Abstract
A 52-year-old woman was diagnosed with chronic myeloid leukemia. Treatment with dasatinib, a second-generation Bcr–Abl tyrosine kinase inhibitor, was initiated, and complete cytogenetic remission was achieved. Two years later, proteinuria occurred, and the urinary protein level increased gradually in the next 3 years. Moreover, the serum creatinine level increased mildly during this period. The urinary protein level reached 2.18 g/gCr; hence, a renal biopsy was conducted. Light microscopy revealed mild proliferation of mesangial cells, and immunofluorescence analysis revealed IgG and C3 depositions in the mesangial area. Electron microscopy revealed electron-dense deposition in the paramesangial area, partial podocyte foot process effacement, and segmental endothelial cell swelling with a slight expansion of the subendothelial space. Dasatinib was discontinued, and within 3 weeks, the proteinuria disappeared, with improvements in her renal function. After switching to bosutinib, a new second-generation of tyrosine kinase inhibitor, the proteinuria remained negative. The rapid cessation of proteinuria following dasatinib discontinuation indicated that proteinuria was induced by the long-term administration of dasatinib. Proteinuria and renal function should be regularly monitored during dasatinib therapy.
Keywords: Dasatinib, Proteinuria, Chronic myelogenous leukemia
Introduction
Chronic myelogenous leukemia (CML) is a clonal myeloproliferative disorder of hematopoietic stem cell origin. It is caused by Bcr–Abl tyrosine kinase induced by the reciprocal chromosomal translocation t(9;22)(q34;q11) called Philadelphia chromosome. The introduction of imatinib, a Bcr–Abl tyrosine kinase inhibitor (TKI), is a major progress in the treatment of CML because imatinib drastically improves patient survival. Dasatinib, a second-generation Bcr–Abl TKI, is a multi-kinase inhibitor that may impede platelet-derived growth factor receptor β, c-kit, and Src family tyrosine kinase. It has been shown to be effective in patients with newly diagnosed, imatinib-resistant or imatinib-intolerant CML in the chronic phase and those with acute lymphoblastic leukemia with Philadelphia chromosome [1–3].
Some studies have reported the occurrence of nephrotic syndrome during dasatinib therapy [4–10]. Here we present a case of dasatinib-induced moderate proteinuria in a patient with CML who received a relatively long-term administration of dasatinib, in which mild, but diverse morphological changes in glomeruli were identified in renal biopsy.
Case
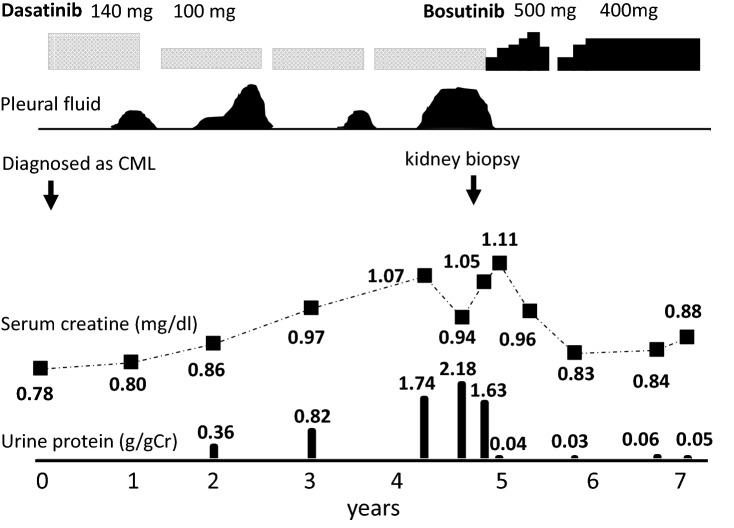
A 52-year-old woman was diagnosed with CML, dasatinib therapy was initiated, and complete cytogenetic remission was observed 3 months later. A possible adverse effect of dasatinib treatment—bilateral pleural effusion—was repeatedly observed 1 year later, and was managed and controlled by a reduction in the dasatinib dose at the first time, temporary withdrawal of dasatinib at each time, and the use of diuretics at each (Fig. 1). Two years later, the patient was positive for proteinuria, whose severity increased gradually in the next 3 years. The patient’s serum creatinine level also increased gradually during that period and pleural effusion recurred simultaneously (Fig. 1). She was admitted to our department 5 years after the initiation of dasatinib therapy. She had a past history of ovarian serous cystadenoma and gastric cancer, and no family history of kidney diseases. Her vital signs, including blood pressure, were normal, and physical examination results were unremarkable. Her laboratory data on admission is shown in Table 1. The serum blood urea nitrogen level was 23 mg/dL, serum creatinine level was 1.05 mg/dL, serum albumin level was 3.6 g/dL, urinary protein level was 1.63 g/gCr, and urinary red blood cell count was 5 cells/high power field. Moreover, her complement activity (CH50) level was within the normal range, and antinuclear antibody was negative.
Fig. 1.
Clinical course of the patient after initiation of dasatinib therapy
Table 1.
Laboratory data on admission
| Urinalysis | |
|---|---|
| Protein (g/gCr) | 1.6 |
| Red blood cell counts (cells/HPF) | 5 |
| White blood cell counts (cells/HPF) | < 1 |
| Cast | (–) |
| NAG-index (U/gCr) | 18 |
| Complete blood counts | |
| White blood cell counts (× 103/μL) | 5.6 |
| Red blood cell counts (× 106/μL) | 4 |
| Hemoglobin (g/dL) | 10.0 |
| Hematocrit (%) | 30 |
| Platelet counts (× 104/μL) | 31 |
| Serum biochemistry | |
| Total protein (g/dL) | 6.6 |
| Albumin (g/dL) | 3.6 |
| Total bilirubin (mg/dL) | 0.3 |
| AST (U/L) | 29 |
| ALT (U/L) | 21 |
| Lactate dehydrogenase (U/L) | 213 |
| Alkaline phosphatase (U/L) | 261 |
| Uric acid (mg/dL) | 5.9 |
| Blood urea nitrogen (mg/dL) | 23 |
| Creatinine (mg/dL) | 1.1 |
| Sodium (mEq/L) | 141 |
| Potassium (mEq/L) | 4.1 |
| Chloride (mEq/L) | 106 |
| Calcium (mg/dL) | 8.9 |
| LDL-cholesterol (mg/dL) | 155 |
| HDL-cholesterol (mg/dL) | 111 |
| Glucose (mg/dL) | 70 |
| Immunological studies | |
| C-reactive protein (mg/dL) | 0 |
| Immunoglobulin G (mg/dL) | 1065 |
| Immunoglobulin A (mg/dL) | 135 |
| Immunoglobulin M (mg/dL) | 66 |
| CH50 (U/mL) | 42 |
| Anti-nuclear antibody (X) | 40 |
HPF high power field, NAG N-acetyl-β-d-glucosaminidase
An ultrasound-guided renal biopsy was performed. Light microscopy specimen contained six glomeruli, with one glomerulus showing global sclerosis, and the remaining showing mild mesangial cell proliferation and matrix expansion (Fig. 2a). Interstitial fibrosis and tubular atrophy were insignificant, with an area of < 10% of that of the whole cortex (not shown). Immunofluorescent staining revealed IgG and C3 deposition mainly in the mesangial area (Fig. 2b), and IgA, IgM, C4, and C1q were negative. Transmission electron microscopy revealed substantial dense deposition in the paramesangial area (Fig. 2c) and a small amount in the subendothelial area (not shown); endothelial cells were mildly swollen (Fig. 2d, e) with a slight expansion of subendothelial space (Fig. 2f); a partial podocyte foot process effacement was also observed (Fig. 2f). In summary, histological examination revealed mild mesangial proliferative glomerulonephritis with IgG and C3 deposition, podocyte foot process effacement, and an endothelial cell injury.
Fig. 2.
Histological findings of the renal biopsy. a Light microscopy (LM) revealed mild and segmental mesangial proliferation and matrix expansion. Periodic acid–Schiff staining, original magnification 400 ×. b Immunofluorescence analysis demonstrated IgG deposition in the mesangial area. Original magnification, 400 ×. c Transmission electron microscopy (EM) revealed proliferation of the mesangial cells with electron-dense deposition in the paramesangial area. Original magnification, 3000 × . d, e Segmental glomerular endothelial cell swelling was observed by LM (d, arrow; methenamine silver periodic acid-Schiff staining) and EM (e). Original magnification, 400 × in (d) and 4000 × in (e). f. Partial podocyte foot process effacement (arrow) and a slight expansion of subendothelial space (arrowhead) were identified by EM. Original magnification, 12,000 × . MC mesangial cells, EC endothelial cells, CL capillary lumen
The clinical course of the patient suggested that dasatinib played a role in the development of proteinuria, and histological analysis supported the secondary glomerulopathy findings; hence, dasatinib was switched to bosutinib, a newly developed TKI targeting Bcr–Abl and Src family tyrosine kinases. The patient’s urinary protein level returned to the normal range within 21 days after dasatinib discontinuation, and her serum creatinine level decreased to 0.83 mg/dL 1 year later. Urinary abnormalities or elevated serum creatinine levels were not observed while the patient was receiving bosutinib treatment (Fig. 1).
Discussion
After the success of imatinib therapy for the treatment of CML, several Bcr–Abl TKIs were developed. Although all these drugs target Bcr–Abl tyrosine kinases, their ability to inhibit other kinases differs, and their unique structures and mechanisms of action induce diverse profiles of adverse events [11, 12]. Dasatinib is generally well tolerated and prompts a lower or comparable incidence of drug-related nonhematologic adverse events than imatinib, except for pleural effusion [13, 14]. Proteinuria was reported in 6 of 34 patients in a phase 1 study; however, it was not highlighted in a phase 3 study [13–15]. To date, 7 cases of dasatinib-induced nephrotic syndrome (5 in adults and 2 in children) have been reported in the scientific literature [4–10]. In addition, 8 cases were reported as dasatinib-induced nephrotic range proteinuria in the European Pharmacovigilance database—EudraVigilance—and The Netherlands Pharmacovigilance Centre Lareb database [16].
Table 2 presents a summary of the seven cases (cases 1–7) described in the literature. The case presented in the present study manifested differences from those described previously [4–10]. First, the urinary protein level was below the nephrotic range, and proteinuria occurred 2 years after the initiation of dasatinib therapy in the present case (Fig. 1), whereas proteinuria was within the nephrotic range in all the cases described in the literature and occurred within 6 months in 5 cases. Second, the serum creatinine levels increased gradually from 0.78 mg/dL to 1.1 mg/mL in 5 years in the present case (Fig. 1), whereas these levels were not available in 6 cases and remained unchanged in 1 case (case 6) before the initiation of dasatinib therapy. We note that the serum creatinine level was higher than that expected from the biopsy findings in our case. One of the possible reasons for the discrepancy might be that the sample size was insufficient to detect the abnormal lesion possibly distributed inhomogeneously. We acknowledge that this is one of the limitations of our report. Another reason might be that endothelial dysfunction may contribute the deterioration of renal function. Third, pleural effusion was observed during dasatinib therapy in the present case (Fig. 1), whereas it was not observed in 2 (cases 1 and 6) cases and not mentioned in the other cases described in the literature. Pleural effusion is a common finding in dasatinib therapy, and it reportedly occurred in approximately 30% of patients in phase 3 trials [14, 17]. Immune-mediated mechanisms were proposed based on the findings that effusion is lymphocyte-predominant exudative and associated with lymphocytosis [18]. Although thoracentesis was not performed in our case, the effusion was assumed to be exudative like previous cases, since hypoalbuminemia was mild and no symptoms of hypervolemia were present. Thus, we suppose that the pleural effusion disappeared by the discontinuation of dasatinib, rather than the treatment with diuretics in our case. Both proteinuria and pleural effusion were observed in the present case, but the mechanisms underlying each may differ because their frequencies were different and pleural effusion was not present in the proteinuria cases described in the literature. Lastly, mesangial immune complex deposition was observed in the present case, whereas it was not observed in 4 renal biopsy cases described in the literature (Table 2) [4, 5, 7, 8]. In case 5, consistent with the diagnosis of fibrillary glomerulonephritis, randomly arranged fibrils (10–20 nm) were observed [8].
Table 2.
Clinical and pathological findings of previously reported 7 cases of dasatinib-induced nephrotic syndrome and our case
| Case No | 1st Author, (Ref. No.) | Agea | Sex | Disease | Duration of DASa | Main findings in renal biopsy | Urinary proteina | Serum Cra | Pleural effusiona | Treatment | Period between withdrawal of DAS and remission |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Wallace, (Ref [4]) | 63 yo | F | CML | 3 Months | Mild increased mesangial matrix, focal corrugation of the glomerular basement membranes and focal foot process effacement | 3.9 g/day | 0.79 mg/dl | (–) | DAS → IMA | 2 Weeks |
| 2 | Ruebner, (Ref [5]) | 3 yo | F | CML | 1 To 2 yearsb | Focal global sclerosis, a few segmental mesangial proliferation and focal foot process effacement | 17 g/gCr | 0.3 mg/dl | NA | DAS + Steroids → partial remission → discontinued DAS | 2 Weeks |
| 3 | Hirano, (Ref [6]) | 64 yo | F | Ph+ALL | > 0.5 Monthc | No renal biopsy | 3.9 g/day | 0.34 mg/dl | NA | Reduced DAS + Steroids → increased proteinuria → discontinued DAS | 1 Week |
| 4 | Lim, (Ref [7]) | 7 yo | M | Ph+ALL | > 1 Monthd | Diffuse l foot process effacement | 5.6 g/day | NA | NA | Discontinued DAS | 1 Week |
| 5 | Ochiai, (Ref [8]) | 40 yo | M | CML | 3 Months | Swelling of endothelial cells, foot process effacement and focal randomly arranged fibril deposition | 12.2 g/gCr | 0.87 mg/dl | NA | DAS → NIL | 2 Weeks |
| 6 | De Luca, (Ref [9]) | 45 yo | F | CML | 6 Months | No renal biopsy | 4.0 g/day | 0.90 mg/dl | (–) | DAS → IMA | 4 Weeks |
| 7 | Mandac Rogulj (Ref [10]) | 33 yo | F | CML | 2 Years | No renal biopsy | 6.19 g/L | NA | NA | Reduced DAS | NAf |
| 8 | Our Case | 57 yo | F | CML | 58 Monthse | Mild mesangial proliferation, immune complex deposition in mesangial area, and focal foot process effacement | 1.63 g/gCr | 1.05 mg/dl | ( +) | DAS → BOS | 3 Weeks |
Ref reference, yo years old, Cr creatinine, CML chronic myelogenous leukemia, Ph+ ALL Philadelphia chromosome-positive acute lymphocytic leukemia, NA not available, DAS dasatinib, IMA imatinib, NIL nilotinib, BOS bosutinib
aAt the time of renal biopsy or at the time of treatment change, when renal biopsy was not performed
bEstimated period based on the clinical history
cProteinuria was detected 2 weeks administration of 140 mg/day of DAS, which was increased from 100 mg/day
dProteinuria was detected 26 days after hematopoietic stem cell transplantation, which was performed after the achieving remission with DAS
eIncluding intermittent withdrawal periods
fUrinary protein returned to normal level by reduction of dasatinib
In all the cases including the present case, except for one (case 7) where the period was not described, proteinuria was not observed within 1 month after dasatinib was discontinued or its dose was reduced. The immediate resolution of proteinuria demonstrated direct and reversible toxicity of dasatinib to the glomerular capillary wall. Corticosteroids were used in 2 cases (cases 2 and 3) described in the literature before dasatinib discontinuation; partial remission was achieved in one case, but no response was reported in the other. A probable mechanism for dasatinib-induced proteinuria is the inhibition of vascular endothelial growth factor (VEGF) signaling pathway by blocking Src family tyrosine kinases [4]. In glomeruli, VEGF is produced from podocytes, and the VEGF receptor is expressed in both podocytes and glomerular endothelial cells. VEGF signals are essential for the normal function of podocytes and endothelial cells, and anticancer therapies targeting VEGF are known to induce proteinuria [19]. Dasatinib inhibits the phosphorylation of downstream molecules of the VEGF signaling pathways, such as focal adhesion kinase (FAK) and paxillin [20]. The finding of Wallace et al. that phosphorylation of FAK and paxillin was decreased in glomeruli and tubules in dasatinib-induced nephrotic syndrome cases was consistent with our hypothesis [4]. However, a recent study by Calizo et al. questioned the association of Src inhibition with the development of proteinuria [21]: they showed that dasatinib therapy induced a podocyte injury by disrupting cytoskeleton and focal adhesion architecture, whereas other TKIs, such as bosutinib, a second-generation TKI, and a potent Src inhibitor, did not. In the present study, proteinuria was not observed after switching to bosutinib, indicating that Src inhibition is not a major mechanism of dasatinib-induced proteinuria. Moreover, they proposed LIM kinase, a key regulator in the Rac/Cdc42 signaling, as a target of dasatinib in a podocyte injury [21]; however, the specific mechanisms remain to be elucidated.
In our case, mesangial cell proliferation with immune-deposits was observed. The case 2 also showed mesangial cell proliferation, but did not have electron-dense deposits (Table 2). Tyrosine kinases, including spleen tyrosine kinase (SYK), Bruton’s tyrosine kinase (BTK), and platelet-derived growth factor receptor (PDGFR) are reportedly involved in various immune-mediated glomerulonephritis, and possible therapeutic effects of TKIs on these glomerulonephritis are suggested [22]. Thus, mesangial cell proliferation in the present case was unlikely caused by inhibition of tyrosine kinases, and other mechanisms, such as immune response to dasatinib could underlie the pathogenesis. We note that the treatment with corticosteroid was partially effective in the case 2. Another possibility is that latent primary mesangial proliferative nephritis was present by chance. Although the mechanism of immune deposition was unclear, the immediate remission of proteinuria after the drug withdrawal indicates that a direct effect of dasatinib on the glomerular capillary wall was more relevant to the proteinuria rather than the mesangial immune deposition.
In summary, we report a case of proteinuria induced by a relatively long-term administration of dasatinib for CML. The renal biopsy revealed mild, but diverse morphological changes in glomeruli, including podocyte, endothelial cell, and mesangial cell injuries with immune complexes deposition. The rapid clinical remission after drug discontinuation even after long-term administration of dasatinib indicated direct and reversible glomerular toxicity of dasatinib. The findings of the present case indicated that regular monitoring of proteinuria and renal function is required over the course of dasatinib therapy.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Informed consent
Informed consent was obtained from the patient for publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, Wang J, Ipina JJ, Kim DW, Ogura M, Pavlovsky C, Junghanss C, Milone JH, Nicolini FE, Robak T, Van Droogenbroeck J, Vellenga E, Bradley-Garelik MB, Zhu C, Hochhaus A. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saglio G, Hochhaus A, Goh YT, Masszi T, Pasquini R, Maloisel F, Erben P, Cortes J, Paquette R, Bradley-Garelik MB, Zhu C, Dombret H. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116:3852–3861. doi: 10.1002/cncr.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foa R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, Elia L, Paoloni F, Fazi P, Cimino G, Nobile F, Ferrara F, Castagnola C, Sica S, Leoni P, Zuffa E, Fozza C, Luppi M, Candoni A, Iacobucci I, Soverini S, Mandelli F, Martinelli G, Baccarani M, Party GALW. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118:6521–6528. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- 4.Wallace E, Lyndon W, Chumley P, Jaimes EA, Fatima H. Dasatinib-induced nephrotic-range proteinuria. Am J Kidney Dis. 2013;61:1026–1031. doi: 10.1053/j.ajkd.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Ruebner RL, Copelovitch L, Evageliou NF, Denburg MR, Belasco JB, Kaplan BS. Nephrotic syndrome associated with tyrosine kinase inhibitors for pediatric malignancy: case series and review of the literature. Pediatr Nephrol. 2014;29:863–869. doi: 10.1007/s00467-013-2696-0. [DOI] [PubMed] [Google Scholar]
- 6.Hirano T, Hashimoto M, Korogi Y, Tsuji T, Miyanaka K, Yamasaki H, Tsuda H. Dasatinib-induced nephrotic syndrome. Leuk Lymphoma. 2016;57:726–727. doi: 10.3109/10428194.2015.1075020. [DOI] [PubMed] [Google Scholar]
- 7.Lim YT, Kim YJ, Park YH, Hah JO, Lee JM. A case of dasatinib-induced nephrotic syndrome in a child with philadelphia chromosome positive acute lymphoblastic leukemia. Yonsei Med J. 2016;57:532–533. doi: 10.3349/ymj.2016.57.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochiai S, Sato Y, Minakawa A, Fukuda A, Fujimoto S. Dasatinib-induced nephrotic syndrome in a patient with chronic myelogenous leukemia: a case report. BMC Nephrol. 2019;20:87. doi: 10.1186/s12882-019-1273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Luca M, Carmosino I, Stefanizz C, Campanelli M, De Angelis F, Cesini L, Latagliata R, Alimena G. Nephrotic proteinuria developed under dasatinib treatment in a patient with chronic myeloid leukemia: a case report and review of the literature. Ann Hematol Oncol. 2016;36:1106. [Google Scholar]
- 10.Mandac Rogulj I, Matisic V, Arsov B, Boban L, Juginovic A, Molnar V, Primorac D. Dasatinib-induced nephrotic syndrome: a case of phenoconversion. Croat Med J. 2019;60:250–254. doi: 10.3325/cmj.2019.60.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuellar S, Vozniak M, Rhodes J, Forcello N, Olszta D. BCR-ABL1 tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. J Oncol Pharm Pract. 2018;24:433–452. doi: 10.1177/1078155217710553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, Kocher T, Superti-Furga G. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 13.Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boque C, Chuah C, Pavlovsky C, Mayer J, Cortes J, Baccarani M, Kim DW, Bradley-Garelik MB, Mohamed H, Wildgust M, Hochhaus A. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123:494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boque C, Shah NP, Chuah C, Casanova L, Bradley-Garelik B, Manos G, Hochhaus A. Final 5-year study results of DASISION: the Dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333–2340. doi: 10.1200/JCO.2015.64.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demetri GD, Lo Russo P, MacPherson IR, Wang D, Morgan JA, Brunton VG, Paliwal P, Agrawal S, Voi M, Evans TR. Phase I dose-escalation and pharmacokinetic study of dasatinib in patients with advanced solid tumors. Clin Cancer Res. 2009;15:6232–6240. doi: 10.1158/1078-0432.CCR-09-0224. [DOI] [PubMed] [Google Scholar]
- 16.Muller-Hansma AHG, van der Lugt J, Zwaan CM. Nephrotic syndrome under treatment with dasatinib: be aware of a possible adverse drug reaction. Neth J Med. 2017;75:428–431. [PubMed] [Google Scholar]
- 17.Shah NP, Guilhot F, Cortes JE, Schiffer CA, le Coutre P, Brummendorf TH, Kantarjian HM, Hochhaus A, Rousselot P, Mohamed H, Healey D, Cunningham M, Saglio G. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood. 2014;123:2317–2324. doi: 10.1182/blood-2013-10-532341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes TP, Laneuville P, Rousselot P, Snyder DS, Rea D, Shah NP, Paar D, Abruzzese E, Hochhaus A, Lipton JH, Cortes JE. Incidence, outcomes, and risk factors of pleural effusion in patients receiving dasatinib therapy for Philadelphia chromosome-positive leukemia. Haematologica. 2019;104:93–101. doi: 10.3324/haematol.2018.188987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ollero M, Sahali D. Inhibition of the VEGF signalling pathway and glomerular disorders. Nephrol Dial Transplant. 2015;30:1449–1455. doi: 10.1093/ndt/gfu368. [DOI] [PubMed] [Google Scholar]
- 20.Liang W, Kujawski M, Wu J, Lu J, Herrmann A, Loera S, Yen Y, Lee F, Yu H, Wen W, Jove R. Antitumor activity of targeting SRC kinases in endothelial and myeloid cell compartments of the tumor microenvironment. Clin Cancer Res. 2010;16:924–935. doi: 10.1158/1078-0432.CCR-09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calizo RC, Bhattacharya S, van Hasselt JGC, Wei C, Wong JS, Wiener RJ, Ge X, Wong NJ, Lee JJ, Cuttitta CM, Jayaraman G, Au VH, Janssen W, Liu T, Li H, Salem F, Jaimes EA, Murphy B, Campbell KN, Azeloglu EU. Disruption of podocyte cytoskeletal biomechanics by dasatinib leads to nephrotoxicity. Nat Commun. 2019;10:2061. doi: 10.1038/s41467-019-09936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma TK, McAdoo SP, Tam FW. Targeting the tyrosine kinase signalling pathways for treatment of immune-mediated glomerulonephritis: from bench to bedside and beyond. Nephrol Dial Transplant. 2017;32:i129–i138. doi: 10.1093/ndt/gfw336. [DOI] [PMC free article] [PubMed] [Google Scholar]