Abstract
Thrombotic microangiopathy (TMA) is generally diagnosed through clinical features characterized as microangiopathic hemolytic anemia, thrombocytopenia, and multiple organ injury, as well as by pathological findings such as vascular damage and endothelial cell injury. Rheumatic and autoimmune diseases could be accompanied by secondary TMA; in fact, systemic lupus erythematosus (SLE) is a common disease associated with secondary TMA, and SLE complicated with TMA has been reported to have a poor prognosis. Although TMA occurs rarely in pediatric SLE patients, it often leads to severe clinical conditions. Here, we report a rare case of severe juvenile-onset SLE complicated with TMA and kidney injury. The 5-year-old patient showed renal dysfunction, thrombocytopenia, hemolytic anemia, nephrotic syndrome, hypocomplementemia, and elevation of anti-dsDNA IgG levels. Kidney biopsy revealed mesangial proliferation and endocapillary proliferation, as well as plumped endothelial cells, with full-house pattern deposits in immunofluorescence study. Combination treatment of methylprednisolone pulse therapy followed by oral prednisolone, mycophenolate mofetil, and plasma exchange was effective, whereas eculizumab did not show therapeutic effects. The patient further showed recurrent deterioration, and we initiated intravenous cyclophosphamide in addition to combination treatment and eventually succeeded in controlling the disease. Genome analysis by whole-exome sequencing revealed no particular gene mutation related to either complement disorders or type-1 interferon. Further elucidations concerning the pathogenic mechanisms causing juvenile-onset SLE are needed to establish an efficient treatment strategy for TMA with SLE.
Keywords: Thrombotic microangiopathy, Systemic lupus erythematosus, Kidney injury, Pediatric rheumatology, Pediatric nephrology
Introduction
Thrombotic microangiopathy (TMA) is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and multiple organ injury [1, 2]. In particular, primary TMA occurs in case of complement disorders—such as atypical hemolytic uremic syndrome, as well as in ADAMTS13 deficiency (also known as thrombotic thrombocytopenic purpura)—whereas secondary TMA is triggered by various factors, such as drugs, bone marrow transplantations, and autoimmune diseases [2, 3]. TMA manifestations generally affect many organs, with the kidney being one of the main involved organs, both in primary and secondary TMA.
Systemic lupus erythematosus (SLE) is an autoimmune disorder affecting multiple organs. SLE is reported to be accompanied by secondary TMA in 0.5–10.0% of the cases [4]; moreover, SLE with TMA has been described as a life-threatening condition [5, 6]. Nonetheless, TMA in pediatric SLE patients is a rather rare occurrence.
Here, we report a case of a pediatric SLE patient presenting with a severe form of secondary TMA, successfully treated using a combination therapy of methylprednisolone pulse therapy, oral steroid, plasma exchange, mycophenolate mofetil, and intravenous cyclophosphamide.
Case report
Before being referred to our hospital, a 5-year-old girl presented to a former hospital complaining of fever and edema. Recent medical history reported periorbital edema and low fever 1 month prior, as well as diarrhea 1 week prior. Furthermore, she had a history of asthma which was treated with montelukast a few years prior, as reported during anamnesis; however, she was not on medication at the time of presentation. In addition, her paternal grandfather was diagnosed with SLE. When she visited a former hospital, she had high fever and edema in the lower extremities, and her systolic blood pressure was 146 mmHg. Urinalysis revealed proteinuria and occult blood, whereas blood cell counts revealed anemia, thrombocytopenia, and schistocytosis. She was suspected of having developed TMA and subsequently admitted to our hospital.
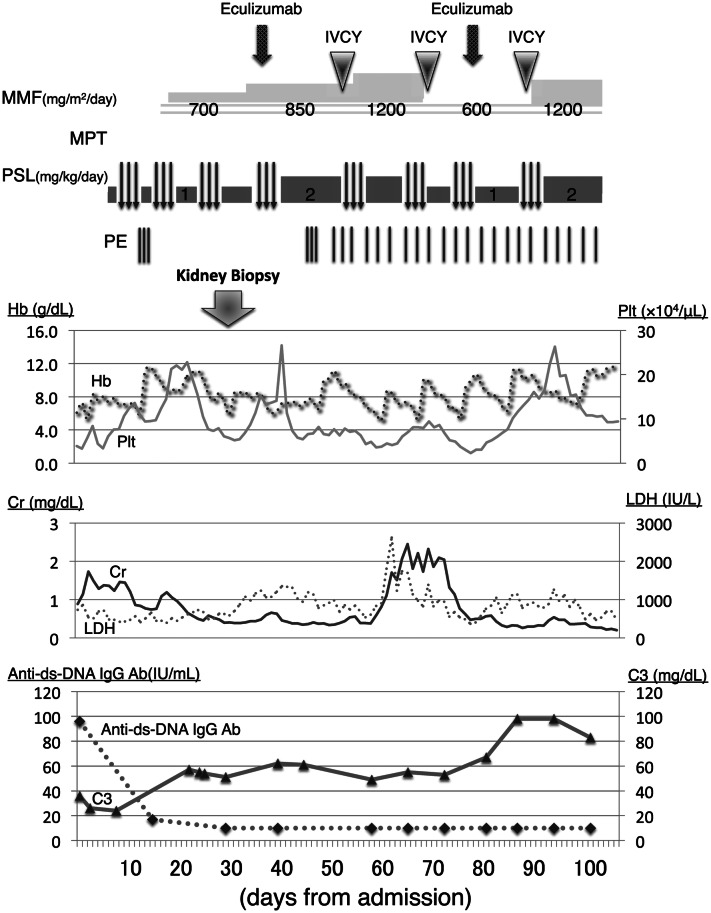
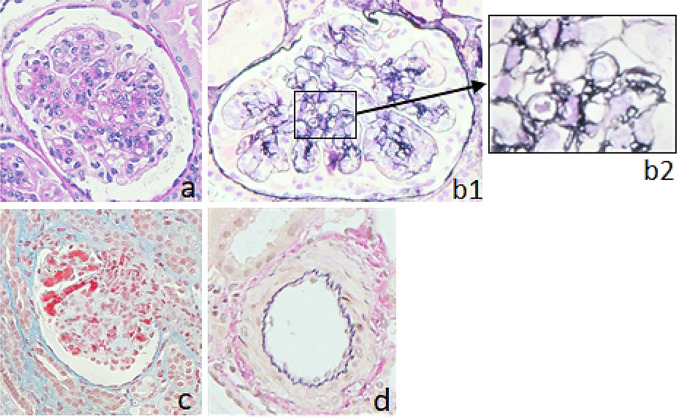
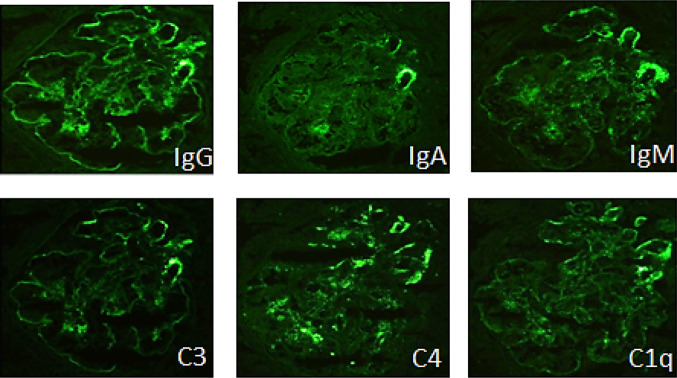
On admission, her body weight was 19.5 kg, which had increased 1.0 kg in the previous month, and her height was 104 cm. Her body temperature was 38.3 °C, blood pressure was 128/98 mmHg with a heart rate of 100 beats per min, respiratory rate was 35 breaths per min, and SpO2 was 99% without oxygen supply. She showed significant edema around the eyes and lower extremities. Although her respiratory sounds were decreased, there were no abnormal heart sounds. Her belly was distended albeit non-tender, and she had no skin rash, joint swelling, or joint pain. She had no abnormalities related to the central nervous system. Although her general conditions were poor, she was fully conscious. Blood cell counts showed that peripheral leucocytes were 8200/µL, with 2591 lymphocytes/µL. The patient’s hematocrit was 19.9%, while red blood cell count was 2,140,000/µL, hemoglobin was 6.1 g/dL, and platelet count was 39,000/µL. Laboratory serological findings were as follows: lactate dehydrogenase (LDH) 735 IU/L, total protein 4.3 g/dL, albumin 1.9 g/dL, creatinine 0.89 mg/dL, urea nitrogen 8.8 mg/dL, potassium 6.0 mEq/L, and brain natriuretic peptide 222.3 pg/mL. In addition, haptoglobin was below 10 mg/dL. Coagulation tests reported that fibrin and fibrinogen degradation products were 321 mg/dL and that D-dimer was 3.65 µg/mL. The serum IgG level was 420 mg/dL, and C3 and C4 were 36 and 5 mg/dL, respectively. CH50 was 13 U/mL, and anti-dsDNA IgG antibody level was 96 IU/mL; in addition, antinuclear antibody titer was 1:160, whereas SS-A antibody titer was 1:4. Other autoimmune antibodies, such as anti-Sm, anti-RNP, PR3-ANCA, and MPO-ANCA, were all negative. Urinalysis showed proteinuria of 21.2 g/day with hematuria and increasing levels of β2-MG to 1418 µg/L (Table 1). A chest X-ray showed pleural effusions and cardiomegaly (i.e., cardiothoracic ratio = 57%), while cardiograms were normal. Ultimately, there were no abnormal findings related to either stool culture or ADAMTS13 activity. Based on these findings, the patient was diagnosed with SLE accompanied by TMA, with kidney injury and nephrotic syndrome. We started a methylprednisolone pulse therapy (MPT), a 3-day pulse as one course in a week, in addition to plasma exchange (PE) during 3 consecutive days and mycophenolate mofetil. Supportive therapy including thrombomodulin alfa, haptoglobin, carperitide, nicardipine, albumin, furosemide, and tolvaptan, was also performed. Additionally, we started administration of amlodipine and nicardipine, because she showed severe hypertension. After having performed three courses of MPT and prednisolone administration between the MPT courses, renal function, as well as thrombocytopenia and hemolytic anemia, noticeably improved (Fig. 1). To evaluate the kidney injury, we performed a kidney biopsy on day 29, which revealed mesangial proliferation in most of the glomeruli, together with severe mesangiolysis; we also found one global sclerosis and one cellular crescent among 45 glomeruli. Moreover, 65% of glomeruli showed endocapillary proliferation, and wire-loop lesion was identified in some glomeruli. Plumped endothelial cells and fibrin depositions were both observed. On the other hand, urinary capillary atrophy and fibrosis of interstitial tissue were rather mild (Fig. 2). On immunofluorescence staining, a full-house pattern was detected (Fig. 3). We diagnosed as lupus nephritis Class IV-S(A) of the ISN/RPS classification, associated with TMA. Three days after the kidney biopsy, blood exams showed recurrence of hemolytic anemia and thrombocytopenia. Serum complements were in the normal range, and anti-dsDNA IgG level was normal. Therefore, we considered TMA as severe, and after having administered a meningococcal vaccine, we subsequently decided to administer eculizumab, an inhibitor of terminal complement activation, on day 40; nonetheless, although having contributed to increasing platelet count, eculizumab did not manage to control TMA. Moreover, the patient was found having pleural and pericardial effusions, as well as ascites after 5 days of eculizumab administration. Therefore, we resumed MPT, PE three times per week, and started intravenous cyclophosphamide therapy (IVCY). After a few weeks, pleural effusion and ascites were decreased. However, as pericardial effusion did not improve, she underwent surgical drainage. Two months after onset, she showed further deterioration of renal function and high LDH, which was accompanied by neither infection nor platelet infusion that could be the trigger for deterioration. We performed a second IVCY and renal function and LDH both improved. She showed severe hypertension and we started administration of nitroprusside. Furthermore, since her platelet counts decreased, we performed a second administration of eculizumab. In total, we performed 28 PEs, 8 MPT courses, and 9 IVCY courses until her laboratory data recovered and disappearance of schizocyte was confirmed on day 100. As for haptoglobin, it took 5 months to normalize. We continued prednisolone and mycophenolate mofetil and later added hydroxychloroquine and tacrolimus as maintenance therapy for SLE. She showed persistent hypertension as systolic blood pressure was 125–130 mmHg, so we started oral amlodipine, which was later switched to nifedipine and azilsartan. Eventually, we analyzed her genome by whole-exome sequencing; interestingly, we found no known mutation related to either SLE, vascular diseases, or kidney diseases. The patient was 7 years and 7 months old at the last observation. Although she continued treatment with prednisolone and immunosuppressants, aside from oral antihypertensive drugs, she showed full recovery with no signs of recurrence and no abnormal laboratory data.
Table 1.
Laboratory data on admission
| CBC | Coagulation | Urinalysis | |||
| WBC | 8200/μL | PT-INR | 0.99 | pH | 6.0 |
| Neu | 62.3% | APTT | 27.9 s | OB | (3+) |
| Lym | 31.6% | Fbg | 321 mg/dL | TP | (4+) |
| Eo | 1.1% | FDP | 16 μg/mL | RBC | 5–9 HPF |
| Ba | 0.1% | D-Dimer | 3.65 μg/mL | WBC | 30–49 HPF |
| Mo | 4.9% | NAG | 35.2 IU/L | ||
| RBC | 214 × 104/μL | Immunology | β2MG | 1418 μg/L | |
| Hb | 6.1 g/dL | C3 | 36 mg/dL | TP/Cr | 21.2 g/g Cr |
| Ht | 19.9% | C4 | 5 mg/dL | ||
| Plt | 3.9 × 104/μL | CH50 | 13 U/mL | ||
| Schizocyte | 1.8% | IgG | 420 mg/dL | ||
| IgA | 116 mg/dL | ||||
| Biochemistry | IgM | 89 mg/dL | |||
| TP | 4.3 g/dL | IC-C1q | 4.2 μg/mL | ||
| Alb | 1.9 g/dL | Rheumatoid factor | 6 IU/mL | ||
| BUN | 52 mg/dL | ANA | × 160 | ||
| Cr | 0.89 mg/dL | Homogeneous | × 160 | ||
| UA | 8.8 mg/dL | Speckled | × 160 | ||
| Na | 143 mEq/L | Anti-dsDNA IgG | 96 IU/mL | ||
| K | 6 mEq/L | Anti-Sm | (–) | ||
| Cl | 117 mEq/L | Anti-RNP | (–) | ||
| Ca | 7.5 mg/dL | Anti-SS-A | × 4 | ||
| P | 4.7 mg/dL | Anti-Scl-70 | (–) | ||
| LDH | 735 IU/L | Anti-RNA polymeraseIII | (–) | ||
| AST | 50 IU/L | Anti-ARS | < 5.0 U/mL | ||
| ALT | 14 IU/L | Anti-cardiolipin IgG antibody | < 8 U/mL | ||
| T-Bil | 0.6 mg/dL | Lupus Anticoagulant | 1.2 | ||
| γ-GTP | 7 U/L | PR3-ANCA | < 1.0 U/mL | ||
| AMY | 93 U/L | MPO-ANCA | < 1.0 U/mL | ||
| CK | 108 IU/L | PA-IgG | 456 ng/107 cells | ||
| Glu | 87 mg/dL | Coombs' Test, Indirect | (–) | ||
| BNP | 222.3 pg/mL | Coombs' Test, Direct | (–) | ||
| CRP | 0.29 mg/dL | HIT antibody | < 6.0 U/mL | ||
| Haptoglobin | < 10 mg/dL | ||||
Fig. 1.
Laboratory data and treatment during the clinical course
Fig. 2.
Light microscopic analysis of a kidney specimen. a Mesangiolysis and endocapillary proliferation. PAS × 400; b1 glomerular basement membrane duplication. PAM × 400; b2 partially enlarged picture of b1; double contour of glomerular basement membrane; c erythrocytes and fibrin deposition in endocapillary. MT × 100. d No abnormalities in blood vessels. EVG × 100
Fig. 3.
Immunofluorescence staining showing deposits’ full-house pattern in the glomerular periphery
Discussion
Juvenile SLE associated with TMA is a very rare condition. We experienced a severe case of TMA with SLE accompanied by nephrotic syndrome and kidney injury, which was successfully treated with a combination therapy of MPT, MMF, PE, and IVCY. Both primary TMA and secondary TMA are diagnosed by clinical and pathological findings [7]; more specifically, clinical findings include thrombocytopenia, microangiopathic hemolytic anemia, and organ injury, whereas pathological findings are defined as vascular damage due to thrombosis of arterioles and capillaries, with characteristic endothelial cell injury and vascular wall abnormalities [2]. It is sometimes difficult to determine whether one is dealing with primary or secondary TMA, especially at its onset. In this case, we first suspected a primary form of TMA, such as Shiga toxin-mediated hemolytic uremic syndrome (HUS), according to the patient’s clinical manifestations, but we were able to promptly diagnose it as SLE with TMA based on laboratory findings, resulting in the immediate initiation of appropriate treatment. Then, we could confirm the diagnosis through the pathological evaluation of the kidney. We would like to emphasize that, in cases of clinical severe conditions, it is extremely important to initiate treatment for TMA before making a definitive histology-based diagnosis.
Furthermore, our patient showed onset of severe TMA simultaneously with the onset of SLE. Sun et al. [5] reported that two-thirds of patients who developed TMA had been already diagnosed with SLE before the onset of the former, whereas in one-third of the cases, the onsets of SLE and TMA were simultaneous. As a consequence, in cases of TMA without past history, the possibility of the patient suffering from a collagen-affecting disease should be taken into account. Moreover, the prognosis of patients who showed TMA as a complication of SLE has been reported to be very poor. Mortality of patients suffering from SLE with TMA at 3 months reached 33.3%, regardless of treatment drugs [5]; patients with thrombotic thrombocytopenic purpura (TTP) and SLE showed 62.5% mortality, sensitively above the 50% mortality rate of TTP patients without SLE [8]. As only a few papers reporting children having both TMA with SLE exist, it is difficult to predict the long-term prognosis for pediatric patients with SLE complicated with TMA. In a study [9] reporting 25 pediatric patients with TTP and SLE, the disease was found to be more severe in this particular population. Therefore, considering the possibility of a poorer prognosis in such cases, adequate treatments should be performed following the most prompt and appropriate approach.
Unfortunately, no reliable treatment strategy for TMA with SLE has been developed yet. As a consequence, patients are generally treated with steroids, including MPT and oral prednisolone, and immunosuppressive agents according to the effective treatments for SLE. In addition, several reports showed the efficacy of PE added to the combination therapy of steroids and immunosuppressants. For instance, during the 1990s, Nesher et al. [6] reported a mortality rate of 25% in patients of TMA with SLE treated by plasma infusion or PE, in contrast to the 57% mortality rate in patients who were not treated by plasma infusion or PE. A recent report [10] indicated that, out of 70 patients who were treated with PE for TMA with SLE, nine patients in severe condition showed complete remission, and only one patient deceased. Therefore, we should not hesitate to perform PE in cases of SLE with TMA. As other treatment options, some papers [6, 11] reported the efficacy of biological agents such as rituximab, an anti-CD20 monoclonal antibody, and eculizumab. In our case, we eventually chose eculizumab due to our patient’s rather severe TMA symptoms as well as the potential risk of serious infections following a 6-month impairment of B cells. However, we found that eculizumab was not effective in controlling disease activity. Even though unregulated complement activation and resistance von Willebrand factor cleavage by ADAMTS13 have both been proposed as pathogenic mechanisms of TMA in SLE patients, such hypotheses have yet to be confirmed [12]. Therefore, further studies concerning the mechanisms of secondary TMA with SLE are needed for allowing a more effective integration of biological agents in the therapeutic strategy for secondary TMA patients.
With regard to kidneys, we would like to point out that the ratio of renal involvement in TMA with SLE is as high as 50–100% [5, 8, 10], and the ratio of progressing end-stage renal disease is as high as 12.9–85.7% [5, 10]. In addition to the nephritis induced by the immunological disorder of SLE, small vascular injury and direct kidney injury caused by thrombosis and by proximal tubular injury due to hemolysis-related circulating heme can be suspected for contributing to the progression of kidney dysfunction. Our patient showed severe renal involvement since the onset of TMA; although normalization of urinary abnormalities was finally obtained after treatment, she still needs careful observation and treatment to maintain her renal function at a sufficient level.
In a Japanese survey [13], the mean age of onset of pediatric SLE is reported to be 11.3 ± 2.7 (range 1.6–15.9) years. Interestingly, the analysis of factors related to treatment resistance revealed that onset age under 10 years is one of the risk factors; our patient was 5 years old at the time of onset, and thus, she had a risk factor for treatment resistance. Nevertheless, the reason for juvenile onset being a risk of treatment resistance is not still completely elucidated. Recently, juvenile-onset SLE has been reported to relate to single-gene mutations [14], leading to the possibility that such SLE cases have a single genomic background underlying their clinical manifestations. We speculated that our patient had gene mutations and subsequently performed a whole-exome sequencing exam, which was negative for mutations related to either complement disorders, type-1 interferon, vascular diseases, or kidney diseases.
In conclusion, SLE patients with TMA have a high risk of developing a severe condition and a subsequently poor prognosis; therefore, prompt diagnosis and appropriate treatment are both extremely important factors in such cases, since they can make a real difference in terms of clinical outcome. Nevertheless, further investigations are needed to elucidate the mechanism underlying pediatric SLE associated with TMA to establish a more efficient treatment strategy for patients in severe clinical condition.
Acknowledgements
We thank Dr Kohsuke Imai, an associate professor in the Department of Community Pediatrics, Perinatal and Maternal Medicine, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, for performing whole-exome sequencing of genome analysis. We also thank the members of the Department of Nephrology and the members of the Medical Engineering Center in Tokyo Medical and Dental University for their help in performing plasma exchanges.
Compliance with ethical standards
Conflict of interest
Research funding: Masaaki Mori, Nippon Kayaku Co., Ltd., Asahi Kasei Pharma Co., AbbVie GK, Mitsubishi Tanabe Pharma Co. Donations: Tomohiro Morio, Astellas Pharma Inc.. Endowed departments by commercial entities: Mariko Mouri, and Masaaki Mori, Chugai Pharmaceutical Co., Ltd., UCB Japan Co. Ltd., CSL Behring K.K., Japan Blood Products Organization, and AYUMI Pharmaceutical Co.
Research involving human participants
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent for publication of this article was obtained from the patient and her parents verbally and by a written document.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347(8):589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 2.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 3.Brocklebank V, Wood KM, Kavanagh D. Thrombotic microangiopathy and the kidney. Clin J Am Soc Nephrol. 2018;13(2):300–317. doi: 10.2215/CJN.00620117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Holanda M, Pôrto L, Wagner T, Christiani L, Palma L. Use of eculizumab in a systemic lupus erythemathosus patient presenting thrombotic microangiopathy and heterozygous deletion in CFHR1–CFHR3. A case report and systematic review. Clin Rheumatol. 2017;36(12):2859–2867. doi: 10.1007/s10067-017-3823-2. [DOI] [PubMed] [Google Scholar]
- 5.Sun F, Wang X, Wu W, et al. TMA secondary to SLE: rituximab improves overall but not renal survival. Clin Rheumatol. 2018;37(1):213–218. doi: 10.1007/s10067-017-3793-4. [DOI] [PubMed] [Google Scholar]
- 6.Nesher G, Hanna VE, Moore TL, Hersh M, Osborn TG. Thrombotic microangiographic hemolytic anemia in systemic lupus erythematosus. Semin Arthritis Rheum. 1994;24(3):165–172. doi: 10.1016/0049-0172(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 7.Arnold DM, Patriquin CJ, Nazy I. Thrombotic microangiopathies: a general approach to diagnosis and management. CMAJ. 2017;189(4):E153–E159. doi: 10.1503/cmaj.160142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letchumanan P, Ng HJ, Lee LH, Thumboo J. A comparison of thrombotic thrombocytopenic purpura in an inception cohort of patients with and without systemic lupus erythematosus. Rheumatology. 2009;48(4):399–403. doi: 10.1093/rheumatology/ken510. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Jiang JJ, Wang CY, Jian S, Zhou Y, Ma MS, et al. Clinical features and prognosis of patients with thrombotic thrombocytopenic purpura associated with systemic lupus erythematosus: a review of 25 cases. Ital J Pediatr. 2019;45(1):55. doi: 10.1186/s13052-019-0641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li QY, Yu F, Zhou FD, Zhao MH. Plasmapheresis is associated with better renal outcomes in lupus nephritis patients with thrombotic microangiopathy: a case series study. Medicine (Baltimore) 2016;95(18):e3595. doi: 10.1097/MD.0000000000003595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sciascia S, Radin M, Yazdany J, et al. Expanding the therapeutic options for renal involvement in lupus: Eculizumab, available evidence. Rheumatol Int. 2017;37:1249–1255. doi: 10.1007/s00296-017-3686-5. [DOI] [PubMed] [Google Scholar]
- 12.Babar F, Cohen SD. Thrombotic microangiopathies with rheumatologic involvement. Rheum Dis Clin N Am. 2018;44(4):635–649. doi: 10.1016/j.rdc.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Takei S. Study on refractory pathology and treatment of childhood systemic lupus erythematosus (SLE): research on diagnosis and treatment of refractory pathology of rheumatic and collagen disease in childhood. 2010 Summary Research Report, pp 74–78. 2011. (article in Japanese)
- 14.Omarjee O, Picard C, Frachette C, Moreews M, Rieux-Laucat F, Soulas-Sprauel P, et al. Monogenic lupus: dissecting heterogeneity. Autoimmun Rev. 2019;18(10):102361. doi: 10.1016/j.autrev.2019.102361. [DOI] [PubMed] [Google Scholar]