Abstract
Purpose
Atherosclerotic cardiovascular disease may share the risk factors for low bone mineral density (BMD), one of which is dyslipidemia. The association between serum cholesterol and BMD remains controversial. Thus, the correlation between serum lipids and BMD in women was explored in the current study.
Materials and Methods
This cross-sectional study included 1116 Chinese female participants. Serum samples were collected to evaluate total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and other laboratory markers. Dual-energy X-ray absorptiometry was used to assess lumbar spine, femoral neck, and total hip BMD.
Results
In the postmenopausal women, a non-linear relationship was detected between TC, LDL-C, HDL-C, and lumbar spine BMD. Using segmented linear regression, the inflection points were 5.86 mmol/L, 3.52 mmol/L, and 2.37 mmol/L, respectively. To the left of the inflection point, the higher the serum lipid level, the lower the value for lumbar spine BMD. To the right of the inflection point, the higher the serum level of TC and LDL-C, the higher the value for lumbar spine BMD. In the premenopausal women, the association between HDL-C and femoral neck BMD was non-linear. In addition, LDL-C had a positive association with BMD of the femoral neck and HDL-C had an inverse association with BMD of the femoral neck in postmenopausal women.
Conclusion
In postmenopausal women, the relationship between TC, LDL-C, HDL-C, and lumbar spine BMD was non-linear. TC, LDL-C, and HDL-C were negatively associated with lumbar spine BMD when the values were less than 5.86 mmol/L, 3.52 mmol/L, and 2.37 mmol/L, respectively. The mechanisms of the association were unclear, and further research is warranted to clarify the relationship.
Keywords: serum lipids, bone mineral density, postmenopausal women, premenopausal women, cholesterol
Introduction
Osteoporosis (OP), which leads to consequent fractures, has become a significant public health concern.1 OP affects more than 200 million people globally, with approximately nine million osteoporotic fractures occurring annually of which 1.6 million affect the hip joint.2 It is primarily caused by suboptimal bone mineral density (BMD). Risk factors for OP include aging, being female, reduced physical activity, low calcium intake, and hypothyroidism.3,4
OP and cardiovascular diseases lead to increased morbidity and fatalities in women. Patients with aortic calcification are at increased risk of hip and vertebral fractures.5 OP patients were demonstrated to be at increased risk of atherosclerotic cardiovascular events, compared to osteopenia patients, after adjusting for cardiovascular risk factors, age, and cardiovascular mortality.6 Epidemiological studies have shown that the two diseases are closely related,7–9 suggesting that there could be a possible link between OP and cardiovascular diseases.
Dyslipidemia is the leading risk factor of cardiovascular diseases and could be responsible for the association between the two disorders.10 Mice studies found that low-density lipoprotein cholesterol (LDL-C) oxidation products suppressed bone formation by inhibiting osteoblast differentiation. LDL oxidation products also facilitated bone marrow stromal cell differentiation into adipocytes rather than osteoblasts.11 In addition, dyslipidemia has been shown to weaken the synthesis and metabolism of parathyroid hormone in bone.12
Dyslipidemia is linked to abnormal bone mass.13–15 However, there are considerable inconsistencies in the findings with regard to the way in which dyslipidemia affects bone mass. Cui et al reported a negative correlation between serum total cholesterol (TC), LDL-C levels, and BMD in premenopausal and postmenopausal women in South Korea.16 Elsewhere, high-density lipoprotein cholesterol (HDL-C) levels were inversely associated with BMD in premenopausal women.17 A population-based study in Spain showed that women taking lipophilic statins had higher bone density in the femoral neck.18 Sivas et al demonstrated that TG was not related to BMD in Turkish postmenopausal women.19 In other research, postmenopausal women with OP were demonstrated to have lower levels of TC and LDL-C and higher levels of HDL than postmenopausal women with normal BMD.20
The relationship between BMD and lipid levels remains unclear. A few large-scale community-based studies have indicated a potential link between BMD and lipids in different subgroups. Therefore, the objective of the current study was to evaluate the relationship between serum TC and other lipid indicators and BMD in pre- and postmenopausal women.
Materials and Methods
Study Population
A cross-sectional study was conducted in four cities in the east, north, south, and southernmost areas in Sichuan Province: Chengdu, Guangyuan, Luzhou, and Xichang from December 2012 to March 2013. Cluster random sampling was applied to urban and rural communities in each target city. From each city, permanent female residents aged approximately 30 years were randomly selected from two urban communities and 1–2 rural communities for inclusion in the study.
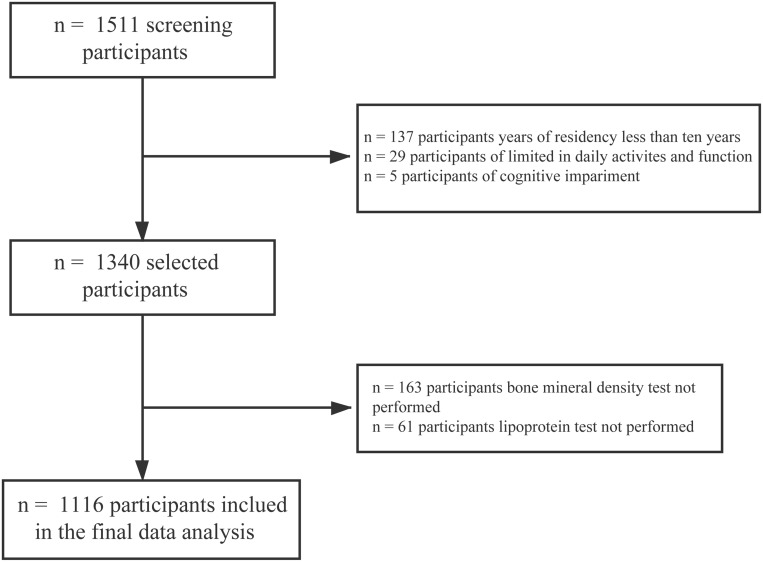
After thorough screening, of 1511 potential participants, 1116 women were included in the final analysis. Exclusion criteria were women who (1) had been living in the city for less than 10 years (n = 137), (2) were severely limited in their daily activities and function (n = 29), (3) were cognitively impaired (n = 5), (4) did not undergo a BMD test (n = 163), and (5) did not undergo a lipoprotein test (n = 61) (Figure 1).
Figure 1.
Flow chart for selecting participants.
The study participants provided written informed consent to participate in the research, which was conducted as per the Helsinki declaration after ethical permission was acquired from the Ethics Review Board of West China Hospital of Sichuan University.
Research Methods
The researchers received standardized training prior to the study to ensure its objectivity and the accuracy of the data. Standardized questionnaires were used to collect the participants’ basic data. The patients’ medical history, which included liver disease, diabetes mellitus, chronic diarrhea, chronic obstructive pulmonary disease, hypertension, and kidney disease, and lifestyle factors (dietary calcium intake, outdoor exercise, smoking, and alcohol consumption) were also obtained. On completion of the questionnaire survey, the data were summarized, checked, reviewed and proofread. Database maintenance was also performed.
The height and weight of the enrolled subjects were measured in the morning on an empty stomach while wearing light clothing and without shoes. Their measurements were obtained twice and then averaged to reduce errors. Body mass index (BMI) was computed as weight divided by height2 (kg/m2).
Laboratory Measurements
Fasting serum samples were obtained to evaluate TC, LDL-C, and HDL-C, and other laboratory markers, such as alanine aminotransferase (ALT), fasting plasma glucose (FBG), and aspartate aminotransferase (AST). Blood glucose and blood lipid were assessed using an Olympus® AU 5400 Automatic Biochemistry Analyser (Olympus Corporation, Japan).The remaining indicators were assessed using standard laboratory methods.
BMD Measurement
The BMD assays were performed by experienced technicians using a GE® Lunar Prodigy Advance Bone Densitometer (Lunar Corp, USA) that utilizes dual-energy X-ray absorptiometry (DXA). The DXA scans were obtained according to the standard scan and analysis protocol specifications of the manufacturer. Quality control was used to achieve stable results. The participants’ femoral neck, lumbar spine (L1–L4), and total hip BMD (g/cm2) measurements were obtained.
Statistical Analysis
The categorical variables are presented as percentages and frequencies, and the continuous variables are displayed as mean ± standard deviation (SD). Univariate rectilinear regression was used to evaluate the association between LDL-C, TC, HDL-C and lumbar spine (L1–L4), femoral neck and total hip BMD. A non-adjusted model and a multivariate-adjusted model were utilized, and the results were reported using unadjusted, minimally adjusted, and fully adjusted analysis, according to the specifications of the STROBE statement.21
Variables in the crude model were not adjusted. However, age and BMI were controlled in the minimally adjusted model. In the fully adjusted model, age, BMI (kg/m2), FBG (mmol/l), 25 (OH) D (nmol/l), ALT (U/L), U/L, AST (U/L), calcium intake (g/week), outdoor time (hours/day), walking time (hours/day), smoking, drinking, hyperlipidemia, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), diabetes mellitus, hyperthyroidism, and chronic diarrhea were controlled.
The generalized additive model (GAM) was used to identify non-rectilinear relationships. If non-rectilinear correlations were identified, the threshold effects of TC, LDL-C, and HDL-C on lumbar spine (L1–L4), total hip, and femoral neck BMD were estimated using piecewise rectilinear regression smoothing. When the ratios of femoral neck, total hip, and lumbar spine BMD to TC, HDL-C, and LDL-C were shown on the smoothed curve, the recursive method was used to automatically calculate the inflection points; the maximum likelihood was used. The p-values were two-sided, and a p-value less than 0.050 denoted statistical significance. The analysis was conducted using R® Statistical Software (The R Foundation) and the Empower Stats (X&Y Solutions Inc., Boston, MA, USA).
Results
Basic Characteristics
The baseline features of the subjects are summarized in Table 1. One thousand one hundred and sixteen women were enrolled (mean age of 58.2 ± 13.9 years), with a BMI of 24.0 ± 3.4 kg/m2. Of these, 360 were premenopausal, and 756 were postmenopausal (premenopausal: postmenopausal = 1.0:2.1). The mean levels of serum TC, LDL-C, and HDL-C were 5.3 ± 1.1, 2.9 ± 0.8, and 1.7 ± 0.4 mmol/L, respectively. The mean lumbar spine (L1–L4), femoral neck, and total hip BMD was 0.937 ± 0.174, 0.805 ± 0.184, and 0.855 ± 0.166 g/cm2, respectively.
Table 1.
Baseline Characteristics of Participants
| Total Patients (n = 1116) | Premenopausal (n = 360) | Postmenopausal (n = 756) | P value | |
|---|---|---|---|---|
| Age (years) | 58.2 ± 13.9 | 43.9 ± 8.3 | 64.9 ± 10.4 | <0.001 |
| BMI (kg/m2) | 24.0 ± 3.4 | 23.5 ± 3.4 | 24.2 ± 3.5 | 0.002 |
| Outdoor time (h/day) | 2.0 (1.0–3.5) | 2.000 (1.000–4.000) | 2.000 (1.000–3.500) | 0.048 |
| Walking time (h/day) | 2.0 (1.0–3.0) | 2.000 (1.000–3.000) | 2.000 (1.000–3.000) | 0.709 |
| Calcium intake, g/week | 2325.7 (1622.0–3527.7) | 2140.9 (1546.0–3250.8) | 2420.4 (1664.9–3653.7) | 0.004 |
| Age at menopause (years) | – | – | 48.3 ± 3.8 | |
| Smoking | 0.438 | |||
| Never | 1099 (98.5%) | 356 (98.9%) | 743 (98.3%) | |
| Current or ever | 17 (1.5%) | 4 (1.1%) | 13 (1.7%) | |
| Drinking | 0.004 | |||
| Never | 1062 (95.2%) | 333 (92.5%) | 729 (96.4%) | |
| Current or ever | 54 (4.8%) | 27 (7.5%) | 27 (3.6%) | |
| Kidney disease | 0.697 | |||
| No | 1085 (97.222%) | 351 (97.5%) | 734 (97.1%) | |
| Yes | 31 (2.778%) | 9 (2.5%) | 22 (2.9%) | |
| Chronic obstructive pulmonary disease (COPD) | <0.001 | |||
| No | 1042 (93.4%) | 351 (97.5%) | 691 (91.4%) | |
| Yes | 74 (6.6%) | 9 (2.5%) | 65 (8.6%) | |
| Diabetes | <0.001 | |||
| No | 1008 (90.3%) | 352 (97.8%) | 656 (86.8%) | |
| Yes | 108 (9.7%) | 8 (2.2%) | 100 (13.2%) | |
| Hyperthyroidism | 0.781 | |||
| No | 1102 (98.7%) | 355 (98.6%) | 747 (98.8%) | |
| Yes | 14 (1.3%) | 5 (1.4%) | 9 (1.2%) | |
| Chronic diarrhea | 0.978 | |||
| No | 1091 (97.8%) | 352 (97.8%) | 739 (97.8%) | |
| Yes | 25 (2.2%) | 8 (2.2%) | 17 (2.2%) | |
| Hyperlipemia | <0.001 | |||
| No | 988 (88.6%) | 346 (96.4%) | 642 (84.9%) | |
| Yes | 127 (11.4%) | 13 (3.6%) | 114 (15.1%) | |
| BMD Total lumbar (g/cm2) | 0.937 ± 0.174 | 1.08 ± 0.138 | 0.869 ± 0.146 | <0.001 |
| BMD Femur neck (g/cm2) | 0.805 ± 0.184 | 0.908 ± 0.162 | 0.756 ± 0.174 | <0.001 |
| BMD Total Hip (g/cm2) | 0.855 ± 0.166 | 0.93 ± 0.185 | 0.82 ± 0.145 | <0.001 |
| TC, mmol/l | 5.3 ± 1.1 | 4.9 ± 1.1 | 5.5 ± 1.1 | <0.001 |
| LDL-C, mmol/l | 2.9 ± 0.8 | 2.7 ± 0.8 | 3.1 ± 0.8 | <0.001 |
| HDL-C, mmol/l | 1.7 ± 0.4 | 1.6 ± 0.4 | 1.7 ± 0.4 | <0.001 |
| FBG, mmol/l | 5.8 ± 2.2 | 5.4 ± 1.3 | 6.0 ± 2.5 | <0.001 |
| 25(OH)D, nmol/l | 44.5 ± 19.0 | 43.4 ± 18.1 | 45.0 ± 19.4 | 0.179 |
| AST, U/L | 25.3 (21.0–30.3) | 24.0 (20.0–28.2) | 26.0 (22.0–31.0) | <0.001 |
| ALT, U/L | 20.4 (16.0–28.0) | 19.1 (15.0–27.0) | 21.0 (16.0–28.2) | 0.053 |
Correlation Between Serum HDL-C, TC, LDL-C and Lumbar Spine BMD (L1–L4)
The relationship between serum levels of HDL-C, TC, LDL-C and BMD at the lumbar spine (L1–L4), femoral neck, and total hip in postmenopausal and premenopausal women was assessed using univariate rectilinear regression. In postmenopausal women, an association was not found between either TC or LDL-C and lumbar spine BMD (L1–L4) in the crude model (β = −0.006, 95% confidence interval [CI]: −0.018 to 0.006, p = 0.311; β = 0.004, 95% CI: −0.012 to 0.02, p = 0.631, respectively). Similarly, a correlation between TC and lumbar spine BMD [L1–L4] was not shown in either the minimally adjusted model (controlled for age and BMI) or the fully adjusted model (β = −0.004, 95% CI: −0.015 to 0.007, p = 0.471; β = −0.003, 95% CI: −0.014 to 0.008, p = 0.594, respectively).
An association was also not established between LDL-C and lumbar spine BMD [L1–L4] in either the minimally adjusted model (controlled for age and BMI) or the fully adjusted model (β = −0.002, 95% CI: −0.017 to 0.013, p = 0.832; β = −0.000, 95% CI: −0.015 to 0.015, p = 0.979, respectively). In addition, HDL-C was shown to negatively correlate with lumbar spine BMD (L1–L4) in the crude model (β = −0.056, 95% CI: −0.084 to −0.028, p =< 0.001). A similar relationship was identified in the minimally adjusted and fully adjusted models (β = −0.031, 95% CI: −0.059 to −0.003, p = 0.03; β = −0.033, 95% CI: −0.061 to −0.004, p = 0.024, respectively). TC, LDL-C, and HDL-C were treated as categorical variables (categorized into quartiles) in the sensitivity assessments. Similar trends were identified using this approach (p-values of 0.239, 0.284, and 0.335; 0.999, 0.337 and 0.454; 0.001, 0.048 and 0.043, respectively) (Table 2).
Table 2.
Multivariate Regression for Effect of TC, LDL-C, and HDL-C on Lumbar BMD in Postmenopausal Women
| Variables | Crude Model | Minimally Adjusted Model | Fully Adjusted Model | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| TC, mmol/l | −0.006 (−0.018, 0.006) | 0.31121 | −0.004 (−0.015, 0.007) | 0.47136 | −0.003 (−0.014, 0.008) | 0.59432 |
| TC (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | −0.009 (−0.043, 0.026) | 0.6238 | −0.008 (−0.041, 0.024) | 0.61999 | −0.002 (−0.036, 0.031) | 0.8829 |
| Q3 | −0.044 (−0.078, −0.011) | 0.00995 | −0.040 (−0.072, −0.008) | 0.0134 | −0.040 (−0.073, −0.008) | 0.01454 |
| Q4 | −0.011 (−0.045, 0.023) | 0.51295 | −0.010 (−0.042, 0.023) | 0.5552 | −0.008 (−0.041, 0.025) | 0.63312 |
| P for trend | 0.23902 | 0.28444 | 0.3348 | |||
| LDL-C, mmol/l | 0.004 (−0.012, 0.020) 0.63116 | −0.002 (−0.017, 0.013) | 0.8322 | −0.000 (−0.015, 0.015) | 0.97906 | |
| LDL-C (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | −0.011 (−0.045, 0.024) | 0.54488 | −0.019 (−0.052, 0.014) | 0.25845 | −0.019 (−0.052, 0.014) | 0.26571 |
| Q3 | −0.019 (−0.052, 0.014) | 0.26184 | −0.035 (−0.067, −0.004) | 0.02951 | −0.032 (−0.064, −0.001) | 0.04686 |
| Q4 | 0.001 (−0.033, 0.035) | 0.95179 | −0.014 (−0.046, 0.019) | 0.40944 | −0.011 (−0.044, 0.021) | 0.49887 |
| P for trend | 0.99904 | 0.3373 | 0.4537 | |||
| HDL-C, mmol/l | −0.056 (−0.084, −0.028) 0.00009 | −0.031 (−0.059, −0.003) | 0.02951 | −0.033 (−0.061, −0.004) | 0.02387 | |
| HDL-C (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | −0.016 (−0.050, 0.017) | 0.33577 | −0.012 (−0.045, 0.020) | 0.45378 | −0.011 (−0.043, 0.021) | 0.50464 |
| Q3 | −0.023 (−0.057, 0.010) | 0.17403 | −0.013 (−0.046, 0.019) | 0.42993 | −0.012 (−0.045, 0.021) | 0.47561 |
| Q4 | −0.057 (−0.089, −0.025) | 0.00046 | −0.031 (−0.063, 0.000) | 0.05077 | −0.033 (−0.065, −0.001) | 0.04141 |
| P for trend | 0.00031 | 0.04836 | 0.04347 | |||
Notes: Crude model: we did not adjust other covariates. Minimally adjusted model: we adjusted age and BMI. Fully adjusted model: we adjusted Age; BMI; FBG, mmol/l; 25(OH)D, nmol/l; ALT, U/L; AST, U/L; Calcium intake, g/week; Outdoor time, h/day; Walking time, h/day; Smoking; Drinking; Chronic Kidney disease (CKD); Chronic obstructive pulmonary disease (COPD); Diabetes; Hyperthyroidism; Hyperlipemia and Chronic diarrhea. CI, confidence interval; Ref, reference.
In premenopausal women, TC, LDL-C, and HDL-C were not shown to have a correlation with lumbar spine BMD (L1–L4) in all the models. These findings were supported using sensitivity analyses (Table 3).
Table 3.
Multivariate Regression for Effect of TC, HDL-C, and LDL-C on Lumber Spine BMD in Premenopausal Women
| Variables | Crude Model | Minimally Adjusted Model | Fully Adjusted Model | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| TC, mmol/l | −0.012 (−0.027, 0.004) | 0.13301 | −0.007 (−0.021, 0.007) | 0.34509 | −0.006 (−0.021, 0.008) | 0.39137 |
| TC (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | −0.012 (−0.052, 0.027) | 0.53523 | −0.022 (−0.056, 0.013) | 0.21969 | −0.020 (−0.056, 0.015) | 0.26229 |
| Q3 | 0.008 (−0.034, 0.050) | 0.7106 | 0.023 (−0.014, 0.061) | 0.22072 | 0.018 (−0.020, 0.057) | 0.35113 |
| Q4 | −0.043 (−0.088, 0.002) | 0.06327 | −0.025 (−0.066, 0.016) | 0.22777 | −0.024 (−0.065, 0.018) | 0.26182 |
| P for trend | 0.19723 | 0.61577 | 0.63612 | |||
| LDL-C, mmol/l | −0.017 (−0.038, 0.004) | 0.11166 | −0.016 (−0.035, 0.003) | 0.10843 | −0.015 (−0.034, 0.005) | 0.13521 |
| LDL (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.006 (−0.032, 0.045) | 0.74357 | −0.012 (−0.047, 0.022) | 0.4852 | −0.012 (−0.047, 0.023) | 0.50624 |
| Q3 | −0.011 (−0.053, 0.031) | 0.61911 | −0.008 (−0.045, 0.030) | 0.69855 | −0.008 (−0.047, 0.030) | 0.67465 |
| Q4 | −0.041 (−0.085, 0.003) | 0.07132 | −0.037 (−0.077, 0.004) | 0.08139 | −0.033 (−0.074, 0.008) | 0.11109 |
| P for trend | 0.08862 | 0.12741 | 0.15223 | |||
| HDL-C, mmol/l | −0.016 (−0.058, 0.026) | 0.44464 | 0.004 (−0.035, 0.043) | 0.83793 | 0.002 (−0.038, 0.042) | 0.91381 |
| HDL (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | −0.017 (−0.059, 0.026) | 0.44213 | −0.006 (−0.044, 0.032) | 0.75195 | −0.004 (−0.042, 0.035) | 0.85452 |
| Q3 | −0.006 (−0.046, 0.034) | 0.77564 | −0.000 (−0.036, 0.036) | 0.98853 | 0.004 (−0.033, 0.040) | 0.84452 |
| Q4 | −0.022 (−0.067, 0.024) | 0.34715 | −0.002 (−0.043, 0.040) | 0.93498 | −0.002 (−0.044, 0.040) | 0.93073 |
| P for trend | 0.46474 | 0.9971 | 0.9536 | |||
Notes: Crude model: we did not adjust other covariates. Minimally adjusted model: we adjusted age and BMI, Fully adjusted model: we adjusted Age; BMI; FBG, mmol/l; 25(OH)D, nmol/l; ALT, U/L; AST, U/L; Calcium intake, g/week; Outdoor time, h/day; Walking time, h/day; Smoking; Drinking; Chronic Kidney disease (CKD); Chronic obstructive pulmonary disease (COPD); Diabetes; Hyperthyroidism; Hyperlipemia and Chronic diarrhea.
Correlation Between Serum TC, LDL-C, HDL-C Levels and Femoral Neck BMD
In postmenopausal women, there was no association between TC and femoral neck BMD in the crude model (β = 0.01, 95% CI: −0.003 to 0.023, p = 0.121). LDL-C was positively associated with femoral neck BMD of (β = 0.03, 95% CI: 0.012 to 0.047, p = 0.001). In contrast, HDL-C was negatively correlated with femoral neck BMD (β = −0.068, 95% CI: −0.1 to −0.037, p = < 0.001). In the minimally and fully adjusted models and after sensitivity analysis (Table 4), the same relationships were established. In premenopausal women, TC, LDL-C, and HDL-C showed no correlation with femoral neck BMD in all the models. Similar trends were reported using sensitivity analysis (Table 5).
Table 4.
Multivariate Regression for Effect of TC, HDL-C, and LDL-C on Femur Neck BMD in Postmenopausal Women
| Variables | Crude Model | Minimally Adjusted Model | Fully Adjusted Model | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| TC, mmol/l | 0.010 (−0.003, 0.023) | 0.12125 | 0.011 (−0.001, 0.024) | 0.07885 | 0.011 (−0.001, 0.024) | 0.08357 |
| TC (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.041 (0.002, 0.080) | 0.0422 | 0.045 (0.007, 0.083) | 0.02153 | 0.049 (0.011, 0.087) | 0.01227 |
| Q3 | 0.033 (−0.006, 0.071) | 0.09935 | 0.035 (−0.003, 0.072) | 0.06976 | 0.039 (0.001, 0.076) | 0.04314 |
| Q4 | 0.045 (0.007, 0.084) | 0.02136 | 0.047 (0.010, 0.084) | 0.0141 | 0.047 (0.010, 0.085) | 0.01329 |
| P for trend | 0.0631 | 0.0541 | 0.05938 | |||
| LDL-C, mmol/l | 0.030 (0.012, 0.047) | 0.00104 | 0.024 (0.007, 0.041) | 0.00619 | 0.023 (0.006, 0.040) | 0.00838 |
| LDL-C (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.031 (−0.008, 0.070) | 0.11541 | 0.024 (−0.014, 0.062) | 0.21553 | 0.020 (−0.018, 0.058) | 0.3032 |
| Q3 | 0.043 (0.005, 0.080) | 0.02683 | 0.028 (−0.009, 0.065) | 0.1351 | 0.026 (−0.011, 0.063) | 0.1738 |
| Q4 | 0.061 (0.023, 0.099) | 0.0016 | 0.047 (0.010, 0.084) | 0.01249 | 0.044 (0.007, 0.081) | 0.01931 |
| P for trend | 0.00158 | 0.01483 | 0.01935 | |||
| HDL-C, mmol/l | −0.068 (−0.100, −0.037) | 0.00002 | −0.047 (−0.078, −0.015) | 0.00386 | −0.041 (−0.073, −0.009) | 0.01257 |
| HDL-C (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | −0.002 (−0.038, 0.035) | 0.92649 | −0.000 (−0.036, 0.036) | 0.99442 | 0.006 (−0.029, 0.042) | 0.72717 |
| Q3 | −0.018 (−0.055, 0.019) | 0.34158 | −0.010 (−0.047, 0.026) | 0.57426 | −0.003 (−0.040, 0.033) | 0.87126 |
| Q4 | −0.070 (−0.105, −0.035) | 0.00009 | −0.048 (−0.083, −0.013) | 0.00698 | −0.041 (−0.077, −0.005) | 0.02434 |
| P for trend | 0.00003 | 0.00415 | 0.01436 | |||
Notes: Crude model: we did not adjust other covariates. Minimally adjusted model: we adjusted age and BMI. Fully adjusted model: we adjusted Age; BMI; FBG, mmol/l; 25(OH)D, nmol/l; ALT, U/L; AST, U/L; Calcium intake, g/week; Outdoor time, h/day; Walking time, h/day; Smoking; Drinking; Chronic Kidney disease (CKD); Chronic obstructive pulmonary disease (COPD); Diabetes; Hyperthyroidism; Hyperlipemia and Chronic diarrhea. CI, confidence interval; Ref, reference.
Table 5.
Multivariate Regression for Effect of TC, HDL-C, and LDL-C on Femur Neck BMD in Premenopausal Women
| Variables | Crude Model | Minimally Adjusted Model | Fully Adjusted Model | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| TC, mmol/l | −0.009 (−0.027, 0.008) | 0.30044 | −0.002 (−0.019, 0.014) | 0.78328 | −0.002 (−0.018, 0.015) | 0.84657 |
| TC (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.050 (0.007, 0.093) | 0.02214 | 0.048 (0.009, 0.087) | 0.01714 | 0.038 (−0.002, 0.078) | 0.06266 |
| Q3 | 0.012 (−0.034, 0.058) | 0.61486 | 0.028 (−0.016, 0.071) | 0.21376 | 0.027 (−0.018, 0.071) | 0.23796 |
| Q4 | −0.041 (−0.092, 0.009) | 0.10951 | −0.022 (−0.070, 0.025) | 0.35497 | −0.016 (−0.063, 0.032) | 0.52242 |
| P for trend | 0.24644 | 0.77539 | 0.91687 | |||
| LDL-C, mmol/l | −0.009 (−0.032, 0.015) | 0.48305 | −0.004 (−0.027, 0.018) | 0.70946 | −0.005 (−0.027, 0.018) | 0.68306 |
| LDL (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.010 (−0.033, 0.054) | 0.63435 | −0.006 (−0.046, 0.034) | 0.77209 | −0.015 (−0.055, 0.026) | 0.47553 |
| Q3 | 0.000 (−0.046, 0.047) | 0.98436 | 0.007 (−0.036, 0.051) | 0.74103 | 0.000 (−0.044, 0.044) | 0.99301 |
| Q4 | −0.041 (−0.092, 0.009) | 0.11023 | −0.031 (−0.080, 0.017) | 0.20738 | −0.027 (−0.075, 0.021) | 0.26675 |
| P for trend | 0.19074 | 0.39877 | 0.40591 | |||
| HDL-C, mmol/l | −0.028 (−0.076, 0.019) | 0.24415 | −0.007 (−0.052, 0.038) | 0.761 | 0.005 (−0.041, 0.051) | 0.82518 |
| HDL (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.015 (−0.031, 0.062) | 0.51079 | 0.024 (−0.019, 0.067) | 0.27658 | 0.026 (−0.017, 0.069) | 0.237 |
| Q3 | −0.006 (−0.050, 0.038) | 0.78824 | 0.005 (−0.037, 0.046) | 0.82301 | 0.015 (−0.027, 0.056) | 0.48213 |
| Q4 | −0.038 (−0.089, 0.013) | 0.14781 | −0.018 (−0.067, 0.031) | 0.47038 | −0.009 (−0.058, 0.040) | 0.72792 |
| P for trend | 0.15712 | 0.48112 | 0.84693 | |||
Notes: Crude model: we did not adjust other covariates. Minimally adjusted model: we adjusted age and BMI. Fully adjusted model: we adjusted Age; BMI; FBG, mmol/l; 25(OH)D, nmol/l; ALT, U/L; AST, U/L; Calcium intake, g/week; Outdoor time, h/day; Walking time, h/day; Smoking; Drinking; Chronic Kidney disease (CKD); Chronic obstructive pulmonary disease (COPD); Diabetes; Hyperthyroidism; Hyperlipemia and Chronic diarrhea.
Correlation Between Serum TC, LDL-C, HDL-C Levels and Total Hip BMD
In postmenopausal women, either TC or LDL-C showed no correlation with total hip BMD in all the models. Similar trends were reported using the sensitivity analysis. In the crude model, HDL-C was shown to have a negative association with total hip BMD (β = −0.044, 95% CI: −0.072 to −0.015, p = 0.003). In the minimally and fully adjusted models, an association was not identified. Similar trends were observed using sensitivity analysis (Table 6). In premenopausal women, TC, LDL-C, and HDL-C showed no correlation with total hip BMD in all the models. Similar trends were reported using sensitivity analysis (Table 7).
Table 6.
Multivariate Regression for Effect of TC, HDL-C, and LDL-C on Total Hip BMD in Postmenopausal Women
| Variables | Crude Model | Minimally Adjusted Model | Fully Adjusted Model | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| TC, mmol/l | −0.001 (−0.012, 0.011) | 0.93165 | 0.000 (−0.011, 0.011) | 0.99993 | −0.001 (−0.012, 0.011) | 0.89663 |
| TC (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.017 (−0.017, 0.052) | 0.32308 | 0.014 (−0.019, 0.047) | 0.4126 | 0.009 (−0.024, 0.042) | 0.59727 |
| Q3 | −0.003 (−0.037, 0.031) | 0.84906 | −0.004 (−0.037, 0.029) | 0.79858 | −0.005 (−0.038, 0.028) | 0.76657 |
| Q4 | 0.006 (−0.028, 0.040) | 0.73376 | 0.004 (−0.029, 0.037) | 0.82476 | −0.003 (−0.035, 0.030) | 0.88118 |
| P for trend | 0.86489 | 0.81617 | 0.61671 | |||
| LDL-C, mmol/l | 0.005 (−0.011, 0.021) | 0.51524 | 0.001 (−0.014, 0.017) | 0.86301 | 0.000 (−0.015, 0.015) | 0.97492 |
| LDL (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.003 (−0.032, 0.038) | 0.87251 | −0.010 (−0.043, 0.024) | 0.58368 | −0.014 (−0.048, 0.020) | 0.4246 |
| Q3 | 0.020 (−0.014, 0.054) | 0.24238 | 0.004 (−0.029, 0.037) | 0.80731 | 0.000 (−0.033, 0.034) | 0.98198 |
| Q4 | 0.008 (−0.026, 0.042) | 0.6391 | −0.006 (−0.039, 0.028) | 0.74078 | −0.010 (−0.043, 0.023) | 0.54066 |
| P for trend | 0.46764 | 0.98147 | 0.7939 | |||
| HDL-C, mmol/l | −0.044 (−0.072, −0.015) | 0.00262 | −0.024 (−0.052, 0.004) | 0.09856 | −0.023 (−0.052, 0.005) | 0.10896 |
| HDL (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | −0.041 (−0.074, −0.007) | 0.01752 | −0.032 (−0.065, 0.001) | 0.05519 | −0.030 (−0.062, 0.003) | 0.07363 |
| Q3 | −0.041 (−0.074, −0.008) | 0.01624 | −0.026 (−0.059, 0.007) | 0.12215 | −0.026 (−0.059, 0.007) | 0.12196 |
| Q4 | −0.056 (−0.087, −0.024) | 0.00056 | −0.034 (−0.066, −0.003) | 0.03351 | −0.035 (−0.066, −0.003) | 0.03187 |
| P for trend | 0.00139 | 0.07073 | 0.05952 | |||
Notes: Crude model: we did not adjust other covariates. Minimally adjusted model: we adjusted age and BMI. Fully adjusted model: we adjusted Age; BMI; FBG, mmol/l; 25(OH)D, nmol/l; ALT, U/L; AST, U/L; Calcium intake, g/week; Outdoor time, h/day; Walking time, h/day; Smoking; Drinking; Chronic Kidney disease (CKD); Chronic obstructive pulmonary disease (COPD); Diabetes; Hyperthyroidism; Hyperlipemia and Chronic diarrhea.
Table 7.
Multivariate Regression for Effect of TC, HDL-C, and LDL-C on Total Hip BMD in Premenopausal Women
| Variables | Crude Model | Minimally Adjusted Model | Fully Adjusted Model | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| TC, mmol/l | 0.013 (−0.009, 0.035) | 0.24955 | 0.010 (−0.012, 0.032) | 0.37078 | 0.005 (−0.017, 0.027) | 0.63903 |
| TC (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.057 (0.003, 0.112) | 0.03957 | 0.039 (−0.013, 0.090) | 0.14703 | 0.028 (−0.026, 0.081) | 0.3096 |
| Q3 | 0.051 (−0.007, 0.109) | 0.08405 | 0.052 (−0.004, 0.108) | 0.06877 | 0.045 (−0.013, 0.103) | 0.13171 |
| Q4 | 0.017 (−0.047, 0.082) | 0.60022 | 0.011 (−0.052, 0.074) | 0.72654 | 0.002 (−0.062, 0.067) | 0.94179 |
| P for trend | 0.25874 | 0.3031 | 0.49422 | |||
| LDL-C, mmol/l | 0.008 (−0.021, 0.038) | 0.58375 | −0.001 (−0.030, 0.029) | 0.96844 | −0.003 (−0.033, 0.026) | 0.82975 |
| LDL (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.057 (0.002, 0.111) | 0.04211 | 0.035 (−0.017, 0.088) | 0.18721 | 0.030 (−0.024, 0.083) | 0.27481 |
| Q3 | 0.052 (−0.006, 0.111) | 0.0823 | 0.046 (−0.010, 0.103) | 0.10831 | 0.032 (−0.025, 0.090) | 0.27317 |
| Q4 | 0.008 (−0.055, 0.072) | 0.79249 | −0.007 (−0.069, 0.055) | 0.82618 | −0.007 (−0.070, 0.056) | 0.83041 |
| P for trend | 0.40574 | 0.65212 | 0.80914 | |||
| HDL-C, mmol/l | −0.032 (−0.090, 0.026) | 0.28283 | −0.002 (−0.060, 0.055) | 0.93745 | −0.002 (−0.061, 0.056) | 0.93483 |
| HDL (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | −0.005 (−0.064, 0.055) | 0.8786 | 0.012 (−0.045, 0.069) | 0.68109 | 0.007 (−0.050, 0.064) | 0.81428 |
| Q3 | −0.020 (−0.076, 0.037) | 0.49717 | −0.003 (−0.057, 0.052) | 0.92356 | 0.010 (−0.046, 0.065) | 0.73139 |
| Q4 | −0.007 (−0.070, 0.056) | 0.83167 | 0.024 (−0.038, 0.086) | 0.44444 | 0.026 (−0.037, 0.089) | 0.42218 |
| P for trend | 0.66455 | 0.61964 | 0.44521 | |||
Notes: Crude model: we did not adjust other covariates. Minimally adjusted model: we adjusted age and BMI. Fully adjusted model: we adjusted Age; BMI; FBG, mmol/l; 25(OH)D, nmol/l; ALT, U/L; AST, U/L; Calcium intake, g/week; Outdoor time, h/day; Walking time, h/day; Smoking; Drinking; Chronic Kidney disease (CKD); Chronic obstructive pulmonary disease (COPD); Diabetes; Hyperthyroidism; Hyperlipemia and Chronic diarrhea.
Non-Rectilinear Relationship Analysis
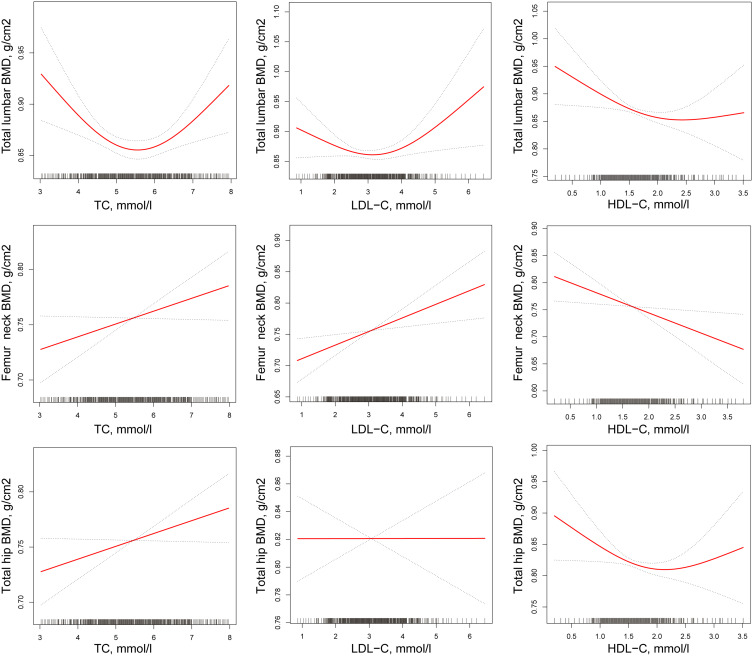
The non-rectilinear relationship between continuous variables, TC, LDL-C, and HDL-C, was evaluated. Consequently, the association between TC, LDL-C, HDL-C and lumbar spine BMD (L1–L4) was found to be non-rectilinear in postmenopausal women (after adjusting for age, BMI, FBG [mmol/l], 25 [OH] D [nmol/l], ALT [U/L], AST [U/L], calcium intake (g/week), outdoor time (hours/day), walking time (hours/day), smoking, drinking, hyperlipidemia, CKD, diabetes mellitus, hyperthyroidism, COPD, and chronic diarrhea). The inflection points were established to be 5.86 mmol/L, 3.52 mmol/L, and 2.37 mmol/L, respectively, using piecewise rectilinear regression. To the left of the inflection points, the β and 95% CI values were −0.029, −0.047 to −0.011; −0.022, −0.044 to −0.001; −0.046, −0.077 to −0.014, respectively. To the right of the inflection points in the relationship curve between either TC or LDL-C and lumbar spine BMD (L1–L4), the β and 95% CI values were 0.046, 0.016 to 0.076; 0.061, 0.013 to 0.109, respectively. However, an association was not found between HDL-C and lumbar spine BMD (L1–L4) to the right of the inflection point (β of 0.094, 95% CI: −0.025 to 0.213). Similarly, a non-rectilinear relationship was not identified between TC, LDL-C, HDL-C and femoral neck BMD, nor between TC, LDL-C, HDL-C and total hip BMD (Table 8 and Figure 2).
Table 8.
Multivariate Regression for Effect of TC, HDL-C, and LDL-C on BMD in Postmenopausal Women
| Linear Regression | Break Point (K) | < K | > K | LLR Test | |
|---|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | P | ||
| Total lumbar BMD, g/cm2 | |||||
| TC, mmol/l | −0.004 (−0.015, 0.007) | 5.86 | −0.029 (−0.047, −0.011) | 0.046 (0.016, 0.076) | <0.001 |
| LDL-C, mmol/l | −0.001 (−0.016, 0.014) | 3.52 | −0.022 (−0.044, −0.001) | 0.061 (0.013, 0.109) | 0.009 |
| HDL-C, mmol/l | −0.033 (−0.062, −0.005) | 2.37 | −0.046 (−0.077, −0.014) | 0.094 (−0.025, 0.213) | 0.036 |
| Femur neck BMD, g/cm2 | |||||
| TC, mmol/l | 0.011 (−0.002, 0.023) | 5.4 | 0.027 (0.001, 0.053) | −0.004 (−0.024, 0.016) | 0.149 |
| LDL-C, mmol/l | 0.022 (0.005, 0.039) | 4.24 | 0.022 (0.003, 0.041) | 0.041 (−0.140, 0.221) | 0.81 |
| HDL-C, mmol/l | −0.041 (−0.073, −0.009) | 2.34 | −0.057 (−0.094, −0.020) | 0.077 (−0.065, 0.220) | 0.133 |
| Total Hip BMD, g/cm2 | |||||
| TC, mmol/l | −0.001 (−0.012, 0.010) | 6.8 | 0.047 (−0.001, 0.094) | 0.081 (−0.008, 0.171) | 0.06 |
| LDL-C, mmol/l | 0.000 (−0.015, 0.015) | 4.22 | −0.003 (−0.020, 0.014) | 0.075 (−0.094, 0.243) | 0.366 |
| HDL-C, mmol/l | −0.023 (−0.052, 0.005) | 2.26 | −0.041 (−0.075, −0.007) | 0.098 (−0.031, 0.228) | 0.063 |
Notes: Multivariate-Adjusted Model adjust for: Age; BMI; FBG, mmol/l; 25(OH)D, nmol/l; ALT, U/L; AST, U/L; Calcium intake, g/week; Outdoor time, h/day; Walking time, h/day; Smoking; Drinking; Chronic Kidney disease (CKD); Chronic obstructive pulmonary disease (COPD); Diabetes; Hyperthyroidism; Hyperlipemia and Chronic diarrhea.
Figure 2.
Multivariate-adjusted smoothing spline plots of BMD by TC, HDL-C, and LDL-C in postmenopausal women. Red dotted lines represent the spline plots of TC, HDL-C, and LDL-C and blue dotted.
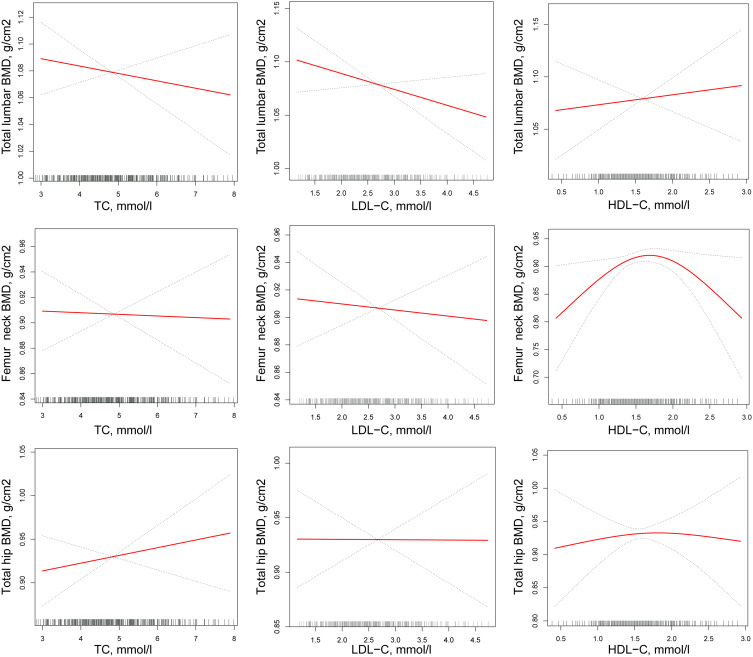
In premenopausal women, a non-rectilinear relationship was not established between either TC and LDL-C and lumbar spine (L1–L4), femoral neck and total hip BMD, nor between HDL-C and either lumbar spine BMD (L1–L4) or total hip BMD. By contrast, the association between HDL-C and femoral neck BMD was non-rectilinear. The inflection point was 1.36 mmol/L. To the left of the inflection point, the β and 95% CI values were 0.205 and 0.058 to 0.351, respectively. To the right of the inflection point, an association linking HDL-C and femoral neck BMD was not established (Table 9 and Figure 3).
Table 9.
Multivariate Regression for Effect of TC, HDL-C, and LDL-C on BMD in Premenopausal Women
| Linear Regression | Break Point (K) | < K | > K | LLR Test | |
|---|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | p | ||
| Total lumbar BMD, g/cm2 | |||||
| TC, mmol/l | −0.006 (−0.021, 0.008) | 4.35 | −0.047 (−0.097, 0.002) | 0.005 (−0.014, 0.025) | 0.073 |
| LDL-C, mmol/l | −0.015 (−0.034, 0.005) | 1.66 | −0.237 (−0.492, 0.019) | −0.009 (−0.030, 0.012) | 0.156 |
| HDL-C, mmol/l | 0.002 (−0.038, 0.042) | 2.24 | 0.020 (−0.026, 0.066) | −0.177 (−0.408, 0.054) | 0.085 |
| Femur neck BMD, g/cm2 | |||||
| TC, mmol/l | −0.002 (−0.018, 0.015) | 5.57 | 0.008 (−0.018, 0.034) | −0.025 (−0.076, 0.025) | 0.295 |
| LDL-C, mmol/l | −0.005 (−0.027, 0.018) | 3.37 | 0.004 (−0.026, 0.035) | −0.038 (−0.119, 0.043) | 0.371 |
| HDL-C, mmol/l | 0.005 (−0.041, 0.051) | 1.36 | 0.205 (0.058, 0.351) | −0.049 (−0.108, 0.010) | 0.005 |
| Total Hip BMD, g/cm2 | |||||
| TC, mmol/l | 0.005 (−0.017, 0.027) | 5.93 | 0.014 (−0.014, 0.043) | −0.037 (−0.123, 0.050) | 0.286 |
| LDL-C, mmol/l | −0.003 (−0.033, 0.026) | 1.81 | −0.127 (−0.377, 0.122) | 0.004 (−0.029, 0.037) | 0.329 |
| HDL-C, mmol/l | −0.002 (−0.061, 0.056) | 1.53 | 0.026 (−0.108, 0.160) | −0.020 (−0.112, 0.073) | 0.564 |
Notes: Multivariate-Adjusted Model adjust for: Age; BMI; FBG, mmol/l; 25(OH)D, nmol/l; ALT, U/L; AST, U/L; Calcium intake, g/week; Outdoor time, h/day; Walking time, h/day; Smoking; Drinking; Chronic Kidney disease (CKD); Chronic obstructive pulmonary disease (COPD); Diabetes; Hyperthyroidism; Hyperlipemia and Chronic diarrhea.
Figure 3.
Multivariate-adjusted smoothing spline plots of BMD by TC, HDL-C, and LDL-C in premenopausal women. Red dotted lines represent the spline plots of TC, HDL-C, and LDL-C and blue dotted.
Discussion
The current study objective was to evaluate the relationship between serum cholesterol profile and BMD in women. To the best of our knowledge, this is the first study to have established a link between serum cholesterol levels and BMD in apparently healthy women via a curvilinear relationship.
Interestingly, in the primary results, TC, LDL-C, and HDL-C were associated with lumbar spine BMD (L1–L4) in postmenopausal women (L1–L4) and this was reflected in a non-rectilinear relationship. The different correlations of LDL-C, TC, and HDL-C with lumbar spine (BMD) (L1–L4) were reported on the two sides of the inflection points. LDL-C, TC, and HDL-C, assessed against the baseline values, were negatively associated with lumbar spine BMD (L1–L4) to the left of the inflection points. However, correlations to the right of the inflection points were positive, except HDL-C. In addition, the non-rectilinear models showed that TC, LDL-C, and HDL-C were not related to femoral neck and total hip BMD, even using sensitivity analysis. In premenopausal women, the association between HDL-C and femoral neck BMD was non-rectilinear, and the correlation to the left of the inflection point was positive.
However, previous studies have mostly focused on the linear relationship between serum lipids and BMD, and overall consensus has not been reached.22–25 The results of a case-control study in which 452 postmenopausal women were assessed between January 2012 and January 2015 indicated that lumbar spine BMD was negatively correlated with LDL levels.26 The findings of a cross-sectional study on 136 postmenopausal Caucasian women who were in good health also revealed that TC levels and hip BMD were positively correlated. Even after controlling for age, BMI, physical activity, energy, calcium, and alcohol intake, the finding remained significant.27 In another cross-sectional study on 170 postmenopausal women aged 50 to 70 years in Iran, performed from March 2013 to September 2013, it was shown that serum lipid levels were independent of lumbar and femoral neck BMD after controlling for BMI.28
The results of numerous studies have demonstrated that the association between serum cholesterol levels and BMD was influenced by gender and ethnicity and whether or not menopause had occurred.29–32 Hence, it was necessary to establish the effect of serum cholesterol levels on BMD in premenopausal and postmenopausal Chinese women.
In the current study, it was established that high level of serum lipids to the left of the inflection point correlated with low lumbar spine BMD in postmenopausal women. Our findings in postmenopausal women are consistent with the data from a different cross-sectional study in which an inverse association was reported between lumbar spine BMD and HDL-C in women of broad-ranging ages.33 The study involved two cohort subjects, a clinical group of women (n = 236) aged 35–81 years in an OP assessment group and a community cohort (n = 265 men, n = 481 women) aged 68–75 years participating in a population-based epidemiological survey. A marked inverse relationship between HDL-C and spinal BMD was reported in the clinical cohort group.
An epidemiological study in South Korea on 355 postmenopausal and 375 premenopausal women showed that serum TC and LDL-C levels had a negative correlation with BMD.16 There were many similarities between the study by Cui et al and the current study. Their study included 867 South Korean women and the current study recruited 1116 Chinese women. Compared with their study, the participants in the current study were drawn from different sources, represented different sectors of the population, and the number of participants was slightly greater.
In terms of differences between the studies, most importantly, they used a general linear model to evaluate the linear relationship between BMD and serum lipids, whereas GAM was used in the present research to determine the non-rectilinear relationship between them; this was perhaps the greatest difference between the two studies. The inflection point of the relationship between serum lipids and BMD was identified in the current study, which was considered to be of invaluable significance and may constitute the real relationship between the two. It is hoped that this will form the basis in future studies for an exploration of the non-linear relationship between serum lipids and BMD.
In the study by Cui et al, the levels of serum TC and LDL-C were inversely associated with BMD in both pre- and postmenopausal women. However, we established that the relationship between TC, LDL-C, HDL-C and lumbar spine BMD was non-linear in postmenopausal women, while in premenopausal women, the association between HDL-C and femoral neck BMD was non-linear. Elsewhere, a weak negative association was found between BMD and TC in older women.34 Although the association between TC and BMD was found to be inversely correlated in other studies, dissimilar to our results, the researchers failed to provide a possible explanation for their findings. To some extent, our results provide evidence of the lipid hypothesis for OP that suggests that LDL atherogenic lipid distribution and oxidation facilitate bone loss. The suppression of cholesterol biosynthesis has also been demonstrated to prevent osteogenic differentiation by inhibiting the mRNA expression of stromal bone marrow cells, which are precursors of osteoblasts, thereby improving BMD and fracture risk.35,36
Paraoxonase is a calcium-dependent esterase and confers HDL antioxidant properties by inhibiting the accumulation of lipid peroxidation products. A study on 100 healthy postmenopausal women found that the concentration of paraoxonase in the osteopenic group was significantly lower than that in the normal group.37 The mechanisms for the negative relationship between serum HDL-C and BMD are unclear. A study on the differentiation of mesenchymal stem cells showed that hydroxysterol stimulated the osteogenic differentiation of bone marrow mesenchymal stem cells, while HDL-C removed oxysterol in the surrounding tissue, indicating that high HDL-C level is not conducive to osteogenic differentiation.38 This may elucidate the negative correlation between serum HDL-C and BMD.
The current study demonstrated that the high levels of serum TC and LDL-C correlated with high lumbar spine BMD to the right of the inflection points. Jeong et al also observed a positive correlation between TC and BMD at different skeletal sites in women.39 This suggests that common mechanisms connect lipids to bone metabolism. Research evidence shows that oxysterols regulate the specialization of mesenchymal stem cells, and this is beneficial to osteoblast differentiation. The researchers hypothesized that osteogenesis of the oxidized sterol was partly moderated by the activation of cyclo-oxygenase and phospholipase A2 in the arachidonic acid cascade and their metabolism to osteogenic prostaglandins and other eicosanoids.38
Subgroup analysis is fundamental in scientific research.40 Unfortunately, most previous studies only used gender as a stratified factor for subgroup analysis, and only a few segregated premenopausal and postmenopausal women, which hindered our exploration of the relationship between serum lipids and BMD. In the present study, a completely different relationship was demonstrated between BMD and serum lipids in pre- and postmenopausal women. In postmenopausal women high LDL-C concentrations correlated with high femoral neck BMD; and high HDL-C concentrations reflected low femoral neck BMD. However, TC, LDL-C, and HDL-C showed no correlation with femoral neck BMD in premenopausal women.
In the study by Cui et al,16 serum TC levels were negatively associated with lumbar BMD in premenopausal women. In the study by Garg et al, a declining trend was demonstrated in relation to the relationship between femoral and lumbar spine BMD and LDL-C in premenopausal women.10 These findings indicate that serum lipids have different effects on BMD at different bone sites in premenopausal women. However, it is difficult to elucidate the effects of serum lipids on BMD in premenopausal women in cross-sectional studies. In addition, there is a scarcity of literature on the impact of serum lipids on BMD in premenopausal women. The differences in the findings of the relationship between serum lipids and BMD between pre- and postmenopausal women may be explained by the following reasons. Firstly, estrogen concentrations in different subjects play an important role. In addition, it is difficult to measure the BMD of lumbar vertebrae in the elderly due to degenerative changes, such as hyperosteogeny, which may cause changes in lumbar BMD unrelated to serum lipids in postmenopausal women. Therefore, it is necessary to evaluate the role of different bone sites and aging when evaluating the relationship between serum lipids and BMD.
Our research had many strengths. Firstly, a generalized rectilinear model was used to assess the rectilinear association between serum lipids and BMD; in addition, a GAM was utilized to assess the non-rectilinear relationship between the two. GAM is advantageous with respect to evaluations of non-rectilinear relationships between response variables and multiple explanatory variables, and it can be effective in the analysis of large data sets.41,42 It accomplishes non-parametric smoothing and fits the spline regression data. In addition, GAM can be effectively applied to analyzing the interaction between influential factors and response variables.43 By contrast, traditional statistical methods are not as effective at determining complex non-linear relationships.44 GAM is also more successful at pinpointing the actual relationship between serum lipids and BMD. Secondly, the data collection, in addition to the estimations made and assessments of the participants, was performed consistently and meticulously in the current study, which was part of a larger project. Thirdly, this was an observational study, with the potential for inevitable potential confounding; thus, rigorous statistical adjustments were utilized to minimize residual confusion. Finally, the use of subgroup analysis achieves better use of the data compared to primary analysis only. In subgroup analysis, the relationship between serum lipids and BMD was different in different groups.
This research also had numerous limitations. Firstly, it utilized a cross-sectional design without a prospective follow-up; thus, the support between the exposure and the outcomes was weak. Secondly, the study population comprised Chinese women only, which limits generalization to other groups. Thirdly, only a small number of patients with hyperlipidemia were used anti-lipid drugs in this research. Adjustments were made for hyperlipidemia but not for the use of anti-lipid drugs. Finally, although adjustments were made for diabetes mellitus, the effects of impaired glucose tolerance were overlooked, which was a limitation of this study.
Conclusion
In postmenopausal women, TC, LDL-C, and HDL-C concentrations were shown to relate negatively to lumbar spine BMD when their respective concentrations were less than 5.86 mmol/L, 3.52 mmol/L, and 2.37 mmol/L. In addition, a non-linear relationship was demonstrated between HDL-C and femoral neck BMD in premenopausal women. The mechanisms that underlie these associations remain unclear; therefore, more well-controlled prospective studies are warranted to establish the actual associations between serum lipids and BMD in women.
Acknowledgment
Thanks to Pianpian Fan for contributing to this article.
Funding Statement
This work was supported by MSD (Project No. IISP-40407), but MSD was not involved in the design and implementation, data collection, analysis, interpretation or article writing of this study.
Abbreviations
BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; TC, total cholesterol; HLD-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; OP, Osteoporosis; BMI, Body Mass Index; ALT, alanine aminotransferase; FBG, fasting plasma glucose; AST, aspartate aminotransferase; SD, standard deviation; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; GAM, generalized additive model; CI, confidence interval.
Data Sharing Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve participant confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Ethics Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical permission was acquired from the Ethics Review Board of West China Hospital of Sichuan University (MISP-40407). Informed consent was obtained from all individual participants included in the study. Consent for publication: Informed consent was obtained from all individual participants included in the study.
Author Contributions
Decai Chen, Chunyan Lu and Qin Wang: designed and conducted the research and critically revised the manuscript. Yong Xu, Hongyi Cao, Xiaohua Xie, Xueyan Wu and Jing Li: collected data, analyzed and interpreted data and critically review article. Qi Zhang and Junteng Zhou: collected data, analyzed the data and performed the statistical analyses, wrote the manuscript and substantially revised the article. We all agreed on the journal to which the article will be submitted, and reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. In addition, we all agree to take responsibility and be accountable for the contents of the article.
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Camacho PM, Petak SM, Binkley N, et al. American association of clinical endocrinologists and American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(Suppl):4. [DOI] [PubMed] [Google Scholar]
- 2.Aaseth J, Boivin G, Andersen O. Osteoporosis and trace elements-An overview. J Trace Elem Med Biol. 2012;26(2–3):149–152. doi: 10.1016/j.jtemb.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 3.Gerber LM, Bener A, Al-Ali HM, Hammoudeh M, Liu LQ, Verjee M. Bone mineral density in midlife women: the Study of Women’s Health in Qatar. Climacteric. 2015;18(2):316e22. doi: 10.3109/13697137.2014.944495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acar B, Ozay AC, Ozay OE, Okyay E, Sisman AR, Ozaksoy D. Evaluation of thyroid function status among postmenopausal women with and without osteoporosis. Int J Gynaecol Obstet. 2016;134(1):53e7. doi: 10.1016/j.ijgo.2015.11.025 [DOI] [PubMed] [Google Scholar]
- 5.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a sitespecific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259(6):598–605. doi: 10.1111/j.1365-2796.2006.01640.x [DOI] [PubMed] [Google Scholar]
- 6.Tanko LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20(11):1912–1920. doi: 10.1359/JBMR.050711 [DOI] [PubMed] [Google Scholar]
- 7.Rosso AL, Wisdom JP, Horner-Johnson W, McGee MG, Michael YL. Aging with a disability: a systematic review of cardiovascular disease and osteoporosis among women aging with a physical disability. Maturitas. 2011;68(1):65–72. doi: 10.1016/j.maturitas.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lian XL, Zhang YP, Li X, Jing LD, Cairang ZM, Gou JQ. Exploration on the relationship between the elderly osteoporosis and cardiovascular disease risk factors. Eur Rev Med Pharmacol Sci. 2017;21(19):4386–4390. [PubMed] [Google Scholar]
- 9.Ageev FT, Barinova IV, Seredenina EM, et al. Osteoporosis and Arterial Stiffness: study of 103 Women With Mild to Moderate Risk of Cardiovascular Disease. Kardiologiia. 2013;53(6):51–58. [PubMed] [Google Scholar]
- 10.Marwaha R, Tandon N, Bhadra K, Mahalle N, Garg MK. Relationship of lipid parameters with bone mineral density in Indian population. Indian J Endocrinol Metab. 2014;18:325–332. doi: 10.4103/2230-8210.131165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parhami F, Tintut Y, Beamer WG, Gharavi N, Goodman W, Demer LL. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16(1):182–188. [DOI] [PubMed] [Google Scholar]
- 12.Sage AP, Lu J, Atti E, et al. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res. 2011;26(6):1197–1206. doi: 10.1002/jbmr.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shuang L, Guo H, Liu Y, et al. Relationships of serum lipid profiles and bone mineral density in postmenopausal Chinese women. Clin Endocrinol (Oxf). 2015;8:53–58. [DOI] [PubMed] [Google Scholar]
- 14.Bijelic R, Balaban J, Milicevic S. Correlation of the Lipid Profile. BMI and Bone Mineral Density in Postmenopausal Women. Mater Sociomed. 2016;28(6):412–415. doi: 10.5455/msm.2016.28.412-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennison EM, Syddall HE, Martin HJ, Martin HJ, Cooper C, the Hertfordshire Cohort Study Group. Lipid profile, obesity and bone mineral density: the Hertfordshire Cohort Study. QJM. 2007;100(5):297–303. doi: 10.1093/qjmed/hcm023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui LH, Shin MH, Chung EK, Lee YH, Kweon SS, Park KS. Association between bone mineral densities and serum lipid profiles of pre- and post-menopausal rural women in South Korea. Osteoporos Int. 2005;16(12):1975–1981. doi: 10.1007/s00198-005-1977-2 [DOI] [PubMed] [Google Scholar]
- 17.Makovey J, Chen JS, Hayward C, Williams FM, Sambrook PN. Association between serum cholesterol and bone mineral density. Bone. 2009;44(2):208–213. doi: 10.1016/j.bone.2008.09.020 [DOI] [PubMed] [Google Scholar]
- 18.Hernández JL, Olmos JM, Romaña G, et al. Bone mineral density in statin users: a population-based analysis from a Spanish cohort. J Bone Miner Metab. 2014;32(2):184–191. doi: 10.1007/s00774-013-0481-6 [DOI] [PubMed] [Google Scholar]
- 19.Sivas F, Alemdaroglu E, Elverici E, Kuluğ T, Özoran K. Serum lipid profile: its relationship with osteoporotic vertebrae fractures and bone mineral density in Turkish postmenopausal women. Rheumatol Int. 2009;29(8):885–890. doi: 10.1007/s00296-008-0784-4 [DOI] [PubMed] [Google Scholar]
- 20.Ersoy GS, Simsek EE, Vatansever D, Kasikci HO, Keser B, Sakin O. Lipid profile and plasma atherogenic index in postmenopausal osteoporosis. North Clin Istanb. 2017;4(3):237–241. doi: 10.14744/nci.2017.61587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ. Strengthening the reporting of observational studies in epidemiology (STROBE), explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diana C, Marlena K, Frances M. Association of Plasma Lipids and Polar Metabolites with Low Bone Mineral Density in Singaporean-Chinese Menopausal Women: A Pilot Study. Int J Environ Res Public Health. 2018;15(5):1045. doi: 10.3390/ijerph15051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panahi N, Soltani A, Ghasem-Zadeh A, et al. Associations between the lipid profile and the lumbar spine bone mineral density and trabecular bone score in elderly Iranian individuals participating in the Bushehr Elderly Health Program: a population-based study. Arch Osteoporos. 2019;14(1):52. doi: 10.1007/s11657-019-0602-5 [DOI] [PubMed] [Google Scholar]
- 24.Wani K, Yakout SM, Ansari MGA. Metabolic Syndrome in Arab Adults with Low Bone Mineral Density. Nutrients. 2019;11(6):1405. doi: 10.3390/nu11061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui R, Zhou L, Zuohong L, Qing L, Zhiming Q, Zhang J. Assessment risk of osteoporosis in Chinese people: relationship among body mass index, serum lipid profiles, blood glucose, and bone mineral density. Clin Interv Aging. 2016;2:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alay I, Kaya C, Cengiz H, Yildiz S, Ekin M, Yasar L. The relation of body mass index, menopausal symptoms and lipid profile with bone mineral density in postmenopausal women. Taiwan J Obstet Gynecol. 2020;59:61–66. doi: 10.1016/j.tjog.2019.11.009 [DOI] [PubMed] [Google Scholar]
- 27.Brownbill RA, Lipid Profile JZILICH. Bone Paradox: higher Serum Lipids are Associated with Higher Bone Mineral Density in Postmenopausal Women. J Women’s HEALTH. 2006;15(3):261–270. doi: 10.1089/jwh.2006.15.261 [DOI] [PubMed] [Google Scholar]
- 28.Ghadiri-Anari A, Mortezaii-Shorokit Z, Modarresi M, Dehghan A. Association of lipid profile with bone mineral density in postmenopausal women in Yazd province. Int J Reprod BioMed. 2016;14(9):597–602. doi: 10.29252/ijrm.14.9.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yulin M, Xianping L, Zhang H, et al. Serum myostatin in central south Chinese postmenopausal women: relationship with body composition, lipids and bone mineral density. Endocr Res. 2016;5:1532–4206. doi: 10.3109/07435800.2015.1044609. [DOI] [PubMed] [Google Scholar]
- 30.Yamauchi M, Yamaguchi T, Nawata K, Tanaka K-I, Takaoka S, Sugimoto T. Increased low-density lipoprotein cholesterol level is associated with non-vertebral fractures in postmenopausal women. Endocrine. 2014;5. doi: 10.1007/s12020-014-0292-0. [DOI] [PubMed] [Google Scholar]
- 31.Dennison EM, Syddall HE, Sayer AA, Martin HJ, Cooper C, the Hertfordshire Cohort Study Group. Lipid profile, obesity and bone mineral density: the Hertfordshire Cohort Study. QJM. 2007;100(5):297–303. doi: 10.1093/qjmed/hcm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saoji R, Das RS, Desai M. Association of high-density lipoprotein, triglycerides, and homocysteine with bone mineral density in young Indian tribal women. Arch Osteoporos. 2018;13(1):108. doi: 10.1007/s11657-018-0525-6 [DOI] [PubMed] [Google Scholar]
- 33.Adami S, Braga V, Zamboni M. Relationship between lipids and bone mass in 2 cohorts of healthy women and men. Calcif Tissue Int. 2004;74(2):136. doi: 10.1007/s00223-003-0050-4 [DOI] [PubMed] [Google Scholar]
- 34.Tanko LB, Bagger YZ, Nielsen SM, Christiansen C. Does serum cholesterol contribute to vertebral bone loss in postmenopausal women? Bone. 2003;32(1):8. doi: 10.1016/S8756-3282(02)00918-3 [DOI] [PubMed] [Google Scholar]
- 35.Parhami F, Jackson SM, Tintut Y. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. 1999;14(12):2067. doi: 10.1359/jbmr.1999.14.12.2067 [DOI] [PubMed] [Google Scholar]
- 36.Parhami F, Tintut Y, Beamer WG, Gharavi N, Goodman W, Demer LL. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16:182. doi: 10.1359/jbmr.2001.16.1.182 [DOI] [PubMed] [Google Scholar]
- 37.TYoldemir D, Yavuz G. Association of serum paraoxonase concentration with serum lipid levels and bone mineral density measurements in early postmenopausal women. CLIMACTERIC. 2014;17:1–6. [DOI] [PubMed] [Google Scholar]
- 38.Kha HT, Basseri B, Shouhed D. Oxysterols regulate differentiation of mesenchymal stem cells: pro-bone and anti-fat. J Bone Mineral Res. 2004;19(5):830–840. doi: 10.1359/jbmr.040115 [DOI] [PubMed] [Google Scholar]
- 39.Jeong IK, Cho SW, Kim SW, et al. Lipid profiles and bone mineral density in pre- and postmenopausal women in Korea. Calcif Tissue Int. 2010;87(6):507–512. doi: 10.1007/s00223-010-9427-3 [DOI] [PubMed] [Google Scholar]
- 40.Chen C, Dai J-L. Triglyceride to high-density lipoprotein cholesterol (HDL-C) ratio and arterial stiffness in Japanese population: a secondary analysis based on a cross-sectional study. Lipids Health Dis. 2018;17(1):130. doi: 10.1186/s12944-018-0776-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guisan A, Edwards TC, Hastie T. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Model. 2002;157(2–3):89–100. doi: 10.1016/S0304-3800(02)00204-1 [DOI] [Google Scholar]
- 42.Wood SN, Goude Y, Shaw S. Generalized additive models for large data sets. J Royal Statistical Soc Series C. 2015;64(1):139–155. doi: 10.1111/rssc.12068 [DOI] [Google Scholar]
- 43.Jia Y, Guan L, Wang Y, Liu G, Lei G, Wen L. Combining Population Growth Model and Generalized Additive Model to Determine Optimal Water Level FOR Waterbird Conservation: A Case Study of Siberian Crane (Leucogeranus Leucogeranus) in Lake Poyang, China. River Res Applications. 2016;32(1):100–109. doi: 10.1002/rra.2840 [DOI] [Google Scholar]
- 44.Rudy ACA, Lamoureux SF, Treitz P, van Ewijk KY. Transferability of regional permafrost disturbance susceptibility modelling using generalized linear and generalized additive models. Geomorphology. 2016;264:95–108. doi: 10.1016/j.geomorph.2016.04.011 [DOI] [Google Scholar]