Abstract
Background
In the past years, different devices have been investigated to help in identifying early decompensation events in patients with heart failure (HF) and reduced ejection fraction (EF), reducing hospital admissions. In this report, we present the first patient experience with levosimendan infusion led by CardioMEMS.
Case summary
A 68-year-old man with HF and reduced EF with more than 20 hospitalizations for exacerbation of HF was enrolled in our HF Clinic from October 2017. Echocardiogram showed a dilated left ventricle with severely reduced EF (29%) and increased pulmonary artery systolic pressure (40 mmHg). From October 2017 to May 2019, the patient went through numerous hospitalizations, despite optimal medical therapy; subsequently, was adopted a strategy of levosimendan infusions guided by CardioMEMS. Levosimendan infusions improved haemodynamic and pressure profiles. The patient was monitored daily by CardioMEMS, and from June to December 2019, he had only two hospitalizations scheduled for levosimendan infusion and none for HF exacerbation.
Discussion
Our case supports the combination of CardioMEMS and levosimendan for the optimal management of patients with advanced HF. These results further strengthen the development of a randomized clinical trial to demonstrate the clinical usefulness of this device in combination with the levosimendan infusion programme in advanced HF patients.
Keywords: Heart failure, Case report, CardioMEMS, Telemonitoring, Remote haemodynamic monitoring, Micro-electrical mechanical system, Levosimendan
Learning points
Signs and symptoms of heart failure (HF) in the advanced stage often do not allow the early recognition of congestion, thus hindering an early intervention with a consequent need for hospitalization.
The CardioMEMS allows early and objective identification of HF exacerbation, even before signs and symptoms occur.
The CardioMEMS optimizes therapy management and the levosimendan infusion.
The combination of CardioMEMS and levosimendan reduces hospitalizations and improves the quality of life in a patient affected by advanced HF.
Introduction
Heart failure (HF) is characterized by structural and/or functional cardiac abnormality, resulting in reduced cardiac output and/or elevated intracardiac pressures at rest or during stress.1 The disease exhibits phases of exacerbation punctuated by periods of clinical stability. During the last three decades, the management of HF made significant progress, focusing on pharmacological2 and device-based therapies3 to meet the challenges of this complex syndrome.4 Implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy devices assess fluid status by intrathoracic impedance monitoring, helping to stratify HF patients’ risk, but not to manage HF treatment with titration guidance like pulmonary artery (PA) pressure. The CardioMEMS (Abbott Medical, Inc., Abbott Park, IL, USA) is an implantable device positioned in the PA able to detect higher cardiac filling pressures, an objective measure of ‘haemodynamic congestion’, estimated to rise more than 2 weeks before the onset of symptomatic clinical congestion.5 Indeed, the CHAMPION trial6 clearly showed a benefit of the pressure-guided therapy in terms of reduced hospitalizations for HF exacerbations. The device is applicable in patients with chronic HF in functional New York Heart Association (NYHA) Class III with at least one admission for HF in the past 12 months.6 The procedure associates with meager device complications rate: ∼2.8%, with the most severe being PA injury and haemoptysis.7,8 Mainly, the haemodynamic data are employed to reduce hospitalizations by modulating the dose of diuretics.6
Levosimendan is a calcium-sensitizer that combines inotropic, vasodilatory, and cardioprotective effects without affecting body oxygen requirements.9 This drug improves symptoms, quality of life, and ejection fraction (EF) of HF patients, with a Class IIb indication.1 In this article, we report an innovative strategy that combines early congestion recognition by CardioMEMS to lead and optimize the levosimendan infusion.
Timeline
| Date | Events |
|---|---|
| 2004 | Diagnosed with dilated cardiomyopathy. |
| From 2004 to 2015 | More than 20 hospitalizations for exacerbation of chronic heart failure (HF) with a progressive reduction of the ejection fraction (EF). |
| 2015 | Balloon angioplasty and stent implantation in circumflex (Cx) artery. Biventricular implantable cardioverter-defibrillator implantation for primary prevention. |
| September 2017 | MitraClip placement for a severe functional mitral regurgitation. |
| October 2017 | First access to our departmental HF Clinic. |
| From October 2017 to May 2019 | The patient went through numerous exacerbations, despite optimal medical therapy. |
| May 2019 | Evidence of reduced EF (20%) and decision to start cyclic levosimendan infusion. |
| June 2019 | CardioMEMS implantation. |
| September 2019 | Scheduled hospitalization for levosimendan infusion. |
| December 2019 | Scheduled hospitalization for levosimendan infusion. |
| From December 2019 to February 2020 | After December 2019, the cardiologist continued to monitor the patient, making therapy adjustments according to diastolic PA pressure. No further levosimendan infusions were made between December 2019 and February 2020. |
Case presentation
A 68-year-old Caucasian male with HF and reduced ejection fraction presented to our HF Clinic in October 2017 with shortness of breath, orthopnoea, and severe reduction in exercise tolerance (symptoms graded as Class III of the NYHA Functional Classification). Physical examination revealed peripheral oedema, increased jugular venous pressure, systolic murmur in the mitral area, and normal peripheral pulses. Blood tests showed brain natriuretic peptide (BNP) 1005 pg/mL (pathological value > 500 pg/mL), haemoglobin 9.5 g/dL (normal range 13.5–17.5 g/dL), and estimated glomerular filtration rate (eGFR) 41 mL/min/1.73 m2 (normal value ≥ 90 mL/min/1.73 m2).
The patient’s comorbidities included: chronic kidney disease stage 3B (eGFR 43 mL/min/1.73 m2) with secondary anaemia, hypothyroidism, dyslipidaemia, carotid atheromasia, chronic obstructive pulmonary disease, sizeable inguinal hernia, hypertension, paroxysmal atrial fibrillation, and obesity.
His cardiovascular history started in 2004 for the appearance of dyspnoea with left ventricle (LV) dilation and reduction of systolic function (EF 42%; normal range 52–72%). Coronary angiography showed no evidence of critical coronary atherosclerotic disease. Despite the optimal medical therapy, from 2004 to 2015, he suffered more than 20 hospitalizations for exacerbation of chronic HF with a progressive reduction of EF. In 2015 for the evidence of EF <35%, he underwent biventricular ICD implantation. Also, he was newly subjected to coronary angiography with the indication of stenting on a critical lesion at the mid-portion in the circumflex. In 2017, severe mitral regurgitation treated by positioning of MitraClip (Abbott Laboratories, Menlo Park, CA, USA); at discharge, the patient was enrolled in our departmental HF Clinic (October 2017).
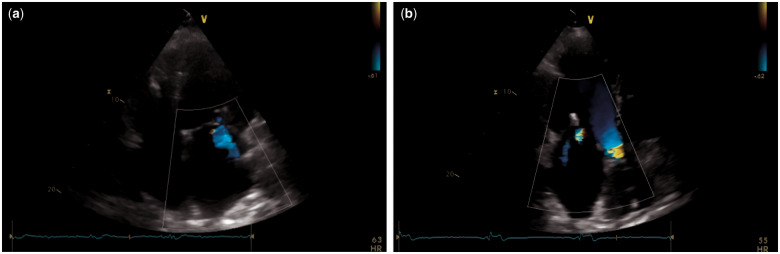
Echocardiogram (October 2017, Figure 1) showed a dilated LV (end-diastolic volume 201 mL; normal range 62–150 mL) with impaired LV systolic function (EF 29%) and global hypokinesia, moderate mitral regurgitation, dilated left atrium (60 mL/m2; normal range 16–34 mL/m2), preserved right ventricle function (Tricuspid annular plane systolic excursion: TAPSE 22 mm; normal value ≥17 mm), and increased PA systolic pressure (40 mmHg; normal value ≤36 mmHg). Pacemaker rhythms on the electrocardiogram.
Figure 1.
Still frame images from transthoracic echocardiogram of October 2017 showing a dilated left ventricle with moderate mitral regurgitation on apical four- (A) and three-chamber (B) views. MitraClips are evident.
From October 2017 to 2019, the patient went through numerous hospitalizations (initially every 2 months, then from January to June 2019 monthly), despite being on optimal guideline-based pharmacological therapy1 at the highest tolerated doses: Carvedilol 6.25 mg b.i.d., Sacubitril/Valsartan 24/26 mg b.i.d., Canrenone 50 mg o.d., and Furosemide 50 mg t.d. Other regular medications consisted of Atorvastatin 40 mg o.d., Allopurinol 150 mg o.d., Levothyroxine 125 mcg o.d., Cordarone 200 mg o.d., Warfarin, and Erythropoietin 6000 IU subcutaneously twice a week.
Another decompensation event followed in May 2019 with evidence of reduced EF (20%). Thus, we decided to start cyclic levosimendan infusion to obtain haemodynamic stability for a more extended period.
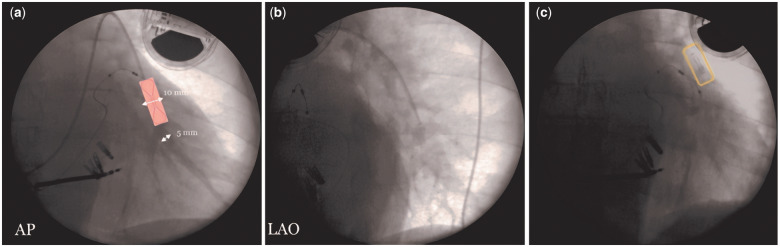
The patient received the first 24-h intravenous infusion of levosimendan (0.1 µg/kg/min) with consequent improvement of EF (35%). Then, the patient attended for CardioMEMS implantation in June 2019 (Figure 2).
Figure 2.
CardioMEMS sensor implantation. (A, B) In the first phase of the procedure, it is necessary to identify the target site for the release of the sensor through an angiography (AP and LAO projections). The target site is a descending vessel positioned as posteriorly as possible. The diameter of this vessel is >7 mm and parallel to the column (<30 degrees of angle) where the sensor body has been positioned; 5–8 mm where the distal loop of the sensor has been positioned. (C) Chest X-ray showing the final position of the device. AP, Antero-Posterior; LAO, Left Anterior Oblique.
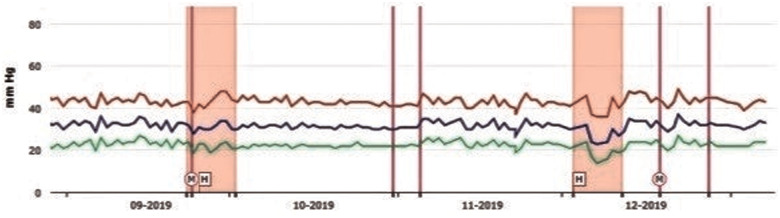
The treating cardiologist with daily reports monitored the patient. If the cardiologist detected a trend towards increasing diastolic PA (PAd) pressures, the patient was contacted, and his medications adjusted to lower PAd to the patient-specific goal. Correctly, the PAd threshold was set at 24 mmHg. If three consecutive readings exceeded this threshold, it was decided for the therapy changes (e.g. increasing the dosage of a diuretic or making a change to any other HF therapies). The dosage of the drug was titrated up to the maximum tolerated dose, including the worsening of renal function. From June 2019 to December 2019, our patient was admitted only two times to the hospital for a 24-h infusion of levosimendan: September (BNP levels before and after infusion: 5198 vs. 620 pg/mL) and December 2019 (4523 vs. 680 pg/mL); explicitly, when the cardiologist noticed a steadily increased PAd, despite the maximum tolerated dose therapy changes, he contacted the patient for hospitalization (Figure 3).
Figure 3.
In September and November 2019, the diastolic PA started to have fluctuations outside the threshold, so remote therapy changes were made, and the two hospitalizations were scheduled (pink squares) for the infusion of levosimendan. Pulmonary artery pressure tracing showing a mean diastolic PA drop after administration of levosimendan. M and/or purple lines, remote therapy modifications; H, hospitalization.
After December 2019, the cardiologist continued to monitor the patient, making therapy adjustments according to PAd. No further levosimendan infusions were made between December 2019 and February 2020.
Discussion
Here, we present the case of an advanced HF patient, whose quality of life was severely compromised by the multiple HF exacerbations. Despite recent advances in medical therapy, it remains an unmet need for adequate monitoring and treatment of HF.10 The CardioMEMS sensor provides noninvasive haemodynamic data (PA pressure waveforms, heart rate, as well as systolic, diastolic, and mean PA pressures),11 allowing clinicians to make therapeutic changes earlier, preventing decompensation and admission to the hospital.11 Indeed, the CHAMPION trial showed a significant reduction in HF hospitalizations, shorter length of stay, and improvement in the quality of life in these patients.6
Similarly, the use of ambulatory haemodynamic monitoring in clinical practise associated with lower HF hospitalization and overall HF costs, supporting the ‘real-world’ effectiveness of this approach to HF management.12 Among eligible patients with HF when compared with standard of care (SoC), the CardioMEMS was cost-effective, with an incremental cost-effectiveness ratio of $44 832 per quality-adjusted life-year.13 Our case report confirmed these observations, with only two hospital admissions after CardioMEMS implantation but none for HF exacerbation.
Our report shares similarities with those of Tschope et al.,14 in particular in the decision for levosimendan infusion. However, our goal was to realize an example of combined strategy CardioMEMS/levosimendan to reduce the number of hospitalization and optimize the levosimendan infusion by daily monitoring of PAd and the tight to tight adjustment of the oral therapy. Indeed, we performed only two levosimendan infusions after CardioMEMS implantation (every 3 months), compared to the closest injections of the LION-HEART study (every 2 weeks for 12 weeks)9 or of the real-world experience reported by Ortis et al. (every 28 days).15 This new concept to associate CardioMEMS with the outpatient levosimendan infusion programme, as well as its role as a bridge to left ventricular assist device-implantation/heart transplantation,14 appears very promising, however, certainly needs additional investigations.
Lead author biography

Dr Valeria Visco is a Cardiology trainee at University Hospital ‘San Giovanni di Dio e Ruggi d’Aragona’ of Salerno Medical School (Italy). Her main research interest is therapy of heart failure, arterial and pulmonary hypertension. Moreover, her research is particularly concerned with telemonitoring and ICT-guided disease management.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report, including the image(s) and associated text, has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR. et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 3. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L. et al. Cardiac Resynchronization-Heart Failure Study I. The effect of cardiac resynchronisation on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 4. Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT. et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition – heart failure (BEAT-HF) randomized clinical trial. JAMA Intern Med 2016;176:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein L. ( Re)discovering the neurohormonal and hemodynamic duality of heart failure. J Am Coll Cardiol 2017;70:1887–1889. [DOI] [PubMed] [Google Scholar]
- 6. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW. et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 7. Vaduganathan M, DeFilippis EM, Fonarow GC, Butler J, Mehra MR.. Postmarketing adverse events related to the CardioMEMS HF system. JAMA Cardiol 2017;2:1277–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pour-Ghaz I, Hana D, Raja J, Ibebuogu UN, Khouzam RN.. CardioMEMS: where we are and where can we go? Ann Transl Med 2019;7:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Comín-Colet J, Manito N, Segovia-Cubero J, Delgado J, García Pinilla JM, Almenar L. et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentre randomised trial. Eur J Heart Fail 2018;20:1128–1136. [DOI] [PubMed] [Google Scholar]
- 10. Assaad M, Sarsam S, Naqvi A, Zughaib M.. CardioMems(R) device implantation reduces repeat hospitalisations in heart failure patients: a single center experience. JRSM Cardiovasc Dis 2019;8:1–7. doi: 10.1177/2048004019833290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ayyadurai P, Alkhawam H, Saad M, Al-Sadawi MA, Shah NN, Kosmas CE. et al. An update on the CardioMEMS pulmonary artery pressure sensor. Ther Adv Cardiovasc Dis 2019;13:1–11. doi: 10.1177/1753944719826826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Desai AS, Bhimaraj A, Bharmi R, Jermyn R, Bhatt K, Shavelle D. et al. Ambulatory hemodynamic monitoring reduces heart failure hospitalisations in “real-world” clinical practice. J Am Coll Cardiol 2017;69:2357–2365. [DOI] [PubMed] [Google Scholar]
- 13. Schmier JK, Ong KL, Fonarow GC.. Cost-effectiveness of remote cardiac monitoring with the CardioMEMS heart failure system. Clin Cardiol 2017;40:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tschope C, Alogna A, Spillmann F, Faragli A, Schmidt G, Blaschke F. et al. The CardioMEMS system in the clinical management of end-stage heart failure patients: three case reports. BMC Cardiovasc Disord 2018;18:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ortis B, Villani A, Oldani M, Giglio A, Ciambellotti F, Facchini M. et al. Intermittent levosimendan infusions in advanced heart failure: a real world experience. J Int Med Res 2017;45:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.