Abstract
Background
Post-cardiac injury syndrome (PCIS) is an inflammatory process that may occur after myocardial infarction, cardiac surgery, percutaneous cardiac interventions or chest trauma. To our knowledge, PCIS following transcatheter mitral valve repair (TMVr) using the MitraClip system has not been reported.
Case summary
A 79-year-old female with chronic heart failure and severe mitral regurgitation received TMVr using the MitraClip system. After the procedure she developed elevated inflammatory markers, pericardial and pleural effusion. Cardiac magnetic resonance provided signs of pericardial and pleural inflammation. After initiating an anti-inflammatory therapy with Aspirin and Colchicine, inflammatory markers decreased markedly, pleural and pericardial effusions were regressive, and the patient showed rapid clinical improvement.
Discussion
Post-cardiac injury syndrome may occur after TMVr and should be considered as a differential diagnosis in patients developing chest pain, signs of pericarditis with or without pericardial effusion and elevated inflammatory markers.
Keywords: Post-cardiac injury syndrome, Transcatheter mitral valve repair, Case report
Learning points
Post-cardiac injury syndrome may occur after transcatheter mitral valve repair (TMVr)
Post-cardiac injury syndrome should be considered as a differential diagnosis in patients developing fever, elevated inflammatory markers, chest pain, pericardial and pleural effusion after TMVr, provided ruling out alternative causes.
Introduction
Post-cardiac injury syndrome (PCIS) represents an acute pericarditis resulting from injury to the pericardium.1 Its mechanism is not well understood. Post-cardiac injury syndrome may develop after myocardial infarction, cardiac surgery or percutaneous cardiac interventions. Most patients present with chest pain, elevation of inflammatory markers as well as pericardial and pleural effusion.2 We present here a case of PCIS after transcatheter mitral valve repair (TMVr) using the MitraClip System.
Timeline
| Presentation of the patient | |
|---|---|
| September 2019 | Main complaint: exertional dyspnoea (New York Heart Association III). |
| CXR: cardiomegaly, pulmonary congestion. | |
| Transthoracic echocardiogram (TTE): dilation of both atria, moderately reduced LVEF (35%), mildly reduced systolic RV function. Severe mitral valve regurgitation (MR), moderate TR, aortic and pulmonary valves with no relevant pathologies. No pericardial effusion. | |
| Cardiac catheterization: no stenosing coronary artery disease. Post capillary pulmonary hypertension. | |
| Intervention | |
| September 2019 | Transcatheter mitral valve repair, implantation of one Clip. |
| Post-procedural course | |
| Day 0 | TTE: No evidence of pericardial effusion immediately after the procedure. |
| Day 1 | TTE: No evidence of pericardial effusion. |
| Day 3 | Patient complained of malaise, fatigue and epigastric pain. |
| C-reactive protein (CRP) 80 mg/L (normal <5 mg/L), procalcitonin (PCT) 0.16 ng/mL (normal <0.5 ng/L). | |
| TTE: Mild circular pericardial effusion (8 mm). | |
| Day 5 | CRP 200 mg/L, PCT 0.17 ng/mL. |
| TTE: Stable pericardial effusion. | |
| Day 7 | CRP 290 mg/L, PCT 0.15 ng/mL. |
| TTE: progress of the pericardial effusion (13 mm). | |
| CT: serous pericardial effusion, bilateral pleural effusion. | |
| Management | Initiation of anti-inflammatory therapy with Aspirin and Colchicine |
| Day 10 | CRP 230 mg/L. |
| TTE: regression of the pericardial effusion (10 mm). | |
| Day 12 | CRP 137 mg/L. |
| Cardiac magnetic resonance: thickening of the pericardium with late gadolinium enhancement and mild pericardial effusion. | |
| Day 15 | CRP 40 mg/L. |
| TTE: no evidence of pericardial effusion. | |
| Discharge of the patient | |
| Follow-up | |
| Day 30 | CRP 1.4 mg/L. |
| TTE: no evidence of pericardial effusion, mild residual MR. | |
| CXR: no pleural effusion | |
Case presentation
A 79-year-old woman was admitted to our cardiology department with progressively worsening dyspnoea (New York Heart Association Classification III) in the last 6 weeks. She suffered from idiopathic dilated cardiomyopathy with moderately reduced systolic left ventricular function for 5 years. Her other medical history included arterial hypertension and permanent atrial fibrillation. Her medication included Candesartan, Carvedilol, Torasemide, Spironolactone, Digoxin in addition to Edoxaban. On admission she had a holosystolic apical murmur radiating to the axilla. Bibasilar crackles were audible and there was bilateral lower extremity pitting oedema. Blood pressure was 130/70 mmHg, heart rate was 65 b.p.m. Electrocardiogram revealed atrial fibrillation, narrow QRS complex (95 ms) with asymmetric T-wave inversion in leads II, III, aVF, and V4–V6, potentially related to digitalis therapy. Chest X-ray showed cardiomegaly with sharp costophrenic angles (Figure 1). NT-pro brain natriuretic peptide (NT-proBNP)-level was elevated (3500 pg/mL). Transthoracic echocardiogram (TTE) revealed dilation of both atria, dilatation of the left ventricle (LV), and moderately reduced left ventricular systolic function with an estimated ejection fraction [left ventricle ejection fraction (LVEF)] of 35%, mild dilation of the right ventricle (RV) with mildly reduced systolic function [right ventricle ejection fraction (RVEF)] and moderate tricuspid regurgitation. In addition, severe mitral valve regurgitation (MR) due to ring dilation and restrictive motion of the posterior mitral leaflet was demonstrated. The aortic and pulmonary valves showed no relevant pathology. Transesophageal echocardiogram (TEE) confirmed the presence of severe functional MR (proximal isovelocity hemispheric surface area radius 10 mm, effective regurgitant orifice 50 mm2, regurgitation volume 66 mL). Coronary angiography excluded stenosing coronary artery disease. Moderate post-capillary pulmonary hypertension was detected by pulmonary artery catheterization (pulmonary artery pressure 40/16/30 mmHg, post-capillary wedge pressure 26 mmHg).
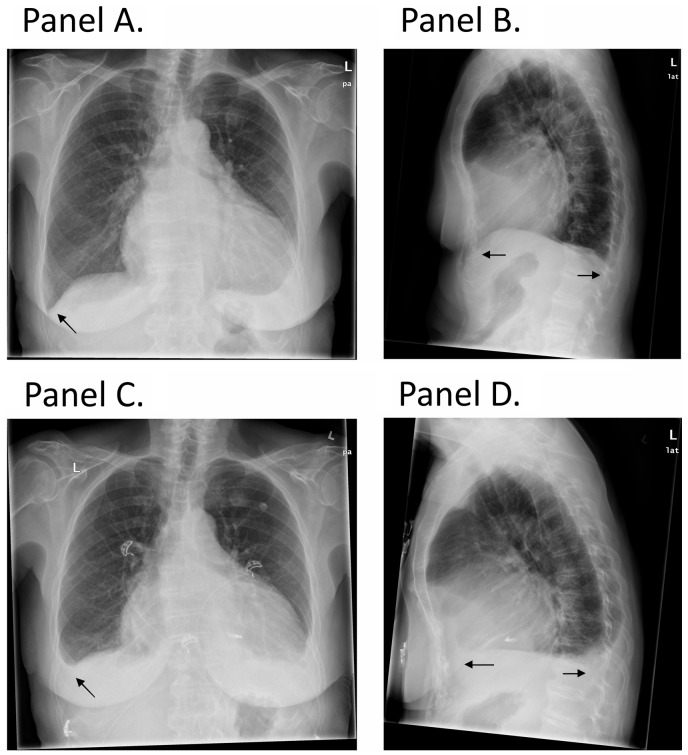
Figure 1.
(A and B) Chest X-ray before transcatheter mitral valve repair demonstrating free costophrenic angles (arrow). (C and D) Chest X-ray on Day 10 after transcatheter mitral valve repair showing the presence of bilateral pleural effusion (arrow).
The case was discussed extensively in our Heart-team. Due to persistence of symptoms despite optimal medical therapy and because of a high surgical risk (EuroScore II of 7%), it was decided to proceed with TMVr.
The procedure was successfully performed and placement of one mitral clip reduced severe MR to mild residual regurgitation. Transthoracic echocardiogram performed immediately, and on Day 1 after the procedure showed no evidence of pericardial effusion. On Day 3, the patient complained of fatigue, malaise and non-specific epigastric pain. Laboratory investigations revealed an elevated C-reactive protein (CRP 70 mg/L) with normal white blood cell count (WBC 9/nL) and normal procalcitonin (PCT 0.16 ng/mL). Chest X-ray detected bilateral pleural effusion without pulmonary congestion or infiltrate (Figure 1). Electrocardiogram did not reveal any new changes. Over the next few days, CRP increased further (200 mg/L on Day 5) while no change of PCT was detected. Blood cultures remained sterile and antibiotics were not applied. TTE on Day 5 revealed a circular mild pericardial effusion (8 mm), which progressed to a moderate effusion (13 mm) on Day 7 (Figure 2). However, the pericardial effusion remained without haemodynamic relevance. Cardiac computed tomography (CT) confirmed the presence of pericardial and pleural effusion. Both had a similar, low density (about 10 Hounsfield units) suggesting serous rather than a haemorrhagic fluid (Figure 3). Cardiac magnetic resonance (CMR) showed thickened pericardium with late gadolinium enhancement (LGE) of both pericardium and pleura, suggesting an acute pleuro-pericarditis (Figure 4).
Figure 2.
(A) Transthoracic echocardiogram in parasternal long-axis view performed before transcatheter mitral valve repair showing no evidence of pericardial effusion. (B) Transthoracic echocardiogram in parasternal long-axis view showing mild pericardial effusion (8 mm) on Day 5 after transcatheter mitral valve repair. (C) Progress of the pericardial effusion (13 mm) on Day 7 after transcatheter mitral valve repair.
Figure 3.
Computed tomography thorax in soft tissue window (A) and in lung window (B) showing pericardial effusion (arrow) and bilateral pleural effusion (arrow). Note the similar density of the pericardial and pleural effusion. Note: local artefact of the MitraClip device.
Figure 4.
Cardiac magnetic resonance four-chamber view 10 min after gadolinium injection showing pericardial inflammation (circular pericardial late gadolinium enhancement and pericardial thickening, arrow). Note: local artefact of the MitraClip device (arrow).
Given the new onset pericardial and pleural effusion, the elevated inflammatory markers and signs of inflammation demonstrated by CMR, the diagnosis of acute pericarditis in the context of PCIS was confirmed. We initiated an anti-inflammatory therapy with high dose Aspirin (3g/day) and Colchicine (0.5 mg/day) under gastric protection with a proton pump inhibitor. Within days after initiating the therapy, CRP decreased, pericardial effusion receded and was no longer detectable after 9 days of treatment. The clinical situation of the patient improved markedly, and she was discharged home. The anti-inflammatory therapy with Aspirin was gradually tapered and reduced by 500 mg every 2 weeks. After 1 month, the patient reported an improvement of her heart failure symptoms especially dyspnoea. TTE showed a proper position of the mitral clip with only mild residual MR (Figure 5). The LVEF and RVEF were unchanged. Other than the previously described moderate tricuspid regurgitation there were no relevant valvular pathologies. Importantly, there was no evidence of pericardial effusion. Chest X-ray did not show any pleural effusion. C-reactive protein level was in the normal range and NT-ProBNP declined to 1900 pg/mL. Aspirin was further reduced and was together with Colchicine discontinued after a total of 3 months. Otherwise, there was no change in the heart failure therapy.
Figure 5.
Transthoracic echocardiogram in four-chamber apical view showing severe mitral valve regurgitation before transcatheter mitral valve repair (left) and minimal mitral valve regurgitation after transcatheter mitral valve repair (right).
Discussion
Pericarditis is the most common pericardial syndrome and has many aetiologies.3,4 Post-cardiac injury syndrome is defined as an acute pericarditis, occurring because of an injury to the pericardium. Owing to the recent increased use of various cardiovascular interventions, the incidence of PCIS is increasing. In the prospective cohort of Gouriet et al.,5 which included 1162 patients with pericarditis, PCIS represented 24% of all cases. The definite pathology of PCIS is not well known. It is thought to be an autoimmune response to a damage of pericardial cells due to myocardial infarction, cardiac surgery, thorax trauma, or cardiac interventions.1 Cases of PCIS were described after coronary interventions, implantation of cardiac devices and transcatheter aortic valve implantation.6–9
However, the diagnosis of PCIS may be challenging. Diagnostic criteria include fever without alternative causes, pleuritic chest pain, friction rub, development of a new or worsening of pericardial effusion in addition to the development of a new or worsening of pleural effusion. At least two of these criteria should be present to confirm the diagnosis.10
In addition to echocardiography, the role of other non-invasive cardiac imaging modalities such as cardiac CT and CMR in the diagnosis of PCIS and pericardial disease is increasing.11 The measured density of pericardial fluid by CT scan may help to determine the type and aetiology of the pericardial effusion. A low density of the pericardial fluid (<20 HU), such as in our case, suggests a serous effusion, whereas a higher density (>30 HU) suggests a haemorrhagic effusion.12 Although it currently does not belong to the diagnostic criteria, the presence of a high pericardial signal in late gadolinium enhanced CMR images suggests an acute inflammation and consequently supports the diagnosis of acute pericarditis.13 The treatment of PCIS, as in acute pericarditis, consists of an anti-inflammatory drug as Ibuprofen or high-dose Aspirin in addition to Colchicine to prevent recurrence.3,4
In our case, clinical complaints of malaise and fatigue accompanied by elevated inflammatory markers suggested primarily a nosocomial infection. However, negative PCT and the negative microbiological cultures made this diagnosis unlikely. The initial absence of pericardial effusion after the intervention and its slow progression in addition to its low density by CT scan argued against a direct injury of heart or its surrounding structures during the procedure. Such injury or a possible perforation should have caused a rapid haemopericardium and eventually cardiac tamponade. Considering the elevated CRP, the development of pericardial effusion as well as the signs of pericardial inflammation by CMR, the diagnosis of acute pericarditis was made. However, in the light of above-mentioned diagnostic criteria,10 the concomitant development of bilateral pleural effusion in addition to the signs of pleural inflammation by CMR proposed the diagnosis of PCIS. The rapid therapeutic response to anti-inflammatory therapy further supports this diagnosis.
As far as we know, this is the first reported case of PCIS after TMVr using MitraClip system.
Conclusion
Post-cardiac injury syndrome may occur after TMVr and should be considered as a differential diagnosis in patients developing elevated inflammatory markers with signs of pleuro-pericarditis after ruling out other causes.
Lead author biography

Mhd Nawar Alachkar is a cardiology resident at the university hospital in Aachen, Germany. In 2018, he was nominated as a young national ambassador of his home country Syria in the acute cardiovascular care association ‘ACCA’.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Imazio M, Hoit BD.. Post-cardiac injury syndromes. An emerging cause of pericardial diseases. Int J Cardiol 2013;168:648–652. [DOI] [PubMed] [Google Scholar]
- 2. Imazio M, Brucato A, Rovere ME, Gandino A, Cemin R, Ferrua S. et al. Contemporary features, risk factors, and prognosis of the post-pericardiotomy syndrome. Am J Cardiol 2011;108:1183–1187. [DOI] [PubMed] [Google Scholar]
- 3. Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J. et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Imazio M, Gaita F, LeWinter M.. Evaluation and treatment of pericarditis: a systematic review. JAMA 2015;314:1498–1506. [DOI] [PubMed] [Google Scholar]
- 5. Gouriet F, Levy P-Y, Casalta J-P, Zandotti C, Collart F, Lepidi H. et al. Etiology of pericarditis in a prospective cohort of 1162 cases. Am J Med 2015;128:784.e1–784.e8. [DOI] [PubMed] [Google Scholar]
- 6. Gao Y, Bishopric NH, Chen H, Li J, Huang Y, Huang H.. Post-cardiac injury syndrome in acute myocardial infarction patients undergoing PCI: a case report and literature review. BMC Cardiovasc Disord 2018;18:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sedaghat-Hamedani F, Zitron E, Kayvanpour E, Lorenz H-M, Katus HA, Meder B.. Post cardiac injury syndrome after initially uncomplicated CRT-D implantation: a case report and a systematic review. Clin Res Cardiol 2014;103:781–789. [DOI] [PubMed] [Google Scholar]
- 8. Sousa C, Martins E, Campelo M, Rangel I, Almeida PB, Maciel MJ.. Post-cardiac injury syndrome following transvenous pacing: case report. Rev Port Cardiol 2014;33:307.e1–307.e4. [DOI] [PubMed] [Google Scholar]
- 9. Llubani R, Böhm M, Imazio M, Fries P, Khreish F, Kindermann I.. The first post-cardiac injury syndrome reported following transcatheter aortic valve implantation: a case report. Eur Heart J Case Rep 2018;2:yty107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imazio M, Brucato A, Ferrazzi P, Spodick DH, Adler Y.. Postpericardiotomy syndrome: a proposal for diagnostic criteria. J Cardiovasc Med (Hagerstown) 2013;14:351–353. [DOI] [PubMed] [Google Scholar]
- 11. Yared K, Baggish AL, Picard MH, Hoffmann U, Hung J.. Multimodality imaging of pericardial diseases. JACC Cardiovasc Imaging 2010;3:650–660. [DOI] [PubMed] [Google Scholar]
- 12. Tomoda H, Hoshiai M, Furuya H, Oeda Y, Mat’sumoto S, Tanabe T. et al. Evaluation of pericardial effusion with computed tomography. Am Heart J 1980;99:701–706. [DOI] [PubMed] [Google Scholar]
- 13. Cosyns B, Plein S, Nihoyanopoulos P, Smiseth O, Achenbach S, Andrade MJ. et al. European Association of Cardiovascular Imaging (EACVI) position paper: multimodality imaging in pericardial disease. Eur Heart J Cardiovasc Imaging 2015;16:12–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.