Abstract
Left renal vein (LRV) transposition is often the preferred treatment for Nutcracker syndrome (NCS). However, pain returns in some patients despite undergoing surgery. One solution to this problem is renal autotransplant. Here we report our initial results of renal autotransplant in patients with persistent flank pain despite a previous LRV transposition. We used the UW-LPHS (Loin Pain Hematuria Syndrome)-test as a diagnostic maneuver to determine who may benefit from renal autotransplant which this procedure subsequently resulted in complete pain resolution in all three patients. All patients underwent successful renal autotransplant and remain pain free. These cases support the UW-LPHS-test as a diagnostic maneuver to determine which patients may benefit from renal autotransplant.
Keywords: autotransplant, renal vein transposition, nutcracker syndrome, loin pain hematuria syndrome
INTRODUCTION
Loin pain hematuria syndrome (LPHS) is a rare clinical entity with a low estimated prevalence of 0.012%.1 Patients typically present with severe loin pain that radiates to the abdomen, medial thigh, or groin.2–4 This pain is often debilitating to the point that patients require narcotics for pain control. Patients may also experience hematuria. Type 1 LPHS results from pathology such as kidney stones or Nutcracker syndrome (NCS) whereas Type 2 LPHS describes a syndrome in which there is no identifiable cause. It is our estimation from our patient population that the cause of LPHS in 40% of patients may be NCS.
Nutcracker syndrome (NCS) results from left renal vein (LRV) compression as it passes between the aorta and superior mesenteric artery (SMA).5,6 LRV compression can result in venous hypertension and the development of varices in the renal pelvis, which subsequently can manifest itself as hematuria, orthostatic proteinuria, and flank pain.7,8 Other symptoms include left-sided varicocele in males, pelvic congestion syndrome in females, and chronic fatigue.9 Duplex scanning, computed tomography scan and magnetic resonance imaging are modalities that can be used to diagnosis NCS. Imaging will reveal LRV narrowing at the aortomesenteric portion, angle between the SMA and aorta less than 41° (normal SMA/aorta angle is 90°), and collateral veins around the renal hilum.8,10
These patients often experience debilitating pain and require operative intervention. Open and endovascular surgical options are utilized including LRV transposition, SMA transposition, renal autotransplant, transluminal balloon angioplasty and stenting, nephropexy, and gonadal-caval bypass.11–15 The most frequently used and effective option for NCS is LRV transposition in which the left renal vein is divided at the level of the inferior vena cava (IVC) and re-anastomosed to the IVC at a level distal to the SMA.8,16,17 Numerous case series have reported long-term outcomes of LRV transposition to be good or excellent in as many as 80% of patients.9,18 However, some patients have persistent flank pain despite a patent vein. Here we present three cases in which renal autotransplant resulted in pain resolution following LRV transposition. All patients agreed to publication of case details.
At our institution, the UW-LPHS test has been developed to determine which patients with LPHS may benefit from renal autotransplant. The UW-LPHS test is often performed under deep sedation or general endotracheal anesthesia due to the hypersensitivity of most patients. Cystoscopy is performed and the bladder is carefully evaluated to rule out any other abnormalities. The ureteral orifice of the painful side is cannulated with a balloon-tipped catheter and a retrograde ureterogram is performed using contrast to assess anatomy. The catheter is then advanced to the kidney and bupivacaine is instilled with the balloon inflated to prevent efflux. Bupivacaine is left to dwell for 5 minutes after which the balloon is deflated and catheter and cystoscope are removed. The patients are not given any additional analgesics. Pain is assessed prior to discharge following the procedure, later in the evening by telephone, and in clinic the following day. Patients who have pain relief for at least 12 hours following the test and have no other anatomical abnormalities are considered to have a positive test and would likely benefit from renal autotransplant.19
CLINICAL CASE
CASE 887
This patient is a 42-year-old female whose loin pain began several years prior to presentation at our institution. She initially was diagnosed with NCS and underwent LRV transposition in 2017. She experienced pain relief for 2 months postoperatively; however, her symptoms returned and progressively worsened. She presented to our institution 4 months after her initial surgery with constant left flank pain, suprapubic pain, and intermittent epigastric pain associated with nausea. Prior to transplant the patient had a widely patent left renal vein. Although previously employed, her severe pain forced her to remain in bed for several days at a time and she ultimately lost her job. She required oxycodone 5 mg every 8 hours as needed and tramadol 50 mg every 4 hours as needed for pain control. The UW-LPHS test in order to determine if LPHS was in fact contributing to her pain.19 Twenty-four hours following the UW-LPHS test, she reported that she was completely pain free. She ultimately underwent left nephrectomy and renal autotransplant. The patient recovered without complication and remains pain free 9 months post-autotransplant.
CASE 672
This patient is a 19-year-old female previously diagnosed with NCS following years of loin pain. Due to her pain severity, she subsequently underwent LRV bypass twice, both of which failed due to venous thrombosis. Following her failed bypasses, she underwent left nephrectomy and remained pain free for approximately 1 year. She presented to our institution due to the return of her symptoms in her right loin. Her past medical history was significant for kidney stones, May-Thurner Syndrome, and Factor V Leiden Deficiency. The patient required a significant amount of narcotics and expressed that she “could not continue to live with this pain”. Upon clinical evaluation, LPHS was found to be a contributing factor to her chronic loin pain. She underwent right renal autotransplant with total ureterectomy. She remains pain free and off narcotics 23 months following her surgery and is employed as an OR technologist.
CASE 866
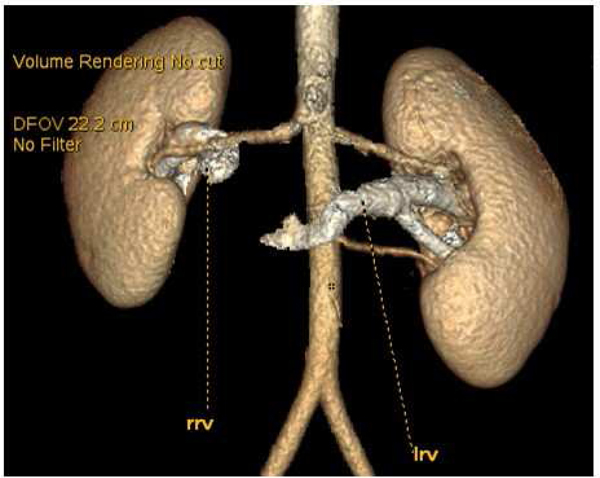
This patient is a 19-year-old female who presented with hematuria, nausea, severe abdominal pain, and weight loss requiring enteral tube feeds, and after extensive work-up was diagnosed with NCS. She underwent renal vein transposition 1 year after her initial development of symptoms. Despite renal vein transposition and dietary modification, her pain continued to worsen. Repeat imaging indicated that her renal vein remained patent (figure 1). Her loin pain left her primarily bedridden despite receiving oxycodone 5 mg every 6 hours for pain control. She underwent the UW-LPHS test and experienced complete resolution of her pain. She subsequently underwent left nephrectomy and renal autotransplant. She is 6 months post-AT and her chronic pain is completely resolved; she has been able to resume a normal life.
Figure 1.
Preoperative imaging demonstrating patent left renal vein (LRV). Three-dimensional reconstruction performed before the patient’s renal autotransplantation shows that the LRV is patent; however, the patient continued to experience severe loin pain. RRV, Right renal vein.
DISCUSSION
Here we present three cases of LPHS in which two of the patients who were diagnosed with NCS underwent successful LRV transposition only to have their pain return soon after operative intervention. These patients were evaluated at our institution and were found to be candidates for renal autotransplant. Selection criteria for patients who are suitable candidates for autotransplant include those with severe pain requiring high doses of analgesics for pain control and those for whom extensive nonsurgical therapies have been unsuccessful.4
There are several critical steps to performing renal autotransplant. Using a midline incision, the major vessels are exposed and the vena cava is carefully dissected so that adequate exposure is completed around the renal vein and contralateral renal vessels. After dissection, the kidney and ureter are removed and cooled with preservation solution. The remaining distal ureter is removed to the bladder. The kidney is then placed in the pelvis with the renal artery anastomosed to the right common iliac artery and the renal vein is anastomosed to the right common iliac vein or distal vena cava. Key to the ability in curing the pain seen in these cases is to re-implant the ureter and remove the distal ureter as was done in all three cases presented here.
Following renal autotransplant, patients have continued to have resolution of their loin pain (mean follow up time 13 months). One explanation for this phenomenon is that in a subset of patients with NCS, LPHS is also caused by ureteral spasm, thus LRV transposition does not fully address the underlying pathophysiology. It is now accepted that NCS causes ureteral spasm, which is categorized as secondary LPHS. The patients presented here likely had secondary (Type I) LPHS as they were all previously diagnosed with NCS at highly experienced centers. The treatment goal for NCS, whether via LRV transposition or other operative techniques, is to reduce LRV hypertension.9 As demonstrated by radiologic methods, the ureter does not have venous outflow. However, the origin of pain in some patients diagnosed with NCS in reality may be the ureter as seen in LPHS, which is diagnosed with the UW-LPHS-test and would not be addressed with LRV transposition. Ureteral spasm seen in LPHS may be due to the continuous passing of stones. The reason renal autotransplant eliminates ureteral spasm is due to total denervation of the kidney and ureter. Additionally, the ureter is re-implanted into the bladder with the distal ureter removed.20
Several published case reports offer support to the hypothesis that ureteral spasm is the origin of pain in LPHS patients. Russell et al. initially presented a case report in which a male patient with typical symptoms of LPHS was trialed on sildenafil (Cialis). This treatment resulted in a pain reduction score by several points.21 Key to this finding is the fact that sildenafil relaxes the smooth muscles of the urogenital system, which subsequently resulted in pain relief in a patient with known LPHS. In our own program, we have developed the UW-LPHS test in order to determine which patients would benefit from renal autotransplant because their pain is due to ureteral spasm. If patients experience pain relief, then this acts as evidence that they would be a good candidate for renal autotransplant. Moving forward, our goal is to utilize the UW-LPHS test to determine if patients have pain secondary to LPHS and to determine if they would benefit from renal autotransplant. Thus, if patients with LPHS caused exclusively by NCS are treated with an intervention that aims to treat LRV hypertension, then their pain should be relieved with LRV transposition. However, a number of patients with NCS who have undergone successful LRV transposition experience pain recurrence suggesting that in addition to renal hypertension, the urogenital system is a contributor to the patient’s pain. It is important to consider renal autotransplant as a reasonable alternative compared to LRV transposition in certain cases. We therefore recommend that every patient undergoing treatment for NCS undergo the UW-LPHS-test in order to determine the most appropriate type of operation.
ACKNOWLEDGEMENTS
The authors would like to thank the UW Transplant Research Training Grant (T32 AI125231) in support of this work.
Footnotes
DISCLOSURES
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Natalie Bath, Department of Surgery, University of Wisconsin School of Medicine and Public Health.
Talal Al-Qaoud, Division of Transplant Surgery, University of Wisconsin School of Medicine and Public Health.
Daniel H. Williams, Department of Urology, University of Wisconsin School of Medicine and Public Health.
Hans W. Sollinger, Division of Transplant Surgery, University of Wisconsin School of Medicine and Public Health.
Robert R. Redfield, III, Division of Transplant Surgery, University of Wisconsin School of Medicine and Public Health.
REFERENCES
- 1.Eisenberg ML, Lee KL, Zumrutbas AE, Meng MV, Freise CE, Stoller ML. Long-term outcomes and late complications of laparoscopic nephrectomy with renal autotransplantation. The Journal of urology. 2008;179(1):240–243. [DOI] [PubMed] [Google Scholar]
- 2.Dube GK, Hamilton SE, Ratner LE, Nasr SH, Radhakrishnan J. Loin pain hematuria syndrome. Kidney international. 2006;70(12):2152–2155. [DOI] [PubMed] [Google Scholar]
- 3.Leaker BR, Gordge MP, Patel A, Neild GH. Haemostatic changes in the loin pain and haematuria syndrome: secondary to renal vasospasm? The Quarterly journal of medicine. 1990;76(281):969–979. [PubMed] [Google Scholar]
- 4.Taba Taba Vakili S, Alam T, Sollinger H. Loin pain hematuria syndrome. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64(3):460–472. [DOI] [PubMed] [Google Scholar]
- 5.Neste MG, Narasimham DL, Belcher KK. Endovascular stent placement as a treatment for renal venous hypertension. Journal of vascular and interventional radiology : JVIR. 1996;7(6):859–861. [DOI] [PubMed] [Google Scholar]
- 6.Grant JCB. A method of anatomy, descriptive and deductive. Baltimore: Williams; 1944. [Google Scholar]
- 7.Zhang H, Li M, Jin W, San P, Xu P, Pan S. The left renal entrapment syndrome: diagnosis and treatment. Annals of vascular surgery. 2007;21(2):198–203. [DOI] [PubMed] [Google Scholar]
- 8.Said SM, Gloviczki P, Kalra M, Oderich GS, Duncan AA, M DF, et al. Renal nutcracker syndrome: surgical options. Seminars in vascular surgery. 2013;26(1):35–42. [DOI] [PubMed] [Google Scholar]
- 9.Reed NR, Kalra M, Bower TC, Vrtiska TJ, Ricotta JJ 2nd, Gloviczki P. Left renal vein transposition for nutcracker syndrome. Journal of vascular surgery. 2009;49(2):386–393; discussion 393–384. [DOI] [PubMed] [Google Scholar]
- 10.Kim KW, Cho JY, Kim SH, Yoon JH, Kim DS, Chung JW, et al. Diagnostic value of computed tomographic findings of nutcracker syndrome: correlation with renal venography and renocaval pressure gradients. European journal of radiology. 2011;80(3):648–654. [DOI] [PubMed] [Google Scholar]
- 11.Barnes RW, Fleisher HL 3rd, Redman JF, Smith JW, Harshfield DL, Ferris EJ. Mesoaortic compression of the left renal vein (the so-called nutcracker syndrome): repair by a new stenting procedure. Journal of vascular surgery. 1988;8(4):415–421. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed K, Sampath R, Khan MS. Current trends in the diagnosis and management of renal nutcracker syndrome: a review. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2006;31(4):410–416. [DOI] [PubMed] [Google Scholar]
- 13.Chiesa R, Anzuini A, Marone EM, Briguori C, Moura MR, Melissano G, et al. Endovascular stenting for the nutcracker phenomenon. Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists. 2001;8(6):652–655. [DOI] [PubMed] [Google Scholar]
- 14.Segawa N, Azuma H, Iwamoto Y, Sakamoto T, Suzuki T, Ueda H, et al. Expandable metallic stent placement for nutcracker phenomenon. Urology. 1999;53(3):631–633. [DOI] [PubMed] [Google Scholar]
- 15.Coolsaet BL. Re: “nutcracker” phenomenon: an unusual cause for renal varicosities with hematuria. The Journal of urology. 1981;125(1):134. [DOI] [PubMed] [Google Scholar]
- 16.Hohenfellner M, Steinbach F, Schultz-Lampel D, Schantzen W, Walter K, Cramer BM, et al. The nutcracker syndrome: new aspects of pathophysiology, diagnosis and treatment. The Journal of urology. 1991;146(3):685–688. [DOI] [PubMed] [Google Scholar]
- 17.Pastershank SP. Left renal vein obstruction by a superior mesenteric artery. Journal of the Canadian Association of Radiologists. 1974;25(1):52–54. [PubMed] [Google Scholar]
- 18.Hartung O, Grisoli D, Boufi M, Marani I, Hakam Z, Barthelemy P, et al. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. Journal of vascular surgery. 2005;42(2):275–280. [DOI] [PubMed] [Google Scholar]
- 19.Sollinger HW, Al-Qaoud T, Bath N, Redfield RR. The “UW-LPHS Test”: A New Test to Predict the Outcome of Renal Autotransplant for Loin Pain Hematuria Syndrome. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zubair AS, Salameh H, Erickson SB, Prieto M. Loin pain hematuria syndrome. Clinical kidney journal. 2016;9(1):128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell A, Chatterjee S, Seed M. Does this case hold the answer to one of the worse types of pain in medicine--that of loin pain haematuria syndrome (LPHS). BMJ case reports. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]