picone P. Potential neurological effects of severe COVID-19 infection. Neurosci Res. 2020;158:1–5. doi: 10.1016/j.neures.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sterne J.A.C., Murthy S., Diaz J.V. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1–13. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gulart A.A., Silva I.J.C.S. Early prone position for COVID-19 patients with severe hypoxia: reduces the mortality but increases the intubation risk. Intensive Care Med. 2020:1–2. doi: 10.1007/s00134-020-06213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zang X., Wang Q., Zhou H., Liu S., Xue X. COVID-19 Early Prone Position Study Group. Efficacy of early prone position for COVID-19 patients with severe hypoxia: a single-center prospective cohort study. Intensive Care Med. 2020;46(10):1927–1929. doi: 10.1007/s00134-020-06182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golestani-Eraghi M., Mahmoodpoor A. Early application of prone position for management of Covid-19 patients. J Clin Anesth. 2020;66:109917. doi: 10.1016/j.jclinane.2020.109917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Food and Drug Administration Emergency use authorization for COVID-19 convalescent plasma, 2020. https://www.fda.gov/media/141477/download Accessed September 10, 2020.
- 49.Raith E.P., Udy A.A., Bailey M. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S2; Items S1-S2; Tables S1-S13.