Abstract
The ultimate goal of phototherapy based on nanoparticles, such as photothermal therapy (PTT) which generates heat and photodynamic therapy (PDT) which not only generates reactive oxygen species (ROS) but also induces a variety of anti-tumor immunity, is to kill tumors. In addition, due to strong efficacy in clinical treatment with minimal invasion and negligible side effects, it has received extensive attention and research in recent years. In this paper, the generations of nanomaterials in PTT and PDT are described separately. In clinical application, according to the different combination pathway of nanoparticles, it can be used to treat different diseases such as tumors, melanoma, rheumatoid and so on. In this paper, the mechanism of pathological treatment is described in detail in terms of inducing apoptosis of cancer cells by ROS produced by PDT, immunogenic cell death to provoke the maturation of dendritic cells, which in turn activate production of CD4+ T cells, CD8+T cells and memory T cells, as well as inhibiting heat shock protein (HSPs), STAT3 signal pathway and so on.
Keywords: pathological mechanism, nanoparticles, photothermal therapy, PTT, photodynamic therapy, PDT
Introduction
Malignant tumors and their metastases have led to a high mortality rate in young people. The key is that many anti-tumor treatments, such as radiotherapy, chemotherapy, molecular-targeted therapy and immunotherapy, have too many systemic side effects; firstly, it causes severe damage to the immune system, and secondly, it also leads to long-term destruction of organ functions.1,2 As a result, few patients can be cured by clinical cancer treatment, and the disease has developed rapidly in recent years. Eventually, it can lead to serious consequences, such as multiple wasting death caused by organ failure, and severe malnutrition, etc.3 In recent years, PDT and PTT have been proposed to inactivate pathogens as new therapeutic regimens for tumor ablation and necrosis. PTT and PDT are mainly composed of near-infrared light (NIR) and nanoparticles, which, respectively, correspond to photosensitive (PS) and photothermal agents. The application principle of PTT lies in the heat generated by gold nanoparticles and the activation of a photothermal agent under specific light wavelengths to kill cancer cells. The anti-tumor effect of PDT is under the interaction of the plasma nano-platform of the local electric field to produce single ROS and free radicals with cytotoxicity, short half-life and small diffusion rate, leading to apoptosis, autophagy and necrosis of tumor cells.4 With the addition of nanoparticles, PS is delivered across the blood-brain barrier, and especially transport drugs to cell chambers such as nuclei.5 This review focuses on the pathological mechanism of PTT and PDT killing tumor tissue.
Progress in Clinical Application of PTT and PDT Based on Nanoparticles
Application Materials
Solid tumors have leaking blood vessels, and the gap between cells is from 100 nm to 780 nm, these permeable blood vessels allow nanoparticles to extravasate into the tumor matrix.6
The photothermal material system has gone through about four generations, as follows: The first generation is precious metal nanoparticles such as Au, Ag, Pt, etc., which have high photothermal conversion efficiency and imaging but are limited to expensive and rare. Gold nanorods depend on adjustable size and aspect ratio and two unique absorption bands are transverse and longitudinal, which make it have unique optical properties.7 However, because of their large surface area, unstable surrounding environment and easy oxidation after long-term exposure to air, their application is limited, so methods to improve the oxygen resistance of metal nanoparticles have been developed such as plasma spraying, phosphating and electrolyte deposition.8 One of the most ideal passivating methods is atomic layer deposition, which provides an ultra-thin layer on metal nanoparticles.9 Using branched poly (vinylamine) (PVA, MW=10k Da) as the best linker, the system was prepared on suitable thin layer hollow gold nanospheres (HAuNS) combined with indocyanine green (ICG) to maintain the fluorescence,10 significantly enhanced intratumoral accumulation, boost photothermal conversion efficiency and synchronous PTT and PDT.11
The second generation is carbon materials, for example, graphene and carbon nanorods, which have large photothermal conversion area but have poor absorption capacity under the NIR.12 Wang et al proposed that an up-conversion Magnetic Agent (FeCUPs) mediated by Hollow carbon spheres provides a dual-function platform of PTT and biological imaging for tumor elimination.13
The third generation is metal and non-metallic compounds such as CuS and ZnS, which are in the most exciting part of the research.14,15 It has high photothermal performance and low cost, can be easy to prepared, but it is usually non-fluorescent and lacks the ability of tumor-targeted ablation, which limits the rapid real-time fluorescence imaging and localization of primary tumors and lymph node metastases.16 Hua Shi et al presented that RGD-CuS-Cy5.5, a kind of fluorescent CuS nanoparticle that combines tumor-targeting ligand RGD and NIR organic dye Cy5.5, for the treatment of sentinel lymph node metastasis of gastric cancer by fluorescence dual-mode imaging.17
The fourth generation is organic and inorganic nanomaterials such as organic semiconducting pronanostimulant (OSPS) and Indoline green, Prussian blue (PB), etc. which are in the key areas for scientists to explore.18 OSPS via singlet oxygen (1O2) as a cutting linker consists of semiconductor polymer nanoparticles (SPN) nucleus which is a PTT agent and immune stimulator. It can produce heat and 1O2 for PDT to achieve combined PTT that means not only suppressing tumor but also generating tumor-associated antigen. Moreover, due to its diffusion of dual electrons, it has strong NIR absorption capability. When exposed to NIR, the cleavage of 1O2-linkers triggers the long-term release of immune stimulator and regulates immune suppression tumor microenvironment. OSPS not only could fall down the expression of metastasis-associated proteins by mediating RNA degradation but also exert synergistic antitumor immunity after excellent PTT, inhibits growth and lung metastasis of primary or distant tumor.19 Experiments have verified that organic nanomaterials possess good biocompatibility, low toxicity and optical stability. Therefore, Liang Cheng et al encouraged researchers to explore more organic nanomaterials for cancer therapy applications.20 PBNPs have strong optical absorbance, excellent photothermal conversion rate and stability in near-infrared spectroscopy, and can be obtained by economical and simple synthesis process. However, due to the lack of functional chemical groups, the ability to combine with other therapeutic molecules is greatly inhibited.21 Sun et al researched that PB@RBC/Ce6NP-mediated PDT/PTT combined therapy has a significant effect on tumor cell necrosis and late apoptosis, because of obvious intratumoral cell uptake and accumulation, it has a great synergistic therapeutic effect on the model of in situ tumor in vivo.22
Meanwhile, PS also experienced three dynasties of exploration during PDT. The father of PS is hematoporphyrin derivative (HPD) which is highly specific to tumor and has obtained worldwide registration examination in lung cancer, esophageal carcinoma and bladder cancer, etc.;23,24 however, its disadvantages include complicated composition, slow excretion and certain light toxicity which require long periods of light protection.25 JANINE R.SHULOK has showed that with increasing HPD dosage and incubation time, mitochondrial damage increased by producing PDT effect.26
Our country independently developed the second generation domestic new photosensitive agent has stable structure strong photosensitive ability, clear quickly, and in terms of curing digestive tract tumor, it has a good curative effect. The representative medicine contains chlorophyll degradation derivatives,27 metal phthalocyanine,28 benzene porphyrin, etc.29 However, biocompatibility is poor, and the target function also needs improving.30
In order to make photosensitizer targeted stronger and more efficient, the third generation PS emerges as the time requires. Generally, it is divided into four categories: a) actively targeting functions, for example, take immune,31 epidermal growth factor receptor (EGFR), low-density lipoprotein (LDL), mRNA, etc., as targeting.32,33
b) It has functions of magnetic orientation and heat therapy: Fe3O4 nanoparticles are widely used in hyperthermia (HPT) therapy for tumor tissues under direct current magnetic field.34 HPT and PDT co-therapy have been successfully combined with HPD or zinc phthalocyanine, etc., in combination therapy.35
c) It has radiation therapy functions: Using these specific luminescent nanoparticles and photosensitizer combinations not only not requires light sources but also improves radiation doses of X-rays.36
d) It has a multifunctional nanoparticle platform for photosensitizer. This nano-platform with polyacrylamide (PAA) core not only makes tumor tissue absorb photosensitizer more specifically but also can realize real-time detection of PDT and dosage; moreover, it evaluates curative effect during and after treatment.37,38
Correlation Between PTT and PDT
This article takes the gold nanorods (AuNRs) as examples. When the AuNRs absorbed by the cell is excited by a light source whose wavelength is close to AuNRs LSP resonance, this enhanced absorption can produce a strong PTT effect, which leads to the damage of cancer cells. By connecting the photosensitizer with the cell’s AuNRs uptake, stimulating the irradiation of the photosensitizer can produce PDT effect, so different effects can be selectively controlled by controlling the intensity of the light source.39 If we choose the excitation wavelength that can excite the photosensitizer and LSP resonance at the same time, the PTT and PDT effects will be produced synchronously, thus further destroying the target cells.40 Ding et al designed Au-Cu9S5, an LSP-enhanced light absorption cross section, applicating of PTT principle, has the thermosensitive nanomaterial potential and imaging ability of X-ray CT.41
Progress in the Field of Oncology
The researchers used this method to transfer the drug from the adnexal lymph nodes to the tumor lymph nodes to treat lymph node metastasis.42 Where there is a rich vascular network on the outside of the tumor, blood can reduce heat accumulation, which reduced the effect of PTT, at the same time, because of the characteristics of PDT, its application outside the blood is very effective. So Bin Liu et al proposed that the prepared multifunctional GNS@CaCO3/Ce6-NK cells have the bimodal functions of fluorescence imagery and enhance the PTT/PDT and immunotherapy of the target tumor tissue.43 It is well known that NKG2D ligands (MHC)I chain-related proteins and UL16-binding proteins are easily activated under any stimulation and are subject to cytotoxicity dominated by NK cells.44 When NK cells are as goods delivery nanoparticles, which aims to prevent virus infection via releasing of a variety of cytokines, such as perforin and granzyme and they have nothing to do with antibodies, antigen presentation or MHC I. In addition, due to the lack of T cell receptor (TCR), on the surface of NK cells, it is not necessary to consider graft and host disease (GVHD).45
Over recent years, with the development of nanoengineering, combining molecular activates cancer immunotherapy has attracted more and more attention, as it can convert cold tumor into hot tumor to apply to PTT and photo-immunotherapy.46 These activated nanoparticles are only stimulated by internal stimulation such as acid PH, oxidation redox potential, hypoxia and overexpression of tumor (caspase and HAase) or external stimulation such as light ultrasound magnetic fields, which cause cascade effects: local reprogramming of tumor microenvironment and activating antitumor immunity while reducing the incidence of immune-associated adverse events.47 But there are two major challenges; generally, in blood circulation processes, a large proportion of nanomedicines are absorbed by nonspecific cells and eventually metabolize in liver, kidney and other organs. Therefore, innovative methods should be studied to reduce the dosage of drug and improve targeting bioavailability. Another challenge is that tumor immune microenvironment is dynamic and changes during treatment, so developing personalized adjustment intervention technologies based on different immune therapy stages is essential.19
Mitochondria are indispensable organelles for cell respiration, which can mediate apoptosis and are important pharmacological targets for clinical tumors.48 Xiaoyan Yang suggested that an imaging-guided mitochondrial targeting PTT/PDT nanosystem is based on functionalized black phosphorus nanoscale (BPNGS).49 Because these materials are lipophilic enough, the carbodiimide reaction between amino groups can penetrate the lipid bilayer and promote the efficient accumulation of reagents in mitochondria.50
Macrophage M1 activates T cells through the expression of IL-12 and IL-23 to play an anti-tumor effect. On the contrary, macrophage M2 synthesizes and secretes nourishing tumor cells and promotes angiogenic cytokines to inhibit T cell proliferation and activation.51
However, the hypoxic microenvironment of the tumor itself polarizes macrophages to M2 phenotype, which seriously hinders tumor immunotherapy.52 Therefore, the reversal principle of hypoxia in tumor microenvironment is helpful to improve the effect of macrophage immunotherapy.53,54 In addition, complete biocompatibility and hydrophobicity by introducing hydrophilic substituents.55 So Wang et al present they constructed a multifunctional Bi/MnPcE4 nanocomposite. In the acidic H2O2 environment of tumor TME, the following reaction processes effectively solved the anoxic environment of PDT, enhanced PTT, and realized in vivo fluorescence/CT/magnetic resonance imaging.56 The basic reaction process is as follows:
Mn2++2H2O2→ Mn(oH)2+2H+ (1)
Mn(OH)2 +H2O2→ MnO2+H2O (2)
MnO2+H2O2+2H+→Mn2++2H2O+O2↑ (3)
Advance for Other Medical Area Treatment
PTT and PDT combination therapy is used in many medical fields, such as. Using the most suitable laser NIR and ICG staining technology to provide a way to control fat cells and decompose fat, while reducing the uploading of waste in fat masses in obese people.57 Apart from that, it also has a crucial therapy of rheumatoid arthritis (RA)58 and melanotic diseases.59 Similarly, P.K. Pandey et al concluded that it has the following advantages: four-fold higher localization and sensitivity and therapeutic efficacy and no hemolytic toxicity and good stability.60 It is suggested that the method can also be applied to different doses of anti-inflammatory and immune regulation and other clinical interventions. Recently, joint application of PDT and PTT is a useful tool for bacterial eradication, especially multi-drug resistant (MDR) bacteria.61 Giza et al propose that Toluidine blue O (TBO) and GNP were used to kill bacteria.62
The Killing Mode of PDT on Tumor Cytokines
The killing effect of photodynamic on tumor cells is mainly through three ways: (1) The direct killing effect on tumor cells leads to its necrosis. (2) ROS produced by photodynamic causes apoptosis by inducing the changes of oxygen free radicals and irreversible damage to cells and microvessels. (3) Dying or dead cells caused by photodynamic stimulate immunogenic cell death, and produce a series of effects and applications in the later stage.
Cell Necrotic
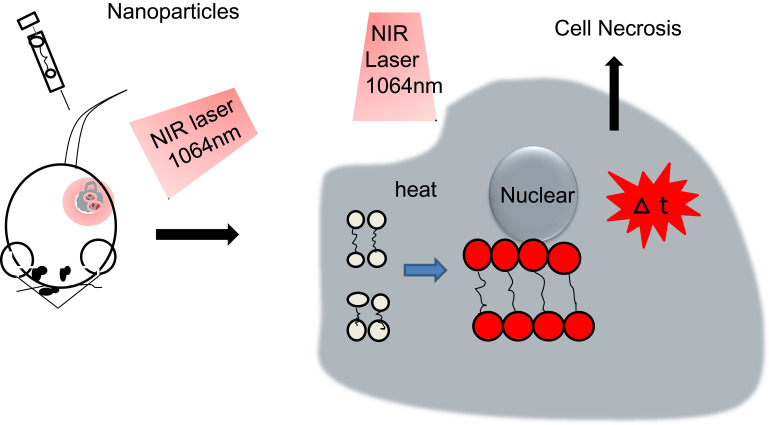
Cell necrosis (NE) is an unscheduled process of cell death, characterized by cytoplasmic expansion, severe organelle damage and plasma membrane rupture.63 It leads to the release of cell contents and inflammation. The PTT effect is due to the LSP resonance of gold nanoparticles.64 The way to cause rapid necrosis or rapid development of late apoptosis is to incubate the cells with only AuNRs and irradiate them with a 1064-nmde laser close to the LAP formant of Au to achieve a separate PTT strategy65 (Figure 1).
Figure 1.
Mechanism of tumor necrosis caused by PTT based on nanomaterials.
Cell Apoptosis
Due to the high metabolism of tumor and the strong ability to stimulate neovascularization, the tumor microenvironment is in a state of hypoxia, which affects the effect of PDT to a great extent. Therefore, Jun Yang et al also presented that a non-oxygen free radical generated nano-system (CuFeSe2-AIPH@BSA) with bimodal absorption in the NIR-II region, achieving deeper tissue penetration and more maximum permissible exposure,66 is used for imaging-guided synergistic hyperthermia and toxic free radical generation in tumor anoxic microenvironments.
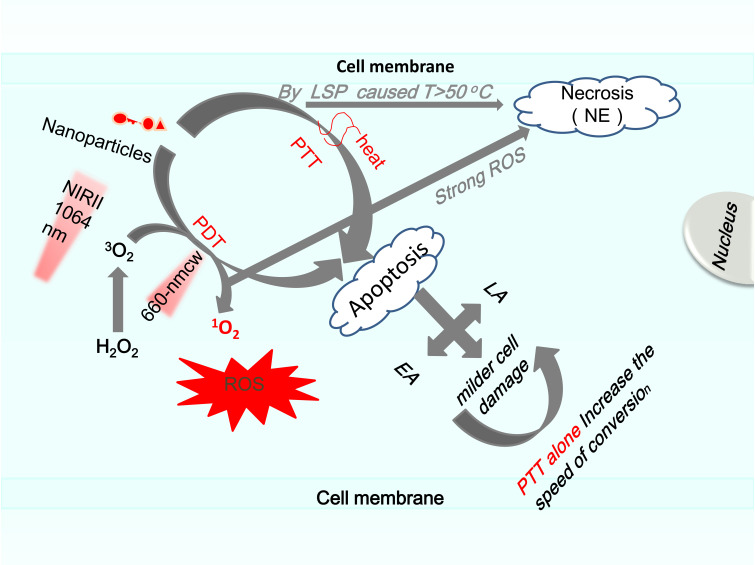
According to the calibration of threshold laser flux, the pathway of cell death and the evolution rate of apoptosis under different cell treatments were evaluated as follows: early apoptosis (EA), necrotic (NE) and late apoptotic (LA). A cell in EA phase presents phosphatidyl serine on the surface of the cell. Compared with the PTT effect, PDT usually shows slighter, which leads to apoptosis. However, when the effect of ROS is too strong, it will cause cell necrosis. Compared with the PDT effect alone, that is, the transition from EA to LA in 30 minutes, only the PTT effect changes faster. Compared with the PT effect alone, when AuNRI is internalized into the cell and adsorbed on the cell membrane, combined with PT and PD effect, the transition rate becomes slower in the early stage. When AuNRIs is not attached to its surface, the transfer rate in the early stage is higher than the PDT effect alone.67 The way to keep the cells in the early stage of apoptosis for a long time is to incubate the cells with AuNRIs connected with AlPcs, and the light of the 1064-nm laser is reached, resulting in the combined effect of PTT and PDT (Figure 2).
Figure 2.
The mechanism of tumor apoptosis and necrosis caused by PTT/PDT/combined therapy based on nanoparticles was explained scientifically.
The heterogeneous ligand-modified nanocarrier activates controlled cargo release through a pH tunable switch, which effectively avoids the side effects of normal tissue, the decomposition of H2O2 in tumor tissue makes it in a weakly acidic microenvironment, so based on this characteristic, a series of PH-sensitive switch therapy measures are designed to specifically kill tumor cells.68 In this study, human serum albumin was used to load IR780, through protein self-assembly to form nanoparticles (HSA-IR780 NPs), which converts part of the energy of the excited singlet into heat by means of vibrational relaxation or other non-radiative transitions.69 At the same time, the singlet also produces reactive oxygen species through interline crossover to a lower energy-excited triplet, which induces oxidation with surrounding biological macromolecules and destroys tumor cells.70
David W.C. Hunt et al found, compared with resting T cells, PDT changes the fluorescence and photodynamic properties of activated T cells due to the ability of PDT to chelate iron in activated T cells with photosensitizer benzoporphyrin derivative monoacid ring A (BPD-MA),71 which may contribute to the immunomodulatory effect of BPD-MA. Therefore, the significant sensitivity of activated T cells to photodynamic inactivation may contribute to the immunomodulatory effect of BPD-MA.72
Immunogenic Cell Death (ICD)
PTT and PDT not only have an obvious curative effect on killing tumor cells but also stimulate a series of immune responses to related apoptotic and necrotic tumor cells and inflammatory cells.73 Recently, it has been found that ROS produced by endoplasmic reticulum stress in PDT to fight against cancer by inducing apoptotic cell death subroutine mode of active immunity, which is called ICD whose prerequisites are calcium reticulin surface exposure (ecto-CA LR), accompanied by IFNG production, ATP secretion, dendritic cell maturation (DCs) and stimulation of T cells.74 After that, injury-related molecular models (DAMPS) can be induced, including calreticulin (CRT), heat shock proteins (HSP70 and HSP90), high mobility box 1 (HMGB1) and so on.75 As an antigen model, it activates the host immune system for anticancer therapy by stimulating the antigen presentation of dendritic cell (DC) and the proliferation of cytotoxic T lymphocytes (CD8+T cells).76 One of the disadvantages of PDT is the oxygen consumption process, so the nanosystem is modified by ER targeting Pardaxin peptides (FAL-ICGHAuNS) and oxygen transfer hemoglobin (FAL-HB liposomes) to reverse hypoxia. ROS generated by PDT in turn trigger ER stress and cause downstream DAMP/danger signal pathway which can be more effectively promoted.77 So Deng et al proposed that Ds-sP/TCPP-TERNPs can selectively accumulate in ER, locally produce ROS, to induce ER stress, magnify ICD and activate immune cells, resulting in enhanced immunotherapy effect.78 In addition, photooxidation of (phox) stress-induced loss of ATP2A2 function, disruption of ER-Ca2+ homeostasis and induction of phox-ER Stress. Phox-ER stress is characterized by activating EIF2AK3-EIF2A-ATF4 branches and ERN1-XBP1 branch of the unfolded protein response and ultimately culminates into BAX and BAK1-dependent mitochondrial apoptosis. In the absence of IL10, it is accompanied by the production of IL1B and induces effective anti-tumor immunity in vivo.79 However, D. Garg et al believe that a large number of experiments, such as ATG5 gene knockout, have proved that under mild hyperthermia ROS-induced autophagy of cancer cells can help to escape the determinants of ICD.80
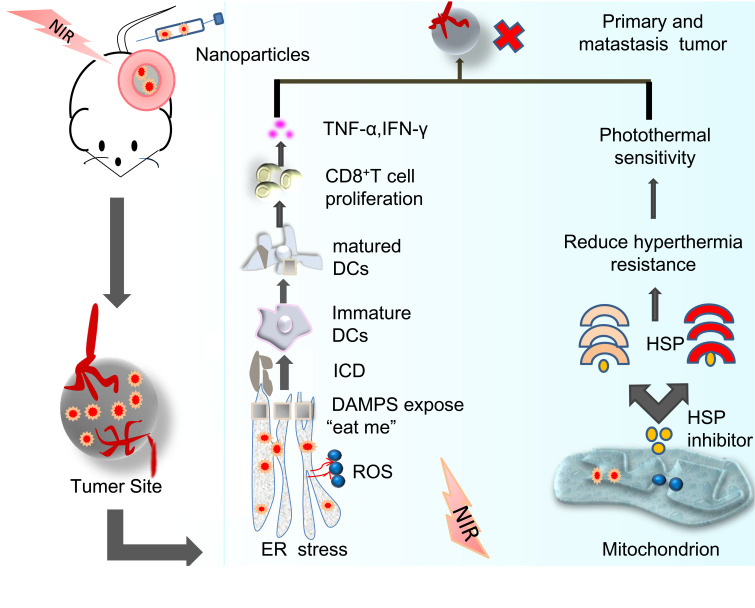
In tumor microenvironment (TME), the high expression of indoleamine 2-dioxygenase induced by IFN-γ leads to excessive consumption of L-tryptophan and accumulation of canine in γ, which can inhibit mTOR pathway to interfere with P-S6K phosphorylation and induce regulatory T cell (Foxp3+Treg), to inhibit CD8+T cell activation,81 which is the main cause of severe immunosuppression. Type I interferon, exogenous and endogenous IFN-α/β play an important role in tumor immune monitoring and tumor control. Up-regulation of IFN-α/β transcription coexists with IRF-3 phosphorylation, which effectively activates STAT182 (Figure3).
Figure 3.
Standardized explanation of anticancer effect induced by endoplasmic reticulum and mitochondria-targeting PTT/PDT therapy and immunogenic cell death.
CD47 is a transmembrane protein overexpressed in most tumors. In order to limit the phagocytic function of macrophages, it interacts with macrophage signal regulatory protein α (sirp α).82 Zhaoming Guo et al developed CD47-targeted Ab-PEG-Bi2Se3 were developed, which can specifically block the crosstalk between CD47 and SIRP α, enhance the phagocytosis of macrophages to tumor cells, and achieve improved PTT.83 Recent reports have found that ICD inducers can kill tumor cells, convert them into vaccines, and release immunostimulatory vaccines. The success of anticancer vaccination is related to the immunogenicity potential of dead/dead cells as an antigen/auxiliary source.84
The Pathway of Tumor Death Caused by PTT and PDT
HSPs (Heat shock proteins), especially HSP70, are ubiquitous molecular chaperones that promote correct protein folding and are more expressive at high temperatures.85 Hsp70 also plays an anti-apoptotic effect by inhibiting the activation of caspase-3 and blocking the stress-activated kinase pathway.86 Down-regulation of HSP70 and BAG3 can reduce the complex formation of anti-apoptosis-related proteins and Wang et al presented that by inhibiting HSP induced by PTT and weakening anti-apoptotic signal, Cantharidin (CTD)-TSL@GNPs obtained efficient PTT effect on A431 cells and had clinically acceptable irradiation power.87 According to the previous work, the inhibition of HSP function can destroy the cell homeostasis and interfere with the integrity of protein interaction, thus reducing the cell thermotolerance and improving the efficiency of photothermal therapy.88
Moustafa R.K. Ali et al presented that in terms of the relative level and results of HSP70, the HSP70 level of Huh7.5 cells was 10 times lower than that of HSC and MCF-7 cells. However, compared with the other two cell lines, the apoptosis of Huh7.5 cells increased significantly after PPT.85 Therefore, inhibition of HSP70 is a recognized target in cancer therapy, which may make cancer cells sensitive to PTT. Hsp72 is also a basic member of the molecular chaperone family. Compared with the low expression in normal cells, the expression level in tumor tissues was significantly increased to avoid the stimulation of apoptosis. So Wang et al designed nanoscale system for HSP72 (SiHSP72)/hyaluronic acid (HA), gold nanoscale (GNS)/SiRNA, was successfully constructed by the layer-by-layer method.89
JAK/STAT, especially STAT3, participates in tumorigenesis and development by regulating TARGE gene: Cell cycle regulators (such as c-fos, meks, cMyc, cyclinD1) and apoptosis inhibitors (such as Survivin, Bcl-xL) are fast signal transduction pathways from extracellular to nuclear.STAT3 should be an oncogene, and its activation and overexpression are related to the malignant transformation of cells.90 The Bcl-2 family is divided into two families in apoptosis: They are anti-apoptotic proteins such as START3 downstream target genes Bcl-2 and Bcl-xl which undergo conformational changes from the cytoplasm to the organelles of the membrane structure, especially the outer membrane of the mitochondria when cells are activated by death signals and pro-apoptotic proteins Bax and Bak when the cell is in a steady state, respectively.91 Their experiments showed that overexpression of STAT3 could significantly reduce the inhibitory effect of ALA-PDT(70). Dose-dependent and light-dependent cellular uptake compound STAT3 dimer has become a sensitive and rapid indicator of the efficacy of PDT in vitro and in vivo and can be used as biomarkers to evaluate and optimize existing and new PDT candidate therapeutic parameters in vivo. Therefore, so Li et al think the combination of ALA-PDT and STAT3-siRNA in the treatment of squamous cell carcinoma has good tumor tissue selectivity, no pain and no scar formation.92 EGFR is a receptor tyrosine kinase that protects apoptosis through important cellular functions such as phosphatidylinositol 3’kinase (PI3K)/AKT, proliferation-mediated cell cycle progression and survival, mitogen-activated protein kinase (MAPK) and STAT3.91 Christine Edmonds also found that PDT stimulates tyrosine phosphorylation and nuclear translocation of EGFR. Therefore, erlotinib which is the inhibition of EGFR signal, Combining PDT, can increase the cytotoxicity of PDT by up-regulating the mechanism of apoptotic cell death.93
In order to target residual tumor cells and metastatic cancer, PTT combined with additional immune intervention is needed to initiate the communication of the whole body’s effective immune system. Zhou et al have also developed interventional photothermal therapy combined with immune adjuvants. It is reported that chitosan is the precursor of GC, which can not only promote the maturation of DC by inducing type I interferon-induced antigen-specific Th1 response but also stimulate the secretion of interferon-γ and tumor necrosis factor-α. However, the immunomodulatory function of GC needs to be further determined in the future.94 Greater maturation of dendritic cells triggered by Yining Zhu designed Al-BSA-Ce6NPs translates into higher levels of tumor and lymph node infiltration through CD8+ and CD4+ T cells which aims to compete for melanoma by albumin-biomineralized nanoparticles to synergize PTT and immunotherapy.95
Conclusion and Future Outlook
In short, phototherapy based on nanoparticles can not only directly eliminate undetectable tumors and metastatic cancers but also treat other diseases, melanoma, reverse drug resistance, etc., and initiate the systemic immune response by regulating the tumor microenvironment. The purpose of tumor treatment is summarized in three levels: stimulating the secretion of cytokines, regulating the death pathway of cancer cells and with the help of follow-up immune adjuvants. Although phototherapy based on nanoparticles and a combination of various treatments have been widely studied, many projects are still in the stage of scholars’ exploration and there are still many deficiencies that need to be improved. For example, the safety of long-term metabolism in human body, biocompatibility and Efficient targeting of target cells by PTT and PDT based on nanoparticles must be considered, and the clinical applicability of their binding mode to various diseases in human body must be further discussed. The intensity and controllability of the induced immune response must also be addressed. In addition, the clarified mechanism of immune response induced by PTT and PDT in vivo is rare, and the clinical application methods based on immunity are not yet fully understood. This new type of PDT, PTT and the combination of various technologies are expected to provide new methods for the treatment of tumors and other medical fields.
Funding Statement
This work was supported by the National Nature Science Foundation of Heilongjiang Province(YQ2020H036), the Postdoctoral Scientific Research Developmental Fund of Heilongjiang Province (LBH-Q18076), the N10 Found project of Harbin Medical University Cancer Hospital (2017-03), the Youth Elite Training Foundation of Harbin Medical University Cancer Hospital (JY2016-06), and the Outstanding Youth Foundation of Harbin Medical University Cancer Hospital (JCQN-2018-05), Special funds of central finance to support the development of local University (2019), Wu-Jieping Medical Foundation (320.6750.19089-22, 320.6750.19089-48).
Disclosure
The authors have declared that no competing interest exists.
References
- 1.Zhang LX, Sun XM, Xu ZP, Liu RT. Development of multifunctional clay-based nanomedicine for elimination of primary invasive breast cancer and prevention of its lung metastasis and distant inoculation. ACS Appl Mater Interfaces. 2019;11(39):35566–35576. doi: 10.1021/acsami.9b11746 [DOI] [PubMed] [Google Scholar]
- 2.Raeesi V, Chou LY, Chan WC. Tuning the drug loading and release of DNA-assembled gold-nanorod superstructures. Adv Mater. 2016;28(38):8511–8518. doi: 10.1002/adma.201600773 [DOI] [PubMed] [Google Scholar]
- 3.Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42(7):804–827. doi: 10.1097/DSS.0000000000000800 [DOI] [PubMed] [Google Scholar]
- 4.Garg AD, Dudek AM, Ferreira GB, et al. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy. 2013;9(9):1292–1307. doi: 10.4161/auto.25399 [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Wen T, Zhao R, et al. Localized electric field of plasmonic nanoplatform enhanced photodynamic tumor therapy. ACS Nano. 2014;8(11):11529–11542. doi: 10.1021/nn5047647 [DOI] [PubMed] [Google Scholar]
- 6.Sun W, Ge K, Jin Y, et al. Bone-targeted nanoplatform combining zoledronate and photothermal therapy to treat breast cancer bone metastasis. ACS Nano. 2019;13(7):7556–7567. doi: 10.1021/acsnano.9b00097 [DOI] [PubMed] [Google Scholar]
- 7.Ge X, Song ZM, Sun L, et al. Lanthanide (Gd(3+) and Yb(3+)) functionalized gold nanoparticles for in vivo imaging and therapy. Biomaterials. 2016;108:35–43. doi: 10.1016/j.biomaterials.2016.08.051 [DOI] [PubMed] [Google Scholar]
- 8.Jiang H, Chen D, Guo D, et al. Zwitterionic gold nanorods: low toxicity and high photothermal efficacy for cancer therapy. Biomater Sci. 2017;5(4):686–697. doi: 10.1039/C6BM00918B [DOI] [PubMed] [Google Scholar]
- 9.Rebollar E, Sanz M, Pérez S, et al. Gold coatings on polymer laser induced periodic surface structures: assessment as substrates for surface-enhanced Raman scattering. Phys Chem Chem Phys. 2012;14(45):15699. doi: 10.1039/c2cp43049e [DOI] [PubMed] [Google Scholar]
- 10.Raut CP, Sethi KS, Kohale BR, Mamajiwala A, Warang A. Indocyanine green-mediated photothermal therapy in treatment of chronic periodontitis: a clinico-microbiological study. J Indian Soc Periodontol. 2018;22(3):221–227. doi: 10.4103/jisp.jisp_128_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Zhang W, Park HB, et al. Indocyanine green and poly I: C containing thermo-responsive liposomes used in immune-photothermal therapy prevent cancer growth and metastasis. J Immunother Cancer. 2019;7(1):220. doi: 10.1186/s40425-019-0702-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazzi A, Fusco L, Khan A, et al. Photodynamic therapy based on graphene and mxene in cancer theranostics. Front Bioeng Biotech. 2019;7:295. doi: 10.3389/fbioe.2019.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Yao C, Shen B, et al. Upconversion-magnetic carbon sphere for near infrared light-triggered bioimaging and photothermal therapy. Theranostics. 2019;9(2):608–619. doi: 10.7150/thno.27952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curcio A, Silva AKA, Cabana S, et al. Iron oxide nanoflowers @ CuS hybrids for cancer tri-therapy: interplay of photothermal therapy, magnetic hyperthermia and photodynamic therapy. Theranostics. 2019;9(5):1288–1302. doi: 10.7150/thno.30238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang S, Deng X, Chang Y, et al. Intelligent hollow Pt-CuS janus architecture for synergistic catalysis-enhanced sonodynamic and photothermal cancer therapy. Nano Lett. 2019;19(6):4134–4145. doi: 10.1021/acs.nanolett.9b01595 [DOI] [PubMed] [Google Scholar]
- 16.Bian Jang MSM, Kyeongeun Song PM, Lee KD, Kwak M. Fucoidan-coated CuS nanoparticles for chemo-and photothermal therapy against cancer. Oncotarget. 2018;9(16):12649–12661. doi: 10.18632/oncotarget.23898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H, Yan R, Wu L, et al. Tumor-targeting CuS nanoparticles for multimodal imaging and guided photothermal therapy of lymph node metastasis. Acta Biomater. 2018;72:256–265. doi: 10.1016/j.actbio.2018.03.035 [DOI] [PubMed] [Google Scholar]
- 18.Song X, Gong H, Liu T, et al. J-aggregates of organic dye molecules complexed with iron oxide nanoparticles for imaging-guided photothermal therapy under 915-nm light. Small. 2014. doi: 10.1002/smll.201401025 [DOI] [PubMed] [Google Scholar]
- 19.Li J, Cui D, Huang J, et al. Organic semiconducting pro-nanostimulants for near-infrared photoactivatable cancer immunotherapy. Angew Chem Int Ed Engl. 2019;58(36):12680–12687. doi: 10.1002/anie.201906288 [DOI] [PubMed] [Google Scholar]
- 20.Cheng L, Yang K, Chen Q, Liu Z. Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer. ACS Nano. 2012;6(6):5605–5613. doi: 10.1021/nn301539m [DOI] [PubMed] [Google Scholar]
- 21.Jing L, Liang X, Deng Z, et al. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials. 2014;35(22):5814–5821. doi: 10.1016/j.biomaterials.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 22.Lihong Sun QL, Hou M, Gao Y, et al. Light-activatable chlorin e6 (Ce6)-imbedded erythrocyte membrane vesicles camouflaged Prussian blue nanoparticles for synergistic photothermal and photodynamic therapies of cancer. Biomater Sci. 2018. [DOI] [PubMed] [Google Scholar]
- 23.Cao LQ, Xue P, Lu HW, Zheng Q, Wen ZL, Shao ZJ. Hematoporphyrin derivative-mediated photodynamic therapy inhibits tumor growth in human cholangiocarcinoma in vitro and in vivo. Hepatol Res. 2009;39(12):1190–1197. doi: 10.1111/j.1872-034X.2009.00569.x [DOI] [PubMed] [Google Scholar]
- 24.Bown SG. Photodynamic therapy in gastroenterology–current status and future prospects. Endoscopy. 1993;25(9):683–685. doi: 10.1055/s-2007-1010433 [DOI] [PubMed] [Google Scholar]
- 25.Yang XM, Luo RC, Ma HJ, et al. Hematoporphyrin derivative-mediated photodynamic therapy for human nasopharyngeal carcinoma: a comparative study with CNE2 and C666-1 cell lines in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27(2):165–167. [PubMed] [Google Scholar]
- 26.Shulok JR, Klaunig JE, Selman SH, Schafer PJ, Goldblatt PJ. Cellular effects of hematoporphyrin derivative photodynamic therapy on normal and neoplastic rat bladder cells. Am J Pathol. 1986;122(2):277–283. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang XH, Zhang LJ, Sun JJ, et al. Photodynamic efficiency of a chlorophyll-a derivative in vitro and in vivo. Biomed Pharmacother. 2016;81:265–272. doi: 10.1016/j.biopha.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 28.Matlou GG, Managa M, Nyokong T. Effect of symmetry and metal nanoparticles on the photophysicochemical and photodynamic therapy properties of cinnamic acid zinc phthalocyanine. Spectrochim Acta A Mol Biomol Spectrosc. 2019;214:49–57. doi: 10.1016/j.saa.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 29.Lu T, Shao P, Mathew I, Sand A, Sun W. Synthesis and photophysics of benzotexaphyrin: a near-infrared emitter and photosensitizer. J Am Chem Soc. 2008;130(47):15782–15783. doi: 10.1021/ja807021n [DOI] [PubMed] [Google Scholar]
- 30.Ménard F, Sol V, Ringot C, et al. Synthesis of tetraglucosyl- and tetrapolyamine-tetrabenzoporphyrin conjugates for an application in PDT. Bioorg Med Chem. 2009;17(22):7647–7657. doi: 10.1016/j.bmc.2009.09.048 [DOI] [PubMed] [Google Scholar]
- 31.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanda T, Sugihara T, Takata T, et al. Low-density lipoprotein receptor expression is involved in the beneficial effect of photodynamic therapy using talaporfin sodium on gastric cancer cells. Oncol Lett. 2019;17(3):3261–3266. doi: 10.3892/ol.2019.10004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheruku RR, Cacaccio J, Durrani FA, et al. Epidermal growth factor receptor-targeted multifunctional photosensitizers for bladder cancer imaging and photodynamic therapy. J Med Chem. 2019;62(5):2598–2617. doi: 10.1021/acs.jmedchem.8b01927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matlou GG, Oluwole DO, Prinsloo E, Nyokong T. Photodynamic therapy activity of zinc phthalocyanine linked to folic acid and magnetic nanoparticles. J Photochem Photobiol B. 2018;186:216–224. doi: 10.1016/j.jphotobiol.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 35.Zhu L, Zhou Z, Mao H, Yang L. Magnetic nanoparticles for precision oncology: theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine (Lond). 2017;12(1):73–87. doi: 10.2217/nnm-2016-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clement S, Chen W, Deng W, Goldys EM. X-ray radiation-induced and targeted photodynamic therapy with folic acid-conjugated biodegradable nanoconstructs. Int J Nanomedicine. 2018;13:3553–3570. doi: 10.2147/IJN.S164967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta A, Wang S, Pera P, et al. Multifunctional nanoplatforms for fluorescence imaging and photodynamic therapy developed by post-loading photosensitizer and fluorophore to polyacrylamide nanoparticles. Nanomedicine. 2012;8(6):941–950. doi: 10.1016/j.nano.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta A, Wang S, Marko A, et al. Polyacrylamide-based biocompatible nanoplatform enhances the tumor uptake, PET/fluorescence imaging and anticancer activity of a chlorophyll analog. Theranostics. 2014;4(6):614–628. doi: 10.7150/thno.8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He L, Mao C, Brasino M, et al. TiO2-capped gold nanorods for plasmon-enhanced production of reactive oxygen species and photothermal delivery of chemotherapeutic agents. ACS Appl Mater Interfaces. 2018;10(33):27965–27971. doi: 10.1021/acsami.8b08868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai X, Gao W, Zhang L, et al. Enabling Prussian blue with tunable localized surface plasmon resonances: simultaneously enhanced dual-mode imaging and tumor photothermal therapy. ACS Nano. 2016;10(12):11115–11126. doi: 10.1021/acsnano.6b05990 [DOI] [PubMed] [Google Scholar]
- 41.Ding X, Liow CH, Zhang M, et al. Surface plasmon resonance enhanced light absorption and photothermal therapy in the second near-infrared window. J Am Chem Soc. 2014;136(44):15684–15693. doi: 10.1021/ja508641z [DOI] [PubMed] [Google Scholar]
- 42.Oladipo AO, Oluwafemi OS, Songca SP, et al. A novel treatment for metastatic lymph nodes using lymphatic delivery and photothermal therapy. Sci Rep. 2017;7(1). doi: 10.1038/srep45459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu B, Cao W, Cheng J, et al. Human natural killer cells for targeting delivery of gold nanostars and bimodal imaging directed photothermal/photodynamic therapy and immunotherapy. Cancer Biol Med. 2019;16(4):2095–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park MJ, Bae JH, Chung JS, Kim SH, Kang CD. Induction of NKG2D ligands and increased sensitivity of tumor cells to NK cell-mediated cytotoxicity by hematoporphyrin-based photodynamic therapy. Immunol Invest. 2011;40(4):367–382. doi: 10.3109/08820139.2010.551435 [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Liu H, Xin J, et al. Chlorin-based photoactivable galectin-3-inhibitor nanoliposome for enhanced photodynamic therapy and NK cell-related immunity in melanoma. ACS Appl Mater Interfaces. 2019;11(45):41829–41841. doi: 10.1021/acsami.9b09560 [DOI] [PubMed] [Google Scholar]
- 46.Zhang C, Pu K. Molecular and nanoengineering approaches towards activatable cancer immunotherapy. Chem Soc Rev. 2020;49(13):4234–4253. doi: 10.1039/C9CS00773C [DOI] [PubMed] [Google Scholar]
- 47.Jiang Y, Zhao X, Huang J, et al. Transformable hybrid semiconducting polymer nanozyme for second near-infrared photothermal ferrotherapy. Nat Commun. 2020;11(1):1857. doi: 10.1038/s41467-020-15730-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen S, Lei Q, Qiu W-X, et al. Mitochondria-targeting “nanoheater” for enhanced photothermal/chemo-therapy. Biomaterials. 2017;117:92–104. doi: 10.1016/j.biomaterials.2016.11.056 [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Guo F, Ji Y, Yu M, Wang J, Li N. Dual-mode imaging guided multifunctional theranosomes with mitochondria targeting for photothermally controlled and enhanced photodynamic therapy in vitro and in vivo. Mol Pharm. 2018;15(8):3318–3331. doi: 10.1021/acs.molpharmaceut.8b00351 [DOI] [PubMed] [Google Scholar]
- 50.Xie Z, Wang D, Fan T, et al. Black phosphorus analogue tin sulfide nanosheets: synthesis and application as near-infrared photothermal agents and drug delivery platforms for cancer therapy. J Mater Chem B. 2018;6(29):4747–4755. doi: 10.1039/C8TB00729B [DOI] [PubMed] [Google Scholar]
- 51.Jiang C, Yang W, Wang C, et al. Methylene blue-mediated photodynamic therapy induces macrophage apoptosis via ROS and reduces bone resorption in periodontitis. Oxid Med Cell Longev. 2019;2019:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han X, Kou J, Zheng Y, et al. ROS generated by upconversion nanoparticle-mediated photodynamic therapy induces autophagy via PI3K/AKT/mTOR signaling pathway in M1 peritoneal macrophage. Cell Physiol Biochem. 2019;52(6):1325–1338. [DOI] [PubMed] [Google Scholar]
- 53.de Oliveira S, da Ordem Trahamane EJ, Monteiro J, et al. Leishmanicidal effect of antiparasitic photodynamic therapy-ApPDT on infected macrophages. Lasers Med Sci. 2017;32(9):1959–1964. doi: 10.1007/s10103-017-2292-9 [DOI] [PubMed] [Google Scholar]
- 54.Hayashi N, Kataoka H, Yano S, et al. A novel photodynamic therapy targeting cancer cells and tumor-associated macrophages. Mol Cancer Ther. 2014;14(2):452–460. doi: 10.1158/1535-7163.MCT-14-0348 [DOI] [PubMed] [Google Scholar]
- 55.Kawczyk-Krupka A, Czuba Z, Szliszka E, Król W, Sieroń A. The role of photosensitized macrophages in photodynamic therapy. Oncol Rep. 2011;26(1):275–280. doi: 10.3892/or.2011.1262 [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Jia T, Sun Q, et al. Construction of Bi/phthalocyanine manganese nanocomposite for trimodal imaging directed photodynamic and photothermal therapy mediated by 808nm light. Biomaterials. 2020;228:119569. doi: 10.1016/j.biomaterials.2019.119569 [DOI] [PubMed] [Google Scholar]
- 57.Yanina IY, Tuchin VV, Navolokin NA, et al. Fat tissue histological study at indocyanine green-mediated photothermal/photodynamic treatment of the skin in vivo. J Biomed Opt. 2012;17(5):058002. doi: 10.1117/1.JBO.17.5.058002 [DOI] [PubMed] [Google Scholar]
- 58.Lu Y, Li L, Lin Z, et al. A new treatment modality for rheumatoid arthritis: combined photothermal and photodynamic therapy using Cu7.2 S4 nanoparticles. Adv Healthc Mater. 2018;7(14):e1800013. doi: 10.1002/adhm.201800013 [DOI] [PubMed] [Google Scholar]
- 59.Costa Lima SA, Reis S. Temperature-responsive polymeric nanospheres containing methotrexate and gold nanoparticles: a multi-drug system for theranostic in rheumatoid arthritis. Colloids Surf B Biointerfaces. 2015;133:378–387. doi: 10.1016/j.colsurfb.2015.04.048 [DOI] [PubMed] [Google Scholar]
- 60.Pandey PK, Maheshwari R, Raval N, Gondaliya P, Kalia K, Tekade RK. Nanogold-core multifunctional dendrimer for pulsatile chemo-, photothermal- and photodynamic- therapy of rheumatoid arthritis. J Colloid Interface Sci. 2019;544:61–77. doi: 10.1016/j.jcis.2019.02.073 [DOI] [PubMed] [Google Scholar]
- 61.ElZorkany HE, Youssef T, Mohamed MB, Amin RM. Photothermal versus photodynamic treatment for the inactivation of the bacteria Escherichia coli and Bacillus cereus: an in vitro study. Photodiagnosis Photodyn Ther. 2019;27:317–326. [DOI] [PubMed] [Google Scholar]
- 62.Chen F, Zang Z, Chen Z, et al. Nanophotosensitizer-engineered Salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials. 2019;214:119226. doi: 10.1016/j.biomaterials.2019.119226 [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Pei J, Cong Z, et al. Development of anisamide-targeted PEGylated gold nanorods to deliver epirubicin for chemo-photothermal therapy in tumor-bearing mice. Int J Nanomedicine. 2019;14:1817–1833. doi: 10.2147/IJN.S192520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwiatkowski S, Knap B, Przystupski D, et al. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049 [DOI] [PubMed] [Google Scholar]
- 65.He Y, Hsiao JH, Yu JH, et al. Cancer cell death pathways caused by photothermal and photodynamic effects through gold nanoring induced surface plasmon resonance. Nanotechnology. 2017;28(27):275101. doi: 10.1088/1361-6528/aa75ad [DOI] [PubMed] [Google Scholar]
- 66.Hoseinpour Jajarm H, Asadi R, Bardideh E, Shafaee H, Khazaei Y, Emadzadeh M. The effects of photodynamic and low-level laser therapy for treatment of oral lichen planus-a systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2018;23:254–260. doi: 10.1016/j.pdpdt.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 67.Tseng H-Y, Chen W-F, Chu C-K, et al. On-substrate fabrication of a bio-conjugated Au nanoring solution for photothermal therapy application. Nanotechnology. 2013;24(6):065102. doi: 10.1088/0957-4484/24/6/065102 [DOI] [PubMed] [Google Scholar]
- 68.Huachao Chen JT, Weijiang H, Guo Z. H2O2‑activatable and O2‑evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J Am Chem Soc. 2014. [DOI] [PubMed] [Google Scholar]
- 69.Alves CG, Lima-Sousa R, de Melo-diogo D, Louro RO, Correia IJ. IR780 based nanomaterials for cancer imaging and photothermal, photodynamic and combinatorial therapies. Int J Pharm. 2018;542(1–2):164–175. doi: 10.1016/j.ijpharm.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 70.Wan SS, Zeng JY, Cheng H, Zhang XZ. ROS-induced NO generation for gas therapy and sensitizing photodynamic therapy of tumor. Biomaterials. 2018;185:51–62. doi: 10.1016/j.biomaterials.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 71.David WC, Granville DJ, Simon Leong JGL. Consequences of the photodynamic treatment of resting and activated peripheral T lymphocytes. Immunopharmacology. 1998;41(1999):31–44. [DOI] [PubMed] [Google Scholar]
- 72.Weng H-Y, Huang T-L, Chang P-Y, Wang J-K. One-year outcome of combination therapy with intravitreal aflibercept and photodynamic therapy for polypoidal choroidal vasculopathy. BMC Pharmacol Toxicol. 2019;20(1). doi: 10.1186/s40360-019-0310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu H, Hu Y, Sun Y, et al. Co-delivery of bee venom melittin and a photosensitizer with an organic-inorganic hybrid nanocarrier for photodynamic therapy and immunotherapy. ACS Nano. 2019;13(11):12638–12652. doi: 10.1021/acsnano.9b04181 [DOI] [PubMed] [Google Scholar]
- 74.Doix B, Trempolec N, Riant O, Feron O. Low photosensitizer dose and early radiotherapy enhance antitumor immune response of photodynamic therapy-based dendritic cell vaccination. Front Oncol. 2019;9:811. doi: 10.3389/fonc.2019.00811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lamberti MJ, Mentucci FM, Roselli E, et al. Photodynamic modulation of type 1 interferon pathway on melanoma cells promotes dendritic cell activation. Front Immunol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta Rev Cancer. 2010;1805(1):53–71. doi: 10.1016/j.bbcan.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 77.Galluzzi L, Kepp O, Kroemer G. Enlightening the impact of immunogenic cell death in photodynamic cancer therapy. EMBO J. 2012;31(5):1055–1057. doi: 10.1038/emboj.2012.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gomes-da-Silva LC, Zhao L, Bezu L, et al. Photodynamic therapy with redaporfin targets the endoplasmic reticulum and Golgi apparatus. EMBO J. 2018;37(13). doi: 10.15252/embj.201798354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crighton D, Wilkinson S, O’Prey J, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126(1):121–134. doi: 10.1016/j.cell.2006.05.034 [DOI] [PubMed] [Google Scholar]
- 80.Rodriguez ME, Cogno IS, Milla Sanabria LS, Moran YS, Rivarola VA. Heat shock proteins in the context of photodynamic therapy: autophagy, apoptosis and immunogenic cell death. Photochem Photobiol Sci. 2016;15(9):1090–1102. doi: 10.1039/C6PP00097E [DOI] [PubMed] [Google Scholar]
- 81.Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300–311. doi: 10.1016/j.jconrel.2020.03.045 [DOI] [PubMed] [Google Scholar]
- 82.Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31(6):539–544. doi: 10.1038/nbt.2576 [DOI] [PubMed] [Google Scholar]
- 83.Guoa Z, Liua Y, Zhoub H, et al. CD47-targeted bismuth selenide nanoparticles actualize improved photothermal therapy by increasing macrophage phagocytosis of cancer cells. Colloids Surf B Biointerfaces. 2019;184(2019):110546. doi: 10.1016/j.colsurfb.2019.110546 [DOI] [PubMed] [Google Scholar]
- 84.Garg AD. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell–driven rejection of high-grade glioma. ScienceTranslationalMedicine. 2016;8(328). [DOI] [PubMed] [Google Scholar]
- 85.Ali MR, Ali HR, Rankin CR, El-Sayed MA. Targeting heat shock protein 70 using gold nanorods enhances cancer cell apoptosis in low dose plasmonic photothermal therapy. Biomaterials. 2016;102:1–8. doi: 10.1016/j.biomaterials.2016.06.017 [DOI] [PubMed] [Google Scholar]
- 86.Wang S, Li L, Ning X, Xue P, Liu Y. pH-activated heat shock protein inhibition and radical generation enhanced NIR luminescence imaging-guided photothermal tumour ablation. Int J Pharm. 2019;566:40–45. doi: 10.1016/j.ijpharm.2019.05.056 [DOI] [PubMed] [Google Scholar]
- 87.Guo Z, Liu Y, Cheng X, et al. Versatile biomimetic cantharidin-tellurium nanoparticles enhance photothermal therapy by inhibiting the heat shock response for combined tumor therapy. Acta Biomater. 2020;110:208–220. doi: 10.1016/j.actbio.2020.03.028 [DOI] [PubMed] [Google Scholar]
- 88.Wang S, Tian Y, Tian W, et al. Selectively sensitizing malignant cells to photothermal therapy using a CD44-targeting heat shock protein 72 depletion nanosystem. ACS Nano. 2016;10(9):8578–8590. doi: 10.1021/acsnano.6b03874 [DOI] [PubMed] [Google Scholar]
- 89.Xu H, Ito T, Tawada A, et al. Effect of hyaluronan oligosaccharides on the expression of heat shock protein 72. J Biol Chem. 2002;277(19):17308–17314. doi: 10.1074/jbc.M112371200 [DOI] [PubMed] [Google Scholar]
- 90.Qiao L, Mei Z, Yang Z, Li X, Cai H, Liu W. ALA-PDT inhibits proliferation and promotes apoptosis of SCC cells through STAT3 signal pathway. Photodiagnosis Photodyn Ther. 2016;14:66–73. doi: 10.1016/j.pdpdt.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 91.Edmonds C, Hagan S, Gallagher-Colombo SM, Busch TM, Cengel KA. Photodynamic therapy activated signaling from epidermal growth factor receptor and STAT3. Cancer Biol Ther. 2014;13(14):1463–1470. doi: 10.4161/cbt.22256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Srivatsan A, Wang Y, Joshi P, et al. In vitro cellular uptake and dimerization of signal transducer and activator of transcription-3 (STAT3) identify the photosensitizing and imaging-potential of isomeric photosensitizers derived from chlorophyll-a and bacteriochlorophyll-a. J Med Chem. 2011;54(19):6859–6873. doi: 10.1021/jm200805y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Floriane Gibaulta MC, Baillya F, Huetb G, Melnyka P, Cotelle P. Non-photoinduced biological properties of verteporfin. Curr Med Chem. 2016;2016(23):1171–1184. doi: 10.2174/0929867323666160316125048 [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Li X, Doughty A, et al. Phototherapy using immunologically modified carbon nanotubes to potentiate checkpoint blockade for metastatic breast cancer. Nanomedicine. 2019;18:44–53. doi: 10.1016/j.nano.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Domvri K, Petanidis S, Anestakis D, et al. Dual photothermal MDSCs-targeted immunotherapy inhibits lung immunosuppressive metastasis by enhancing T-cell recruitment. Nanoscale. 2020;12(13):7051–7062. doi: 10.1039/D0NR00080A [DOI] [PubMed] [Google Scholar]