Abstract
We report here two cases of membranoproliferative glomerulonephritis that developed during treatment of rheumatoid arthritis with tocilizumab. In both cases, the initial findings were proteinuria and haematuria, followed by development of bilateral lower leg oedema. One of the patients was weakly positive for anti-nuclear antibody; both had hypocomplementaemia. The patients’ renal impairment gradually resolved with discontinuation of tocilizumab followed by treatment with moderate doses of oral prednisolone. Pathological examination of renal biopsies resulted in diagnoses of immunocomplex glomerulonephritis and immunofluorescence staining revealed depositions of IgG, IgA, and IgM, accompanied by C3. Tocilizumab rarely induces autoimmune disorders; therefore, the underlying mechanism is unknown. One patient with immunocomplex glomerulonephritis that may have been associated with tocilizumab therapy for rheumatoid arthritis has been reported previously; that patient and our two are similar in their clinical courses and pathological findings. We conclude that such glomerulonephritis can occur during tocilizumab treatment, but this is rare. Clinicians should be aware of the possibility of paradoxical development of autoimmune diseases during tocilizumab therapy.
Keywords: Tocilizumab, Glomerulonephritis, Rheumatoid arthritis
Introduction
Tocilizumab is a humanized monoclonal anti-interleukin (IL)-6 receptor antibody that acts as an IL-6 antagonist. It is used worldwide to treat adults with moderate-to-severe active rheumatoid arthritis (RA) [1]. Because IL-6 is a pleiotropic pro-inflammatory cytokine that is involved in diverse physiological processes, tocilizumab is also used to treat Castleman disease [2, 3], other cytokine release syndromes, for example TAFRO (thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly) syndrome [4], and the glomerulonephritis that can accompany these diseases [5–9]. Although tocilizumab is generally well tolerated, adverse reactions, including upper respiratory tract infection and hypercholesterolaemia, have been reported [1]. The rate of development of anti-drug antibodies is low [10]; this phenomenon may reflect the effects of tocilizumab-mediated IL-6 blockade of B-cell responses and the function of follicular helper CD4 T cells [11]. Very recently, new-onset cutaneous sarcoidosis during tocilizumab treatment for giant cell arteritis was reported with three other cases that had been described previously [12]; this was considered to be a quasi-paradoxical adverse drug reaction.
In this case report, we describe two similar patients with concomitant immune-complex glomerulonephritis that developed during tocilizumab therapy for RA; both patients were managed in our hospital. The histological findings and clinical courses of our patients and another previously reported patient [13] are similar, indicating that tocilizumab may induce paradoxical adverse drug reactions, including the development of immunocomplex glomerulonephritis.
Case report
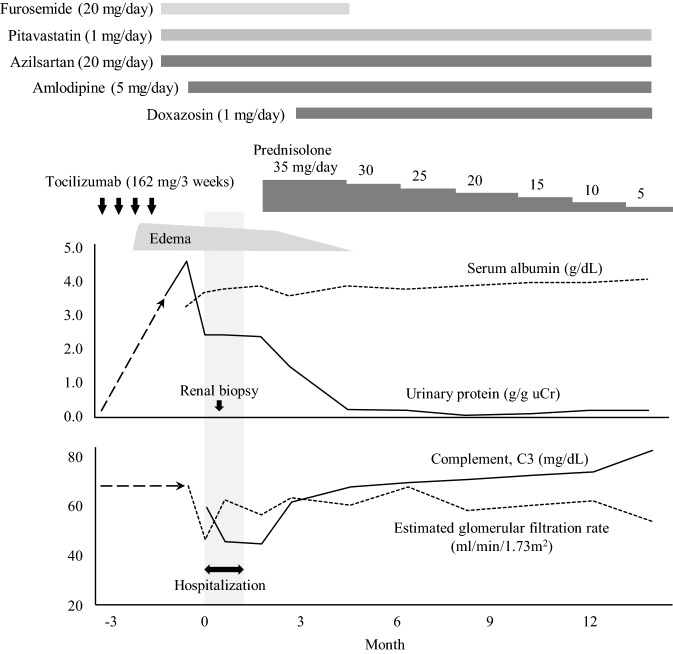
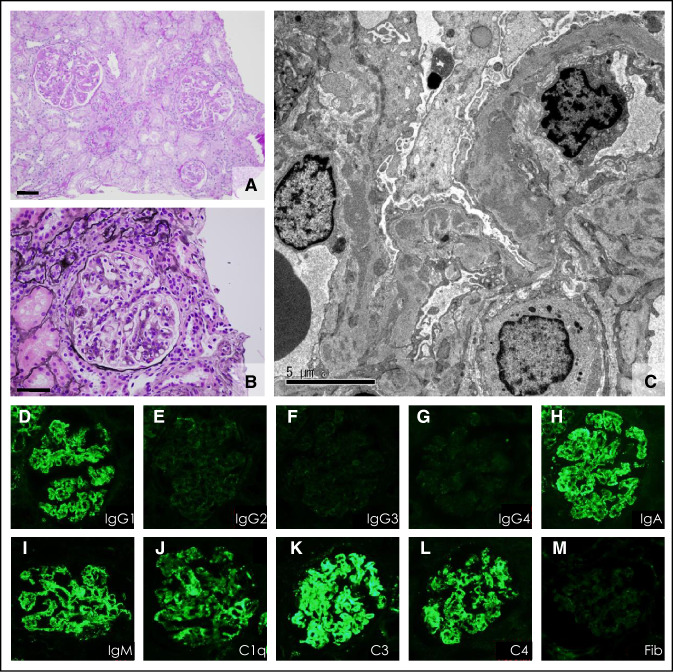
Case 1: A 48-year-old Japanese woman with a 13-year history of RA was admitted to our hospital because of oedema and proteinuria. Tocilizumab treatment (162 mg every 2–3 weeks) had been started 3 years before admission, because etanercept had been ineffective in controlling her arthritis. Previous investigations had revealed no proteinuria or haematuria and her serum creatinine concentration had always been within normal limits. Three months prior to admission, she developed bilateral leg oedema (Fig. 1). On physical examination on admission, she exhibited no evidence of synovitis, the only clinical abnormality being pitting oedema of both legs. Her height was 154.6 cm, weight 66.1 kg, and blood pressure 126/81 mmHg. Urinalysis revealed proteinuria of 2.38 g/gCr and minor haematuria, and the 24-h urinary protein was 3508 mg (Table 1). Her complete blood count and serum creatinine were within the normal range, as shown in Table 1; circulating cryoglobulins, anti-nuclear antibody (ANA; 1:40, homogenous), and anti-Sm antibodies were all weakly detectable. Serum C3 and C4 concentrations were low (Table 1), whereas anti-ds-DNA antibodies, hepatitis B surface antigen, hepatitis C antibodies, and serum/urinary paraprotein were all negative. A renal biopsy was performed to determine the cause of this patient’s nephrotic range proteinuria. Pathological examination of the biopsy revealed that all glomeruli showed mesangial proliferation and capillary wall thickening and that some glomeruli contained small fibrocellular crescents (Fig. 2a, b). Tubular atrophy surrounded by interstitial inflammation and fibrosis (< 25% in area) were also observed. The arteries were slightly sclerotic, but free from vasculitis. Direct fast scarlet staining was negative for amyloid deposition. Direct immunofluorescent staining revealed deposits of IgG (IgG1 dominant; Fig. 2d–g), IgA, and IgM, together with complement along the capillary walls and in the mesangial area (Fig. 2h–m). Electron microscopy also revealed mesangial, subendothelial, and subepithelial electron-dense deposits (Fig. 2c). A diagnosis of membranoproliferative glomerulonephritis, type III, caused by immunocomplex deposition, was made. Tocilizumab therapy was discontinued and prednisolone 35 mg/day started (Fig. 1), after which her anti-Sm antibodies became undetectable, and C3 and C4 concentrations increased. Her proteinuria and haematuria both resolved and her disease did not relapse despite tapering her prednisolone dose to 5 mg/day (Fig. 1).
Fig. 1.
Clinical course of Case 1. Gray blocked area indicates hospital stay
Table 1.
Laboratory findings on admission in Case 1, a 48-year-old woman
| Urine tests | |||
| pH | 5.5 | Albumin (g/dL) | 3.2 |
| Specific gravity | 1.015 | Aspartate aminotransferase (IU/L) | 41 |
| Protein (g/gCr) | 2.38 | Alanine aminotransferase (IU/L) | 30 |
| Glucose | ( −) | Lactate dehydrogenase (IU/L) | 207 |
| Red blood cell (/HPF) | 5–9 | Alkaline phosphatase (IU/L) | 164 |
| White blood cell (/HPF) | 5–9 | Blood urea nitrogen (mg/dL) | 18.9 |
| N-Acetyl-β-d-glucosaminidase (IU/L) | 18.6 | Creatinine (mg/dL) | 0.72 |
| β2-Microglobulin (mg/L) | 625 | eGFR (ml/min/1.73m2) | 67.6 |
| 24-h urine collection test | Uric acid (mg/dL) | 6.6 | |
| Protein (mg/day) | 3508 | Sodium (mEq/L) | 140 |
| Potassium (mEq/L) | 4.2 | ||
| Blood tests | Chloride (mEq/L) | 105 | |
| White blood cells (/µL) | 4050 | Calcium (mg/dL) | 8.9 |
| Neutrophils (%) | 64.2 | Phosphate (mg/dL) | 3.4 |
| Lymphocytes (%) | 26.2 | HbA1c (%) | 5.2 |
| Monocytes (%) | 6.9 | Triglyceride (mg/dL) | 135 |
| Eosinophils (%) | 2.2 | Total cholesterol (mg/dL) | 201 |
| Basophils (%) | 0.5 | High-density lipoprotein cholesterol (mg/dL) | 56 |
| Red blood cells (× 104/µL) | 350 | Low-density lipoprotein cholesterol (mg/dL) | 118 |
| Hemoglobin (g/dL) | 11.3 | C-reactive protein (mg/dL) | 0.84 |
| Hematocrit (%) | 32.7 | Complement C3 (mg/dL) | 60.0 |
| Platelets (× 104/µL) | 21.6 | Complement C4 (mg/dL) | 11.0 |
| Thyroid stimulating hormone (µIU/mL) | 3.49 | ||
| Free triiodothyronine (pg/mL) | 2.42 | ||
| Free thyroxine (ng/dL) | 0.99 | ||
Fig. 2.
Renal biopsy findings in Case 1, a 48-year-old, woman (a–m). a, b Light microscope images with periodic acid–Schiff and periodic acid-methenamine-silver staining, respectively, showing mesangial proliferation, thickening of the capillary walls, and small crescent formation. c Electron micrograph showing obvious electron-dense deposits in the glomerular basement membrane. d–m Direct immunofluorescent antibody staining demonstrated marked immunocomplex depositions in the glomerulus. d–m IgG (IgG1–IgG4), IgA, IgM, C1q, C3, C4, and Fib (fibrinogen): labels indicate antigens. Bar scale = 50 µm (a and b), 5 µm (c)
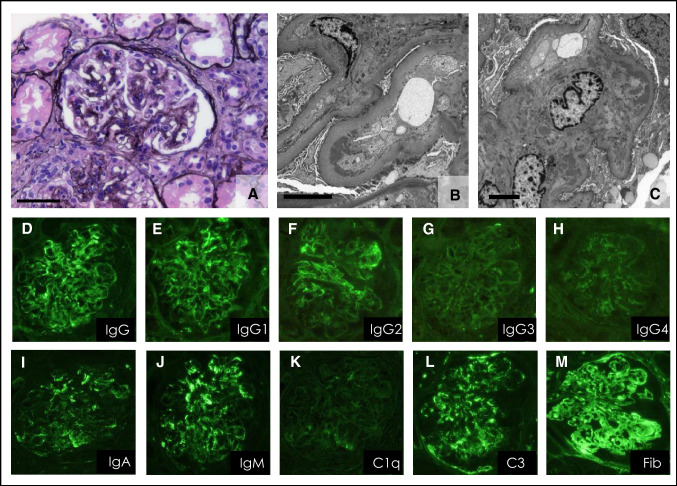
Case 2: A 74-year-old Japanese man who had been diagnosed with RA 25 years previously was admitted to our hospital because of leg oedema, proteinuria, and renal insufficiency. He had originally been taking low-dose prednisolone (5–10 mg/day) and etanercept. Two years prior to admission, tocilizumab (560 mg, every 4 weeks) had been substituted for etanercept because of uncontrolled arthritis. At that time, he had no proteinuria or haematuria, his serum creatinine concentration was 0.86 mg/dL, and serum complement concentrations were also within the normal range (C3 98.6 mg/dL; C4 9.8 mg/dL). Five months prior to admission, he had developed bilateral leg oedema, persistent proteinuria (1 + to 2 + on dipstick; 3.35 g/gCr), and haematuria (10–19 red blood cells/high power field), all of which worsened over time without the development of any other symptoms besides leg oedema. On admission, urinalysis revealed proteinuria of 2.67 g/gCr and moderate haematuria of 20–29 red blood cells per high power field (Table 2). A complete blood count revealed the following: red blood cells, 351 × 104/L; hemoglobin, 11.8 g/dL; and haematocrit, 35.4%. The serum creatinine and albumin concentrations were 1.55 mg/dL and 3.2 mg/dL, respectively. His serum C3 (71.8 mg/dL) and C4 (9.6 mg/dL) concentrations were low (Table 2). Trace amounts of circulating cryoglobulins were found. ANA, anti-ds-DNA antibodies; anti-Sm antibodies, hepatitis B surface antigen, hepatitis C antibodies, and serum/urinary paraprotein were all negative. Renal biopsy revealed that almost all glomeruli displayed mesangial proliferation accompanied by segmental capillary thickening and fibrocellular crescents (Fig. 3a). Immunostaining revealed deposits of immunoglobulins and C3 along capillary walls and in the mesangial area (Fig. 3d–m). Electron microscopy also revealed mesangial and subendothelial electron-dense deposits (Fig. 3b, 3c). These findings led to a diagnosis of membranoproliferative glomerulonephritis type I caused by immune-complex deposition. Tocilizumab was discontinued and prednisolone 30 mg/day was started. Unfortunately, we were unable to follow the patient up, because he died shortly thereafter from severe infection.
Table 2.
Laboratory findings on admission in Case 2, a 74-year-old man
| Urine tests | |||
| pH | 5.5 | Albumin (g/dL) | 3.2 |
| Specific gravity | 1.009 | Aspartate aminotransferase (IU/L) | 25 |
| Protein (g/gCr) | 2.67 | Alanine aminotransferase (IU/L) | 26 |
| Glucose | ( −) | Lactate dehydrogenase (IU/L) | 185 |
| Red blood cell (/HPF) | 20–29 | Blood urea nitrogen (mg/dL) | 34.0 |
| White blood cell (/HPF) | 1–4 | Creatinine (mg/dL) | 1.55 |
| N-Acetyl-β-d-glucosaminidase (IU/L) | 8.2 | eGFR (ml/min/1.73m2) | 34.9 |
| β2-Microglobulin (mg/L) | 55 | Uric acid (mg/dL) | 9.7 |
| Sodium (mEq/L) | 142 | ||
| Blood tests | Potassium (mEq/L) | 39 | |
| White blood cells (/µL) | 8900 | Chloride (mEq/L) | 103 |
| Neutrophils (%) | 87.0 | Calcium (mg/dL) | 8.7 |
| Lymphocytes (%) | 10.0 | Phosphate (mg/dL) | 2.8 |
| Monocytes (%) | 2.0 | HbA1c (%) | 5.4 |
| Eosinophils (%) | < 1.0 | Triglyceride (mg/dL) | 93 |
| Basophils (%) | 1.0 | Total cholesterol (mg/dL) | 212 |
| Red blood cells (× 104/µL) | 351 | Low-density lipoprotein cholesterol (mg/dL) | 102 |
| Hemoglobin (g/dL) | 11.8 | C-reactive protein (mg/dL) | < 0.1 |
| Hematocrit (%) | 35.4 | Complement C3 (mg/dL) | 71.8 |
| Platelets (× 104/µL) | 18.4 | Complement C4 (mg/dL) | 9.6 |
Fig. 3.
Renal biopsy findings in Case 2, a 74-year-old man (a–m). a Light microscopic images with periodic acid-methenamine-silver staining. b, c Electron micrograph showing electron-dense deposits in the subendothelial space. d–m Direct immunofluorescent staining revealed immunocomplex depositions in the glomeruli. d–m IgG (IgG1 to IgG4), IgA, IgM, C1q, C3, and Fib (fibrinogen): labels indicate antigens. Bar scale = 50 µm (a), 5 µm (b and c)
Discussion
We report here two cases of membranoproliferative glomerulonephritis that developed during tocilizumab therapy for active RA. In Case 1, renal impairment developed with slight but significant aberrations in ANA; additionally, complement concentrations had decreased during tocilizumab treatment in both patients. In Case 1, patient symptoms and laboratory findings resolved after cessation of tocilizumab. Unfortunately, we were unable to follow up Case 2 for a sufficient period, because he died of infection. Because we detected no other evidence suggestive of lupus, it is likely that tocilizumab induced these patients’ immune-complex glomerulonephritis. The pathological findings by both light and electron microscopy and on immunohistochemical staining were similar in our two patients. The first such case was reported in 2013 [13]. It resembled our cases in clinical course and histological findings; however, our Case 1 is the first reported patient in whom subclasses of IgG deposition in the glomeruli were evaluated.
The use of biologic drugs has been linked with the paradoxical development of systemic and organ-specific autoimmune processes, including those that are described as biologics-induced autoimmune renal disorders. The biologic drug most frequently associated with the development of these renal diseases is etanercept (51.7%), followed by adalimumab (31.0%) in 29 cases [14]. Almost half (44.8%) of reported cases showed only renal-associated symptoms, including lower limb oedema (38.5%), haematuria (69.2%), and proteinuria in the nephrotic range (53.8%), without any other organ manifestations such as purpura [14]. Impaired renal function with high serum creatinine concentrations was present in 37.5% of reported cases [14]. As to histopathological findings, lupus nephritis and necrotizing crescentic glomerulonephritis have been the major histological findings in biologics-induced autoimmune renal disorders. These patients have clinical and serological characteristics of drug-induced systemic lupus erythematosus and antineutrophilic cytoplasmic antibody (ANCA)-associated systemic vasculitis with high titres of ANA and ANCA, respectively. However, a few cases of membranous glomerulopathy and mesangial proliferative glomerulonephritis have been reported in patients who manifested only renal disorders; in such cases, auto-antibodies such as ANA and ANCA have usually been negative or only weakly positive [14].
Tumor necrosis factor (TNF) antagonists, another type of biological agent used to treat RA, can induce autoimmune diseases such as vasculitis, lupus-like syndrome, and immune-mediated glomerulonephritis [14, 15]. In contrast, tocilizumab-induced autoimmune disorders have rarely been reported. The first case of tocilizumab-associated immunocomplex renal disease was reported in 2013 [13]; a 61-year-old woman with RA who developed bilateral leg oedema and persistent proteinuria and haematuria 6 months after commencing tocilizumab treatment. ANA (1:40) and anti-ds-DNA antibodies were weakly positive; serum complement concentrations were low, and her serum creatinine had gradually increased. A renal biopsy revealed membranoproliferative glomerulonephritis with cellular crescents accompanied by deposits of IgG, IgA, IgM, C3, C4, and C1q. After withdrawal of tocilizumab followed by administration of oral prednisolone, urinalysis normalized and serum creatinine concentrations decreased to within the normal range within 6 months [13]. The authors postulated that the renal immune deposits consisted of auto-antibodies because of the full-house pattern found on glomerular immunofluorescent staining. In this regard, immune complexes associated with tocilizumab should have shown selective IgG1 deposition, because tocilizumab is a humanized antibody with the IgG1 Fc domain.
Our Case 1 had markedly dominant IgG1 deposition in her glomeruli, suggesting that her condition differed from primary membranoproliferative glomerulonephritis and lupus nephritis, because IgG3 and IgG2, respectively, are known to be dominant in such cases. In our Case 2, IgG1 was slightly more plentiful than IgG2; in addition, as in Case 1, IgG3 and IgG4 were not identified in the glomeruli. Given that our patients’ immunocomplex glomerulonephritis developed during tocilizumab therapy for active RA, it is possible that the immune complexes in these patients contained tocilizumab itself, accompanied by soluble IL-6 receptor. Serum soluble IL-6 receptor concentrations should be increased in such patients, possibly resulting in the formation of circulating immunocomplexes of tocilizumab and soluble IL-6 receptor. These immunocomplexes could be trapped in the glomeruli, after which the deposits would probably be activated via the complement activation pathway. However, it has been reported that tocilizumab decreases complement without immune-complex formation. Tocilizumab is assumed to suppress a cascade of acute inflammatory responses, resulting in decreased complement and other acute-phase proteins [16]. Histological examination has suggested that complement is probably activated locally in the kidney; however, decreases in serum complement are not necessarily associated with nephritis. Another theory relates to the well-known fact that blockade of IL-6 receptor signals, for instance by tocilizumab, can regulate autoimmune diseases including RA and this is accompanied by an increase in regulatory T cells [17, 18]. Alternatively, IL-6 plays a major role in maintaining immature dendritic cells via the same signal pathway through phosphorylation of signal transducer and activator of transcription 3 (STAT3). Indeed, IL-6 knockout mice have increased numbers of mature dendritic cells, indicating that IL-6 blocks maturation of dendritic cells in vivo. Dendritic cell-mediated T-cell activation is also reportedly enhanced in IL-6 knockout mice [19]. Of course, these cascades play important roles, mainly in recognition of foreign antigens and induction of immune responses to them. However, what is important is that the function of IL-6 and IL-6 receptors, or the effects of inhibiting IL-6 receptors, can be complex and multifaceted, as shown by the example of the effects on T cells. Further discussion concerning the details of the mechanism requires accumulation and analysis of more similar cases.
The factors common to these cases include the following: (1) tocilizumab had been administered for several months or longer, after which glomerulonephritis had developed gradually with a significant amount of proteinuria and haematuria; (2) the proteinuria was accompanied by mild hypocomplementaemia and occasionally positive auto-antibodies; (3) renal biopsy revealed membranoproliferative glomerulonephritis-like lesions associated with deposition of immunocomplex, occasionally showing a “full-house” pattern; and (4) the symptoms and laboratory findings gradually improved with moderate prednisolone doses and tocilizumab withdrawal. For all these reasons, we conclude that membranoproliferative glomerulonephritis can occur during tocilizumab treatment, that it is rare, and that the precise mechanism is unknown. Clinicians should be aware of the possible paradoxical development of autoimmune diseases during tocilizumab therapy.
Acknowledgements
We thank Dr Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this case report.
Funding
No specific funding was received from any bodies in the public, commercial, or not-for-profit sectors in relation to the work described in this manuscript.
Compliance with ethical standards
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77(17):1865–1879. doi: 10.1007/s40265-017-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koga T, Sumiyoshi R, Kawakami A, Yoshizaki K. A benefit and the prospects of IL-6 inhibitors in idiopathic multicentric Castleman's disease. Mod Rheumatol. 2019;29(2):302–305. doi: 10.1080/14397595.2018.1532383. [DOI] [PubMed] [Google Scholar]
- 3.Maeshima A, Nakasatomi M, Henmi D, Yamashita S, Kaneko Y, Kuroiwa T, et al. Efficacy of tocilizumab, a humanized neutralizing antibody against interleukin-6 receptor, in progressive renal injury associated with Castleman's disease. CEN Case Rep. 2012;1(1):7–11. doi: 10.1007/s13730-012-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai K, Maeda T, Kuriyama A, Shimada N, Notohara K, Ueda Y. TAFRO syndrome successfully treated with tocilizumab: a case report and systematic review. Mod Rheumatol. 2018;28(3):564–569. doi: 10.3109/14397595.2015.1120389. [DOI] [PubMed] [Google Scholar]
- 5.Ebisawa K, Masamoto Y, Tokushige J, Nishi H, Honda K, Hinata M, et al. Tocilizumab for focal segmental glomerulosclerosis secondary to multicentric Castleman's disease. Ann Hematol. 2019;98(8):1995–1997. doi: 10.1007/s00277-019-03616-y. [DOI] [PubMed] [Google Scholar]
- 6.Iijima T, Suwabe T, Sumida K, Hayami N, Hiramatsu R, Hasegawa E, et al. Tocilizumab improves systemic rheumatoid vasculitis with necrotizing crescentic glomerulonephritis. Mod Rheumatol. 2015;25(1):138–142. doi: 10.3109/14397595.2013.874748. [DOI] [PubMed] [Google Scholar]
- 7.Iwai A, Naniwa T, Tamechika S, Maeda S. Short-term add-on tocilizumab and intravenous cyclophosphamide exhibited a remission-inducing effect in a patient with systemic lupus erythematosus with refractory multiorgan involvements including massive pericarditis and glomerulonephritis. Mod Rheumatol. 2017;27(3):529–532. doi: 10.3109/14397595.2014.990409. [DOI] [PubMed] [Google Scholar]
- 8.Otani N, Morishita Y, Oh I, Saito O, Takemoto F, Muto S, et al. Successful treatment of a mesangial proliferative glomerulonephritis with interstitial nephritis associated with Castleman's disease by an anti-interleukin-6 receptor antibody (tocilizumab) Intern Med. 2012;51(11):1375–1378. doi: 10.2169/internalmedicine.51.6555. [DOI] [PubMed] [Google Scholar]
- 9.Sumida K, Ubara Y, Suwabe T, Hayami N, Hiramatsu R, Hasegawa E, et al. Complete remission of myeloperoxidase-anti-neutrophil cytoplasmic antibody-associated crescentic glomerulonephritis complicated with rheumatoid arthritis using a humanized anti-interleukin 6 receptor antibody. Rheumatology (Oxford) 2011;50(10):1928–1930. doi: 10.1093/rheumatology/ker222. [DOI] [PubMed] [Google Scholar]
- 10.Burmester GR, Choy E, Kivitz A, Ogata A, Bao M, Nomura A, et al. Low immunogenicity of tocilizumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76(6):1078–1085. doi: 10.1136/annrheumdis-2016-210297. [DOI] [PubMed] [Google Scholar]
- 11.Sigaux J, Hamze M, Daien C, Morel J, Krzysiek R, Pallardy M, et al. Immunogenicity of tocilizumab in patients with rheumatoid arthritis. Joint Bone Spine. 2017;84(1):39–45. doi: 10.1016/j.jbspin.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Del Giorno R, Iodice A, Mangas C, Gabutti L. New-onset cutaneous sarcoidosis under tocilizumab treatment for giant cell arteritis: a quasi-paradoxical adverse drug reaction. Case report and literature review. Ther Adv Musculoskelet Dis. 2019;11:1759720X19841796. doi: 10.1177/1759720X19841796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo Y, Mizoguchi F, Kohsaka H, Ito E, Eishi Y, Miyasaka N. Tocilizumab-induced immune complex glomerulonephritis in a patient with rheumatoid arthritis. Rheumatology (Oxford) 2013;52(7):1341–1343. doi: 10.1093/rheumatology/kes403. [DOI] [PubMed] [Google Scholar]
- 14.Piga M, Chessa E, Ibba V, Mura V, Floris A, Cauli A, et al. Biologics-induced autoimmune renal disorders in chronic inflammatory rheumatic diseases: systematic literature review and analysis of a monocentric cohort. Autoimmun Rev. 2014;13(8):873–879. doi: 10.1016/j.autrev.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-Casals M, Brito-Zeron P, Munoz S, Soria N, Galiana D, Bertolaccini L, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007;86(4):242–251. doi: 10.1097/MD.0b013e3181441a68. [DOI] [PubMed] [Google Scholar]
- 16.Romano C, Del Mastro A, Sellitto A, Solaro E, Esposito S, Cuomo G. Tocilizumab reduces complement C3 and C4 serum levels in rheumatoid arthritis patients. Clin Rheumatol. 2018;37(6):1695–1700. doi: 10.1007/s10067-018-3992-7. [DOI] [PubMed] [Google Scholar]
- 17.Khoury T, Molho-Pessach V, Ramot Y, Ayman AR, Elpeleg O, Berkman N, et al. Tocilizumab promotes regulatory T-cell alleviation in STAT3 gain-of-function—associated multi-organ autoimmune syndrome. Clin Ther. 2017;39(2):444–449. doi: 10.1016/j.clinthera.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi J, Hashizume M, Kaneko Y, Yoshimoto K, Nishina N, Takeuchi T. Peripheral blood CD4(+)CD25(+)CD127(low) regulatory T cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: increase in regulatory T cells correlates with clinical response. Arthritis Res Ther. 2015;17:10. doi: 10.1186/s13075-015-0526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173(6):3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]