Abstract
Background
In cardiac resynchronization therapy, left ventricular (LV) lead placement at the desired position may be difficult due to abnormal coronary sinus (CS) and lateral vein anatomy. We present a case with difficult anatomy in which we used ‘an indigenous snare’ made from hardware used for coronary angioplasty procedures, which is available in any cardiac catheterization laboratory.
Case summary
A 52-year-old man presented with dyspnoea due to chronic heart failure was evaluated for cardiac resynchronization therapy. The LV lead was difficult to advance into the only target lateral branch of the CS due to a combination of angulation and proximal stenosis. Balloon dilation was tried first, but we failed to track the LV lead. We formed a venovenous loop, advancing the coronary guidewire 0.014″ into the posterolateral vein; subsequently into the middle cardiac vein via a collateral. The wire was advanced into the CS and then to superior vena cava. The guidewire then snared through the same left subclavian vein and exteriorized by using indigenous snare. Over this loop, the LV lead of the cardiac resynchronization therapy with defibrillator device was implanted successfully.
Discussion
We have used the snare technique, with the use of a snare prepared from a coronary guidewire. Use of such an indigenous snare has not been described before in the literature. The hardware used in this case is routinely used for coronary angioplasty procedures in all catheterization labs. The importance of our case is that no special hardware like dedicated snare was required to negotiate the LV lead at its desired location.
Keywords: Case report, Cardiac resynchronization therapy (CRT), Left ventricular lead, Coronary sinus anatomy, Indigenous snare, Ischaemic cardiomyopathy
Learning points
In case of difficulty in tracking the left ventricular (LV) lead into the lateral vein during cardiac resynchronization therapy, snaring the distal end of the coronary guidewire with the use of an indigenous snare and forming a venovenous loop can be used to aid its placement.
A good contrast injection to fully opacify the collateral vein, sound knowledge of the hardware to be used and its utility in specific situations like tortuosity, small-sized veins, etc. are the key to a successful implant of the LV lead.
Introduction
When patients undergo cardiac resynchronization therapy (CRT), the left ventricular (LV) lead is the most important, requiring precise placement.1 A meta-analysis performed in 2016 showed that the rates of CRT failure were 5.4% in procedures performed before 2005 and 2.4% after 2005, with CRT failure in 30–53% of patients due to failure to navigate through coronary sinus (CS) and lateral vein.2
This report describes a patient in whom it was difficult to navigate the LV lead into the lateral vein. The distal end of the guidewire was snared with the help of an ‘indigenous snare’, forming a venovenous loop that aided passage of the LV lead. This manoeuvre obviated the need for specialized hardware.
Timeline
| Day 0 | Middle-aged man presented with New York Heart Association functional Class III dyspnoea. Known hypertensive and diabetic with history of coronary artery bypass grafting done a year back |
| Day 1 |
Evaluated for cardiac resynchronization therapy implanatation On guideline-directed medical therapy (GDMT); left ventricular ejection fraction = 15–20%; left bundle branch block with QRS duration of 180 ms |
| Day 5 9:15 a.m. | Coronary sinus venogram—single lateral vein with proximal obstruction and unfavourable angulation |
| Day 5 9:20 a.m. | Balloon dilation assisted tracking of lead tried—failed |
| Day 5 9:30 a.m. | Indigenous snare prepared |
| Day 5 10:00 a.m. | Venovenous loop formed with coronary wire through lateral vein → middle cardiac vein → superior vena cava |
| Day 5 10:15 a.m. | End of wire caught and exteriorized |
| Day 5 10:30 a.m. | Left ventricular lead tracked into desired position with help of this venovenous loop |
Case presentation
A 52-year-old man, with a history of diabetes and hypertension for 9 years, had been diagnosed with ischaemic cardiomyopathy and had undergone coronary artery bypass grafting 1 year earlier. After surgery, there was mild improvement in symptoms but no improvement in LV systolic function, with an LV ejection fraction (LVEF) of 15–20%. Moreover, he continued to have New York Heart Association Class III dyspnoea despite being on adequate medication. An electrocardiogram showed a sinus rhythm heart rate of 78 b.p.m. and left bundle branch block, with a QRS duration (QRSd) of 180 ms.
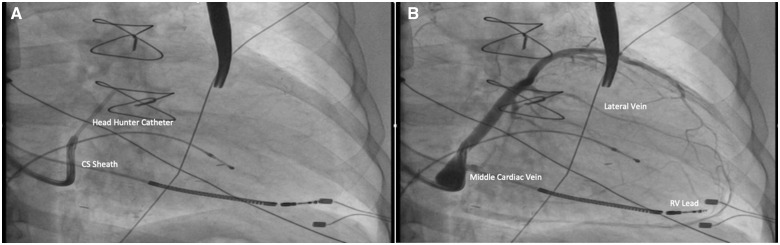
Cardiac resynchronization therapy was performed under local anaesthesia and intravenous sedation with midazolam and fentanyl. After dissection of the subclavian pocket in the prepectoral fascia, the right ventricular (RV) lead was positioned in the RV apex, followed by a selective CS angiogram with a coronary sheath. A superselective 5 F Headhunter catheter was used to enter the lateral vein. Contrast injection revealed a single small lateral branch with angulation and proximal stenosis (Figure 1). The branch was wired with a 0.014″ coronary guidewire (RunThrough NS Floppy, Terumo Corporation, Tokyo) and pre-dilated with a 2.5 × 12 mm balloon (Sprinter Legend, Medtronic, MN, USA) at 14 atm for 1 min. An attempt to negotiate an LV lead was not possible due to an unfavourable angle. A venovenous loop was therefore formed to support lead tracking.
Figure 1.
Coronary sinus venogram. (A) Superselective catheter. (B) Venogram showing a single lateral vein.
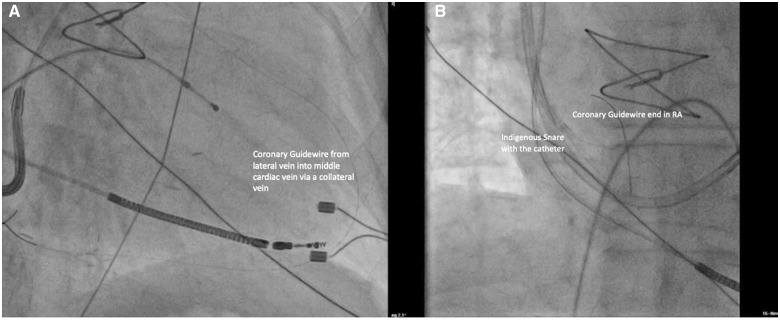
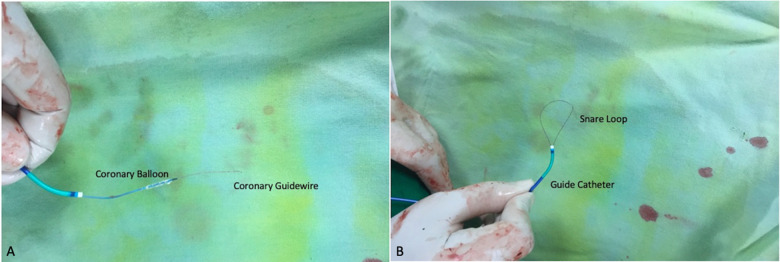
The 0.014″ coronary guidewire wire was passed into the distal segment of the lateral vein. The collateral connecting the lateral vein and the middle cardiac vein (MCV) was identified after injection of contrast. The wire was subsequently advanced into the collateral while rotating (clockwise-anticlockwise) to ensure that the tip was freely moving. An adequately sized, less tortuous collateral was identified for wiring. The wire was tracked via the collateral into the MCV and eventually into the CS. It was pushed gently at the wall to form a knuckle and then advanced into the right atrium, and then further into the superior vena cava (Figure 2A). Advancement of the wire did not need any extra-support tools as it was not very tortuous and could be tracked easily. The distal end of this guidewire was caught with an indigenous snare in the high right atrium (Figure 2B). This indigenous snare was prepared using a 6 F Judkins JR3.5 guide, a 0.014″ coronary guidewire (RunThrough NS) and a 2 × 15 mm angioplasty balloon (Sprinter balloon, Medtronic, MN, USA). The second guidewire was passed with the balloon and its distal end was reintroduced into the tip of the guide catheter to form a loop. The balloon was inflated at the tip to entrap and fix the distal end of the coronary guidewire (Figure 3).
Figure 2.
Passage of a coronary guidewire. (A) Illustration showing a coronary wire in the middle cardiac vein via a collateral. (B) Illustration showing the wire in the high right atrium and superior vena cava.
Figure 3.
Indigenous snare. (A) Preparation of the snare with a coronary balloon and workhorse coronary guidewire. (B) Snare ready for use.
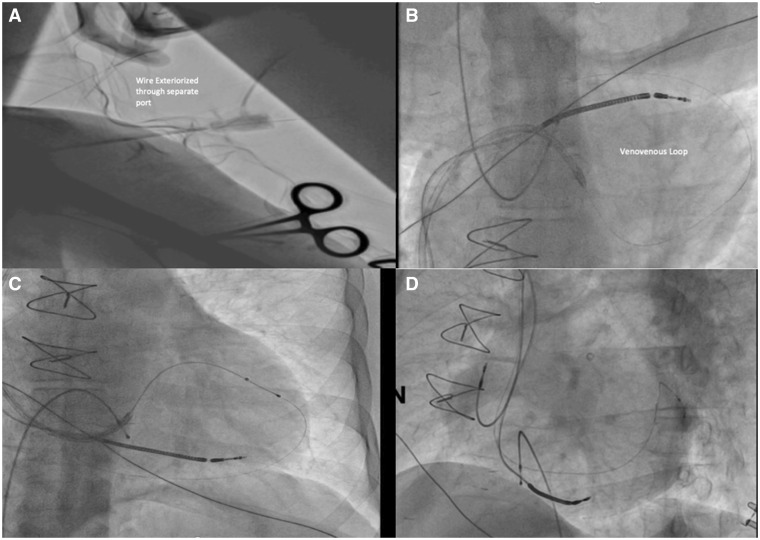
The distal end of the first coronary guidewire was snared, pulled out through the left subclavian vein and externalized through a separate sheath (Supplementary material online, Video S1) in the infraclavicular pocket, forming a venovenous loop (Figure 4A and B). Both ends of the guidewires were placed under the control of the operator. The LV lead could then be advanced easily over the venovenous loop and positioned very stably in the vein ahead of the tortuous segment (Figure 4C). After implantation of the LV lead, the guidewire was carefully pulled out from the leads under fluoroscopic control. Fluoroscopy confirmed good separation of both RV and LV leads (Figure 4D), with the thresholds of the right atrial, RV, and LV leads after implantation being 0.9, 0.7, and 1.5 V, respectively, at pulse widths of 0.4, 0.4, and 1 ms, respectively. The patient was asymptomatic and did not complain of any pain or palpitations during the procedure, nor were any arrhythmias observed. The total fluoroscopy time was 13 minutes and the total volume of contrast agent was 120 mL.
Figure 4.
Formation of a venovenous loop and passage of the left ventricular lead. (A) Exteriorization. (B) Formation of the loop. (C) Passage of the left ventricular lead over the venovenous loop. (D) Good separation of the left ventricular and right ventricular leads.
Eleven months later, the patient is being maintained on heart failure medications. He showed significant symptomatic improvement after the procedure. His QRSd had improved to 90 ms with good R wave in V1. Echocardiography showed improved LVEF (25–30%).
Discussion
Due to problematic lateral vein anatomy, implantation of the LV lead can be challenging in some patients undergoing CRT. Many techniques have been found to minimize difficulties in placement of the LV lead, with percutaneous LV lead placement being preferred in patients with compromised left ventricle to avoid the need for surgical epicardial placement.1
An early study found that the main reason for difficult lead implantations was variability of the CS anatomy.3 Moreover, manipulation of the CS sheath caused CS perforations in 2.8–5% of patients.4 Techniques to negotiate LV leads have therefore been developed to avoid these manipulations. One new method for LV lead implantation involved the use of an anchor balloon and pre-shaped guidewire assisted venoplasty, with or without stent placement.5
Another method for LV lead implantation involves the ‘Worley loop technique’.6 The hardware essential for successful positioning of the LV lead using this technique has been described in detail.7 In brief, the guidewire is advanced into one of the accessible branches of the CS (e.g. the MCV) and then back into the CS via a collateral vein and a chosen lateral vein to form a venovenous loop (antidromic technique). Alternatively, as in our patient, the guidewire can be passed into the chosen lateral vein with difficult anatomy, then into CS via a collateral and another vein, such as the MCV or the anterior vein (the orthodromic technique). The lead is advanced over the soft end of the wire to the desired position and the wire is then removed. Care must be taken while manipulating the wire via the small collateral veins to avoid the risk of perforation. Injection of contrast is necessary to visualize the entire course of the collateral vein. The collateral should not be too tortuous, as that can impede the advancement of the wire. Coated/slimy tip wires, such as Fielder FC and Choice PT wires, are preferred for tracking through tortuous collateral veins. The wire must be rotated in the vein so that the tip is free and does not get stuck while being advanced. No force should be applied while advancing the wire, thereby avoiding perforation or creation of a false track. If the collateral vein can accommodate the loop, the tip of the wire should be looped while it is being advanced through the collateral vein. Wires with tapered tips and/or stiff tip support should be avoided while advancing the wires through the collaterals. The position of the wire in the MCV and its entry into the main body of the CS should be confirmed by fluoroscopy. Our patient did not require any extra-support tools to pass the wire through the collaterals. In some patients, however, sub-select guide catheters or microcatheters can aid the passage of the wire through very tortuous veins by providing more support. Use of a coronary wire with a soft tip but extra support may aid in its passage by straightening the bends in the vein. For example, a Whisper extra-support guidewire and a microcatheter were shown to be successful in a similar patient (Table 1).8
Table 1.
Suggested hardware for engaging the coronary sinus and subsequent snaring technique
| No. | Type of hardware | Hardware | |
|---|---|---|---|
| 1 | CS Access Catheters | Worley’s Advanced [Merit Medical (South Jordan, UT, USA)] | Standard and Jumbo Curve |
| Boston Scientific (Marlborough, MA, USA) | 6F Runway MP2 (Multipurpose 2) | ||
| Medtronic (Minneapolis, MN, USA) | 5/6F MB1 Z2 Guiding Catheter (Medtronic Vascular Z26MB1), Medtronic Launcher MB2 Attain Command Hook XL and Deflectable Coronary Sinus Cannulation Catheters [Attain Command catheter in the present patient] |
||
| Cordis (Milpitas, CA, USA) | 5/6F Vista Brite Tip MPB 1 | ||
| St. Jude’s Medical (Saint Paul, MN, USA) | CPS Direct Extra Wide | ||
| 2 | Vein Selector/Sub-select | Worley’s [Merit Medical (South Jordan, UT, USA)] | Standard, Vert, Hook Type; Headhunter [Merit Medical (South Jordan, UT, USA)]—used in the present patient |
| Medtronic (Minneapolis, MN, USA) | Select II | ||
| St. Jude’s Medical (Saint Paul, MN, USA) | CPS Aim Extra Wide | ||
| 3 | Wires | Worley’s [Merit Medical (South Jordan, UT, USA)] | 0.014″ ChoICE PT—light and extra support and 300 cm; 0.018 V–18 Control Wire Guidewire; 0.035″ 180 cm Cook Amplatz Extra Stiff Wire Guide; 0.035″ 180 cm Angled polymer tip hydrophilic wire |
| Terumo (Shibuya, Tokyo, Japan) | RunThrough NS—used in the present patient | ||
| Asahi (Seto-shi, Aichi, Japan) | Fielder FC | ||
| 4 | Microcatheters | Antidromic | 0.020″ tipped Merit (South Jordan, UT, USA) SureCross Support Catheter |
| Orthodromic | 0.020″ (1.8F) tipped Terumo (Shibuya, Tokyo, Japan) Finecross Coronary Microcatheter; 0.020″ (1.3F) tipped Asahi (Seto-shi, Aichi, Japan) Corsair Coronary Microcatheter | ||
| 5 | Snares | Antidromic | 4F Merit Medical (South Jordan, UT, USA) MeritOneSnare ONE 1000; Medtronic (Minneapolis, MN, USA) Amplatz Gooseneck Microsnares |
| Orthodromic | Medtronic (Minneapolis, MN, USA) Amplatz Gooseneck snares; Indigenous snare (as described in the present patient) | ||
| Femoral Snares | Cook (Bloomington, IN, USA) Needle’s Eye Snare (13 and 20 mm) | ||
| 6 | Venoplasty Balloons | Bard (Murray Hill, New Providence, NJ, USA) | CQ7564 and CQ7594 Conquest PTA balloon dilatation catheter |
| Cordis (Milpitas, CA, USA) | PowerflexPro OTW Balloon Catheter | ||
Because the venoplasty technique was unsuccessful in the present patient, a technique using a snare prepared from a coronary guidewire was performed. To the best of our knowledge, no previous study has described the use of this type of indigenous snare. The hardware used in this case was identical to that routinely used for coronary angioplasty procedures and is available in all catheterization laboratories. To prevent venous tearing or perforation, excessive force should not be used while delivering pressure over the lead. An Amplatz Gooseneck snare has been used to snare the distal end of the wire.8 A stepwise approach to the preparation of the snare is depicted in Figure 5. This indigenous snare method may be useful in settings where special snares are unavailable.
Figure 5.
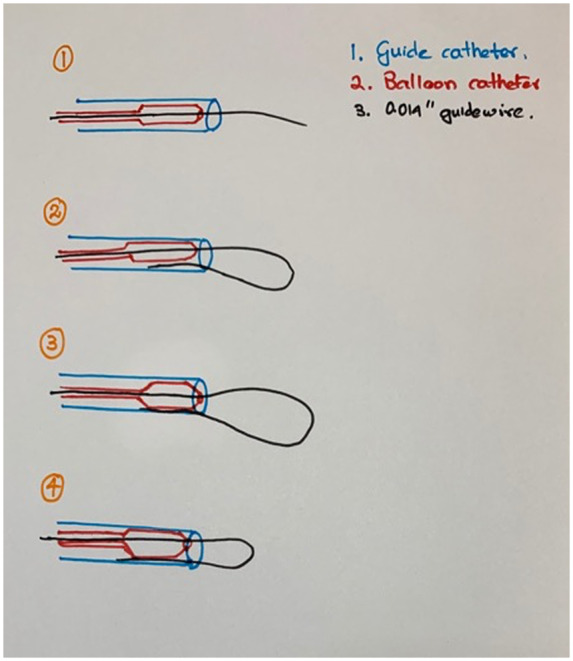
Four-step approach to preparation of the snare. Step 1: Insertion of the 0.014″ guidewire with the collapsed balloon into the guide catheter. Step 2: Looping of the wire so that its distal end is reintroduced into the guiding catheter. Step 3: Inflation of the balloon, so that the distal end of the wire is caught between the inner wall of the guiding catheter and the outer wall of the inflated balloon. Step 4: Retraction of the 0.014″ wire to reduce the size of the loop or further insertion of the wire to increase the size of the loop.
Conclusions
Difficulties negotiating LV leads in patients undergoing resynchronization therapy are due primarily to abnormal anatomy of the CS and lateral vein. Various methods may help guide the lead to a desired and stable position. One such method is using an indigenous snare and coronary angioplasty hardware.
Lead author biography

Muni Venkatesa Reddy is working as an additional chief health director cardiology at Jagjivan Ram Western Railway Headquarters Hospital, Mumbai. He has done post-graduation in medicine and subspecialized in the field of cardiology. He has more than 20 years’ experience in the field of vascular interventions—cardiac and peripheral both including endovenous LASER ablation of chronic venous disorders. He is a member of many national and international intervention societies. He has presented many cases and topics in national and international conferences. He has shared many sessions with leading faculties during scientific meetings.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: A written, informed consent has been obtained from the patient for the publication of this case report. The patient identification has been removed from the images and video that has been attached with the manuscript. No data have been altered or falsified while doing the same. As it has been informed before, the video which has been shared, belongs to another patient and the required consent has been obtained from the other patient as well.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Singhal M, Rohit MK, Barwad P.. Cardiac resynchronisation therapy: an approach to difficult left ventricular lead placement. Eur J Arrhythmia Electrophysiol 2015;1:25–26. [Google Scholar]
- 2. Gamble JHP, Herring N, Ginks M, Rajappan K, Bashir Y, Betts TR.. Procedural success of left ventricular lead placement for cardiac resynchronization therapy: a meta-analysis. JACC Clin Electrophysiol 2016;2:69–77. [DOI] [PubMed] [Google Scholar]
- 3. Stellbrink C, Breithardt O-A, Hanrath P.. Technical considerations in implanting left ventricular pacing leads for cardiac resynchronization therapy. Eur Heart J Suppl 2004;6:D43–D46. [Google Scholar]
- 4. Purerfellner H, Nesser HJ, Winter S, Schwierz T, Hornell H, Maertens S; European EASYTRAK Registry. Transvenous left ventricular lead implantation with the EASYTRAK lead system: the European experience. Am J Cardiol 2000;86:157K–164K. [DOI] [PubMed] [Google Scholar]
- 5. Worley SJ. Implant venoplasty: dilation of subclavian and coronary veins to facilitate device implantation: indications, frequency, methods, and complications. J Cardiovasc Electrophysiol 2008;19:1004–1007. [DOI] [PubMed] [Google Scholar]
- 6. Worley SJ, Gohn DC, Pulliam RW.. Gooseneck snare for LV lead placement in difficult venous anatomy. Pacing Clin Electrophysiol 2009;32:1577–1581. [DOI] [PubMed] [Google Scholar]
- 7. Worley SJ. Challenging implants require tools and techniques not tips and tricks. Card Electrophysiol Clin 2019;11:75–87. [DOI] [PubMed] [Google Scholar]
- 8. Nath RK, Raj A, Parvatagouda C, Pandit N.. Veno-venous loop through coronary sinus for LV lead placement during cardiac resynchronization therapy. Indian Heart J 2016;68:S212–S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.