Abstract
Background and Objectives:
The respiratory tract is the most common site for developing fungal infections. People who have a weakened immune system are more susceptible to respiratory system involvement with fungi. Fungal infections of the respiratory tract are largely unrecognized and their true burden is elusive. Therefore, the aim of the current study was to evaluate the clinical spectrum, demographic characteristics, risk factors, and etiology of fungal respiratory infections in 384 patients hospitalized in pulmonary units of Razi hospital, Guilan province, Iran.
Materials and Methods:
A total of 384 lung specimens (192 Bronchoalveolar lavages (BAL) and 192 sputa) were obtained from patients who met the inclusion criteria. All samples were analyzed by direct microscopy and culture. Fungal identification was accomplished by internal transcribed spacer (ITS) and beta-tubulin sequencing. Also, in patients suspected to invasive pulmonary aspergillosis BAL specimens were tested for galactomannan (GM) antigen. According to the host factors (clinical symptoms, radiology findings and predisposing factors which were defined as inclusion criteria), and the positive results in direct examination, culture and serology (GM for aspergillosis) the infection was confirmed.
Results:
Fungal respiratory infection was confirmed in 137 cases (35.67%) including 86 (62.77%) males and 51 (37.23%) females and the highest prevalence of infection was found in the age group of 46–72 years (n=75, 54.74%). Cough (n=129, 94.16%), dyspnea (n=111, 81.02%), purulent sputum (n=85, 62.04%) and weight loss (n=77, 56.2%) were the predominant symptoms. Tuberculosis (n=34, 24.81%), taking chemotherapy regimen (n=30, 21.89%) and diabetes mellitus (n=27, 19.70%) were the predominant underlying conditions. Candida albicans (37.22%) and Candida tropicalis (21.89%) represent the two most commonly isolated species in the current study. Furthermore, according to revised EORTC/MSG (2008) definitions for invasive fungal infections, from 5 cases of pulmonary aspergillosis, 2 (40%) cases of probable invasive pulmonary aspergillosis (IPA) and 3 (60%) cases of possible IPA were diagnosed.
Conclusion:
According to the results of this study, infected infants with congenital CMV infection could identify at early stage by testing Guthrie cards (within 21 days of life). Furthermore, since there is a lack of CMV knowledge in our population, educating and effective counseling by obstetricians/gynecologists to the pregnant women are recommended.
Keywords: Bronchoalveolar lavage, Sputum, Candidiasis, Fungal respiratory infections, Invasive pulmonary aspergillosis, Galactomannan antigen
INTRODUCTION
Respiratory tract infections are globally responsible for one-third of infectious disease-associated mortality, accounting for 4.3 million annual deaths. Despite treatment, most invasive pulmonary fungal infections are associated with high mortality rates of > 50% (1, 2). The air we breathe is filled with thousands of fungal spores (conidia). After inhalation these tiny elements, hosts may have no symptoms or may cough up blood or have a fever or chest pain or may have symptoms ranging from allergies to life-threatening invasive mycoses (3, 4). The outcome depends on the immune status of the host. During recent decades, fungal respiratory infections are being recognized with increasing frequency in the world in parallel with an expanding population of immunocompromised patients, such as those with human immunodeficiency virus (HIV), cancer and transplant patients who are taking certain immunosuppressive drugs (5).
In Iran, the prevalence of candidiasis has doubled in some hospitals, especially in intensive care units. In most Iranian reports in the past two decades, Aspergillus has been the second most common cause of invasive fungal infections that have been isolated from patients (6).
In the last decade, less well-known molds, such as members of the Fusarium and Scedosporium genera, and newly described Aspergillus species are increasingly found to cause invasive pulmonary infection. Also, Cryptococcus gattii has emerged in North America as a cause of pulmonary infection (7).
The incidence and etiology of pulmonary fungal infections can vary in various types of patient’s hospital settings, and geographical locations. In one study, Aspergillus spp. were isolated from 33% (86/251 cases) of lung-transplantation recipients, which involved colonization (n=50), tracheobronchial lesions (n=17) or invasive aspergillosis (n=19) (8). Also, invasive pulmonary aspergillosis mortality of neutropenic patients was 40 to 60% in early reports (9). Candida infection was reported as the most dominant pulmonary fungal infection in patients with non-hematologic malignant tumors and in non-lung solid organ transplant recipients. The diagnosis of this serious infection is so problematic and it can annually cause many deaths around the world.
Taken together, the clinicians must remain vigilant for invasive and serious fungal lung infections even to individuals who were considered only moderately immune-compromised. The purpose of the current study was to determine the clinical spectrum and etiology of fungal respiratory infections and identify the underlying risk factors in 384 hospitalized patients in Razi hospital, Rasht, Iran.
MATERIALS AND METHODS
Ethics statement.
Informed consent to take part in the study has been obtained from subjects or their guardians prior to sample collection. This study was approved by ethical committee of Tehran University of Medical Sciences (the number of Ethics Committee protocol: IR.TUMS.SPH.REC.1397.002).
Sampling.
This descriptive cross-sectional study was carried out over a period of two years, from October 2017 to October 2019 in Razi hospital, the lung care center of Guilan province, Iran. The presence of two or more following conditions was used as inclusion criteria in this study:
(1) Patients who displayed at least one of the following host factors: receiving chemotherapy within the last 3 months before admission in order to treat solid tumors, chronic obstructive pulmonary disease (COPD), steroid use: at least 4 mg methylprednisolone (or equivalent) per day for at least 7 days in the past 3 weeks before admission or a cumulative dose of at least 250 mg of methylprednisolone (or equivalent) in the past 3 months before enrollment and recipient of any other immunosuppressive treatment (tacrolimus, cyclosporine, methotrexate, cyclophosphamide, and sirolimus).
(2) Patient with clinical symptoms indicative of pulmonary fungal diseases according to a pulmonary diseases specialist opinion (dyspnea, cough, high and persistent fever, recurrent fever, chest pain, purulent sputum, weight loss, night fever, hemoptysis, rhinitis, and wheezing)
(3) Patients with suspicious radiographic findings indicative of pulmonary fungal diseases according to a pulmonologist opinion.
Patients who had taken any systemic antifungal agents before enrollment for treating infections other than pulmonary fungal disease were excluded from the study in order to prevention of false-negative results.
A total of 384 lung specimens including 192 Bronchoalveolar lavage (BAL) and 192 sputum samples were obtained from patients hospitalized in pulmonary units of Razi hospital. Demographic features including age, gender, underling disease and patient’s clinical manifestations (fever, purulent sputum, dyspnea, etc.) were recorded. Samples were collected in sterile dry containers and transported to laboratory. Sputum samples were concentrated and BAL samples were centrifuged and the deposit was used for examination. For direct microscopic examination, the samples were dissolved in KOH 10% solution and observed under a microscope for fungal elements. Calcofluor white staining (Sigma, Deisenhofen, Germany) was done to detect mycelial and yeast cells and Indian ink was used to check for Cryptococcus neoformans (10, 11). All specimens were cultured on Sabouraud Dextrose Agar (SDA) with chloramphenicol and Brain Heart Infusion (BHI) agar media (Merck, Germany). Culture media were surveyed after incubation at 30 and 37°C for 48–72 hours. Culture media for those cases with no growth were maintained up to two weeks. Any growth obtained was further identified by its rate of growth, colony morphology and lactophenol cotton blue mounts. Slide culture was performed as required (12, 13). Yeast isolates were identified based on production chlamydoconidia in cornmeal agar (Becton, France) and colony color on chromogenic CHROMagar Candida medium (CHROMagar, Paris, France) (14). Also, for all patients with a positive direct examination of the BAL sample in addition to isolation of Aspergillus species in culture who were suspected to invasive pulmonary aspergillosis (IPA) BAL specimens were tested for galactomannan (GM) antigen with ELISA technique. Furthermore, for confirmation of diagnosis, all isolates were subjected to PCR and sequencing techniques.
BAL GM test.
Platelia Aspergillus GM EIA (Bio-Rad, France) was used to measure the galactomannan of lavage samples according to the manufacturer procedures (15). Briefly, 300 μl of serum or BAL fluid was added to 100 μl of treatment solution, boiled for three minutes at 104°C and then centrifuged for 10 minutes in 10000× g. Next, 50 μl of supernatant and 50 μl of conjugate were mixed and incubated in microtiter plates precoated with monoclonal antibody EB-A2 for 90 minutes at 37°C. The plates were washed five times; after which they were incubated with 200 μl of tetramethylbenzadine in the dark for 30 minutes. The reaction was stopped by 100 μl of sulfuric acid and absorbance at 450 and 620 nm read using a plate reader. Positive and negative controls were included in each assay. Results were recorded as an index relative to the optical density (OD) of the cut-off control. The GM of lavage and serum was considered positive when OD index was ≥ 0.5. All positive cases were repeated in the same sample before they were considered positive.
Molecular technique.
DNA Extraction: Fungal genomic DNA was extracted from harvested colonies using glass bead disruption method (16).
PCR conditions and sequencing.
PCR amplification for each isolate was performed as described previously (17, 18). To discriminate Aspergillus isolates at the species level the Beta tubulin gene of Aspergillus species was amplified using the forward (Bt2a: 5′-GGTAACCAAATCGGTGCTGCTTTC-3′) and reverse (Bt2b: 5-ACCCTCAGTGTAGTGACCCTTGGC-3) primers. Also, for identification of other fungal isolates in the species level the universal primers used for fungal amplification were ITS1 (5′TCC GTA GGT GAA CCT GCG G 3′), which hybridizes at the end of 18S rDNA, and ITS4 (5′TCC TCC GCT TAT TGA TAT GC 3), which hybridizes at the beginning of 28S rDNA (Life Technologies, Barcelona, Spain).
Positive PCR products were sent for sequencing at Bioneer Advanced Nucleic Acids core facility. Sequences were then parsed from the coting and separately used to perform individual nucleotide–nucleotide searches using the BLASTn algorithm at the NCBI website (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Fungal identifications were made based on maximum identities ≥ 99% and query coverage ≥ 98% with this method. All sequences were deposited in Gen-Bank under the accession number reported in Table 1.
Table 1.
GenBank accession numbers of DNA sequences included in this study.
Statistical tests.
The data analysis was performed by SPSS software (V.20). The study was assessed by using standard Chi-squared and 95% Confidence intervals (CI). Statistically, P value < 0.5 was considered as significant difference or correlation.
RESULTS
Totally 384 hospitalized patients (including 238 Males and 146 females) suspected to fungal respiratory infections according to inclusion criteria were enrolled. Of this population, in 137 patients (35.67%), respiratory fungal infection was proved. The patients were within the age range of 1–86 years and the highest prevalence of respiratory fungal infections was found in the age group of 46–72 years (n=75, 54.74%) and showed the age of subjects was not significantly effective on the incidence of fungal respiratory infections (P=0.546) (Table 2).
Table 2.
The distribution of 137 patients with pulmonary fungal infections hospitalized in pulmonary units based on age groups, gender and underlying diseases
| Patients | P-valuea | ||||||
|---|---|---|---|---|---|---|---|
| Number | Percentage | ||||||
| Age groups (years) | 1–45 | 33 | 24.08 | 0.546 | |||
| 46–72 | 75 | 54.74 | |||||
| 73–86 | 29 | 21.16 | |||||
| Gender | Female | 86 | 62.77 | 0.8111 | |||
| Male | 51 | 37.23 | |||||
| Underlying diseases | Tuberculosis | 34 | 24.81 | <0.001 | |||
| Breast cancer | 9 | 6.56 | |||||
| Prostate cancer | 7 | 5.1 | |||||
| Solid tumors | Ovarian cancer | 30 | 6 | 21.86 | 4.37 | ||
| Lung cancer | 4 | 2.92 | |||||
| Esophageal adenocarcinoma | 3 | 2.1 | |||||
| Osteosarcoma | 1 | 0.72 | |||||
| Diabetes mellitus | 27 | 19.70 | |||||
| Kidney failure | 10 | 7.29 | |||||
| Heart failure and hypertension | 10 | 7.29 | |||||
| Respiratory failure | 10 | 7.29 | |||||
| Rheumatoid arthritis | 3 | 2.18 | |||||
| Autoimmune diseases | Multiple Sclerosis | 8 | 2 | 5.83 | 1.46 | ||
| Systemic lupus erythematosus | 2 | 1.46 | |||||
| Pemphigus vulgaris | 1 | 0.72 | |||||
| Without underlying disease | 4 | 2.92 | |||||
| Iron deficiency anemia | 2 | 1.46 | |||||
| AIDS b | 1 | 0.72 | |||||
| Asthma | 1 | 0.72 | |||||
| Total | 137 | 100 | |||||
P-value, probability value;
AIDS, Acquired immunodeficiency syndrome common symptoms.
The ratio of infected females to males was 51 (37.23%) to 86 (62.77%) and statistical analysis could not find any association between the distribution of hospitalized patients with proven respiratory fungal infections and patient’s gender (P= 0.811) (Table 2).
Previous history of tuberculosis, taking chemotherapy regimens at the time of study (in order to treat solid tumors) and diabetes mellitus were found in 24.81%, 21.86% and 19.70% of patients, respectively. These predisposing factors were significantly associated with the occurrence of respiratory system involvement with fungi (P=0.000) (Table 2).
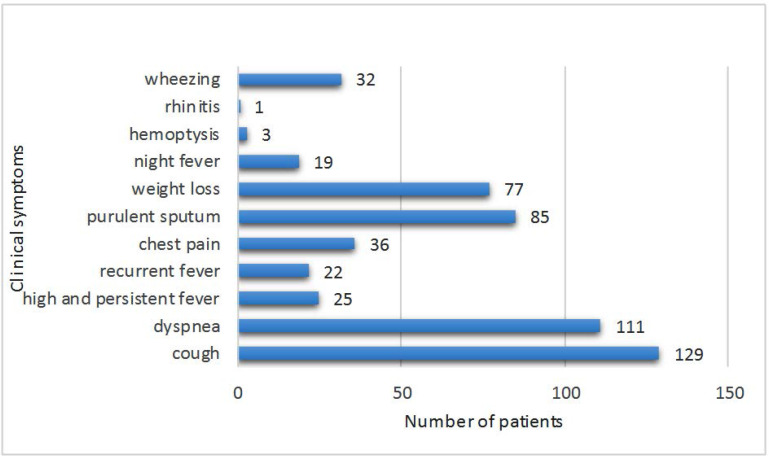
Among 137 positive cases, cough (n=129, 94.16%), dyspnea (n=111, 81.02%), purulent sputum (n=85, 62.04%) and weight loss (n=77, 56.2%) were the most there was a significant relationship between developing respiratory fungal infections and clinical symptoms (P=0.000) (Fig. 1).
Fig. 1.
The clinical spectrum and different symptoms among 137 hospitalized patients with respiratory fungal infections
Statistical analysis showed that Candida albicans (37.22%) and Candida tropicalis (21.89%) represent the two most commonly isolated species in the current study followed by Candida glabrata (12.4%), Candida krusei (5.83%), Candida parapsilosis (5.1%), Trichosporon asahii (2.18%), Geothricum candidum (2.18%), Aspergillus flavus (2.18%), Rhizopus orizae (0.72%), Aspergillus niger (0.72), Aspergillus fumigatus (0.72%) and Alternaria alternata (0.72%). Also, mixed fungal elements in some examined specimens were detected in this study (Table 3).
Table 3.
The frequency distribution of fungal elements isolated from 137 patients with pulmonary fungal infections hospitalized in pulmonary units
| Isolated fungi | Frequency | Percent |
|---|---|---|
| Candida albicans | 51 | 37.22 |
| Candida tropicalis | 30 | 21.89 |
| Candida glabrata | 17 | 12.4 |
| Candida krusei | 8 | 5.83 |
| Candida parapsilosis | 7 | 5.1 |
| Candida glabrata + Candida albicans | 5 | 3.56 |
| Rhodotorula mucilaginosa + Candida albicans | 4 | 2.92 |
| Trichosporon asahii | 3 | 2.18 |
| Geotrichum candidum | 3 | 2.18 |
| Aspergillus flavus | 3 | 2.18 |
| Rhizopus oryzae | 1 | 0.72 |
| Aspergillus niger | 1 | 0.72 |
| Aspergillus fumigatus | 1 | 072 |
| Alternaria alternata | 1 | 0.72 |
| Candida krusi + Candida parapsilosis | 1 | 0.72 |
| Rhodotorula mucilaginosa + Trichosporon asahii | 1 | 0.72 |
| Total | 137 | 100 |
In this study, criteria such as presence of invasive forms (numerous budding yeasts, pseudohyphae and true hyphae) in direct examination or significant growth of pure creamy mucoid colonies on culture media were considered to distinguish between Candida colonization and infection of the respiratory tract in patients who met the inclusion criteria (1, 19). According to these defined criteria from 137 patients with respiratory fungal infections, in 124 cases (90.51%) respiratory candidiasis was reported. In 46 cases (37.1%) forming pseudohyphae in direct smear and in 33 cases (26.61%) forming pseudohyphae in direct smear along with significant growth of pure creamy mucoid colonies on culture media were observed in mycological tests of patients with respiratory tract candidiasis (Table 4).
Table 4.
Frequency distribution of respiratory tract candidiasis in 137 hospitalized patients with respiratory fungal infections base on defined criteria for Candida respiratory infection in direct examination and culture.
| Criteria for Candida respiratory infection in direct examination and culture | Frequency | Percent |
|---|---|---|
| Forming pseudohyphae in direct smear | 46 | 37.1 |
| Significant growth of pure creamy mucoid colonies on culture media | 29 | 23.39 |
| Forming numerous budding yeasts in direct smear | 12 | 9.68 |
| Forming pseudohyphae in direct smear + significant growth of pure creamy mucoid colonies on culture media | 33 | 26.61 |
| Forming numerous budding yeasts in direct smear + significant growth of pure creamy mucoid colonies on culture media | 4 | 3.22 |
| Total | 124 | 100 |
From 8 patients suspected to pulmonary aspergillosis, 5 cases (62.5%) of IPA were reported. All of them had positive results in direct examination with forming branching septate hyphae along with positive results in culture media. In 3 patients (2.19%) Aspergillus flavus was the etiologic agent of invasive aspergillosis, in one patient (0.72%) A. fumigatus was the etiologic agent and in one other patient (0.72%) A. niger was the cause of respiratory infection. A. flavus was the most common cause of invasive aspergillosis in present study.
Also during the period of this study GM tests were performed for all patients suspected to invasive aspergillosis. In this EIA test the OD index of 0.5 or more was taken as positive result (Table 5).
Table 5.
GM cut-off value in BAL related to 8 patients suspected to invasive pulmonary aspergillosis hospitalized pulmonary units based on gender
|
Gender GM a cut-off value In BAL b specimens |
Women | Men | Total | |||
|---|---|---|---|---|---|---|
| number | % | number | % | number | % | |
| <0.5 | 2 | 25 | 2 | 25 | 4 | 50 |
| ≥0.5 | 1 | 12.5 | 3 | 37.5 | 4 | 50 |
| Total | 3 | 37.5 | 5 | 62.5 | 8 | 100 |
GM, galactomannan;
BAL, Bronchoalveolar lavage
It should be noted that in the current study, all sequencing results were consistent with macroscopic and microscopic identifications of the isolates.
According to revised EORTC/MSG (2008) definitions for invasive fungal infections (20), from 5 cases of pulmonary aspergillosis, 2 (40%) cases of probable IA and 3 (60%) cases of possible IA were diagnosed in this study (Table 6).
Table 6.
Detailed information of each patient involved with invasive pulmonary aspergillosis categorized based on EORTC/MSC a (2008) criteria
| Row | Age (years) | Gender | Underlying disease | Symptoms | GM c level in BAL d specimen | CT e scan | Direct examination | Culture | IPAf |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | Male | AIDSb | Cough, dyspnea, weight loss | 0.7 | - | Branching septate hyphae | A. fumigatus | Possible |
| 2 | 53 | Male | Solid tumor | Recurrent fever, chest pain, cough | 2.3 | Nodular lesions | Branching septate hyphae | A. flavus | Probable |
| 3 | 35 | Female | Lupus | Cough, dyspnea, Purulent sputum | 0.5 | - | Branching septate hyphae | A .flavus | Possible |
| 4 | 63 | Female | Solid tumor | Cough, Purulent sputum, dyspnea | 0.2 | - | Branching septate hyphae | A. niger | Possible |
| 5 | 71 | Male | Solid tumor | Hemoptysis, dyspnea, chest pain | 2.1 | Cavity | Branching septate hyphae | A. flavus | Probable |
EORTC/MSC; European Organization for Research and Treatment of Cancer and Mycoses Study Group;
AIDS, Acquired immunodeficiency syndrome;
GM, galactomannan;
BAL, Bronchoalveolar lavages;
CT scan, computerized tomography scan;
IPA, Invasive pulmonary aspergillosis
Also in this study, from 137 hospitalized patients with proven respiratory fungal infections, one case of mucormycosis (0.72%) in a 76 years old man with prostate cancer was reported. After culture on SC medium and sequencing, Rhizopus oryzae was reported as the etiologic agent. Also one case of Alternaria alternata (0.72%) was isolated from the BAL sample belonged to a30 years old woman with a history of chronic nasal congestion and drainage, asthma and allergic rhinitis from 8 years ago. Also, 3 cases (2.19%) of geotrichosis due to Geotrichum candidum and 4 cases (2.92%) of trichosporonosis due to Trichosporon asahii, were reported in this study.
DISCUSSION
In the course of their lives humans often come into contact with fungi that are present in the biosphere and the lungs are organs especially predisposed for fungal infections. Most fungal infections occur after inhalation of ubiquitous mycelial fungi in the environment. Also some yeast and yeast-like organisms are opportunistic fungal agents found as part of the normal microflora in human respiratory tract. Respiratory tract infections are globally responsible for one-third of infectious disease–associated mortality, accounting for 4.3 million annual deaths (2, 21). Despite treatment, most invasive fungal infections are associated with high mortality rates of > 50% (2, 22). In this study we evaluated the spectrum of fungal organisms causing infection in 384 patients hospitalized in pulmonary units and in 137 (35.67%) cases respiratory fungal infection was confirmed. In agreement with our results Basiri Jahromi et al. reported the same prevalence for fungal respiratory infections in Iran (23). Also Roohani AH et al. reported a higher prevalence of 26.7% for respiratory fungal infections in immunocompromised patients in India (24). While the Egyptian study of Ahmed and colleagues showed that the prevalence of fungal pneumonias in respiratory intensive care units was 66.67% (25). This inconsistency may be a result of the different patterns of age grouping, diversity in study designs and the different inclusion criteria for patients defined by each research group.
Most of patients in current study (54.74 %) were at an age range of 46–72 years old. Older adults become more susceptible to infections due to predisposing factors such as diabetes, renal insufficiency and arthritis. Also, when people age, there is immunosenescence, which means that the immune system doesn’t function as well or as vigorously. The combination of predisposing factors and the decrease in activity of the immune system can make these age group more prone to infections (26).
In this work, in accordance with the results of another report (23) the ratio of infected females to males was 51 (37.23%) to 86 (62.77%) and there was no significant difference in the prevalence of fungal respiratory infection between the genders. Similar exposure to pollution sources due to equal socio-demographic condition, occupations and responsibilities for men and women in society could be the reason for the similarity observes in the incidence of fungal respiratory infection between two gender groups. Cough (n=129, 94.16%), dyspnea (n=111, 81.02%), purulent sputum (n= 85, 62.04%) and weight loss (n=77, 56.2%) were the most common symptoms among 137 patients with fungal respiratory infection. Considering the fact that fungal respiratory infections often cause symptoms that are similar to other illnesses, such as the flu or tuberculosis, clinical and laboratory findings should be used simultaneously for making the final decision on drug administration.
Tuberculosis was the main predisposing factor observed in 24.81% of patient in current study. Tuberculosis (TB) is still one of the biggest killers among the infectious diseases especially in developing countries like Iran (27). This country shares geographic borders with three countries in which, tuberculosis is endemic: Afghanistan, Iraq and Pakistan. In addition, Iran is in a close association with other countries where tuberculosis is highly prevalent, i.e. China, India, Nepal, Bosnia, Bangladesh, Tajikistan, Sri Lanka and Azerbaijan. It should be noted that the percentage of mycotic infections increase in pulmonary tuberculosis patients. The results of different studies showed that physicians should pay particular attention to fungal co-infection with pulmonary TB (28). A history of taking chemotherapy regimens (21.89%) and diabetes mellitus (19.70%) were the other common underlying conditions for developing fungal respiratory infections. Studies showed that after intensive chemotherapy, the estimated risk of developing invasive pulmonary fungal infection is about 5%, and the reported mortality ranges from 30 to 80% (29). On the other hand, diabetic patients are very often prone to fungal infections, because of higher blood glucose levels which help for the growth of fungi. Diabetics have an immune system with a lower ability to respond to and deal with infections of any type. This means they are more prone to illnesses than the general population. Diabetes can contribute to the development of fungal/bacterial/viral pneumonia, tuberculosis and chronic obstructive pulmonary disease (COPD) (30).
During the laboratory analysis of specimens, a set of 165 fungal isolates including 152 (92.12%) yeasts and 13 (7.88%) molds were recovered from 137 patients. Candioda albicans (n=62, 37.57%) and Candida tropicalis (n=33, 20%) were the most common isolated species in this study. In Accordance with our results, Spahr J et al. reported that Candida albicans and Candida tropicalis are the most important causes of pulmonary involvement (31). Candida species are normal microflora of mucosal surfaces like respiratory tract. In this anatomical site, there is a balance between normal fungal flora and normal bacterial flora. When this balance is disturbed, Candida colonization is replaced by infection due to the creation of invasive forms (forming budding yeast cells, pseudohyphae and true hyphae in the specimen or by significant growth in the form of pure creamy mucoid colonies on culture media (1).
Also in current study, from 5 patients with IPA, in 3 patients (60%) Aspergillus flavus was the etiologic agent of invasive aspergillosis. Since in the most of studies, Candida albicans and Aspergillus flavus were specified as the most prevalent fungal etiology of respiratory infections (31–34), uncommon species like Geothricum candidum, Trichosporon asahii, mucorales fungi and non-albicans Candida species should not be ignored. Given that some uncommon species are intrinsically resistant to routine antifungal drugs, they could cause treatment failure and should be taken into account.
Also according to revised EORTC/MSG (2008) definitions for invasive fungal infections, from 5 cases of invasive pulmonary aspergillosis, 2 (40%) cases of probable IPA and 3 (60%) cases of possible IPA were diagnosed in this study. Studied showed that with increased use of immunosuppressive agents in recent years, the incidence rates of IPA have increased up to 30% in some centers (35–38). A BAL or serum enzyme-linked immunosorbent assay (ELISA) can help us for early diagnosis of IA with galactomannan (GM) detecting, which is a major constituent of Aspergillus cell walls. Raoul Herbrecht et al. in an assay evaluated over a 4-year reported a large-scale evaluation of Aspergillus GM ELISA (39). They stated that of the 67 probable cases of IA, 19 cases were defined by a single positive sputum or tracheal aspiration culture. Also in a large study of GM ELISA performed in allo-HSCT patients, 19 (76%) of the 25 patients that had proven aspergillosis and 14 (93%) of the 15 patients that had probable aspergillosis had positive antigenemia tests (40). To the best of our knowledge, this is the first study on the epidemiology, clinical spectrum, underlying conditions, and demographic characteristics associated with pulmonary fungal infections in Guilan province, located in Iran’s northern region.
CONCLUSION
The prevalence of pulmonary fungal infections in Guilan province was 35.67%. Tuberculosis, receiving chemotherapy within the last 3 months before admission in order to treat solid tumors and diabetes mellitus were important risk factors, and Candida albicans was the most common fungal species responsible for pulmonary fungal infection. The highest prevalence of pulmonary fungal infections was found in the age group of 46–72 years and in male patients. Many physicians missed fungal pulmonary infection because it does not show specific clinical manifestations. Given that some of uncommon causal agents of fungal pulmonary infections are intrinsically resistant to routine antifungal drugs and could cause treatment failure, mycological examinations should be considered for proper treatment.
ACKNOWLEDGEMENTS
This work was supported by the funding from Tehran University of Medical Sciences, Tehran, Iran (grant number: 43972). The authors would like to thank all the staff members of pulmonary units of Razi hospital, Rasht, Guilan.
REFERENCES
- 1.Topely Wilsons. (2005). Microbiology and microbial infections. 10nd ed Hodder Arnold Publisher; London, UK. [Google Scholar]
- 2.Wilson LS, Reyes CM, Stolpman M, Speckman J, Allen K, Beney J. The direct cost and incidence of systemic fungal infections. Value in Health 2002; 5:26–34. [DOI] [PubMed] [Google Scholar]
- 3.Panda B. Fungal infections of lungs: the emerging scenario. Indian J Tuberc 2004; 51:63–69. [Google Scholar]
- 4.Lacey J. (1981). The aerobiology of conidial fungi. In: Biology of conidial fungi. ED, Academic Press; 1st ed Austin, Texas, UK, pp. 373–416. [Google Scholar]
- 5.Roeder A, Kirschning CJ, Rupec RA, Schaller M, Korting HC. Toll-like receptors and innate antifungal responses. Trends Microbiol 2004; 12:44–49. [DOI] [PubMed] [Google Scholar]
- 6.Jahromi SB, Khaksar AA. Deep-seated fungal infections in immunocmpromised patients in Iran. Iran J Allergy Asthma Immunol 2005; 4:27–32. [PubMed] [Google Scholar]
- 7.Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology 2012; 17:913–926. [DOI] [PubMed] [Google Scholar]
- 8.Sole A, Morant P, Salavert M, Peman J, Morales P. Aspergillus infections in lung transplant recipients: risk factors and outcome. Clin Microbiol Infect 2005; 11:359–365. [DOI] [PubMed] [Google Scholar]
- 9.Tripathy U, Yung GL, Kriett JM, Thistlethwaite PA, Kapelanski DP, Jamieson SW. Donor transfer of pulmonary coccidioidomycosis in lung transplantation. Ann Thorac Surg 2002; 73:306–308. [DOI] [PubMed] [Google Scholar]
- 10.Cheesbrough M. (2006). District laboratory practice in tropical countries. 2nd ed Cambridge university press; Norfolk, England. [Google Scholar]
- 11.Collee TG, Mackie TJ, McCartney JE. (1996). Mackie & McCartney practical medical microbiology. 14nd ed Harcourt Health Sciences; Churchill Livingstone, New York. [Google Scholar]
- 12.Abe M, Ogawa Z, Tanuma H, Kume H. [Study of mycological examination methods in clinical laboratories--specimen pretreatment and isolation]. Nihon Ishinkin Gakkai Zasshi 2009; 50:235–242. [DOI] [PubMed] [Google Scholar]
- 13.Procop GW, Koneman EW. (2016). Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. Koneman E. W., 7nd ed, Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- 14.Agarwal S, Manchanda V, Verma N, Bhalla P. Yeast identification in routine clinical microbiology laboratory and its clinical relevance. Indian J Med Microbiol 2011; 29:172–177. [DOI] [PubMed] [Google Scholar]
- 15.Lai C, Hsu H, Lee L, Hsueh P. Assessment of Platelia Aspergillus enzyme immunoassay for the diagnosis of invasive aspergillosis. J Microbiol Immunol Infect 2007;40: 148–153. [PubMed] [Google Scholar]
- 16.Harju S, Fedosyuk H, Peterson KR. Rapid isolation of yeast genomic DNA: Bust n’Grab. BMC Biotechnol 2004; 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeates C, Gillings M, Davison A, Altavilla N, Veal D. Methods for microbial DNA extraction from soil for PCR amplification. Biol Proced Online 1998; 1:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reischer GH, Lemmens M, Farnleitner A, Adler A, Mach RL. Quantification of Fusarium graminearum in infected wheat by species specific real-time PCR applying a TaqMan Probe. J Microbiol Methods 2004; 59:141–146. [DOI] [PubMed] [Google Scholar]
- 19.Badiee P. Evaluation of human body fluids for the diagnosis of fungal infections. Biomed Res Int 2013; 2013: 698325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis 2008; 46:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N Engl J Med 2012; 366:454–461. [DOI] [PubMed] [Google Scholar]
- 22.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med 2012; 4:165rv13. [DOI] [PubMed] [Google Scholar]
- 23.Basiri Jahromi SH, Khaksar AA. Respiratory fungal infections in specimens referred to the Pasteur Institute of Iran, 1994–2001. Res Med 2004; 28:265–268. [Google Scholar]
- 24.Roohani AH, Fatima N, Shameem M, Khan HM, Khan PA, Akhtar A. Comparing the profile of respiratory fungal pathogens amongst immunocompetent and immunocompromised hosts, their susceptibility pattern and correlation of various opportunistic respiratory fungal infections and their progression in relation to the CD4+ T-cell counts. Indian J Med Microbiol 2018; 36:408–415. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed M, Farghaly A, Raafat R, Abd Elsattar W. Study of the prevalence and pattern of fungal pneumonias in respiratory intensive care units. Egypt J Bronchol 2019; 13:545–550. [Google Scholar]
- 26.Gavazzi G, Krause K-H. Ageing and infection. Lancet Infect Dis 2002; 2:659–666. [DOI] [PubMed] [Google Scholar]
- 27.Rafiee S, Besharat S, Jabbari A, Golalipour F, Nasermoaadeli A. Epidemiology of tuberculosis in northeast of Iran: a population-based study. Iran J Med Sci 2009; 34:193–197. [Google Scholar]
- 28.Fontalvo DM, Jiménez Borré G, Gómez Camargo D, Chalavé Jiménez N, Bellido Rodríguez J, Cuadrado Cano B, et al. Tuberculosis and fungal co-infection present in a previously healthy patient. Colomb Med 2016; 47:105–108. [PMC free article] [PubMed] [Google Scholar]
- 29.Joos L, Tamm M. Breakdown of pulmonary host defense in the immunocompromised host: cancer chemotherapy. Proc Am Thorac Soc 2005; 2:445–448. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez LC, Dal Nogare A, Hsia C, Arauz C, Butt I, Strowig SM, et al. Relationship between diabetes control and pulmonary function in insulin-dependent diabetes mellitus. Am J Med 1991; 91:371–376. [DOI] [PubMed] [Google Scholar]
- 31.Spahr J, Weiner DJ, Stokes DC, Kurland G. (2019). Pulmonary disease in the pediatric patient with acquired immunodeficiency states. In: Kendig’s Disorders of the Respiratory Tract in Children. Elsevier: pp. 923–943. [Google Scholar]
- 32.Zarrinfar H, Saber S, Kordbacheh P, Makimura K, Fata A, Geramishoar M, et al. Mycological microscopic and culture examination of 400 bronchoalveolar lavage (BAL) samples. Iran J Public Health 2012; 41: 70–76. [PMC free article] [PubMed] [Google Scholar]
- 33.Zanganeh E, Zarrinfar H, Rezaeetalab F, Fata A, Tohidi M, Najafzadeh MJ, et al. Predominance of non-fumigatus Aspergillus species among patients suspected to pulmonary aspergillosis in a tropical and subtropical region of the Middle East. Microb Pathog 2018; 116:296–300. [DOI] [PubMed] [Google Scholar]
- 34.Hedayati MT, Mayahi S, Denning DW. A study on Aspergillus species in houses of asthmatic patients from Sari City, Iran and a brief review of the health effects of exposure to indoor Aspergillus. Environ Monit Assess 2010; 168: 481–487. [DOI] [PubMed] [Google Scholar]
- 35.Dykewicz CA. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis 2001; 33:139–144. [DOI] [PubMed] [Google Scholar]
- 36.Verweij PE, Stynen D, Rijs A, De Pauw BE, Hoogkamp-Korstanje J, Meis J. Sandwich enzyme-linked immunosorbent assay compared with Pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. J Clin Microbiol 1995; 33:1912–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 2006; 91:1068–1075. [PubMed] [Google Scholar]
- 38.Ozçelik T, Ozkalemkaş F, Kocaeli H, Altundal Y, Ener B, Ali R, et al. Successful treatment of neuroaspergillosis in a patient with acute lymphoblastic leukemia: role of surgery, systemic antifungal therapy and intracavitary therapy. Mikrobiyol Bul 2009; 43:499–506. [PubMed] [Google Scholar]
- 39.Herbrecht R, Letscher-Bru V, Oprea C, Lioure B, Waller J, Campos F, et al. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J Clin Oncol 2002; 20:1898–1906. [DOI] [PubMed] [Google Scholar]
- 40.Sulahian A, Tabouret M, Ribaud P, Sarfati J, Gluckman E, Latgé JP, et al. Comparison of an enzyme immuno-assay and latex agglutination test for detection of galactomannan in the diagnosis of invasive aspergillosis. Eur J Clin Microbiol Infect Dis 1996; 15:139–145. [DOI] [PubMed] [Google Scholar]