Abstract
Background
In-stent restenosis is a difficult percutaneous scenario if calcific neoatherosclerosis is the underlying aetiology.
Case summary
A 69-year-old diabetic woman with a previous percutaneous coronary intervention on the left anterior descending coronary artery was readmitted for non-ST-elevation myocardial infarction. In-stent restenosis due to calcific neoatherosclerosis was observed by intracoronary imaging during the intervention. Intravascular lithotripsy was used successfully to fracture the underlying calcific plaque. However, the balloon ruptured during treatment although this did not damage the artery.
Discussion
Intravascular lithotripsy is a promising tool for the treatment of extremely calcified lesions including calcific neoatherosclerosis of in-stent restenosis. Balloon rupture is a complication of this new percutaneous treatment that has not previously been described.
Keywords: Restenosis, Plaque modification, Intracoronary lithotripsy, Drug-eluting stent, Case report
Learning points
In-stent restenosis by underlying calcific neoatherosclerosis is a challenging percutaneous scenario.
Rotational atherectomy may be performed with caution due to risk of burr stuck if associated under-expansion of the stent is present.
Intracoronary lithotripsy may be included into the tools to treat extremely calcified lesions.
Intracoronary imaging should be performed to understand the aetiology of the in-stent restenosis and guide the treatment.
Introduction
Coronary calcification is a strong predictor of major adverse cardiac events after percutaneous coronary intervention (PCI) because it complicates device delivery, expansion, and apposition, predisposing to stent failure.1 Intracoronary lithotripsy is a new tool for treatment of extremely calcified coronary lesions by fracturing the calcified plaque, thus allowing mean area gain and facilitating stent apposition and expansion.2,3 However, balloon rupture may occur during treatment. Prompt detection of this complication is needed so that rapid measures can be taken to avoid damage to the vessel.
Timeline
| 69-Year-old obese, diabetic, and asthmatic woman | |
|---|---|
| 2003 | Non-ST-elevation myocardial infarction (NSTEMI) Killip Class I: coronary angiography showed one vessel disease treated with left anterior descending coronary artery (LAD) stenting |
| 2016 | NSTEMI Killip Class I: coronary angiography showed non-significant in-stent restenosis and severe lesion at a marginal branch of left circumflex treated with drug-eluting stent |
| 2019 | NSTEMI Killip Class III: coronary angiography showed diffuse in-stent LAD restenosis with positive haemodynamic invasive testing. Intracoronary imaging with intravascular ultrasound revealed severe calcified neoatherosclerosis. Plaque modification with intracoronary lithotripsy was performed. Balloon rupture occurred during treatment and distal embolization resolved with the retrieval of the balloon and intracoronary nitroprussiate administration |
| 2020 | Uneventful at 12 months of follow-up |
Case presentation
The patient was a 69-year-old woman, with diabetes mellitus, obesity, and asthma. In 2003, she had a non-ST-elevation myocardial infarction (NSTEMI) that was treated with a PCI at the proximal and mid-left anterior descending coronary artery (LAD) stent at another facility. In 2016, she was readmitted for a new NSTEMI. Coronary angiography showed a 45% in-stent restenosis and an 85% lesion at a marginal branch, which was treated with 2.75 mm × 26 mm drug-eluting stent (DES). She did not attend post-hospitalization and was lost to follow-up. In January 2019, she was again readmitted for NSTEMI Killip Class III.
On physical examination, the patient was found to have hypertension (non-invasive arterial pressure was 147/75 mmHg), tachycardia (96 b.p.m.), a basal oxygen saturation of 94%, bilateral basal crackles, no cardiac murmurs, and absence of limb oedema. Echocardiography was not performed due to a poor acoustic window.
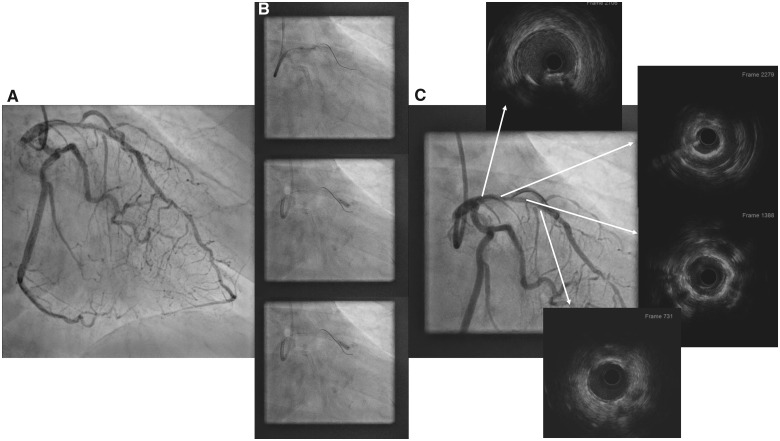
Coronary angiography showed a moderate restenosis of the LAD stent with no other significant lesion (Figure 1A and Supplementary material online, Video S1). During the diagnostic catheterization, invasive left ventricular (LV) end-diastolic pressure was measured revealing high LV filling pressure (20 mmHg). An invasive haemodynamic evaluation with resting full-cycle ratio (RFR) was performed, finding a significant LAD ischaemia (RFR 0.75) with a clear step-up into mid- and proximal LAD. Two wires were inserted: one into the LAD and a second wire to protect the first diagonal branch. In contrast to other plaque modification devices, intravascular lithotripsy may be used safely with several wires left in place. Attempts to expand the proximal part of the LAD stent and the proximal LAD were unsuccessful using a progressive 3.0 high-pressure cutting balloon and a 3.5 non-compliant high-pressure balloon. Intravascular ultrasound (IVUS) showed there was neoatherosclerosis with a 360° calcific arch at the proximal part of the LAD stent and at the proximal LAD; there was no fracture on the calcific arch or dissection at the proximal LAD (Supplementary material online, Video S2). The proximal reference vessel size was 3.5 mm × 3.6 mm (Figure 1B).
Figure 1.
Left coronary angiography (A) showing a diffuse in-stent restenosis involving proximal and mid-left anterior descending coronary artery. Initial treatment with a 3.0 mm cutting balloon and 3.5 mm non-compliant balloon that did not reach adequate expansion (B). Intravascular ultrasound performed after unsuccessful attempts to dilate left anterior descending coronary artery showed a 360°-calcific neoatherosclerosis involving the mid- and proximal part of the stent with a minimal diameter of 2 mm (C).
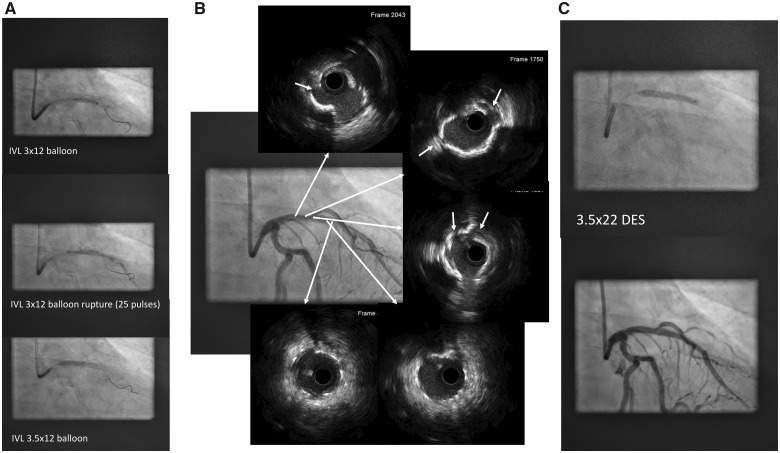
Intravascular lithotripsy was planned in order to treat the calcified neoatherosclerosis and prevent sudden closure of the first diagonal branch. Initially, lithotripsy with a 3.0 × 12 balloon at the mid-distal part of the stent was performed. As a good expansion was achieved with two cycles of pulses, the balloon was then retrieved to the central and proximal part of the stent. However, the balloon ruptured at the third cycle of pulses (Supplementary material online, Video S3), although it had been applied at the recommended pressure (4 atm). The emission of pulses was immediately stopped (Figure 1C) with distal downstream embolization of balloon contents. Transient ST-elevation on electrocardiogram and a drop in the blood pressure were observed and resolved with retrieval of the balloon and application of intracoronary nitroprusside. A coronary angiography showed that there was no distal dissection or vessel rupture (Supplementary material online, Video S4). Then, another 3.5 × 12 lithotripsy balloon at proximal LAD was used to treat the proximal in-stent and proximal LAD (Figure 2A). Following lithotripsy, an IVUS showed multiple fractures of the 360° calcific neoatherosclerosis plaque (Figure 2B, white arrows and Supplementary material online, Video S5). Then, a 3.5 mm × 22 mm DES was implanted covering the proximal previous stent to the LAD ostium, expansion was good, and the final result was excellent (Figure 2C and Supplementary material online, Video S6).
Figure 2.
Initial inflation of the lithotripsy balloon at the mid-stent left anterior descending coronary artery and rupture at 25 pulses with dye leakage in distal left anterior descending coronary artery (A). Adequate expansion of the 3.5 mm × 12 mm lithotripsy balloon at the proximal part of the left anterior descending coronary artery stent. Angiography and intravascular ultrasound performed after the lithoplasty showing calcium rupture and absence of vessel perforation or distal dissection (B). Final result after implantation of a 3.5 mm × 22 mm drug-eluting stent (C).
The patient is on double antiplatelet therapy (with a recommended duration of at least 12 months) and is still uneventful 12 months after the intervention.
Discussion
In-stent restenosis due to calcific neoatherosclerosis is a challenging scenario in which different PCIs such as rotational and orbital atherectomy, intravascular lithotripsy, and intracoronary laser have been tested.4–6 The aim of all of these techniques is to adequately modify the calcific plaque in order to obtain a sufficient minimal lumen area and an expandable intima. Intravascular lithotripsy enables to treat calcific lesions in arteries larger than 2.5 mm without dislodgement of debulked material, through a semi-compliant balloon. However, if the lesion is extremely severe or there is tortuosity at the proximal part of the artery, the lithotripsy balloon may not advance into the lesion. In this case, a mother-in-child catheter may help to advance the balloon into the lesion7 but if this technique is not successful, an alternative therapy (rotational atherectomy) may be used.
So far, few complications had been reported from patients treated with this new technology.2–9 However, here we report the rupture of the balloon in the treatment of an in-stent restenosis lesion at the third cycle of pulses. Balloon laceration may be induced by fractured calcium from the atherosclerotic plaque, leading to balloon rupturing during the treatment. Intracoronary lithotripsy emitters stop as soon as there is no fluid inside the balloon; and then the propagation of the pressure waves decreases and finally stops. Therefore, continuous fluoroscopy is advisable for early detection of balloon leakage in order to rapidly stop the emission of pulses and protect the vessel from further damage.
Conclusion
Intracoronary lithotripsy is a new technology that is becoming an essential tool to treat extremely calcified coronary lesions. However, this procedure must still prove clinical benefits and safety in large series with different types of calcific lesions. The larger series of studies are needed in order to confirm the good results of small series of cases and to assess the rate of complications, such as the balloon rupture reported in this case study.
Lead author biography

Helena Tizón-Marcos is an interventional cardiologist interested in cardiac imaging.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Genereux P, Madhavan MV, Mintz GS, Maehara A, Palmerini T, Lasalle L, Xu K, McAndrew T, Kirtane A, Lansky AJ, Brener SJ, Mehran R, Stone GW.. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J Am Coll Cardiol 2014;63:1845–1854. [DOI] [PubMed] [Google Scholar]
- 2. Ali ZA, Brinton TJ, Hill JM, Maehara A, Matsumura M, Karimi Galougahi K, Illindala U, Götber M, Whitbourn R, Van Mieghem N, Meredith IT, Di Mario C, Fajadet J.. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: first description. JACC Cardiovasc Imaging 2017;10:897–906. [DOI] [PubMed] [Google Scholar]
- 3. Ali ZA, Nef H, Escaned J, Werner N, Banning AP, Hill JM, De Bruyne B, Montorfano M, Lefevre T, Stone GW, Crowley A, Matsumura M, Maehara A, Lansky AJ, Fajadet J, Di Mario C.. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenosis. Circ Cardiovasc Interv 2019;12:e008434. [DOI] [PubMed] [Google Scholar]
- 4. Chen G, Zrenner B, Pyxaras SA.. Combined rotational atherectomy and intravascular lithotripsy for the treatment of severely calcified in-stent neoatherosclerosis: a mini-review. Cardiovasc Revasc Med 2019;20:819–821. [DOI] [PubMed] [Google Scholar]
- 5. Ocaranza-Sánchez R, Abellás-Sequeiros RA, Galvão-Braga C, Trillo-Nouche R, González-Juanatey JR.. Excimer laser coronary atherectomy during percutaneous coronary intervention. Rev Esp Cardiol (Engl Ed) 2016;69:867–868. [DOI] [PubMed] [Google Scholar]
- 6. Salazar C, Escaned J, Tirado G, Gonzalo N.. Recurrent restenosis caused by severe calcific neoatherosclerosis treated with intravascular lithotripsy. EuroIntervention 2019; doi: 10.4244/EIJ-D-19-00268. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7. Rodríguez Costoya I, Tizón-Marcos H, Vaquerizo Montilla B, Salvatella Giralt N, Martí Almor J, Millán Segovia R.. Litoplastia coronaria: experiencia inicial en lesiones calcificadas. Rev Esp Cardiol 2019;72:788–790. [DOI] [PubMed] [Google Scholar]
- 8. Wong B, El-Jack S, Newcombe R, Glenie T, Armstron G, Cicovic A, Khan A.. Shockwave intravascular lithotripsy of calcified coronary lesions in ST-elevation myocardial infarction: first-in-man experience. J Invasive Cardiol 2019;31:E73–E75. [DOI] [PubMed] [Google Scholar]
- 9. Venuti G, D'Agosta G, Tamburino C, La Manna A.. Coronary lithotripsy for failed rotational atherectomy, cutting balloon, scoring balloon, and ultra-high-pressure non-compliant balloon. Catheter Cardiovasc Interv 2019;94:E111–E115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.