Abstract
Parvovirus B19 (PVB19) has been known to cause acute glomerulonephritis and nephrotic syndrome with various renal histologic patterns, such as endocapillary glomerulonephritis and collapsing glomerulopathy. Remission is achieved spontaneously or by treatment with steroid and/or immunosuppressants in most patients, except those with sickle cell anemia or two APOL1 risk alleles. In this study, we report the case of a previously healthy 5-year-old boy with infection-related glomerulonephritis (IRGN) associated with PVB19 that progressed to end-stage renal disease (ESRD). He presented with macrohematuria, nephrotic-range proteinuria, and progressive renal dysfunction despite treatment with methylprednisolone pulse therapy, plasmapheresis, and intravenous immunoglobulin. The kidney biopsy specimens exhibited endocapillary infiltration and mesangiolysis with cellular crescent formation. Immunofluorescence analysis revealed that IgA was dominantly positive in the glomeruli, with some co-localized with KM55, which is a specific monoclonal antibody for galactose-deficient IgA1 (Gd-IgA1). The intensity of the KM55 signal in the present patient was weaker than that in patients with IgA nephropathy. To our knowledge, this is the first report of IRGN associated with PVB19 that progressed to ESRD without any underlying diseases. Further investigations are needed to determine the significance of IgA and Gd-IgA1 deposition in IRGN associated with PVB19.
Keywords: Infection-related glomerulonephritis, Parvovirus B19, Galactose-deficient IgA1
Introduction
Parvovirus B19 (PVB19) is the etiological agent of erythema infectiosum, which has been found in association with various kidney diseases, such as acute glomerulonephritis and nephrotic syndrome. Kidney specimens from infected patients display various pathological findings, such as endocapillary glomerulonephritis and collapsing focal segmental glomerulosclerosis [1, 2]. Immunofluorescence analysis revealed subendothelial granular deposits of C3 and IgG in the glomeruli [1]. Previous reports illustrated that patients with sickle cell anemia [3] and one patient with two APOL1 risk alleles [4] progressed to end-stage renal disease (ESRD), whereas remission can occur spontaneously or by treatment with steroids and immunosuppressive therapies in individuals with no underlying diseases [1]. In this study, we report the case of a 5-year-old boy with no underlying diseases who exhibited endocapillary glomerulonephritis with IgA-dominant deposition associated with PVB19 that rapidly progressed to ESRD.
Case report
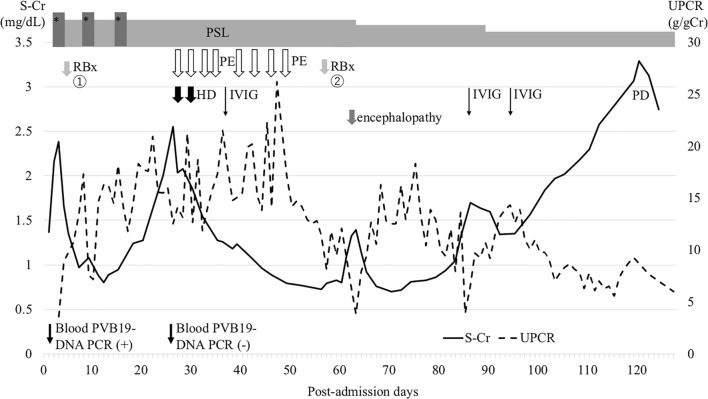
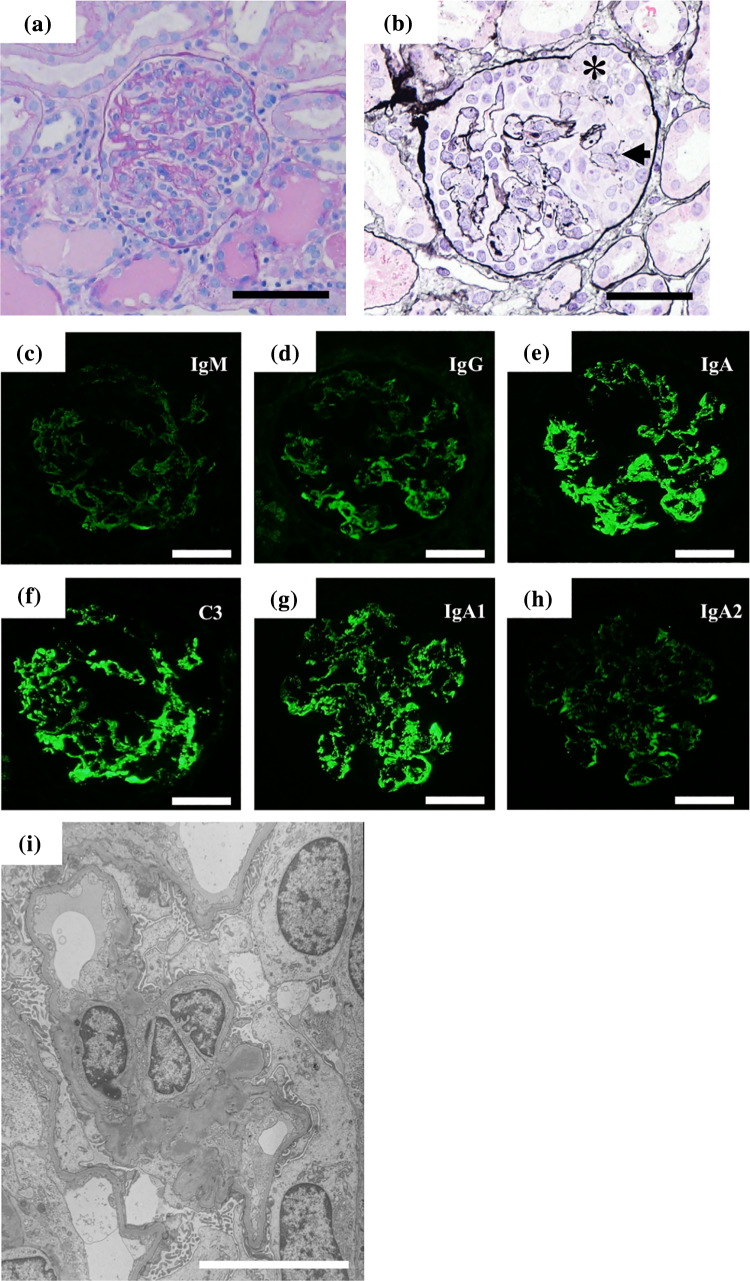
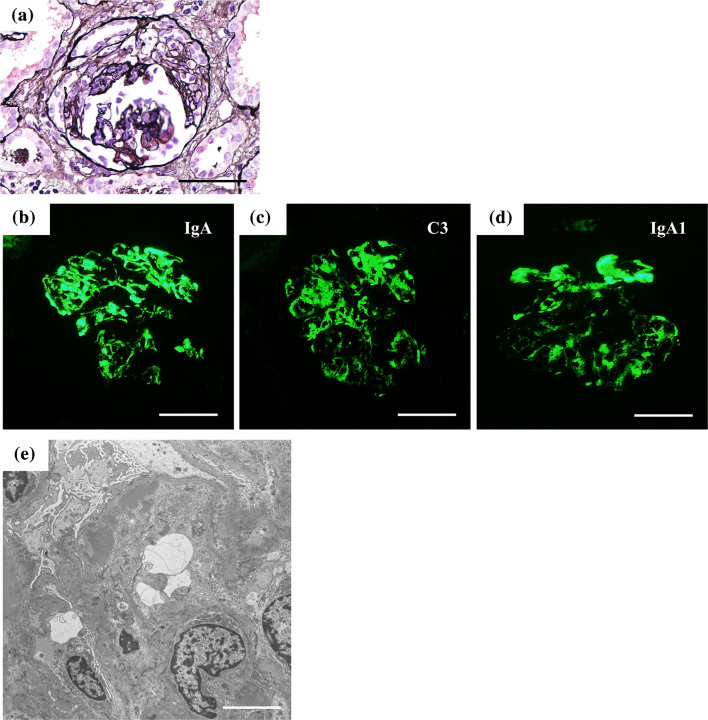
A previously healthy 5-year-old boy presented with macrohematuria followed by erythema on his cheeks and vomiting. He had no family history of kidney disease. Urinary analyses performed at the age of 3 years under the mass screening program in Japan revealed no abnormalities. Four days later, he was admitted due to pretibial edema, proteinuria, and persistent macrohematuria. The initial laboratory evaluation revealed a normal hemoglobin level of 11.6 g/dL, a slightly decreased albumin level of 3.1 g/dL, increased levels of blood urea nitrogen, and creatinine at 74.9 and 1.94 mg/dL, respectively, a potassium level of 4.8 mmol/L, and normal serum complement levels. Antibodies against hepatic B virus and hepatic C virus, as well as antistreptolysin-O titers and antistreptokinase antibodies, were negative. Serum PVB19 DNA PCR and anti-PVB19 IgM antibody were positive. Serum antinucleus, anti-ds-DNA, antineutrophil cytoplasmic, and anti-glomerular basement membrane antibodies were negative. Urinary analysis revealed heavy proteinuria (3+, urinary protein/creatinine ratio = 9.05 g/gCr) and hematuria (occult blood 3+, red blood cells > 100/high power field) with no active casts. Ultrasonography revealed increased echogenicity in both kidneys. Figure 1 shows the clinical course of the present case. The patient was treated with a course of intravenous methylprednisolone pulse therapy [500 mg (29.4 mg/kg) × 3 days] followed by daily oral prednisolone (2 mg/kg body weight). On day 4 after admission, open kidney biopsy was performed. The specimen contained 118 glomeruli, 18 of which (15.3%) had cellular crescents. Light microscopy revealed prominent endocapillary proliferation and mesangiolysis with occasional tuft rupture (Fig. 2a, b), but tubulointerstitial changes were not observed. Immunofluorescence analysis revealed that signals for IgA (Fig. 2e) and C3 (Fig. 2f) were co-dominantly positive in the mesangial area and along the capillary walls. Signals for C1q were negative. Immunofluorescence analysis of IgA subclasses revealed IgA1-dominant deposition (Fig. 2g, h). Electron microscopy showed electron-dense deposits in the subendothelial and mesangial areas (Fig. 2i), but not in the subepithelial area. No humps were observed. Podocyte hypertrophy, foot process effacement, and microvilli degeneration were also observed, which were suggestive of podocyte injury (Fig. 2i). PVB19 DNA was detected in the kidney specimen via PCR. Diagnosis of IgA-dominant infection-related glomerulonephritis (IRGN) associated with PVB19 was made on the basis of the serological and pathological findings. After a course of intravenous methylprednisolone pulse therapy, the patient’s serum creatinine level decreased. Therefore, two additional courses of intravenous methylprednisolone pulse therapy were administered. Serum anti-PVB19 IgG antibody positivity was detected on day 25 after admission, and serum PVB19 DNA was negative on day 27 after admission (Table 1). However, the patient’s serum creatinine level increased again, which prompted us to perform eight sessions of plasma exchange to remove circulating immune complexes. Consequently, the patient’s serum creatinine level decreased from 2.5 to 0.7 mg/dL, whereas macrohematuria and heavy proteinuria persisted and urinary protein to creatinine ratio was 8.1 g/gCr. We performed follow-up biopsy on day 54 after admission. The specimen contained 13 glomeruli, three (23%) of which exhibited fibrocellular crescent formation (Fig. 3a). Global sclerosis was observed in eight (62%) glomeruli. However, there were no findings suggestive of thrombotic microangiopathy, such as endothelial cell swelling and thrombosis in the capillary lumen. Tubulointerstitial infiltration and fibrosis were not observed. Immunofluorescence analysis revealed persistent IgA (IgA1 dominant) and C3 co-dominant depositions in the mesangial area and along the capillary walls (Fig. 3b–d). Electron microscopy revealed persistent electron-dense deposits in the subendothelial and mesangial areas and podocyte injury (Fig. 3e).
Fig. 1.
The clinical course of the patient. Three courses of intravenous methylprednisolone pulse therapy followed by daily oral prednisolone were administered, which resulted in a transient decrease in serum creatinine level. Eight sessions of plasma exchange also resulted in a transient effect on improvement of renal function, but the patient had persistent nephrotic-range proteinuria and progressed to end-stage renal disease. PSL prednisolone, RBx renal biopsy, PE plasma exchange, mPSL methylprednisolone, IVIG intravenous immunoglobulin, UPCR urinary protein to creatinine ratio, S-Cr serum creatinine level, HD hemodialysis, PD peritoneal dialysis. *methylprednisolone pulse therapy
Fig. 2.
Kidney biopsy findings of the first biopsy. Light microscopy uncovered diffuse endocapillary hypercellularity and mesangiolysis (a) periodic acid-Schiff stain; b periodic acid silver-methenamine stain. The arrow indicates a rupture of the capillary loop (b). The asterisk indicates cellular crescent (b). Immunofluorescence study for IgM (c), IgG (d), IgA (e) and C3 (f) showed IgA-dominant depositions in the mesangial areas and along the capillary walls. Signals for C3 was also intense. Immunostaining of IgA subclasses showed intense signals for IgA1 (g), while signals for IgA2 were trace (h). Scale bars = 50 μm. i Electron microscopy showed electron-dense deposits in the subendothelial and mesangial areas. Podocyte hypertrophy, foot process effacement, and microvilli degeneration were also observed (Scale bar = 20 μm)
Table 1.
Laboratory test results of serum anti-parvovirus B19 (PVB19) specific IgM and IgG antibodies, serum PVB19 DNA, serum IgA, and Gd-IgA1
| Days after admission | |||
|---|---|---|---|
| Four (After a course of mPSL pulse therapy) | 23 (before PE) | 120 (end-stage kidney disease) | |
| Anti-PVB19-IgM antibody (EIA) | 9.06 | 7.64 | 1.92 |
| Anti-PVB19-IgG antibody (EIA) | ND | 11.5 | 3.34 |
| Serum PVB19-DNA (PCR) | Positive | Negative | ND |
| Serum IgA (mg/dL) | 163 | ND | 135 |
| Serum Gd-IgA1 (μg/mL) | 2.8 | ND | 1.1 |
PVB19 parvovirus B19, EIA enzyme immunoassay, Gd-IgA1 galactose-deficient IgA1, mPSL methylprednisolone, PE plasma exchange, ND not done
Fig. 3.
Kidney biopsy findings of the follow-up biopsy. Light microscopy uncovered fibrocellular crescent formation (a). Periodic acid-Schiff stain; Intense signals were detected for IgA (b), C3 (c), and IgA1 (d) in the mesangial areas and along the capillary walls. Scale bars = 50 μm. e Foot process effacement, microvilli degenerations, and persistent electron-dense deposits in the subendothelial and mesangial areas were observed on electron microscopy (Scale bar = 20 μm)
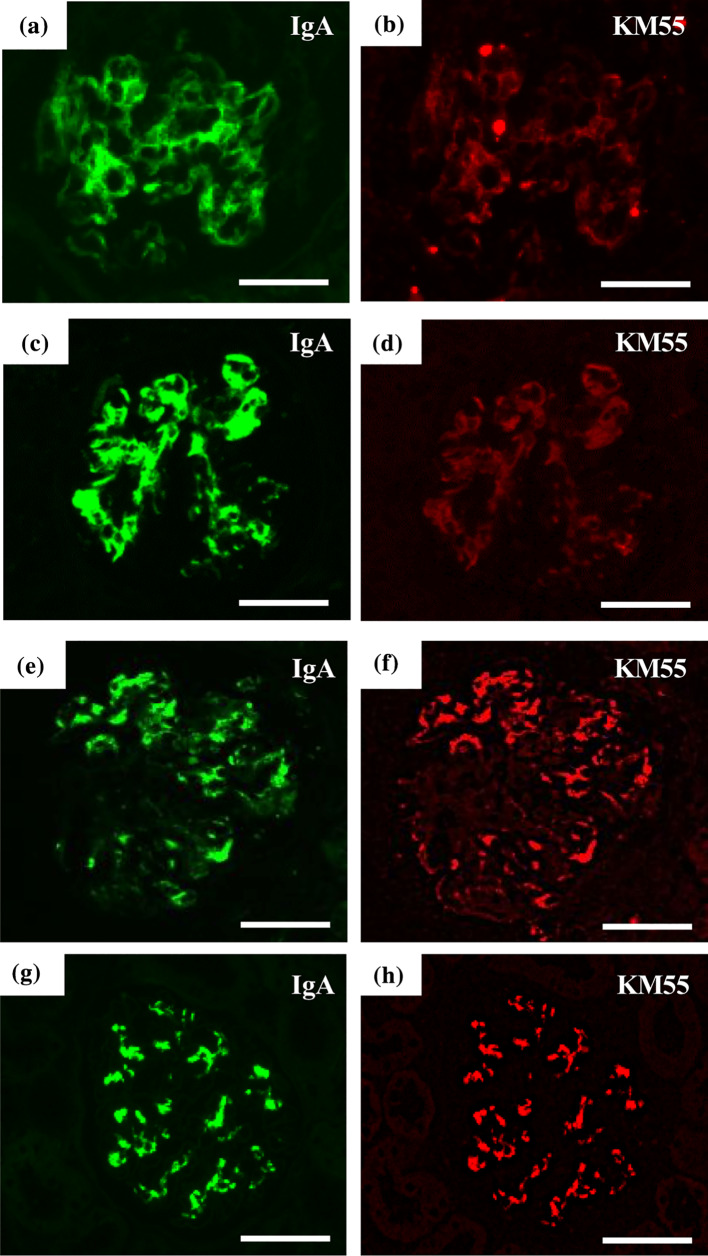
On day 60 after admission, the patient presented with headache, vomiting, and decreased urine volume. His systolic blood pressure was 158 mmHg. He developed impaired consciousness and rightward deviation of the eye despite an intravenous infusion of nicardipine hydrochloride. Brain MRI revealed cytotoxic edema in the left temporal cortex. Bacterial culture, herpes simplex virus DNA PCR, and PVB19 DNA PCR of cerebrospinal fluid were negative. His mental status improved without any neurological sequelae, and follow-up brain MRI revealed no abnormalities, which was compatible with a diagnosis of hypertensive encephalopathy. Further treatment with methylprednisolone pulse therapy and plasma exchange, which could affect hemodynamics was not performed. His renal function continued to deteriorate despite the administration of intravenous immunoglobulin, and the patient eventually developed ESRD 4 months after onset. Hematuria and proteinuria persisted until 2 months after initiation of peritoneal dialysis. To investigate the significance of IgA1 deposition in kidney biopsy specimens, immunohistochemical analysis of galactose-deficient IgA1 (Gd-IgA1) was performed using an anti-Gd-IgA1 monoclonal antibody (KM55). As a result, some amount of IgA was co-localized with KM55 in the glomeruli of both the first and follow-up biopsy specimens (Fig. 4a–d). Signals for KM55 in both the first and follow-up biopsy specimens were weaker than those for KM55 in the glomeruli of patients with IgA nephropathy (Fig. 4b, d, f, h). The patient’s serum IgA level at admission was 163 mg/dL. After one course of methylprednisolone pulse therapy, his serum Gd-IgA1 was 2.8 μg/mL, which further decreased to 1.1 µg/mL when the patient developed ESRD. The patient’s serum IgA level at the time ESRD developed was 135 mg/dL (Table 1).
Fig. 4.
Immunofluorescence study of IgA and KM55. Immunofluorescence study of IgA and KM55 were performed simultaneously in the first and follow-up biopsy specimens of the present case (a–d) and in glomeruli of two patients with IgA nephropathy (e–h). Some amount of IgA (a) was co-localized with KM55 (b) in the glomeruli of the first biopsy specimen. The follow-up biopsy specimen showed similar findings (c, d). IgA and KM55 were co-localized in two patients with IgA nephropathy (e–h). Signals for KM55 in both the first and the follow-up biopsy specimens of the present case were weaker than those in the glomeruli of patients with IgA nephropathy (b, d, f, h). Scale bars = 50 μm
Discussion
Nasr et al. described the diagnostic criteria of IRGN, in which at least three of the following clinical and pathological findings were required: (1) clinical or laboratory evidence of infection preceding or at the onset of glomerulonephritis; (2) decreased serum complement; (3) endocapillary proliferative and exudative glomerulonephritis; (4) C3-dominant or co-dominant deposition in glomeruli on immunofluorescence staining; and (5) hump-shaped subepithelial deposits on electron microscopy [5]. The present case fulfilled criteria 1, 3, and 4, which was suggestive of a diagnosis of IRGN. The present case showed IgA-dominant deposition in the glomeruli as revealed via immunofluorescence analysis. Previous reports stated that IgA was positive in the glomeruli of 13 (54%) of 24 patients with IRGN associated with PVB19 [1, 6–8]. However, they did not exhibit IgA-dominant deposition. Therefore, this is the first report to describe IgA-dominant IRGN associated with PVB19.
IgA nephropathy and IgA vasculitis with nephritis facilitated differential diagnoses in the present case. Suzuki et al. reported that Gd-IgA1 depositions were observed in the glomerulus of the patients with IgA nephropathy and IgA vasculitis specifically by immunohistochemistry using KM55 [9]. In our patient, the signals for KM55 were weaker than those in patients with IgA nephropathy, and only a part of IgA was co-localized with KM55. In addition, the patient’s serum IgA level was not elevated when he developed ESRD, whereas his serum Gd-IgA1 level was decreased. These findings suggest that the pathogenesis of glomerulonephritis in our patient is different from that in patients with IgA nephropathy or IgA vasculitis with nephritis. In addition, the patient’s clinical course was unusual for IgA nephropathy and IgA vasculitis with nephritis because it rapidly progressed to ESRD despite treatment with methylprednisolone pulse therapy and plasma exchange [10]. Nevertheless, exacerbation of IgA nephropathy or IgA vasculitis induced by PVB19 infection cannot be excluded, because IgA nephropathy and IgA vasculitis with nephritis presenting as a rapidly progressive form of glomerulonephritis have been described [10, 11].
Previous reports indicated that IRGN associated with PVB19 in patients with sickle cell anemia and a patient carrying two APOL1 risk alleles progressed to severe proteinuria and progressive chronic kidney disease [3, 4]. Contrarily, in patients with IRGN associated with PVB19 without any underlying diseases, partial or complete remission with normal kidney function was achieved spontaneously or following immunosuppressive treatment with prednisolone, cyclosporine, and methylprednisolone pulse therapy [1, 12, 13]. Therefore, the clinical course of the present case was exceptional because PVB19 infection caused rapid progression to ESRD despite the absence of underlying diseases and treatment with methylprednisolone pulse therapy and plasma exchange. The present patient showed crescent formation in the glomeruli. To date, two patients with crescentic glomerulonephritis induced by PVB19 have been reported. Spontaneous remission was achieved in one patient, and partial remission with mild proteinuria and normal kidney function was achieved via methylprednisolone pulse therapy in the other patient [12, 13]. In the present case, there were pathological findings suggestive of podocyte injury such as hypertrophy, foot process effacement, and microvilli degeneration on electron microscopy, which may have contributed to heavy proteinuria. Reportedly, IgA-dominant IRGN often manifests as heavy proteinuria and AKI, and the prognosis is worse than that related to other types of IRGN [14]. Renal histology is characterized by diffuse proliferative glomerulonephritis and crescent formation [14], which was also observed in our patient. Persistent IgA deposition in the glomeruli observed on electron microscopy and immunofluorescence analysis despite the treatment with methylprednisolone pulse therapy and plasma exchange may also have contributed to the rapid progression to ESRD. In addition, the present case exhibited persistent positivity for PVB19 DNA in the second kidney biopsy specimen. It has been described that PVB19 DNA was detected in 25% of kidney specimens from control subjects such as patients with hematuria, minimal change disease, and thin basement membrane disease, in addition to a normal portion of nephrectomies for renal cell carcinoma [2]. Therefore, it is unclear whether the persistent detection of PVB19 DNA in kidney specimens was associated with the pathogenesis of rapid progression to ESRD in the present case. With regard to treatment, it has been described that a course of intravenous methylprednisolone pulse therapy and administration of calcineurin inhibitors successfully ameliorated proteinuria and kidney function in IRGN associated with PVB19 [6]. However, we could not use a calcineurin inhibitor because of the risk of hypertension. Cyclophosphamide might have been a treatment option for rapidly progressive glomerulonephritis, although caution is needed for side effects such as bone marrow toxicity and gonadal toxicity in the presence of renal dysfunction, mandating appropriate dose adjustment [15].
IgA-dominant deposition in IRGN occurs via the pathway of major histocompatibility complex (MHC) class II molecules on the membrane of antigen-presenting cells binding with pathogenic proteins. This forms an MHC class II complex, which binds to the T cell antigen receptor Vβ region, inducing massive T-cell activation and cytokine bursts and activating B cells to produce polyclonal IgA [16]. Tzang et al. demonstrated that purified IgG from rabbits immunized with VP1 protein, a component of PVB19, upregulated MHC class II molecule expression and TNF-α production in endothelial cells [17]. This mechanism might explain the pathogenesis of IgA production induced by PVB19 infection in our patient. The recent studies revealed the involvement of Toll-like receptor 9 (TLR9) in the pathogenesis of IgA nephropathy. Suzuki et al. conducted a quantitative analysis of mRNAs of TLRs in the splenocytes of ddY mice, which spontaneously develop IgA nephropathy [18]. Mice with IgA nephropathy that were housed in conventional conditions had more severe renal injuries and higher levels of TLR9 transcripts than their counterparts that were housed in specific pathogen-free conditions [18]. Furthermore, the researchers found that nasal challenge with cytosine linked to a guanine by a phosphate-bound oligodeoxynucleotide (CpG-ODN), which is a ligand for TLR9, aggravated renal injury and increased serum and mesangial IgA levels [18]. The consensus sequence of CpG-ODN is located in the PVB19 [19]. In addition, it has been reported that TLR9 protein expression was increased significantly in COS-7 cells transfected with a component of the PVB19 DNA genome [20]. Therefore, it may be presumed that PVB19 infection induced Gd-IgA1 production via the TLR9 pathway, although it is unclear whether Gd-IgA1 contributed to the progression of glomerulonephritis in the present case.
In conclusion, we reported a case of IRGN associated with PVB19 that progressed to ESRD without any underlying diseases. Further investigations are needed to and determine the significance of IgA and Gd-IgA1 deposition in patients with IRGN associated with PVB19.
Acknowledgements
This work was supported by MEXT/JSPS KAKENHI (Grant number 18K07857 to M. H). The authors would like to thank MARUZEN-YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei-honyaku/) for the English language editing.
Abbreviations
- PVB19
Parvovirus B19
- IRGN
Infection-related glomerulonephritis
- ESRD
End-stage renal disease
- Gd-IgA1
Galactose-deficient IgA1
- PCR
Polymerase chain reaction
- TLR
Toll-like receptor
- CpG-ODN
Cytosine linked to a guanine by a phosphate-bound oligodeoxynucleotide
Compliance with ethical standards
Conflict of interest
The authors confirm that they have nothing to declare.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from the patient whose case in reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marco H, Guermah I, Matas L, et al. Postinfectious glomerulonephritis secondary to erythrovirus B19 (parvovirus B19): case report and review of the literature. Clin Nephrol. 2016;85:238–244. doi: 10.5414/CN108694. [DOI] [PubMed] [Google Scholar]
- 2.Mudgul A, Nast CC, Bagga A, et al. Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int. 2001;59:2126–2133. doi: 10.1046/j.1523-1755.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 3.Wierenga KJ, Pattison JR, Brink N, et al. Glomerulonephritis after human parvovirus infection in homozygous sickle-cell disease. Lancet. 1995;19:475–476. doi: 10.1016/S0140-6736(95)91324-6. [DOI] [PubMed] [Google Scholar]
- 4.Besse W, Mansour S, Jatwani K, Nast CC, Brewster UC. Collapsing glomerulopathy in a young woman with APOL1 risk alleles following acute parvovirus B19 infection: a case report investigation. BMC Nephrol. 2016;17:125. doi: 10.1186/s12882-016-0330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasr SH, D’Agati VD. IgA–dominant postinfectious glomerulonephritis: a new twist on an old disease. Nephron Clin Pract. 2011;119:18–25. doi: 10.1159/000324180. [DOI] [PubMed] [Google Scholar]
- 6.Hara S, Hirata M, Ito K, et al. Post-infectious acute glomerulonephritis with podocytopathy induced by parvovirus B19 infection. Pathol Int. 2018;68:190–195. doi: 10.1111/pin.12643. [DOI] [PubMed] [Google Scholar]
- 7.Takeda S, Takaeda C, Takazakura E, Haratake J. Renal involvement induced by human parvovirus B19 infection. Nephron. 2001;89:280–285. doi: 10.1159/000046086. [DOI] [PubMed] [Google Scholar]
- 8.Ieiri N, Hotta O, Taguma Y. Characteristics of acute glomerulonephritis associated with human parvovirus B19 infection. Clin Nephrol. 2005;64:249–257. doi: 10.5414/CNP64249. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Yasutake J, Makita Y, et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactosedeficient IgA1–oriented pathogenesis. Kidney Int. 2018;93:700–705. doi: 10.1016/j.kint.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Hattori M, Ito K, Konomoto T, Kawaguchi H, Yoshioka T, Khono M. Plasmapheresis as the sole therapy for rapidly progressive Henoch–Schönlein purpura nephritis in children. Am J Kidney Dis. 1999;33:427–433. doi: 10.1016/S0272-6386(99)70178-2. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu A, Takei T, Moriyama T, Itabashi M, Uchida K, Nitta K. Clinical and pathological studies of IgA nephropathy presenting as a rapidly progressive form of glomerulonephritis. Intern Med. 2013;52:2489–2494. doi: 10.2169/internalmedicine.52.0420. [DOI] [PubMed] [Google Scholar]
- 12.Uchida T, Oda T, Watanabe A, et al. Transition from endocapillary proliferative glomerulonephritis to membranoproliferative glomerulonephritis in a patient with a prolonged human parvovirus B19 infection. Clin Nephrol. 2014;82:62–67. doi: 10.5414/CN107822. [DOI] [PubMed] [Google Scholar]
- 13.Nakazawa T, Tomosugi N, Sakamoto K, et al. Acute glomerulonephritis after human parvovirus B19 infection. Am J Kidney Dis. 2000;35:E31. doi: 10.1016/S0272-6386(00)70070-9. [DOI] [PubMed] [Google Scholar]
- 14.Bu R, Li Q, Duan Z, et al. Clinicopathologic features of IgA-dominant infection-associated glomerulonephritis: a pooled analysis of 78 cases. Am J Nephrol. 2015;41:98–106. doi: 10.1159/000377684. [DOI] [PubMed] [Google Scholar]
- 15.Ponticelli C, Escoli R, Moroni G. Does cyclophosphamide still play a role in glomerular diseases? Autoimmun Rev. 2018;17:1022–1027. doi: 10.1016/j.autrev.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Koyama A, Kobayashi M, Yamaguchi N, et al. Glomerulonephritis associated with MRSA infection: a possible role of bacterial superantigen. Kidney Int. 1995;47:207–216. doi: 10.1038/ki.1995.25. [DOI] [PubMed] [Google Scholar]
- 17.Tzang BS, Tsai CC, Chiu CC, Shi JY, Hsu TC. Up-regulation of adhesion molecule expression and induction of TNF-α on vascular endothelial cells by antibody against human parvovirus B19 VP1 unique region protein. Clin Chim Acta. 2008;395:77–83. doi: 10.1016/j.cca.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Suzuki Y, Narita I, et al. Toll–like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol. 2008;19:2384–2395. doi: 10.1681/ASN.2007121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo YM, Ishii K, Horikawa M, et al. CpG–ODN 2006 and human parvovirus B19 genome consensus sequences selectively inhibit growth and development of erythroid progenitor cells. Blood. 2010;115:4569–4579. doi: 10.1182/blood-2009-08-239202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu GJ, Tzang BS, Tsai CC, et al. Effects of human parvovirus B19 on expression of defensins and toll-like receptors. Chin J Physiol. 2011;54:367–376. doi: 10.4077/CJP.2011.AMK075. [DOI] [PubMed] [Google Scholar]