Abstract
Background:
Inhibition of biofilm formation is essential for the prevention and treatment of urinary tract infection. This study was aimed to identify the probiotic potential of Lactobacillus strains isolated from kefir and evaluate their antimicrobial and antibiofilm activities against Uropathogenic Escherichia coli (UPEC).
Methods:
Twelve Lactobacillus strains were evaluated. Antimicrobial and antibiofilm activities of Cell Free Supernatant (CFS) of the Lactobacillus strains against UPEC isolates were evaluated by agar well diffusion method and crystal violet assay, respectively. Probiotic potential of selected isolates was assessed by analyzing their tolerance to acidic pH and bile salts, auto-aggregation ability, co-aggregation with Escherichia coli (E. coli) and hemolytic activity. The isolates were identified by phenotypic and 16S rRNA gene sequencing.
Results:
The CFS of all lactobacilli strains was able to inhibit UPEC isolates even after neutralization. Four out of 12 isolates inhibited the biofilm formation by UPEC in the range 62–75%. The viability under acidic condition varied among the isolates ranging from 6–89.8%. All the isolates could tolerate the 0.3% bile and eight isolates showed the adaptation time of less than 1 hr. All the strains exhibited co-aggregation with E. coli. Auto-aggregation was highly correlated with co-aggregation of all lactobacilli strains with E. coli (r=0.889, p<0.001). The isolates with satisfactory probiotic potential and higher ability of biofilm inhibition and antibacterial activity belonged to the species Lactobacillus rhamnosus and Lactobacillus paracasei.
Conclusion:
All four selected probiotic strains exhibited antimicrobial and antibiofilm activities, which suggest potential applications for controlling or preventing infections caused by UPEC.
Keywords: Kefir, Lactobacillus paracasei, Lactobacillus rhamnosus, Probiotics, Urinary tract infection, Uropathogenic E. coli
Introduction
Urinary Tract Infection (UTI) is one of the most common bacterial infections that annually affect 150 million people worldwide. Among the microorganisms associated with UTI, uropathogenic Escherichia coli (UPEC) is the most important cause of community-acquired (90%) and nosocomial UTIs (50%) 1. UPEC forms microcolonies known as biofilms on the surface of urethral catheters, as well as on mucosa of urinary bladder 2. Biofilm protects bacteria against host immune response and antimicrobial therapy by encapsulating them in an extracellular matrix. In addition, close association of bacteria enables easy transfer of resistance determinants between the bacteria residing in the biofilm 2. This will improve drug resistance and makes treatment of UTIs more difficult.
With the emergence and spread of new antibiotic resistant isolates, antibiotic free treatments have gained popularity in recent years. Among these treatment approaches, the use of probiotics is a promising alternative for control of UTIs. It has been stated that some probiotics are able to adhere to uroepithelial cells and inhibit the growth of pathogenic bacteria 3. In addition, oral administration of lactobacilli can colonize these microorganisms in urinary tract after intestinal colonization 3.
Probiotics are defined as live microorganisms that, when applied in adequate amounts, provide health benefits to their host 4. Among the probiotic microorganisms, Lactic Acid Bacteria (LAB) have a long history of safe use in fermented and non-fermented food. Although a considerable number of probiotic microorganisms are commercially available all over the world, screening for new strains is still of great industrial interest.
Kefir is one of the most potent sources of probiotics. It is a popular fermented milk which confers various beneficial health 5. Kefir is traditionally made by kefir grains as starter. The kefir grains are composed of polysaccharide and protein matrix which is populated by a diverse group of LAB and yeast 5. The beneficial effects of kefir is not only due to the bioactive peptide and soluble polysaccharide (Kefiran), but also its undefined microbial composition and secondary metabolites 6. Previous reports described the antimicrobial activity of LAB isolates from kefir against pathogenic bacteria 7–9. However, no report has documented the antibiofilm activity of kefir isolates against UPEC. The purpose of this study was to identify and characterize the probiotic potential of LAB isolated from kefir and to investigate the antimicrobial and antibiofilm properties of the isolates against UPEC.
Materials and Methods
Microorganisms and growth conditions
For the isolation of LAB strains, 10 g of kefir grains from Kefirnoosh Company (Iran) were added to 200 ml of sterilized milk and incubated at 21°C for 24 hr. The grains were then passed through a sterilized strainer and homogenized with 90 ml of sterile saline containing 0.9% NaCl and 0.1% bacto peptone (Difco Laboratories). Serial dilutions were made using the homogenized suspensions of kefir grains in sterilized saline and aliquots were spread plated onto the Man, Rogosa and Sharpe (MRS) agar (Merk, Germany). The plates were incubated in anaerobic condition (Gaspak EZ, Difco) at 30°C for 48 hr. After incubation, the bacterial colonies were picked and streaked on a fresh MRS agar plate. Catalase negative, gram positive and rod shape isolates were identified as lactobacilli and stocked in skim milk with 20% (v/v) glycerol (Merk, Germany) at −80°C.
For UPEC isolation and cultivation, urine samples from the patients with urinary tract infection referred to Imam Khomeini hospital, Karaj, Iran were cultured on MacConkey agar (Merck, Germany) and incubated at 37°C for 24 hr. The identification of the isolates was performed using the biochemical tests 10. Escherichia coli (E. coli) PTCC 1399 was purchased from the Iranian Research Organization for Science and Technology (IROST), grown in Trypticase Soy Broth (TSB) (Merck, Germany), and incubated at 37°C under aerobic conditions. All E. coli strains were maintained at −80°C in the TSB containing 20% (v/v) glycerol.
Antimicrobial susceptibility testing of UPEC isolates
The antimicrobial susceptibility testing was performed by Kirby Bauer's disk diffusion method on Mueller-Hinton agar (Merk, Germany) according to the Clinical and Laboratory Standard Institute (CLSI) guidelines 11, using 8 antibiotics including Ampicillin (10 μg), Cefotaxime (30 μg), Ceftazidime (30 μg), Imipenem (10 μg), Gentamicin (10 μg), Amikacin (30 μg), Tetracycline (30 μg) and Ciprofloxacin (5 μg) (MAST, UK).
Antimicrobial activity of culture supernatant of LAB strains
Antimicrobial activity of cell free supernatant (CFS) of lactobacilli strains against UPEC isolates was assessed by agar well diffusion method as described previously 12. Overnight culture of LAB strains was centrifuged at 9000×g for 10 min at 4°C and CFS was sterilized using syringe filters (0.22 μm pore size). Then, 100 μl of each UPEC isolates (0.5 McFarland turbidity) was spread onto the Mueller-Hinton agar plate and 6 mm wells were cut with a sterilized Pasteur pipette. Next, 100 μl of CFS was poured into well and the plates were incubated at 37°C for 24 hr. The zone of inhibition around the wells was measured in millimeters. To counteract the effect of reduced pH on antimicrobial activity, the pH of CFS was adjusted to 6.5 (NaOH 1N, Merk) and antimicrobial activity was determined as mentioned above. E. coli PTCC 1399 was used as the control in the experiments.
Biofilm formation by UPEC isolates
Biofilm formation was assessed in 96-well micro-titer plates, according to the method described by Ste-panović et al 13. Briefly, overnight culture of E. coli strains was diluted to get 0.5 McFarland turbidity. After that, 1:100 dilution of this suspension was prepared in fresh Luria-Bertani (LB) medium (Merck, Germany) and 100 μl of diluted suspension was poured into the well of microtiter plate and the plates were incubated at 37°C for 48 hr. The attached cells were then fixed by 200 μl of methanol (96%) (Merk, Germany) for 15 min. Then, 150 μl of 2% crystal violet was added to each well and the plates were incubated for 15 min at room temperature. Finally, 150 μl of 33% acetic acid was added to each well and the OD550 was measured using a microtiter-plate reader (Bio-Rad, USA). The isolates, which had the highest biofilm formation potential, were used for antibiofilm assay.
Inhibition of biofilm formation
Inhibition of biofilm formation by CFS of LAB strains was performed as described previously by Kaur et al with some modification 14. Briefly, 100 μl of Brain Heart Infusion (BHI) broth (Merck, Germany) was added to each well of a microtiter plate. Then, 100 μl of CFS was added to each well except for negative control, which contained MRS broth. At the final step, 20 μl of overnight culture of E. coli isolates (OD595=0.1) was added to each well and the plates were incubated at 37°C for 48 hr. The biofilm formation was determined as described above. The percentage of biofilm inhibition was calculated by the following equation:
Percentage inhibition=100–[(OD595 of experimental wells ×100)/OD595 of negative control well].
Probiotic potential of lactobacilli
Acid and bile tolerance of LAB isolates: Overnight cultures of LAB strains were centrifuged (Hettich, Germany) at 7500×g for 5 min at 4°C. The pellet was then resuspended in phosphate-saline buffer (10 mM Na2HPO4, 1 mM KH2PO4, 140 mM NaCl, 3 mM KCl) (PBS pH=6.5) at a concentration of 109 Colony-Forming Units (CFU) ml−1. Cell suspension was diluted 1×10−1 in MRS broth at pH=3 and incubated for 3 hr at 30°C. Samples were taken at 0 and 3 hr and serially diluted in physiological saline solution. The pH tolerance of the isolates was evaluated by counting the viable cells on MRS agar plates after 48 hr incubation at 30°C. The survival rate of LAB was estimated as follows:
The effect of bile salts on the growth rate of LAB strains was evaluated as described previously 16. Briefly, overnight cultures of LAB isolates were inoculated (1%) into the MRS broth in the presence (Test) and absence (Control) of 0.3% Oxgall (Sigma-Aldrich, USA). The tubes were then incubated at 37°C for 9 hr and the Optical Density (OD) at 620 nm was measured every hour. Tolerance to bile salts was determined based on the time needed to increase the absorbance at 620 nm by 0.3 units in MRS broth with and without 0.3% bile salts. The difference between times (hr) to obtain 0.3 units between the culture media was considered as the Adaptation Time (AT).
Auto-aggregation assay
Overnight culture of bacteria was centrifuged at 5000×g for 20 min at 4°C and the pellet was resuspended in PBS pH=7 (108 CFU.ml−1). The suspension was incubated for 24 hr at 30°C and the absorbance was recorded before and after incubation at 600 nm. The auto-aggregation percentage was determined as [1−(At/A0)×100] where At represents the absorbance at 24 hr and A0 absorbance at time 0 17.
Co-aggregation assay
LAB suspensions were prepared (108 CFU.ml−1) as described above. A bacterial suspension of E. coli (PTCC 1399) was also prepared at the same concentration and mixed with equal volumes (500 μl) of the LAB strains. The mixture was then incubated at 37°C without shaking and the OD 600 nm was recorded after 24 hr. The co-aggregation percentage was determined as follows:
[(Apathog+ALAB)/2-(Amix)/(Apathog+ALAB)/2]×100, where Apathog and ALAB are the OD of tubes containing pathogens or LAB strains respectively, and Amix represents the OD of the mixture 18.
Hemolytic activity
Overnight culture of LAB strains was plated on blood agar plates containing 5% (v/v) sheep blood and incubated at 30°C for 24–48 hr. No hemolysis was scored as negative (−) and alpha or beta hemolysis was recorded based on greenish or clear zone around the isolates, respectively.
Identification of LAB isolates by 16S rRNA gene sequencing and phenotypic test
The LAB strains were identified by using 16S rRNA gene sequencing. Genomic DNA of the isolates was extracted using SinaPure DNA extraction kit (Sinaclone, Iran) and 16S rRNA gene was amplified by prokaryotic universal primers (27F; 5′-AGAGTTTGA TCCTGGCTCAG-3′ and 1492R; 5′-GGTTACCTTG TTACGACTT-3′ as described previously 8. The PCR products were purified and sequencing was performed by Microgene Company (Korea). The sequences were then aligned with blast program (http://www.ncbi.nlm.nih.gov/BLAST/) to determine the closest known phylogenetic relatives of the sequenced gene. The phenotypic tests including growth at different temperatures and carbohydrate fermentation were performed to confirm the identification 19.
Statistical analysis
Statistical analysis was carried out by SPSS software (Version 20). All the data are represented as mean±standard deviation (SD) of three independent experiments. The significant differences between mean values were determined by Tukey's test. Correlation coefficients were calculated between auto-aggregation and co-aggregation, and biofilm inhibition ability and antagonistic activity. p<0.05 was considered significant.
Results
Biochemical identification of UPEC and antimicrobial susceptibility testing
All the urine isolates formed typical red colonies on MacConkey agar indicating fermentation of lactose. All the isolates were indole and methyl red positive, Voges-Proskauer, citrate and urea negative, and motile. Accordingly, all were confirmed as E. coli. The results of antibiotic susceptibilities of UPEC isolates are shown in table 1. Five out of 12 isolates were multidrug resistant and exhibited resistance to at least one agent in three or more antimicrobial categories. These isolates were used for evaluation of antimicrobial activity of lactobacilli isolates.
Table 1.
Antibiotic susceptibility pattern of UPEC isolates
| Antibiotics | UPEC isolates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC1 | EC2 | EC3 | EC4 | EC5 | EC6 | EC7 | EC8 | EC9 | EC10 | EC11 | EC12 | |
| Ampicillin | R | R | R | S | R | R | S | R | R | S | R | R |
| Gentamicin | S | S | R | R | S | S | S | S | R | R | S | S |
| Amikacin | R | S | S | S | S | R | S | S | S | S | R | R |
| Imipenem | R | S | S | S | S | S | S | S | S | R | R | S |
| Tetracycline | R | R | S | S | R | S | R | S | R | R | R | R |
| Cefepime | S | S | S | S | S | S | S | R | S | S | R | S |
| Ceftazidime | S | S | S | R | S | S | R | S | R | S | R | R |
| Ciprofloxacin | R | S | S | S | S | S | S | S | S | R | S | R |
Denotes for Resistant (R), Intermediate (I) and Susceptible (S).
Antimicrobial activity of CSF of probiotics
In the present study, 12 catalase negative, gram positive and rod shape strains were isolated from kefir grains and their antimicrobial activities were assessed against UPEC isolates. The UPEC isolates showed sensitivity to CFS of all probiotics (Table 2). Seven out of 12 lactobacilli strains showed high antagonistic activity against all UPEC isolates (inhibition zone of 13.6–15.9 mm). The CFS of all lactobacilli isolates was also active against the control strain. Among the isolates, strain LAB2 showed the highest antibacterial activity against EC10 (p<0.05). The CFS of all LAB strains also displayed antagonistic activity even after neutralization.
Table 2.
Antagonistic activity of lactobacilli CFS against UPEC isolates by agar well diffusion method
| Lactobacilli strains | Zones of inhibition (mm)± S.D | |||||
|---|---|---|---|---|---|---|
| E. coli PTCC 1399 | EC1 | EC9 | EC10 | EC11 | EC12 | |
| LAB1 | 14.3±1.2a | 15.1±.07 a | 14.45±0.2 ab | 14.3±0.2 bc | 14.9±0.2 a | 15.1±0.2 a |
| LAB2 | 12.2±1.3ab | 14.3±0.3 bc | 14.7±0.14 ab | 15.9±0.14 a | 14.5±0.35 a | 15.3±0.14 a |
| LAB3 | 10.3±1.1bcd | 12.4±0.1d | 12.7±0.3 de | 13.2±0.3de | 12.9±0.14 b | 13.3±0.07cd |
| LAB4 | 12.3±0.6ab | 14.6±0.1 bc | 14.4±0.2 ab | 14.7±0.14 bc | 15. ±0.2 a | 15.3±35 a |
| LAB5 | 12.7±0.6ab | 14.3±0.1 bc | 14±0.35 bc | 14.2±0.07 bc | 13.6±0.14 b | 14.2±0.07 abc |
| LAB6 | 8.7±0.6d | 11.3±0.2e | 11.4±0.3f | 11.9±0.2 f | 11.2±0.3c | 11.3±0.07e |
| LAB7 | 12.3±1.1ab | 14.3±0.7 bc | 14±0.3 bc | 14.9±0.07b | 15.2±0.3a | 14.8±0.07 ab |
| LAB8 | 10.7±0.6bcd | 12.3±0.2d | 12.1±0.3 ef | 12.7±0.14ef | 12.7±0.2b | 12.8±0.07d |
| LAB9 | 9.3±0.6cd | 12.3±0.2d | 12.7±0.14de | 12±0.2f | 13.2±0.3 b | 13.7±0.21 bcd |
| LAB10 | 11.7±0.6bc | 14.4±0.7 bc | 14.6±0.2 ab | 13.9±0.2cd | 14.7±0.07 a | 14.3±0.85 abc |
| LAB11 | 14.3±1.1a | 14.9±0.2 ab | 15.4±0.3a | 15±0.3b | 15.2±0.2a | 14.9±0.21 ab |
| LAB12 | 11.3±0.6bc | 13.4±0.1c | 13.25±0.2cd | 12.7±0.2 ef | 13.3±0.14 b | 13.3±07cd |
Data are expressed as mean±standard deviation (n=3). Means within the same column with different superscript letters are statistically different based on Tukey's test (p<0.05).
Antibiofilm activity of CFS against UPEC
Before determining the inhibitory effect of CFS on biofilm formation by UPEC, the biofilm forming ability of all E. coli isolates was tested. Four out of 12 E. coli isolates displayed the highest biofilm forming potential (p<0.05) and were used for evaluation of antibiofilm activity of LAB strains (Figure 1).
Figure 1.
The biofilm formation ability of UPEC isolates. Data are expressed as mean±standard deviation (n=3). Different letters indicate significant differences between E. coli isolates based on Tukey's test (p<0.05).
The CFS of all LAB strains resulted in more than 30% inhibition of biofilm formation by E. coli isolates (Table 3). Seven out of 12 strains exhibited more than 50% inhibition against all four urinary isolates and the highest inhibition was observed by LAB7 and LAB4 (more than 70% for both) (p<0.05). Pearson correlation coefficient analysis showed no correlation between biofilm inhibition and antagonistic activity of lactobacilli strains against E. coli isolates (r=0.297, p>0.05).
Table 3.
The inhibitory activity of CFS against biofilm formation by UPEC isolates
| Isolates | Inhibition of biofilm formation (%) | |||
|---|---|---|---|---|
| EC9 | EC10 | EC11 | EC12 | |
| LAB1 | 48±2 ef | 47±4.4 de | 46±3f | 50. 7±1.5def |
| LAB2 | 62.3±2.5c | 64.3±1.1 b | 65.7±2.5c | 65±2 c |
| LAB3 | 32.3±2g | 31.3±0.6 f | 34±1g | 31.3±3 g |
| LAB4 | 71.7±1.5 ab | 73±1 a | 72.7±2.5 ab | 73. 7±1.5 ab |
| LAB5 | 55.3±2.3e | 55±2.6 c | 56.3±1.5d | 56±2.6 d |
| LAB6 | 50±2de | 50±1.5 cd | 52.3±0.6 de | 51±3.6 def |
| LAB7 | 73.7±1.5 a | 73.3±0.6 a | 73. 7±1.5 a | 75. 7±3.2a |
| LAB8 | 43±2f | 43.3±1.1 e | 44±2f | 43.3±2.1 f |
| LAB9 | 47.7±1.5 ef | 48±1 de | 44. 7±1.5f | 48.3±2.5 def |
| LAB10 | 67.3±1.5bc | 69.7 a | 66. 7±1.5 bc | 67.3±3.5 bc |
| LAB11 | 48.7±3ef | 50±1 cd | 46.3±2.5 ef | 47.3±2.1ef |
| LAB12 | 51.3±1.5 de | 54±1 c | 52.3±3 de | 54±2.6 de |
Data are expressed as mean±standard deviation (n=3). Means within the same column with different superscript letters are statistically different based on Tukey's test (p<0.05).
Probiotic properties of lactobacilli
Acid and bile tolerance: Acid and bile tolerance of the isolates are shown in table 4. For two strains (LAB2 and LAB10) survival at pH=3 was significantly higher (p<0.05). LAB4, LAB7 and LAB12 showed the moderate acid tolerance and the lowest acid tolerance was observed for LAB3 and LAB11 (p<0.05) after 3 hr of exposure to pH=3 (Table 4).
Table 4.
Acid and bile salts tolerance of LAB strains
| Isolates | Acid tolerance (survival %) | Bile tolerance (Time needed for increasing 0.3 units) | ||
|---|---|---|---|---|
| MRS (h) | MRS+0.3% bile (h) | Adaptation time (h) | ||
| LAB1 | 21.52±6.5def | 2.93±0.09 | 3.74±0.1 | .79±0.16b |
| LAB2 | 89.79±4a | 4.04±0.05 | 4.5±0.04 | .42±0.06bcd |
| LAB3 | 7.31±1.45ef | 4.5±0.04 | 6.36±0.12 | 2.05±0.15a |
| LAB4 | 48.41±8.1bc | 3.79±0.14 | 4.06±0.05 | .36±0.05bcd |
| LAB5 | 22.4±7.27def | 3.55±0.06 | 5.57±0.09 | 2.02±0.16a |
| LAB6 | 24.42±3.2 def | 4.51±0.17 | 6.68±0.03 | 2.18±0.17a |
| LAB7 | 52.51±8.1b | 3.64±0.02 | 4.02±0.03 | .37±0.05bcd |
| LAB8 | 44.11±4.5ef | 4.67±0.02 | 6.68±0.04 | 2.00±0.2a |
| LAB9 | 28.4±2.54cde | 4.04±0.03 | 4.67±0.02 | .62±0.04b |
| LAB10 | 80.07±12.63a | 3.69±0.04 | 4.15±0.04 | .46±0.1bc |
| LAB11 | 5.89±1.35f | 3.9±0.07 | 4.66±0.04 | .75±0.11b |
| LAB12 | 32.77±2.37bcd | 2.95±0.05 | 3.57±0.09 | .62±0.13b |
Data are expressed as mean±standard deviation (n=3). Means within the same column with different superscript letters are statistically different based on Tukey's test (p<0.05).
All the isolates could tolerate the 0.3% bile for 3 hr. Also, 8 out of 12 isolates showed the shortest adaptation time (p<0.05) of less than 1 hr (Table 4).
Auto-aggregation, co-aggregation and safety assessment
The auto-aggregation and co-aggregation of the LAB strains are presented in figure 2. Strains LAB2 and LAB7 showed the highest auto-aggregation (58.9 and 64.42%, respectively, p<0.05). All LAB strains exhibited co-aggregation with E. coli. The co-aggregation values of LAB strains varied between 33–64.7%. Four out of 12 isolates showed the highest co-aggregation values (p<0.05). To examine whether the autoaggregation and co-aggregation abilities of the strains with E. coli were related phenotypes, the Pearson correlation coefficient (r) was conducted. Statistically significant correlation was observed between auto-aggregation and co-aggregation of all lactobacilli strains with E. coli (r=0.889, p<0.001). Hemolytic activity was not detected in any LAB strains.
Figure 2.
Auto-aggregation (A) and co-aggregation of LAB strains with E. coli (B). Data are expressed as mean±standard deviation (n=3). Different letters indicate significant differences between isolates based on Tukey's test (p<0.05).
Identification of the isolates
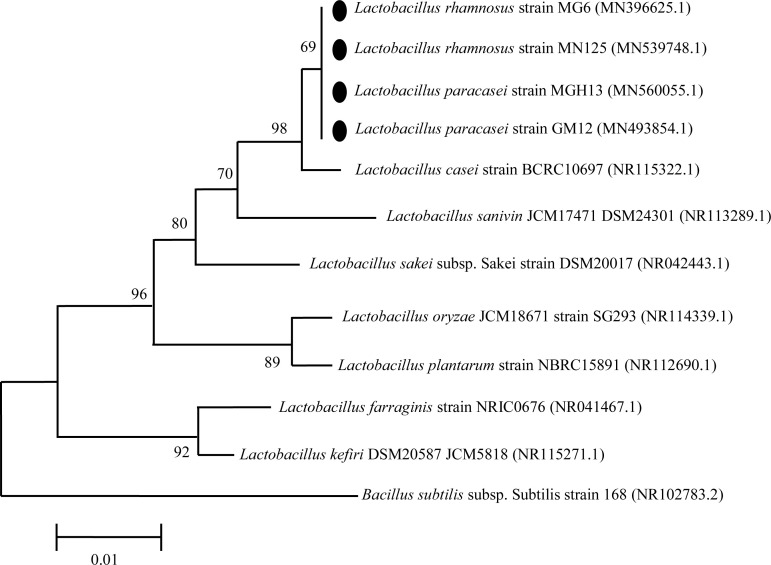
Based on acid and bile tolerance, auto-aggregation and co-aggregation properties and antimicrobial and antibiofilm activities, four isolates (LAB2, LAB4, LAB7 and LAB10) were selected for phenotypic and molecular identification. The 16S rDNA of LAB2, LAB4, LAB7 and LAB10 isolates was sequenced, and the isolates were identified by alignment of 16S rDNA sequences as Lactobacillus paracasei (L. paracasei) strain GM12 (Genbank accession no. MN493854), L. paracasei strain MGH13 (Genbank accession no. MN560055), Lactobacillus rhamnosus (L. rhamnosus) strain MG6 (Genbank accession no. N396625) and Lactobacillus rhamnosus (L. rhamnosus) strain MN125 (Genbank accession no. MN539748), respectively. Strains identified as L. rhamnosus were able to grow at 45°C and ferment mannitol, sorbitol, ribose and sucrose, but not xylose. In comparison, isolates identified as L. paracasei were not able to ferment any of the tested carbohydrates and grow at 45°C. The results of phenotypic testing confirmed the molecular identification by 16S rRNA gene sequencing. The phylogenetic relationship of selected Lactobacillus strains among the members of the genus Lactobacillus is shown in figure 3.
Figure 3.
Phylogenetic relationship of selected strains among members of the genus Lactobacillus in the neighbor joining phylogenetic tree. The tree was generated on the basis of 16S rDNA sequences. Accession numbers are in parentheses. Bacillus subtilis was used as out groups.
Discussion
Nowadays, inhibition of biofilm formation by pathogenic bacteria has become an attractive therapeutic target 20. UPEC is well known for its ability to form strong biofilm on the surface of urethral catheter and bladder epithelial cells 2. The results obtained in the present study also confirmed the biofilm forming abilities of UPEC isolates in 96-well microtiter plates. Biofilm formation plays a significant role in uropathogenicity 21. In addition, biofilms are resistant to the action of antimicrobial drugs as most of the available antimicrobial agents are active only against the planktonic forms of bacteria. Therefore, probiotic strains that have both anti-biofilm and antimicrobial activities against UPEC can be of clinical importance.
In this study, 12 lactobacilli strains were screened for their antimicrobial potential against UPEC isolates, out of which seven isolates showed high antagonistic activity against all UPEC isolates. Different species of lactobacilli are known to produce compounds with antimicrobial properties, including low molecular weight compounds, antimicrobial peptides (Bacteriocins) and organic acids 22. It seems that the antimicrobial activity of the lactobacilli strains isolated in this study was not due to the overproduction of acid, since antimicrobial activity was evident even after neutralizing CFS. It can be concluded that other molecules may also be involved in their inhibitory activity against UPEC isolates.
The antibiofilm activity of lactobacilli strains against pathogenic bacteria has been investigated previously. It has been reported that CFS of fecal lactobacilli isolates was able to prevent the Vibrio cholerae biofilm formation by more than 90% 14. Similarly, the antibiofilm activity of Lactobacillus plantarum (L. plantarum) and Lactobacillus pentosus (L. pentosus) CFS against Pseudomonas aeruginosa and Klebsiella pneumoniae has been documented 23. In the present study, the LAB isolates from kefir grains were screened for their biofilm inhibition potential against uropathogenic E. coli. The antibiofilm activity of lactobacilli strains against ciprofloxacin resistant UPEC has been discussed previously 24. However, no documented report concerning the inhibitory activity of LAB isolates from kefir grains against UPEC isolates is available. In the present study, the CFS of all kefir isolates caused reduction in the biofilm formation of all uropathogenic strains. However, biofilm inhibition ability varied significantly among lactobacilli strains (p<0.05). Among the lactobacilli strains, L. rhamnosus MG6 and L. paracasei MGH13 showed the highest biofilm inhibition potential. This is the first study concerning the antibiofilm activity of kefir isolates against UPEC isolates. It has been documented that lactobacilli CFS contains a variety of bioactive compounds including biosurfactant and exopolysaccharides, which can prevent biofilm formation of pathogenic bacteria 25. The amount of biosurfactant or exopolysaccharide produced by each isolate, which depends on its genetic potency, can affect the amount of its antibiofilm activity. The antibiofilm activity of CFS may also be due to its antimicrobial activity, which inhibits bacterial growth and biofilm formation. However, in the present study, no correlation between biofilm inhibition and antagonistic activity against E. coli isolates was observed (r=0.279, p>0.05).
In this study, lactobacilli strains were screened for their probiotic properties such as acid and bile tolerance, two key features that enable them to survive and grow in the gastrointestinal conditions 26. The acidic conditions of the stomach are the primary defense mechanism that probiotics must overcome. In the present study, most of the lactobacilli strains showed resistance to low pH. The ability of lactobacilli to survive at low pH was also reported in the previous studies 15,26. In our study, the survival ability of the lactobacilli strains varied significantly (p<0.05). In this regard, Tokatl et al documented the strain specific survival of lactobacilli originated from traditional pickles 26.
Bile resistance is an important feature for the selection of bacteria, since gut contains high concentrations of bile salts, which are toxic for bacteria 27. In our study, all of the selected isolates were able to grow in 0.3% of bile salts, similar to the concentration found in the small intestine 8. However, the time required for growing the isolates in culture media with and without bile salts varied between the strains. Our results are in agreement with the previous study, which demonstrated that lactobacilli strains possessed high resistance to 0.3% of bile salts 28.
Colonization of probiotic bacteria in the gut is considered as a desirable feature 29. In this regard, adhesion to the intestinal epithelial cells, which is an essential step for colonization, is an important factor in selecting a strain as probiotic 26. Auto-aggregation of probiotic bacteria is used as a measurement directly related to adhesion capacity of probiotic bacteria to cell monolayers 30. In the present study, significant differences (p<0.05) in auto-aggregation values were observed between lactobacilli strains. Among the lactobacilli isolates, L. rhamnosus MG6 and L. paracasei GM12 showed the highest auto-aggregation activity (p<0.05). Ramos et al also demonstrated variation in auto-aggregation among lactobacilli strains 31. Autoaggregation is a phenomenon that has previously been shown to be dependent on production of exopolysaccharide (EPS). The amount of EPS produced by each isolate reflects the rate of auto-aggregation 32.
Co-aggregation between lactobacilli and pathogenic bacteria provides a barrier that prevents their adhesion to the urinary and intestinal epithelial cells. All the lactobacilli isolated in this study exhibited interaction with E. coli. However, co-aggregation percentages varied significantly (p<0.05). Among the lactobacilli isolates, four strains (LAB2, LAB4, LAB10 and LAB7), identified as strains of L. paracasei and L. rhamnosus, showed high percentages of co-aggregation with E. coli in the range of 58–68%. In a study conducted by Tareb et al, the co-aggregation percentage of L. rhamnosus CNCM-I-3698 and L. farciminis CNCM-I-3699 with E. coli was found to be 38.2 and 34.5, respectively 33. Comparing to their results, L. rhamnosus strains isolated in this study showed the higher percentages of coaggregation potential. The co-aggregation potential of L. rhamnosus and L. paracasei strains isolated in this study may be an effective host defense mechanism against infections caused by E. coli. In addition, the coaggregation phenomenon can cause the probiotic to be in close contact with the pathogenic bacteria and thus increase the efficiency of the antimicrobial compounds produced by the probiotics.
In the present study, strains with strong autoaggregation ability were also well co-aggregated with E. coli. The correlation coefficients between autoaggregation and co-aggregation showed that autoaggregation was highly correlated with co-aggregation of all lactobacilli strains with E. coli (r=0.889, p<0.01). Our findings suggest that auto-aggregation ability is related to co-aggregation property of each lactobacilli isolates.
In this study, four Lactobacillus strains, including two L. paracasei strains (LAB2 and LAB4) and two L. rhamnosus strains (LAB7, LAB10) were selected and identified by molecular methods. The phylogenetic tree obtained in this study, showed the close relationship between our isolates and Lactobacillus casei (L. casei). It has been stated that L. paracasei, L. rhamnosus and L. casei are phenotypically and phylogenetically closely related; and are regarded as the L. casei group. Members of this group are widely used in fermented dairy products and food supplements 34.
Conclusion
In the present study, four lactobacilli strains with satisfactory probiotic potential were isolated from kefir grains. The isolates exhibited high auto-aggregation and co-aggregation activities and were able to inhibit biofilm formation by uropathogenic E. coli. Our findings suggest that Lactobacillus strains isolated in this study may be promising probiotics for prevention and treatment of UTIs. However, in vivo studies are necessary for future applications of these microorganisms as probiotics.
Acknowledgement
This study is derived from an M.Sc thesis, supported by Islamshahr branch, Islamic Azad University, Islamshahr, Iran.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1. Toval F, Köhler CD, Vogel U, Wagenlehner F, Mellmann A, Fruth A, et al. Characterization of Escherichia coli isolates from hospital inpatients or outpatients with urinary tract infection. J Clin Microbiol 2014; 52( 2): 407– 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eberly A, Floyd K, Beebout C, Colling S, Fitzgerald M, Stratton C, et al. Biofilm formation by uropathogenic Escherichia coli is favored under oxygen conditions that mimic the bladder environment. Int J Mol Sci 2017; 18 (10): 2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zuccotti GV, Meneghin F, Raimondi C, Dilillo D, Agostoni C, Riva E, et al. Probiotics in clinical practice: an overview. J Int Med Res 2008; 36( Suppl 1): 1A– 53A. [DOI] [PubMed] [Google Scholar]
- 4. Quigley EMM. Prebiotics and probiotics in digestive health. Clin Gastroenterol Hepatol 2019; 17( 2): 333– 344. [DOI] [PubMed] [Google Scholar]
- 5. Leite AMdO, Miguel MAL, Peixoto RS, Rosado AS, Silva JT, Paschoalin VMF. Microbiological, technological and therapeutic properties of kefir: a natural probiotic beverage. Braz J Microbiol 2013; 44( 2): 341– 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nielsen B, Gürakan GC, Ünlü G. Kefir: a multifaceted fermented dairy product. Probiotics Antimicrob Proteins 2014; 6( 3–4): 123– 135. [DOI] [PubMed] [Google Scholar]
- 7. Chifiriuc MC, Cioaca AB, Lazar V. In vitro assay of the antimicrobial activity of kephir against bacterial and fungal strains. Anaerobe 2011; 17( 6): 433– 435. [DOI] [PubMed] [Google Scholar]
- 8. Leite AM, Miguel M, Peixoto R, Ruas-Madiedo P, Paschoalin V, Mayo B, et al. Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. J Dairy Sci 2015; 98( 6): 3622– 3632. [DOI] [PubMed] [Google Scholar]
- 9. Zheng Y, Lu Y, Wang J, Yang L, Pan C, Huang Y. Probiotic properties of Lactobacillus strains isolated from Tibetan kefir grains. PloS One 2013; 8( 7): e69868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Versalovic J, Carroll K, Funke G, Jorgensen J, Landry M, Warnock D. Manual of clinical microbiology. systems for detection and identification of bacteria and yeasts. 10th ed Vol. 1 ASM Press; 2011; 18– 19. [Google Scholar]
- 11. Patel J, Cockerill F, III, Eliopoulos G, Jenkins S, Lewis J, Limbago B, et al. M100 Performance standards for antimicrobial susceptibility testing. United State: Clinical and Laboratory Standards Institute; 2017: 240. [Google Scholar]
- 12. González L, Sandoval H, Sacristán N, Castro J, Fresno J, Tornadijo M. Identification of lactic acid bacteria isolated from Genestoso cheese throughout ripening and study of their antimicrobial activity. Food Control 2007; 18( 6): 716– 722. [Google Scholar]
- 13. Stepanović S, Vuković D, Hola V, Bonaventura GD, Dj-ukić S, Ćirković I, et al. Quantification of biofilm in mic-rotiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007; 115( 8): 891– 899. [DOI] [PubMed] [Google Scholar]
- 14. Kaur S, Sharma P, Kalia N, Singh J, Kaur S. Anti-biofilm properties of the fecal probiotic Lactobacilli against Vibrio spp. Front Cell Infect Microbiol 2018; 8: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Argyri AA, Zoumpopoulou G, Karatzas KAG, Tsakalidou E, Nychas GJE, Panagou EZ, et al. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol 2013; 33( 2): 282– 291. [DOI] [PubMed] [Google Scholar]
- 16. Guo Z, Wang J, Yan L, Chen W, Liu XM, Zhang HP. In vitro comparison of probiotic properties of Lactobacillus casei Zhang, a potential new probiotic, with selected probiotic strains. LWT-Food Science and Technology 2009; 42( 10): 1640– 1646. [Google Scholar]
- 17. Collado MC, Meriluoto J, Salminen S. Adhesion and aggregation properties of probiotic and pathogen strains. European Food Research and Technology 2008; 226( 5): 1065– 1073. [Google Scholar]
- 18. García-Cayuela T, Korany AM, Bustos I, de Cadiñanos LPG, Requena T, Peláez C, et al. Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res Int 2014; 57: 44– 50. [Google Scholar]
- 19. De Vos P, Garrity GM. Bergey's manual of systematic bacteriology: New York: Springer; 2009. 1450 p. [Google Scholar]
- 20. Bjarnsholt T, Ciofu O, Molin S, Givskov M, Høiby N. Applying insights from biofilm biology to drug development-can a new approach be developed? Nat Rev Drug Discov 2013; 12( 10): 791– 808. [DOI] [PubMed] [Google Scholar]
- 21. Boll EJ, Struve C, Boisen N, Olesen B, Stahlhut SG, Krogfelt KA. Role of enteroaggregative Escherichia coli virulence factors in uropathogenesis. Infect Immun 2013; 81( 4): 1164– 1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salminen S, Von Wright A. Lactic acid bacteria: microbiological and functional aspects: CRC Press; 2004. 656 p. [Google Scholar]
- 23. Rao KP, Chennappa G, Suraj U, Nagaraja H, Raj AC, Sreenivasa M. Probiotic potential of Lactobacillus strains isolated from sorghum-based traditional fermented food. Probiotics Antimicrob Proteins 2015; 7( 2): 146– 156. [DOI] [PubMed] [Google Scholar]
- 24. Yeganeh M, Hosseini H, Mehrabian S, Torbati ES, Zamir SM. Antibiofilm effects of Lactobacilli against ciprofloxacin-resistant uropathogenic Escherichia coli strains in pasteurized milk. Applied Food Biotechnology 2017; 4( 4): 241– 50. [Google Scholar]
- 25. Sharma D, Saharan BS. Functional characterization of biomedical potential of biosurfactant produced by Lactobacillus helveticus. Biotechnol Rep (Amst) 2016; 11: 27– 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tokatlı M, Gülgör G, Bağder Elmacı S, Arslankoz İşleyen N, Özçelik F. In vitro properties of potential probiotic indigenous lactic acid bacteria originating from traditional pickles. BioMed Res Int 2015; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmadova A, Todorov SD, Choiset Y, Rabesona H, Zadi TM, Kuliyev A, et al. Evaluation of antimicrobial activity, probiotic properties and safety of wild strain Enterococcus faecium AQ71 isolated from Azerbaijani Motal cheese. Food Control 2013; 30( 2): 631– 641. [Google Scholar]
- 28. Floros G, Hatzikamari M, Litopoulou-Tzanetaki E, Tzanetakis N. Probiotic and technological properties of facultatively heterofermentative lactobacilli from Greek traditional cheeses. Food Biotechnol 2012; 26( 1): 85– 105. [Google Scholar]
- 29. Iñiguez-Palomares C, Pérez-Morales R, Acedo-Félix E. Evaluation of probiotic properties in Lactobacillus isolated from small intestine of piglets. Rev Latinoam Microbiol 2007; 49( 3–4): 46– 54. [Google Scholar]
- 30. Bautista-Gallego J, Arroyo-López F, Rantsiou K, Jiménez-Díaz R, Garrido-Fernández A, Cocolin L. Screening of lactic acid bacteria isolated from fermented table olives with probiotic potential. Food Res Int 2013; 50( 1): 135– 142. [Google Scholar]
- 31. Ramos CL, Thorsen L, Schwan RF, Jespersen L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol 2013; 36( 1): 22– 29. [DOI] [PubMed] [Google Scholar]
- 32. Sorroche FG, Spesia MB, Zorreguieta Á, Giordano W. A positive correlation between bacterial autoaggregation and biofilm formation in native Sinorhizobium meliloti isolates from Argentina. Appl Environ Microbiol 2012; 78( 12): 4092– 4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tareb R, Bernardeau M, Gueguen M, Vernoux JP. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J Med Microbiol 2013; 62( 4): 637– 649. [DOI] [PubMed] [Google Scholar]
- 34. Huang CH, Li SW, Huang L, Watanabe K. Identification and classification for the Lactobacillus casei group. Front Microbiol 2018; 9: 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]