Abstract
Moxetumomab pasudotox is a second-generation recombinant immunotoxin against CD22 on B-cell lineages. Antileukemic activity has been demonstrated in children with chemotherapy-refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL), with variable responses. Here, we report in vitro and in vivo evaluation of moxetumomab pasudotox treatment of human cell lines and patient-derived cells as a preliminary study to understand characteristics of sensitivity to treatment. Binding, internalization, and apoptosis were evaluated using fluorescently tagged moxetumomab pasudotox. Studies in NOD-scid IL2Rgnull mice showed a modest survival benefit in mice engrafted with 697 cells but not in NALM6 or the two patient-derived xenograft models.
Keywords: acute lymphoblastic leukemia, immunotherapy
1 |. INTRODUCTION
A human cluster of differentiation antigen (CD) 22 is a B-lymphoid lineage-specific marker expressed on both normal and malignant B cells. Because treatment and cure for many CD22-expressing hematologic malignancies continue to be an unmet need, recombinantly engineered monoclonal antibodies against CD22 hold promise as therapeutic agents.1,2 Moxetumomab pasudotox (CAT-8015 or HA22, Moxetumomab) is an affinity-optimized second-generation recombinant immunotoxin consisting of an Fv fragment of the mouse anti-hCD22 monoclonal antibody (RFB4) conjugated with a truncated form of Pseudomonas exotoxin A, PE38.3 Since CD22 is expressed in a lineage-restricted fashion along the B-cell developmental cascade, moxetumomab is being evaluated for treatment against a number of B-cell malignancies, including B-cell precursor acute lymphoblastic leukemia (BCP-ALL), where approximately 90% of patient blasts are CD22 positive.4 In hairy cell leukemia, a comparatively more differentiated CD22-expressing malignancy than BCP-ALL, treatments including moxetumomab achieved excellent results.5 Considerable intrapatient variability in moxetumumab-induced cytotoxicity was observed in a study of 35 primary patient samples, suggesting that variables in underlying BCP-ALL biology might be impacting the response.6 In a phase I trial of 23 children with BCP-ALL, the clinical burden was only transiently reduced when anti-CD22 immunotoxin was given as a single agent, and there was no obvious influence of CD22 density on clinical activity.7 To investigate the best potential setting for the inclusion of moxetumomab into clinical practice, we utilized BCP-ALL cell lines and primary patient samples to assess the effects of binding and internalization on ex vivo killing activity. Although we found conditions for which moxetumomab effectively killed BCP-ALL cells ex vivo, it did not impact survival in our patient-derived xenograft (PDX) models.
2 |. RESULTS
Moxetumomab was originally developed using the disulfide-stabilized Fv region from the mouse monoclonal antibody RFB4 and selected for its high-specificity binding to human CD22.3 For these studies, moxetumomab was produced at MedImmune (Gaithersburg, MD, USA) using good clinical practice manufacturing techniques.8 Primary BCP-ALL patient samples were obtained at the time of diagnosis with written, informed consent, in accordance with the Declaration of Helsinki. With local institutional approval (ARF 200240), NOD.Cg-Prkdcscid scid Il2rgtm1Wjl/SzJ (NSG) female mice (8–10 weeks old; Jackson Laboratory) were housed in a pathogen-free American Association for Accreditation of Laboratory Animal Care (AAALAC)-accredited facility and applied for PDX models. Additional details regarding the 697 and NALM6 BCP-ALL cell lines, antibodies, reagents, techniques, and statistical analyses can be found in the online Materials and Methods (Supplementary Information S1). The clinical, cellular, and cytogenetic details for the primary patient samples and cell lines are provided in Table 1.
TABLE 1.
Characteristics of BCP-ALL cell line and patient samples
| BCP-ALL cell line/sample | Age | Sex | CNS status | Initial WBC (×103) | Ploidy | Cytogenetic profile | Stage of maturation arrest | hCD22/cell by RFB4 antibody | NCI risk status; treatment course |
|---|---|---|---|---|---|---|---|---|---|
| Cell line 697 | 12 | M | - | - | ~1.0 | 46 (45–48)<2n>XY, t(1;19)(q23;p13), del(6)(q21) (E2A-PBX1) | Pre-B | 44,467 ± 814 | NA |
| Cell line NALM6 | 19 | M | - | - | ~1.0 | 46 (43–47)<2n>XY, t(5;12)(q33.2;p13.2) | Pre-B | 35,907 ± 3,305 | NA |
| Patient 238-13 | 18 | M | CNS2b | 692 | 1.0 | 46,XY,t(4;11)(q21;q23)[cp6]/49,XY,+X,+1,t(4;11)(q21;q23),+21[5]/46,X,-Y,t(4;11)(q21;q23),+mar[3] (MLL-AF4) | Pro-B | 3,257 ± 296 | Very high risk; COG AALL1131 |
| Patient 280-13 | 2 | M | CNS2a | 22 | 0.92 | 46, XY No FISH evidence for triple trisomies, MLL-R, BCR-ABL1 or E2A-PBX1 |
Pre-B | 21,253 ± 1721 | Standard risk; COG AALL0932 |
CNS, central nervous system; COG, Children’s Oncology Group; FISH, fluorescent in-situ hybridization; pre-B, precursor-B cells; pro-B, pro-B cells; NA, not available; WBC, white blood cell count.
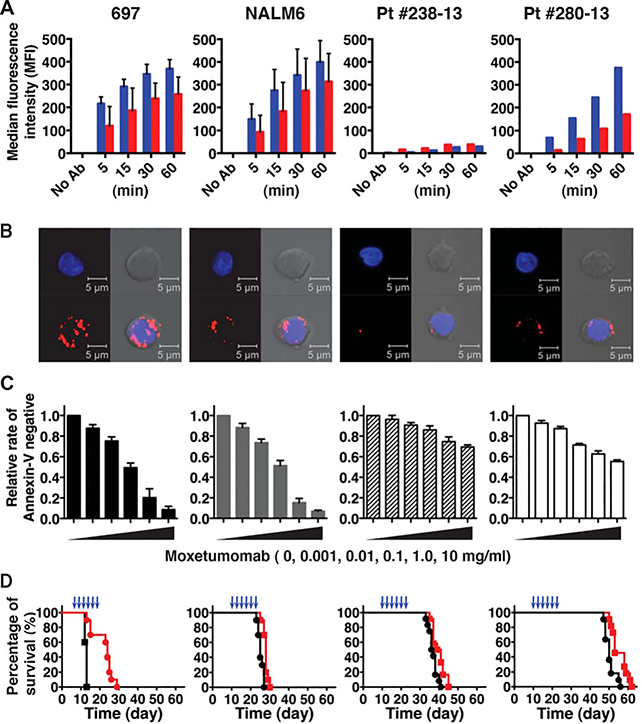
Two cell lines and five patient blasts expressed common BCP-ALL features, including varying levels of CD22 (Table 1 and Supplementary Table S1). Live cells from both cell lines, as well as patient 280–13, bound significant amounts of fluorescently labeled moxetumomab (moxetumomab-AF488) in a time-dependent manner (blue bars, Fig. 1A). In contrast, there was a relatively little accumulation of moxetumomab-AF488 in cells derived from patient 238–13, which expressed only nearly 3,000 receptors/blast. The data from these two patient cells are thus representative of two clinical subsets: patient 280–13 is typical of cases with higher CD22 expression (>20,000), while patient 238–13 represents cases with very low CD22 expression. Three additional patient samples, with CD22 expression ranging from 32,000–45,000 receptors per cell, showed similar binding and internalization of fluorescent moxetumomab (Supplementary Table S1).
FIGURE 1.
Differential moxetumomab binding, internalization, and efficacy for BCP-ALL cell lines and patient samples, as labeled at the top. (A) Moxetumomab-AF488 internalization was determined by flow cytometry after indicated incubation time, +/− acidic buffer wash to remove surface-bound antibodies. Red bars, acid-stripped (internalized); blue bars; unstripped (surface + internalized). Cell lines were evaluated as Mean with standard deviation (SD) from two independent experiments with triplicates. The patient-derived leukemia blasts were evaluated with triplicates directly after enrichment process. (b) Confocal images of moxetumomab-AF594 internalization by BCP-ALL cells (red, lower left images in each panel). Cells were incubated with moxetumomab-AF594 for 30 min, followed by acid stripping to remove surface-bound labeled immunotoxin. Leukemia nuclei were stained with DAPI (blue, top left images). Phase contrast images are shown at top right, with merged images in the lower right. Images acquired with 40× objective; scale bars = 5.0 mm. Representative images are shown from three independent experiments. (c) The bar graphs summarize relative frequency of moxetumomab-resistant BCP-ALL cells, determined as Annexin-V negative after 66 hr incubation with moxetumomab-AF594. Data are reported as means with SDs from three independent experiments. (d) NSG mice were injected with BCP-ALL cells (10 × 106, IV) on day 0. Treated groups were administered moxetumomab pasudotox (697 cells, 300 mg/kg; NALM6 and patient-derived cells, 400 mg/kg, IV) for a total of six doses, with the timing indicated by blue arrowheads. Survival was significantly increased in the treated group of 697-xenografted mice (P< 0.0001, Kaplan–Meier log–rank test). Data represent the combined results of two independent experiments with five to six mice for each group (total n = 10–12)
We also used an acid-stripping protocol to examine the accumulation of internalized moxetumomab-AF594 in BCP cell lines and patient 280–13 (red bars, Fig. 1A and confocal images in Fig. 1B). Note that, due to the sparse cytoplasm in these leukemia cells, labeling for endosomal compartments is often near the cell boundary. Having demonstrated differential binding, internalization, and processing of moxetumomab, we tested how efficiently moxetumomab could induce apoptosis of BCP-ALL cells. After endocytosis, moxetumomab must successfully navigate to the endoplasmic reticulum where the proteolytically cleaved Pseudomonas exotoxin translocates into the cytosol to inhibit protein synthesis and induce apoptosis.9 As shown in Figure 1C, reduction in Annexin V-negative cells indicates the reduced viability of moxetumomab-treated BCP-ALL cells. Importantly, most Annexin V-positive cells were also AF594-positive by flow cytometry analysis (data not shown), consistent with direct causality between moxetumomab uptake and loss of cell viability. The two cell lines were more susceptible to moxetumomab in vitro treatment than either of the patient-derived cells (Fig. 1C). As might be expected, the lowest killing was observed for cells from patient 238–13, having relatively low CD22 expression and poor internalization. Despite expressing relatively higher levels of CD22 and corresponding internalization, blasts from patient 280–13 exhibited some resistance to apoptosis than cell lines.
We next evaluated a treatment of six doses of moxetumomab administered by tail vein injection every other day for its effect on reducing leukemia burden in mice engrafted with BCP-ALL cell lines and patient samples. In comparison to the 697 model, median survival in the NALM6 model was not increased significantly by moxetumomab treatment (control: 25 days vs. moxetumomab: 28 days; Fig. 1D). For mice engrafted with patient 280–13 cells and patient 238–13 cells, the effects of treatment were even less beneficial, with percentage tumor burden remaining at high levels in bone marrow, spleen, and liver (data not shown). Only in 697 cells, which showed the highest expression and internalization of moxetumomab-AF594, showed a significantly (P < 0.0001) improved survival following treatment of moxetumomab (Fig. 1D). Thus, we found that one cycle of moxetumomab treatment can decrease the CD22+ leukemia burden in animal models established with CD22+ high blasts. However, durable responses in vivo were limited to models (i.e., 697 cells) in which moxetumomab binding and internalization are clearly measurable in vitro.
3 |. DISCUSSION
For effective cell killing, internalized moxetumomab–immunotoxin must enter endocytic compartments, be cleaved by furin to release the exotoxin portion of the molecule, and traverse through Golgi and endoplasmic reticulum compartments, followed by translocation into the cytoplasm. Once in the cytoplasm, it inhibits protein translation via adenosine diphosphate-ribosylation of histidine on dipthamide of elongation factor 2.9 Leukemic survival advantages may also be linked to additional mechanisms, including the rate of proliferation, antiapoptotic proteins, ATPase binding cassette protein-mediated pump activity, treatment timing, initial burden, chemotherapy-dependent resistance mechanisms, and other unknown, case-by-case factors that influence efficacy.10,11 Our in vitro and in vivo studies confirm that moxetumomab-mediated killing of BCP-ALL cells is partially correlated with CD22 expression, where the CD22lo blasts from patient 238–13 had both low uptake and poor killing. As observed by Haso et al.,12 cell lines typically expressed relatively higher levels of CD22 (6,485–54,878, in six cell lines) in comparison with patient samples (713–9,380, median 4,546 receptors/cell), as described in an anti-CD22 immunotoxin phase I clinical trial of 23 subjects having refractory/progressive BCP-ALL.6 Despite apparent binding and internalization, we did not appreciate a significant benefit in our PDX models, similar to the responses observed in early-phase clinical trials.7 We confirm here that CD22 expression level is most critical for the early steps of moxetumomab binding and internalization. In addition to CD22 internalization and turnover rates, multiple intracellular trafficking steps are necessary to result in cleavage and production of active toxin. These cellular biologic factors influencing moxetumomab resistance are particularly difficult to measure in an in vivo setting. Other factors, including leukemia doubling time, additional gene mutations, immune response, and influence from the local microenvironment, likely contribute to reduced response to moxetumomab in PDX models. While a phase I trial for relapsed/refractory hairy cell leukemia moxetumomab achieved an overall response rate of 86%, and a complete remission in 46% of patients,5 additional work in BCP-ALL may be of questionable significance. Both patients from whom we developed our PDX models remain in first complete remission, without the use of any targeted therapies. However, our modest PDX sample size prevents us from reaching definitive conclusions about which patient subsets might still benefit from such therapy. Further studies with a larger number of patient samples, representing a broader range of cytogenetic alternations and risk groups, will be required for definitive conclusions with respect to the therapeutic promise of moxetumomab.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank members of the Wilson and Lidke labs for helpful discussion and acknowledge the shared resources of the UNM Animal Resource Facility and Comprehensive Cancer Center (Microscopy and Flow Cytometry). This work was funded in part by MedImmune (Gaithersburg, MD, USA) and pilot funds from P50 GM085273.
Abbreviations
- BCP-ALL
B-cell precursor acute lymphoblastic leukemia
- PDX
patient-derived xenograft
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Sullivan-Chang L, O’Donnell RT, Tuscano JM Targeting CD22 in B-cell malignancies: current status and clinical outlook. Biodrugs. 2013;27:293–304. [DOI] [PubMed] [Google Scholar]
- 2.Hoelzer D. Anti-CD22 therapy in acute lymphoblastic leukaemia. Lancet Oncol. 2012;13:329–331. [DOI] [PubMed] [Google Scholar]
- 3.Alderson RF, Kreitman RJ, Chen T, et al. CAT-8015: a second-generation Pseudomonas exotoxin A-based immunotherapy targeting CD22-expressing hematologic malignancies. Clin Cancer Res. 2009;15:832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah NN, Stevenson MS, Yuan CM, et al. Characterization of CD22 expression in acute lymphoblastic leukemia. Pediatric Blood Cancer. 2015;62:964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreitman RJ, Tallman MS, Robak T, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol.2012;30:1822–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mussai F, Campana D, Bhojwani D, et al. Cytotoxicity of the anti-CD22 immunotoxin HA22 (CAT-8015) against paediatric acute lymphoblastic leukaemia. Br J Haematol. 2010;150:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wayne AS, Kreitman RJ, Findley HW, et al. Anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: preclinical studies and phase I clinical trial. Clin Cancer Res. 2010;16:1894–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linke T,Aspelund MT,Thompson C,et al. Development and scale-up of a commercial fed batch refolding process for an anti-CD22 two chain immunotoxin. Biotechnol Prog. 2014;30:1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weldon JE, Pastan I. A guide to taming a toxin–recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011;278:4683–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter SS, Lovato DM, Khawaja HM, et al. High-throughput screening for daunorubicin-mediated drug resistance identifies mometasone furoate as a novel ABCB1-reversal agent. J Biomol Screen. 2008;13:185–193. [DOI] [PubMed] [Google Scholar]
- 11.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30:1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.