Abstract
Acute kidney injury (AKI) is an abrupt and usually reversible decline in the glomerular filtration rate (GFR). Patients with AKI must be evaluated promptly to determine cause. Different disorders can BE associated with AKI, and biopsy is the most accurate instrument for diagnosis of different types of diseases. We report a case of 69-year-old woman. In history, type II diabetes mellitus and arterial hypertension admitted to our hospital for the evaluation of leg pain, asthenia, diarrhea, and malaise. She was in the treatment with metformin and ARB. Laboratory data revealed renal failure: serum creatinine (Scr 16.5 mg/dl, BUN 280 mg/dl) hyperkalemia and severe anemia (Hb 7.8 g/dl). Renal ultrasound displayed preserved kidneys size. An X-ray of backbone showed fracture. She underwent hemodialysis in urgency regimen. After some days, urine output began to improve up to 1200 cc/24 h. we find proteinuria in nephrotic range. Renal function remained compromised (sCr 8.5 mg/dl, BUN 150 mg/dl). For the evaluation of renal disease, the patient underwent a kidney biopsy. Histological examination findings showed overlapping changes composed of three concurrent pathologic findings: cast nephropathy, diabetes, and light chain deposition disease. After the renal biopsy, therapy with bortezomib, thalidomide, and steroid were administered. At the same time, plasma exchange was carried out. Clinical response occurred with partial recovery of renal function (Scr 3.5 mg/dl eGFR), and dialysis treatment was stopped.
Keywords: Multiple myeloma, Light chain deposition disease, Plasma exchange, Diabetic nephropathy, Acute renal failure
Introduction
Acute kidney injury (AKI) is an abrupt and usually reversible decline in the glomerular filtration rate (GFR). Multiple etiologies can cause AKI; therefore, it may be incorrect to treat AKI as a single disease. The KDIGO guidelines specified that patients with AKI must be evaluated promptly to determine the cause [1]. To assess and evaluate acute renal failure due to parenchymal damage, it is crucial to exclude pre-renal disease (assess and optimize volume status) and post-renal (exclude obstructions of the urinary tract).
Different parenchymal disorders can be associated with AKI, for example renal vascular disease (vasculitis, malignant hypertension or microangiopathy associated with hemolytic anemia), glomerular disease (primary or secondary glomerulonephritis (due to systemic disease), and tubular and interstitial disease (drug toxicity, cast nephropathy in multiple myeloma).
A kidney biopsy is the most accurate instrument for the diagnosis of different types of renal diseases during AKI. Moreover, different types of diseases can be contemporaneously present in patients with an acute injury. Correct diagnosis is crucial for appropriate treatment in these complex cases. A kidney biopsy may also provide information for management and outcome [2].
We report the case of a woman with multiple myeloma and renal failure who had three concurrent pathological findings on the renal biopsy: cast nephropathy, light chain deposition disease, and diabetic nephropathy.
Clinical case
A 69-year-old woman with a history of type II diabetes mellitus in oral antidiabetic therapy (metformin) and arterial hypertension in therapy with angiotensin receptor blocker (ARB) was admitted to our hospital for the evaluation of leg pain, asthenia, diarrhoea, and malaise. She has a history of chronic kidney disease (CKD) G3A1, 3 months before her serum creatinine was 1.3 mg/dl. Vital signs were stable. Physical examination was pertinent for dehydration. The laboratory data were significant for severe normocytic normochromic anemia Hb 5.8 g/dl and for renal failure: serum creatinine (sCr) 16.5 mg/dl, BUN 140 mg/dl, with over imposed hyperkalemia (7.8 mEq/l) and metabolic acidosis (pH 7.22, lactic acid 2.5 mEq/l).
Renal ultrasound examination demonstrated normal morphology of the kidneys and no bilateral parenchymal hyperechogenicity. No calico-pielic dilatations. Column X-ray showed a biconcave lens fracture.
Owing to hyperkalemia and anuria even after saline infusions and diuretic therapy an hemodialysis was started as an urgent regimen. Some hours later, the hemodialysis urine output started to improve without diuretic therapy.
24-h urine collection (2.5 l/24 h) showed proteinuria of 6 g; urinary albumin was 0.9 g strongly suggesting the presence of a paraprotein in the urine [3]. Renal function remained impaired (sCr 8.5 mg/dl, BUN 110 mg/dl). Corrected calcium for albuminemia was normal (9.6 mg/dl). Lactate dehydrogenase (LDH) was normal as well as haptoglobin, so we exclude an hemolytic cause of anemia.
Further investigations revealed an abnormal monoclonal peak in the gamma region on serum protein electrophoresis; M protein on SPEP was 0.1 g/dl. Elevated k FLC 20.18 mg/l, elevated ƛ FLC 9329 mg/l, and an abnormal k/ƛ FLC ratio 0.0021 on immunofixation. Bone marrow examination revealed scattered as well as clusters of plasma cells which accounted for 72% of marrow nucleated cells with lambda light chain restriction.
For the evaluation of renal disease: AKI and new onset of proteinuria in patients with type 2 diabetes mellitus with chronic kidney disease G2A2 historically, a kidney biopsy was performed.
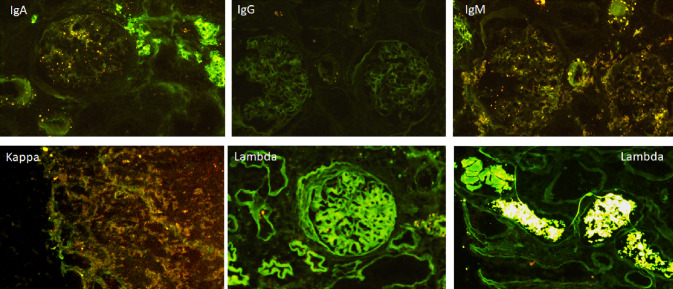
Pathological findings showed three distinct features: (1) numerous periodic acid-Schiff-negative fractured casts surrounded by macrophages in the distal tubular lumen (2), diffuse interstitial fibrosis and acute tubulopathy, light chain related, with fragmentation, and desquamation of tubular cells and loss of tubular integrity resulting from cell injury, and (3) mesangial matrix expansion and mesangial cell proliferation with early nodular lesions (Fig. 1). The tubular interstitial lesions were consistent with myeloma cast nephropathy; while the nodular glomerular lesions had several differential diagnoses: light-chain amyloidosis, membranoproliferative glomerulonephritis, monoclonal immunoglobulin deposition disease (MIDD), and diabetic nephropathy. The immunofluorescence staining showed lambda light chain deposition inside glomeruli and linear staining along TBM (Fig. 2) Congo red staining was negative. Electron microscopy showed dense deposition in subendothelial spaces, and a diffuse glomerular capillary basement membrane thickness of 619.48 nm ± 108.88 nm (Fig. 1) in areas without deposits, a hallmark of diabetic nephropathy. The arteries displayed media and intima fibrosis, while arterioles showed severe diffuse hyalinosis. Taken together, these findings led to the diagnosis of the patient with myeloma cast nephropathy (Durie–Salmon Stage IIIB—International staging system Stage III) [4], light chain deposition disease (LCDD) and diabetic nephropathy with early nodular lesion (stage III sec Cohen Tervaert) [5].
Fig. 1.
a PAS 10× glomerulus with mesangial expansion; it also evident tubular atrophy and arteriolar hyalinosis. b PAS 20×: nodular glomerulopathy. c Electron microscopy: thickened GBM without any evidence of deposits (619.48 nm ± 108.88 nm). d In other area of the same glomerulus GBM showed dark electron-dense deposits
Fig. 2.
IFD: light chain deposition disease. Linear staining along glomerular basement membrane (GBM) tubular, basement membrane (TBM) and even mesangial staining of lambda light chain. Casts of ƛ light chain inside tubules. IgA, IgG, IgM, and kappa light chain appear negative
After renal biopsy, we performed a combination therapy using steroids, bortezomib, thalidomide, and we additionally performed plasma exchange (6 therapeutic exchanges) with exchange of 1.5 plasma volumes using albumin replacement solution, with reduction in FLC up to 776 mg/dl (> 90% to the basal value). We observed a good clinical response; partial recovery renal function and dialysis treatment was stopped (Fig. 3).
Fig. 3.

Serum-free lambda light chain concentrations. Changes in concentration with PEX and chemotherapy during the follow-up with concomitant creatinine reduction. The concentration at the start of the first pulse of dexamethasone is also shown
At her most recent follow-up, approximately 1 year after treatment, her Scr is stable at 4.5 mg/dL, and her FLC levels are in the normal range.
Discussion
Multiple myeloma (MM) is a hematologic cancer characterized by an abundant monoclonal clone of plasma cells in the bone marrow [6]. The most common cause of acute kidney injury (AKI) in patients with MM is cast nephropathy, especially in patients requiring renal replacement therapy (RRT). Tubular deposition of casts made of Tamm–Horsfall protein and free light chains (FLCs) are the cause of renal damage [7]. Kidney involvement is a common sequela of MM and, besides the cast nephropathy, in patients with a long history of myeloma, other histological changes may co-exist in the same patient such as cast nephropathy (CN), amyloidosis, and MIDD [8]. MIDD are a group of diseases caused by light or heavy chain deposition or both of them in basement membranes [9].
We report an unusual case of renal failure caused by coexistent histological changes: cast nephropathy (CN), LCDD over imposed to diabetic nephropathy.
Nodular lesions impose a complex different diagnosis for pathologists and only with results of light microscopy taken together with IFD and TEM it is possible to make the correct definition of diagnosis. Although light microscopy could not distinguish between the diabetic nodular lesion and light chain nephropathy (LCDD), the result of lambda in IF staining, as well as dark electron-dense deposits showed in some areas of the glomerular basement membrane, definitely contributed to the final diagnosis as LCDD.
Light chains have different local effects within the kidney. Monoclonal light chains form casts in distal tubules and collecting ducts to block the urine flux, and do not affect glomeruli such as light chains involved in CN (both kappa and lambda chains) and they have been referred to as ‘tubulopathic’ light chains. In contrast, light chains in LCDD (mostly kappa chains) have been termed ‘glomerulopathic’ light chains because they interact with mesangial cells within glomeruli and cause mesangial expansion and the mesangial matrix can take on a nodular aspect [9]. MIDD are a group of diseases caused by light or heavy chain deposition or both of them in basement membranes.
The diabetic glomerulopathy is typically characterized by thickening of the glomerular basement membrane. Other significant diabetic glomerular changes include mesangial expansion, which can be diffuse or nodular (often termed “Kimmelstiel–Wilson nodules”), podocyte injury, and glomerular sclerosis. Arteriolar hyalinosis and arteriosclerosis of larger vessels are common, likely representing the combined effect of hyperglycemia and hypertension. Tubulointerstitial fibrosis usually occurs after the initial glomerular lesions. Tervaert reported morphological classification of the renal changes in diabetic nephropathy, and it is widely shared [5].
In our case, kidney biopsy confirmed its crucial role in a correct and detailed diagnosis and choice of therapy. As a matter of fact, it revealed different lesions of kidney involvement of myeloma (cast nephropathy and LCDD); different patterns of disease can co-exist in long-lasting monoclonal dysproteinemia [9]. Noticeably, the renal biopsy allowed us to made the correct diagnosis of acute renal injury in a myeloma patient with associated a diabetic nephropathy.
In conclusion, the acute renal failure was due to cast nephropathy over-imposed to other histological lesions: LCDD and diabetic nephropathy. Only with the renal biopsy, it was possible to detect and specify this intricate clinical pattern and an appropriate successful therapy was started with partial recovery of renal function and discontinuation of dialysis treatment.
Conclusion
Kidney biopsy is the most accurate instrument to make a diagnosis of parenchymal disease-causing AKI. In this clinical case, it was crucial for diagnosis and treatment of systemic disease with elevated one-year mortality like MM. Instead, our patient’s case illustrates the complexity of light chain pathophysiology and emphasizes that further studies examining the molecular characteristics and pathologic effects of light chains are needed.
Author contributions
DM and SF wrote the paper, treated and followed the patient and revisited the case. EC reviewed samples and reported pathology results. ART was the electron microscopist who read TEM. FRDR e CM were the physicians of the patient and completed data and helped in writing the draft. All authors read and signed the final paper.
Compliance with ethical standards
Conflict of interest
The authors have no disclosures to declare.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
David Micarelli, Email: davidmicarelli@gmail.com.
Emanuela Cristi, Email: emanuela.cristi@gmail.com.
Anna Rita Taddei, Email: artaddei@unitus.it.
Francesca Romana Della Rovere, Email: F.rdr72@gmail.com.
Caterina Mercanti, Email: mercanticaterina@libero.it.
Sandro Feriozzi, Email: sandro.feriozzi@gmail.com.
References
- 1.KDIGO AKI definition. Kidney Int Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luciano RL, Moeckel GW. Update on the native kidney biopsy: core curriculum 2019. Am J Kidney Dis. 2019;73(3):404–415. doi: 10.1053/j.ajkd.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Leung N, Gertz M, Kyle RA, et al. Urinary albumin excretion patterns of patients with cast nephropathy and other monoclonal gammopathy-related kidney diseases. Clin J Am Soc Nephrol. 2012;7:1964–1968. doi: 10.2215/CJN.11161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 5.Cohen Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 6.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 7.Clyne DH, Pesce AJ, Thompson RE. Nephrotoxicity of Bence Jones proteins in the rat: importance of protein isoelectric point. Kidney Int. 1979;16:345–352. doi: 10.1038/ki.1979.137. [DOI] [PubMed] [Google Scholar]
- 8.Leung N, Bridoux F, Batuman V, et al. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 2019;15(1):45–59. doi: 10.1038/s41581-018-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seth S, Rajkumar SV, D’Agati V, et al. Heterogeneity of monoclonal immunoglobulin associated renal diseases. J Am Soc Nephrol. 2018;29:1810–1823. doi: 10.1681/ASN.2017121319. [DOI] [PMC free article] [PubMed] [Google Scholar]