Abstract
Introduction:
Klotho and Dipeptidyl Peptidase-4 (DPP4) are two proteins that modulate inflammatory pathways. We investigated the association between circulating klotho and DPP4 activity and their relationship with inflammatory cytokines, miR-29a, and miR-195 in Alzheimer Disease (AD).
Methods:
This study was conducted on 16 AD patients and 16 healthy age-matched controls. Plasma levels of tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β, interleukin-6 (IL-6), klotho, and DPP4 were measured by enzyme-linked immunosorbent assay. Plasma expression of miR-29a and miR-195 were also measured and compared by a real-time polymerase chain reaction.
Results:
There was a significant increase in TNF-α (p=0.006), IL-1β (p=0.012), and IL-6 (p=0.012) levels in the AD subjects compared with controls. Also, we found a decrease in plasma levels of klotho and an increase in plasma levels of DPP4 in the AD group that was not significant compared with the controls. Lower expression of miR-29a (P=0.009) and higher expression of miR-195 (P=0.003) were observed in the AD group that was significant than controls. Further analysis showed a negative correlation between klotho and plasma levels of IL-6 (r=−0.58, p=0.01). Also, there was a positive correlation between plasma DPP4 activity and TNF-α levels (r=0.50, P=0.04) and IL-1β (r=0.62, P=0.01). Likewise, plasma klotho concentration showed a negative correlation with the age of AD subjects (r=−0.56, P=0.02).
Conclusion:
TNF-α, IL-1β, and IL-6 are involved in AD pathophysiology, and dysregulation of DPP4 and klotho may be associated with the inflammatory response of AD. Down-regulation of miR-29a and up-regulation of miR-195 indicated the role of miRNAs in the AD process.
Keywords: Alzheimer Disease (AD), Klotho, Dipeptidyl Peptidase-4, Inflammatory cytokines, MicroRNAs
Highlights
Plasma levels of IL-1β, IL-6 and TNF-α increase in the Alzheimer disease patients compared to healthy controls.
Plasma levels of klotho proteins decrease while DPP4 increase in the Alzheimer disease patients compared to healthy controls.
miR-29a is down-regulated while miR-195 is up-regulated in the Alzheimer disease patients compared to healthy controls.
Increased DPP4 and decreased klotho concentrations are associated with dysregulation of inflammatory cytokines.
Plain Language Summary
Alzheimer Disease (AD) is the most common cause of dementia in the elderly and is complicated with neural inflammation in the brain tissue. Inflammatory cytokines such as Tumour Necrosis Factor-α (TNF-α), interleukin-1-beta (IL-1β) and interleukin-6 (IL-6) are the most potent inflammatory cytokines and produced by activated microglia and astrocytes in the brain. Our findings showed that TNF-α, IL-1β and IL-6 increased in AD patients. Also, plasma klotho, which is an anti-aging protein decreased while DPP-4 increased in AD subjects compared to the healthy controls. Furthermore, circulating miR-29a showed down regulation while miR-195 showed up regulation in the AD patients. Down-regulation of miR-29a and up-regulation of miR-195 indicate contribution of miRNAs to process of AD.
1. Introduction
Alzheimer Disease (AD) is a common age-related disease that causes memory loss, the decline in cognitive function, and progressive disability (Liu & Chan, 2014; Motta, Imbesi, Di Rosa, Stivala, & Malaguarnera, 2007). Neural inflammation is a hallmark of neurodegenerative diseases, such as AD, which is defined by the stimulation of astrocytes and microglia, and the activation of pro-inflammatory agents. Tumour necrosis factor-α (TNF-α) and Inter-leukin 1-beta (IL-1β) are the first two cytokines in the inflammatory cascade, which are released locally and stimulate the production of IL-6 (de Gonzalo-Calvo et al., 2010). From the biological point of view, TNF-α, IL-1β, and IL-6 are the most pivotal inflammatory cytokines involved in the AD process produced by activated microglia and astrocytes in the nervous system (Dursun et al., 2015; Faria et al., 2014).
Recent studies have reported klotho protein as an anti-aging factor with neuroprotective effects through the alleviation of oxidative stress in the neurodegenerative process (Sedighi et al., 2019; Brobey, Dheghani, Foster, Kuro-o, & Rosenblatt, 2015; Kuang et al., 2014). Klotho is a pivotal regulator of the Insulin/insulin-like Growth factor-1 (IGF-1) signaling pathway, which is proposed as an evolutionary mechanism involved in the oxidative stress process and aging. Hence, klotho might diminish oxidative stress and progress of AD through the negative regulation of insulin/IGF-1 signaling cascade, as well as the alleviation of oxidative stress in the brain tissue (Utsugi et al., 2000).
Moreover, the Dipeptidyl Peptidase-4 (DPP4) enzyme has shown to induce inflammation and oxidative stress through its interaction with IL- 12 or IGF-II. Numerous investigations have reported the relationship between increased DPP4 activity and the pathogenesis of insulin resistance and metabolic syndrome (Zheng, Chen, Liu, Gao, & Tian, 2015; Zheng, Chen, Liu, Gao, & Tian, 2014). A recent investigation by Wronkowitz et al showed that MAPK and NF-kB signaling pathways are activated by DPP4 leading to the promotion of inflammation and proliferation of human vascular smooth muscle cells (Wronkowitz et al., 2014).
With the emergence of microRNAs (miRNAs) as potential diagnostic factors for neurological diseases, it has documented that several miRNAs, such as miR-29a and miR-195 were dysregulated in AD process (Basak, Patil, Alves, Larsen, & Møller, 2016; Delay, Mandemakers, & Hébert, 2012). Biologically, miR-29a directly targets β- site amyloid precursor protein cleaving enzyme (BACE1), which is directly involved in the process of Amyloid-beta (Aβ) formation. Also, miR-195 is involved in the oxidative stress process through the dysregulation of inflammatory agents (Garza-Manero, Pichardo-Casas, Arias, Vaca, & Zepeda, 2014; Shi et al., 2013). Also, miR-195 inversely modulates the protein level of BACE1 and Aβ accumulation (Zhu et al., 2012).
Considering the role of klotho and DPP4 in the regulation of the inflammatory process, the present study was conducted to evaluate the comparatively the levels of inflammatory cytokines, including IL-1β, IL-6, TNF-α in the plasma of AD patients and assess the relationship between plasma DPP4 and klotho activity and the abovementioned cytokines. Moreover, miR-29a and miR-195, which are involved in the regulation of AD mechanisms through targeting inflammatory cytokines were measured and compared in the AD and healthy subjects to investigate the possible relationship between miRNAs quantities and inflammatory cytokines levels.
2. Methods
2.1. Subjects
This study was conducted on cases referring to our neurological research institute. The informed consent was obtained from the subjects and the study was approved by our local Ethics Committee at Iran University of Medical Sciences (IR.IUMS #26942). Subjects were divided into two groups: AD and healthy control (age-matched control) groups. All subjects who had diabetes mellitus and hypertension were excluded from the study. Diagnosis of the AD was done by the neurologist at the clinic and the Mini-Mental State Examination (MMSE) was performed to assess participants with mild cognitive impairment. Further assessment was done to exclude subjects with previous brain attack or abnormality from the study.
2.2. Sample collection and ELISA analysis
After an overnight fast, 5 ml of venous blood samples were taken from the participants and collected in heparin lithium tubes. The blood samples were centrifuged at 1500 g for 15 min at room temperature, and after isolation of the serum, the levels of IL-1β, IL-6, TNF-a, klotho, and DPP4 were measured by Enzyme-Linked Immunosorbent Assay (ELISA) (R&D Systems, USA) and the commercial kit according to the manufacturer’s instructions (BioTek, Winooski, Vermon USA).
2.3. Quantitative real-time PCR
Total RNA was isolated from the plasma sample using Qiazol (Qiagene, Germany) based on the manufacturer’s instruction. The purity and concentration of total RNA were evaluated by the absorbance ratio at 260/280 nm by the NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). The TaqMan Advanced miRNA cDNA Synthesis Kit (Applied Biosystems, USA) was used to synthesize microRNA. The Taqman advanced miRNA assays (Thermo Fisher Scientific, USA) were subsequently implemented to assess the expression of miRNAs based on the instruction provided by the manufacturer and included the specific Stem-Loop primer and probe for each microRNA. Real-time Polymerase Chain Reaction (PCR) was run in an ABI StepOne (Applied Biosystems, USA) system using RealQ Plus 2x Master Mix Green (Amplicon, Denmark) for 40 cycles (15 s at 95°C, 15 s at 60°C, and 30 s at 72°C). Hsa-miR-195-5P (MIMAT0000461, miRBase) and hsa-mir-29a (MI0000087, miRBase) profiling were performed using specific MiScript Primer Assays.
The amplicon sizes for the hsa-mir-29a were 80 bp and 82 bp for the hsa-miR-195-5P. The following primers were used for the PCR:
Hsa-miR-195-5P forward, 5’ CGCAGAGCTAGCAGCACAG-3’and reverse, 5’ GTGCAGGGTCCGAGGTAT-3’; hsa-mir-29a forward, 5’-GACTCGTAGCACCATCTG-3’ and reverse, 5’ GTGCAGGGTCCGAGGTAT-3’. The 2-ΔΔCt method was used to quantify the relative levels of gene expression.
2.4. Statistical analysis
Data were analyzed using the SPSS V. 22 (SPSS Inc., Chicago IL, USA) statistical software package. Continuous values were expressed as Mean±Standard Deviation (SD) and analyzed using the Student’s t-test. The Pearson chi-square test was used to examine the categorical data. The correlation coefficient was used to determine the significant contribution of the variable parameters to the constant parameter. P-value of <0.05 was considered as statistically significant.
3. Results
Our study population consisted of 32 subjects, including 12 males (37.5%) and 20 females (62.5%). Table 1 summarizes the demographic data and clinical characteristics of the subjects in the healthy control and AD groups. Both groups were homogenized for age, gender, comorbidities, and consumption of nonsteroidal anti-inflammatory drugs. The plasma IL-1β, IL-6, and TNF-α levels in both groups are shown in Figure 1. The plasma IL-1β levels were significantly higher in AD patients than in the control group (P=0.006). Likewise, the IL-6 and TNF-α levels showed a significant rise in AD patients compared with the control group (P=0.012 and P=0.021, respectively).
Table 1.
Characteristics of the participants in the two studied groups (n=16)
| Variables | Mean±SD/No. (%) | |||
|---|---|---|---|---|
| AD | HCs | |||
| Age (y) | 78.06±5.1 | 75.08±4.6 | ||
| Gender | Male | 6 (37.5) | 6 (37.5) | |
| Female | 10 (62.5) | 10 (62.5) | ||
| Hypertension | 5 (31) | 4 (25) | ||
| Diabetes Mellitus | 2 (12.5) | 3 (19) | ||
| NSAIDs consumption | 1 (6) | 2 (12.5) | ||
AD: Alzheimer Disease; HCs: Healthy Controls; NAIDs: Nonsteroidal Anti-inflammatory Drugs
Figure 1.
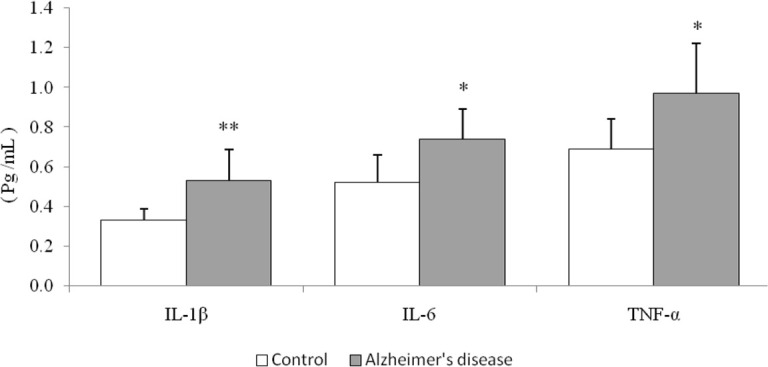
Plasma levels of IL-1β, IL-6 and TNF-α in AD patients (n=16) compared with the age-matched controls (n=16) * P< 0.05; ** P< 0.01
IL-1β: Interleukine-1beta; IL-6: Interleukine-6; TNF-α: Tumor necrosis factor-alfa
As depicted in Figure 2, the plasma level of klotho was lower, whereas the plasma level of DPP4 was higher in AD patients in comparison with the control group. However, differences between the two groups based on klotho and DPP4 were not significant (P>0.05).
Figure 2.
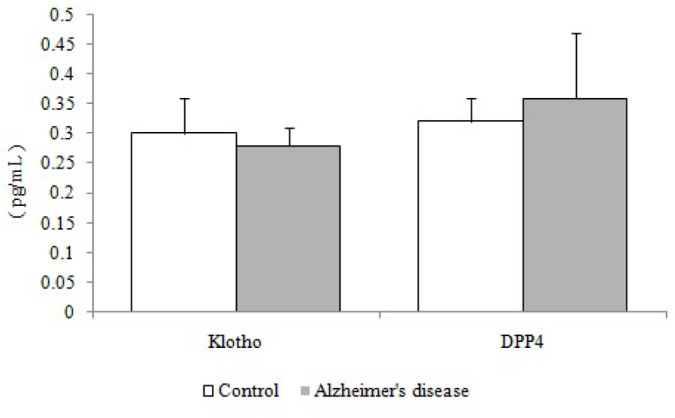
Plasma klotho and DPP4 levels in patients with AD (n=16) compared with the age-matched controls (n=16)
DPP-4: Dipeptidyl Peptidase-4
As illustrated in Figure 3, the results of our study showed that the plasma concentration of miR-29a significantly diminished in the AD group compared with the control group (P=0.009). In contrast, the plasma levels of mir-195 showed a remarkable increase in the AD group compared with the healthy subjects (P=0.003).
Figure 3.
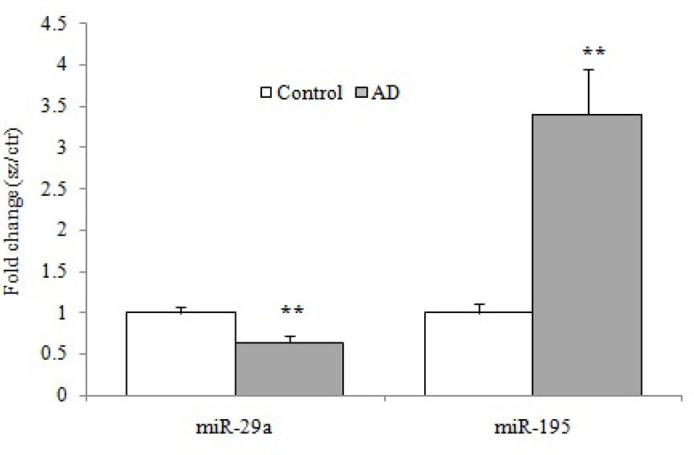
The relative expression of plasma level of miR-29a and miR-195 by the quantitative real-time polymerase chain reaction of patients with AD (n=16) compared with the healthy age-matched controls (n=16)
** P<0.01
The correlation analysis of plasma variables showed a remarkable positive correlation between plasma levels of DPP4 and IL.1β (r=0.62, P=0.01) and TNF-α (r=0.50, P=0.04) in the AD group. Also, a significant negative correlation was found between plasma levels of klotho and IL-6 (r= −0.58, P=0.01) in the AD patients (Table 2). Further analysis disclosed an inverse correlation between plasma levels of klotho and age of AD patients (r=−0.56, P= 0.02) (Figure 4).
Table 2.
The correlation between klotho, DPP-4, miR-29a, miR-195, and cytokines in patients with AD
| Plasma Variables | IL-1β | IL-6 | TNF-α | |||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Klotho | 0.18 | 0.49 | −0.58 | 0.01* | −0.11 | 0.66 |
| DPP-4 | 0.62 | 0.01* | 0.45 | 0.07 | 0.50 | 0.04* |
| miR-29a | 0.15 | 0.57 | −0.25 | 0.34 | 0.48 | 0.056 |
| miR-195 | −0.25 | 0.33 | −0.01 | 0.96 | −0.35 | 0.18 |
IL-1β: Interleukine-1beta; IL-6: Interleukine-6; TNF-α: Tumor necrosis factor-alfa; DPP-4: Dipeptidyl peptidase-4
Correlation was significant at P<0.05 level
Figure 4.
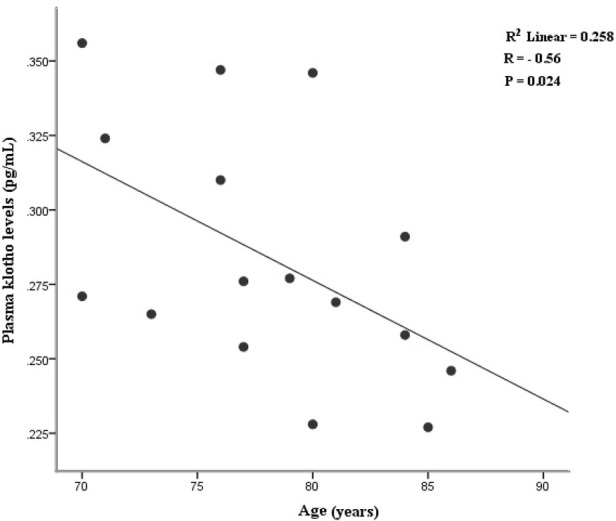
Plasma levels of klotho demonstrated a negative correlation with the age of patients with AD
4. Discussion
In this study, we assessed circulating biomarkers of neuroinflammation, including IL-1β, IL-6, and TNF-α in AD subjects compared with healthy controls to evaluate biochemical changes in AD pathophysiology. Also, we evaluated the plasma levels of klotho, DPP4, miR-29a, and miR-195 in both groups to compare them between AD and healthy subjects to find the possible relationship between plasma biomarkers and inflammatory cytokines levels.
Senescence is a physiological process, which is associated with inflammation. Inflammatory factors, such as interleukins promote the development of senescence (Liu, Wu, Ren, & Gu, 2011). IL-1β plays a pivotal role in the onset and development of various types of neurodegenerative diseases, such as AD. IL-1β can activate other cell types, especially astrocytes and microglia in the neural system to further induction of cytokine release (Calsolaro & Edison, 2016). Accordingly, IL-1β induces IL-6 production, enhances inducible nitric oxide synthase activity, and stimulates the production of macrophage colony-stimulating factor (Rubio-Perez & Morillas-Ruiz, 2012). Our results indicated the elevated levels of IL-1β in AD subjects in comparison with healthy subjects (P<0.01), which is in parallel with other investigations that reported elevated IL-1β in the plasma and Cerebrospinal Fluid (CSF) of AD subjects (Angelopoulos et al., 2008; Öztürk et al., 2007; Rao, Kellom, Kim, Rapoport, & Reese, 2012; Yasutake, Kuroda, Yanagawa, Okamura, & Yoneda, 2006). IL-6 is another inflammatory cytokine that plays a crucial role in both the development and differentiation of the central nervous system (Chen et al., 2012).
Physiologically, IL-6 enhances the activation of microglia that leads to the induction of tau protein phosphorylation and synthesis of acute-phase proteins in neural tissues. Functionally, the activated IL-6 by IL-1β stimulates microglia and astrocytes that can stimulate the production of proinflammatory cytokines and C-reactive protein in AD patients (Querfurth & LaFerla, 2010; Quintanilla, Orellana, González-Billault, & Maccioni, 2004). Based on our findings, plasma levels of IL-6 showed an up-regulation in AD subjects in comparison with the age-matched control group, which is consistent with other reports indicating an elevated level of IL-6 in AD patients (Baranowska-Bik et al., 2008; Bermejo et al., 2008; Cojocaru, Cojocaru, Miu, & Sapira, 2011; Galimberti et al., 2008; Licastro et al., 2000).
Klotho has described as an anti-aging factor with age-regulating effects and acts as a neuroprotective agent via preventing neural cells from oxidative stress (Shiozaki et al., 2008; Uchida et al., 2001). Our results showed a negative correlation between plasma levels of klotho and the age of AD patients (P<0.05). This finding is consistent with the results reported by Semba et al. indicating an inverse correlation between age and CSF klotho levels (Semba et al., 2014). Reduced klotho levels in aged brain white matter may be responsible for myelin degeneration and age-associated cognitive decline in AD patients (Chen et al., 2013; Shardell et al., 2015).
DPP4 is a serine protease enzyme with catalytic activity on the chemokines and inflammatory cytokines located on the surface of various types of cells. DPP4 stimulates T-cell maturation and migration, inflammatory agents release, and induction of cytotoxic T lymphocyte through an IL-12-dependent mechanism (Zheng et al., 2014; Zheng et al., 2016). The Spearman correlation between DPP4 and cytokines concentration in our study disclosed a positive correlation between DPP4 and IL-1β and TNF-α in the AD group (Figure 4). These findings are in parallel with Zheng et al. findings who reported that the elevated plasma DPP4 activities lead to an increase in inflammation and oxidative stress in the peripheral circulation (Zheng et al., 2015). Also, DPP4 inhibition is associated with a decline in the inflammatory cytokines via the mitigation of IL-1β, IL-6, and TNF-α release, which are increased in the AD.
Blood plasma is one of the most important sources of circulatory miRNAs in the human body. miRNAs are known as a novel type of gene expression regulatory agents that inhibit the translation process of mRNAs encoding proteins and have been proposed as noninvasive factors for the diagnosis of many neurodegenerative diseases (Kumar et al., 2013; Kumar & Reddy, 2016). Based on some evidence, multiple miRNAs are associated with the initiation and development of sporadic AD. Although Yang et al. reported similar blood levels of miR-29a in the AD group (Yang et al., 2015), our findings showed a remarkable down-regulation of miR-29a in AD patients in comparison with age-matched controls, which is in agreement with other studies (Geekiyanage, Jicha, Nelson, & Chan, 2012; Hebert et al., 2008). Functionally, miR-29a inversely targets BACE1 expression and may be directly involved in the production of the Aβ protein from its amyloid precursor protein (Hebert et al., 2008). Interestingly, miR-195 in our research showed a marked up-regulation in the AD group compared with the controls. Consistent with our results, MA et al. reported that the overexpression of miR-195 leads to increased levels of IL-1β and IL-6 in cells (Ma et al., 2018). Moreover, Shi et al. also reported that the up-regulated miR-195 can elevate pro-inflammatory cytokines of IL-1β and TNF-α in microglia and promote neuroinflammation (Shi et al., 2013).
Our findings proved concurrent evidence suggesting the potential role of inflammatory cytokines, such as IL-1β, IL-6, and TNF-α in the pathogenesis of AD. Moreover, it is suggested that increased DPP4 and decreased klotho levels in plasma may be associated with neural inflammation and cognitive impairment in AD subjects. Taken together, based on our results, the down-regulation of miR-29a and up-regulation of miR-195 can indicate the role of miRNAs in the AD process, and consequently, they can be used as potential diagnostic markers.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Research and Ethics Committee (Code: IR.IUMS.REC 1395.9321559001) at Iran University of Medical Sciences, Tehran, Iran.
Footnotes
Funding
This research was extracted from a PhD. thesis of Mohsen Sedighi and it was financially supported by the Iran University of Medical Sciences, Tehran, Iran (Grant No.: 94-04-30-26942).
Authors' contributions
Conceptualization, investigation: Mohsen Sedighi, Tourandokht Baluchnejadmojarad, and Mehrdad Roghani; Writing -original draft: Mohsen Sedighi; Writing - review & editing: Mohsen Sedighi, Mehrdad Roghani; Funding acquisition and supervision: Tourandokht Baluchnejadmojarad; Methodology: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Angelopoulos P., Agouridaki H., Vaiopoulos H., Siskou E, Doutsou K., Costa V., et al. (2008). Cytokines in Alzheimer Disease and vascular dementia. International Journal of Neuroscience, 118(12), 1659–72. [DOI: 10.1080/00207450701392068] [PMID ] [DOI] [PubMed] [Google Scholar]
- Baranowska-Bik A., Bik W., Wolinska-Witort E., Martynska L., Chmielowska M., Barcikowska M., et al. (2008). Plasma beta amyloid and cytokine profile in women with Alzheimer Disease. Neuro Endocrinology Letters, 29(1), 75–9. [PMID ] [PubMed] [Google Scholar]
- Basak I., Patil K. S., Alves G., Larsen J. P., Møller S. G. (2016). microRNAs as neuroregulators, biomarkers and therapeutic agents in neurodegenerative diseases. Cellular and Molecular Life Sciences, 73(4), 811–27. [DOI: 10.1007/s00018-015-2093-x] [PMID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo P., Martín-Aragón S., Benedí J., Susín C., Felici E., Gil P., et al. (2008). Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer Disease. Immunology Letters, 117(2), 198–202. [DOI: 10.1016/j.imlet.2008.02.002] [PMID ] [DOI] [PubMed] [Google Scholar]
- Brobey R. K., Dheghani M., Foster P. P., Kuro-O M., Rosenblatt K. P. (2015). Klotho regulates 14-3-3ζ monomerization and binding to the ASK1 signaling complex in response to oxidative stress. PLoS One, 10(10), e0141968. [DOI: 10.1371/journal.pone.0141968] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calsolaro V., Edison P. (2016). Neuroinflammation in Alzheimer Disease: Current evidence and future directions. Alzheimer’s & Dementia, 12(6), 719–32. [DOI: 10.1016/j.jalz.2016.02.010] [PMID ] [DOI] [PubMed] [Google Scholar]
- Chen C. D., Sloane J. A., Li H., Aytan N., Giannaris E. L., Zeldich E., et al. (2013). The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. Journal of Neuroscience, 33(5), 1927–39. [DOI: 10.1523/JNEUROSCI.2080-12.2013] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Y., Chen T. F., Lai L. C., Chen J. H., Sun Y., Wen L. L., et al. (2012). Sequence variants of interleukin 6 (IL-6) are significantly associated with a decreased risk of late-onset Alzheimer Disease. Journal of Neuroinflammation, 9, 21 [DOI: 10.1186/1742-2094-9-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojocaru I. M., Cojocaru M., Miu G., Sapira V. (2011). Study of interleukin-6 production in Alzheimer Disease. Romanian Journal of Internal Medicine, 49(1), 55–8. [PMID ] [PubMed] [Google Scholar]
- de Gonzalo-Calvo D., Neitzert K., Fernández M., Vega-Naredo I., Caballero B., García-Macía M., et al. (2010). Differential inflammatory responses in aging and disease: TNF-α and IL-6 as possible biomarkers. Free Radical Biology and Medicine, 49(5), 733–7. [DOI: 10.1016/j.freeradbiomed.2010.05.019] [PMID ] [DOI] [PubMed] [Google Scholar]
- Delay C., Mandemakers W., Hébert S. S. (2012). MicroRNAs in Alzheimer Disease. Neurobiology of Disease, 46(2), 285–90. [DOI: 10.1016/j.nbd.2012.01.003] [PMID ] [DOI] [PubMed] [Google Scholar]
- Dursun E., Gezen-Ak D., Hanağası H., Bilgiç B., Lohmann E., Ertan S., et al. (2015). The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer Disease, mild cognitive impairment or Parkinson’s disease. Journal of Neuroimmunology, 283, 50–7. [DOI: 10.1016/j.jneuroim.2015.04.014] [PMID ] [DOI] [PubMed] [Google Scholar]
- Faria M. C., Gonçalves G. S., Rocha N. P., Moraes E. N., Bicalho M. A., Cintra M. T. G., et al. (2014). Increased plasma levels of BDNF and inflammatory markers in Alzheimer Disease. Journal of Psychiatric Research, 53, 166–72. [DOI: 10.1016/j.jpsychires.2014.01.019] [PMID ] [DOI] [PubMed] [Google Scholar]
- Galimberti D., Venturelli E., Fenoglio C., Guidi I., Villa C., Bergamaschini L., et al. (2008). Intrathecal levels of IL-6, IL-11 and LIF in Alzheimer Disease and frontotemporal lobar degeneration. Journal of Neurology, 255(4), 539–44. [DOI: 10.1007/s00415-008-0737-6] [PMID ] [DOI] [PubMed] [Google Scholar]
- Garza-Manero S., Pichardo-Casas I., Arias C., Vaca L., Zepeda A. (2014). Selective distribution and dynamic modulation of miRNAs in the synapse and its possible role in Alzheimer Disease. Brain Research, 1584, 80–93. [DOI: 10.1016/j.brainres.2013.12.009] [PMID ] [DOI] [PubMed] [Google Scholar]
- Geekiyanage H., Jicha G. A., Nelson P. T., Chan C. (2012). Blood serum miRNA: Non-invasive biomarkers for Alzheimer Disease. Experimental Neurology, 235(2), 491–6. [DOI: 10.1016/j.expneurol.2011.11.026] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert S. S., Horré K., Nicolaï L., Papadopoulou A. S., Mandemakers W., Silahtaroglu A. N., et al. (2008). Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer Disease correlates with increased BACE1/beta-secretase expression. Proceedings of the National Academy of Sciences of the United States of America, 105(17), 6415–20. [DOI: 10.1073/pnas.0710263105] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang X., Chen Y. S., Wang L. F., Li Y. J., Liu K., Zhang M. X., et al. (2014). Klotho upregulation contributes to the neuroprotection of ligustilide in an Alzheimer Disease mouse model. Neurobiology of Aging, 35(1), 169–78. [DOI: 10.1016/j.neurobiolaging.2013.07.019] [PMID ] [DOI] [PubMed] [Google Scholar]
- Kumar P., Dezso Z., MacKenzie C., Oestreicher J., Agoulnik S., Byrne M., et al. (2013). Circulating miRNA biomarkers for Alzheimer Disease. PLoS One, 8(7), e69807. [DOI: 10.1371/journal.pone.0069807] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Hemachandra Reddy P. (2016). Are circulating microRNAs peripheral biomarkers for Alzheimer Disease? Biochimica et Biophysica Acta, 1862(9), 1617–27. [DOI: 10.1016/j.bbadis.2016.06.001] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licastro F., Pedrini S., Caputo L., Annoni G., Davis L. J., Ferri C., et al. (2000). Increased plasma levels of interleukin-1, interleukin-6 and α-1-antichymotrypsin in patients with Alzheimer Disease: Peripheral inflammation or signals from the brain? Journal of Neuroimmunology, 103(1), 97–102. [DOI: 10.1016/S0165-5728(99)00226-X] [DOI] [PubMed] [Google Scholar]
- Liu F., Wu S., Ren H., Gu J. (2011). Klotho suppresses RIGI-mediated senescence-associated inflammation. Nature Cell Biology, 13(3), 254–62. [DOI: 10.1038/ncb2167] [PMID ] [DOI] [PubMed] [Google Scholar]
- Liu L., Chan C. (2014). The role of inflammasome in Alzheimer Disease. Ageing Research Reviews, 15, 6–15. [DOI: 10.1016/j.arr.2013.12.007] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Yao H., Yang Y., Jin L., Wang Y., Wu L., et al. (2018). miR-195 suppresses abdominal aortic aneurysm through the TNF-α/NF-κB and VEGF/PI3K/Akt pathway. International Journal of Molecular Medicine, 41(4), 2350–8. [DOI: 10.3892/ijmm.2018.3426] [PMID ] [DOI] [PubMed] [Google Scholar]
- Motta M., Imbesi R., Di Rosa M., Stivala F., Malaguarnera L. (2007). Altered plasma cytokine levels in Alzheimer Disease: Correlation with the disease progression. Immunology Letters, 114(1), 46–51. [DOI: 10.1016/j.imlet.2007.09.002] [PMID ] [DOI] [PubMed] [Google Scholar]
- Öztürk C., Özge A., Yalın O. Ö., Yılmaz İ. A., Delialioglu N., Yıldız Ç., et al. (2007). The diagnostic role of serum inflammatory and soluble proteins on dementia subtypes: correlation with cognitive and functional decline. Behavioural Neurology, 18(4), 207–15. [DOI: 10.1155/2007/432190] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth H. W., LaFerla F. M. (2010). Alzheimer Disease. The New England Journal of Medicine, 362(4), 329–44. [DOI: 10.1056/NEJMra0909142] [PMID ] [DOI] [PubMed] [Google Scholar]
- Quintanilla R. A., Orellana D. I., González-Billault C., Maccioni R. B. (2004). Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Experimental Cell Research, 295(1), 245–57. [DOI: 10.1016/j.yexcr.2004.01.002] [PMID ] [DOI] [PubMed] [Google Scholar]
- Rao J. S., Kellom M., Kim H. W., Rapoport S. I., Reese E. A. (2012). Neuroinflammation and synaptic loss. Neurochemical Research, 37(5), 903–10. [DOI: 10.1007/s11064-012-0708-2] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Perez J. M., Morillas-Ruiz J. M. (2012). A review: Inflammatory process in Alzheimer Disease, role of cytokines. The Scientific World Journal, 2012, 756357. [DOI: 10.1100/2012/756357] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedighi M., Baluchnejadmojarad T., Fallah S., Moradi N., Afshin-Majdd S., Roghani M. (2019). Klotho ameliorates cellular inflammation via suppression of cytokine release and upregulation of miR-29a in the PBMCs of diagnosed Alzheimer Disease patients. Journal of Molecular Neuroscience, 69(1), 157–65. [DOI: 10.1007/s12031-019-01345-5] [PMID ] [DOI] [PubMed] [Google Scholar]
- Semba R. D., Moghekar A. R., Hu J., Sun K., Turner R., Ferrucci L., et al. (2014). Klotho in the cerebrospinal fluid of adults with and without Alzheimer Disease. Neuroscience Letters, 558, 37–40. [DOI: 10.1016/j.neulet.2013.10.058] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shardell M., Semba R. D., Rosano C., Kalyani R. R., Bandinelli S., Chia C. W., et al. (2015). Plasma klotho and cognitive decline in older adults: Findings from the InCHIANTI Study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 71(5), 677–82. [DOI: 10.1093/gerona/glv140] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G., Shi J., Liu K., Liu N., Wang Y., Fu Z., et al. (2013). Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia, 61(4), 504–12. [DOI: 10.1002/glia.22451] [PMID ] [DOI] [PubMed] [Google Scholar]
- Shiozaki M., Yoshimura K., Shibata M., Koike M., Matsuura N., Uchiyama Y., et al. (2008). Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neuroscience, 152(4), 924–41. [DOI: 10.1016/j.neuroscience.2008.01.032] [PMID ] [DOI] [PubMed] [Google Scholar]
- Uchida A., Komiya Y., Tashiro T., Yorifuji H., Kishimoto T., Nabeshima Y., et al. (2001). Neurofilaments of klotho, the mutant mouse prematurely displaying symptoms resembling human aging. Journal of Neuroscience Research, 64(4), 364–70. [DOI: 10.1002/jnr.1087] [PMID ] [DOI] [PubMed] [Google Scholar]
- Utsugi T., Ohno T., Ohyama Y., Uchiyama T., Saito Y., Matsumura Y., et al. (2000). Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism, 49(9), 1118–23. [DOI: 10.1053/meta.2000.8606] [PMID ] [DOI] [PubMed] [Google Scholar]
- Wronkowitz N., Görgens S. W., Romacho T., Villalobos L. A., Sánchez-Ferrer C. F., Peiró C., et al. (2014). Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochimica et Biophysica Acta, 1842(9), 1613–21. [DOI: 10.1016/j.bbadis.2014.06.004] [PMID ] [DOI] [PubMed] [Google Scholar]
- Yang G., Song Y., Zhou X., Deng Y., Liu T., Weng G., et al. (2015). MicroRNA-29c targets β-site amyloid precursor protein-cleaving enzyme 1 and has a neuroprotective role in vitro and in vivo. Molecular Medicine Reports, 12(2), 3081–8. [DOI: 10.3892/mmr.2015.3728] [PMID ] [DOI] [PubMed] [Google Scholar]
- Yasutake C., Kuroda K., Yanagawa T., Okamura T., Yoneda H. (2006). Serum BDNF, TNF-α and IL-1β levels in dementia patients. European Archives of Psychiatry and Clinical Neuroscience, 256(7), 402–6. [DOI: 10.1007/s00406-006-0652-8] [DOI] [PubMed] [Google Scholar]
- Zheng T., Chen T., Liu Y., Gao Y., Tian H. (2015). Increased plasma DPP4 activity predicts new-onset hypertension in Chinese over a 4-year period: Possible associations with inflammation and oxidative stress. Journal of Human Hypertension, 29(7), 424–9. [DOI: 10.1038/jhh.2014.111] [PMID ] [DOI] [PubMed] [Google Scholar]
- Zheng T., Gao Y., Baskota A., Chen T., Ran X., Tian H. (2014). Increased plasma DPP4 activity is predictive of prediabetes and type 2 diabetes onset in Chinese over a four-year period: Result from the China National Diabetes and Metabolic Disorders Study. The Journal of Clinical Endocrinology & Metabolism, 99(11), E2330–4. [DOI: 10.1210/jc.2014-1480] [PMID ] [DOI] [PubMed] [Google Scholar]
- Zheng T., Qin L., Chen B., Hu X., Zhang X., Liu Y., et al. (2016). Association of plasma DPP4 activity with mild cognitive impairment in elderly patients with type 2 diabetes: Results from the GDMD study in China. Diabetes Care, 39(9), 1594–601. [DOI: 10.2337/dc16-0316] [PMID ] [DOI] [PubMed] [Google Scholar]
- Zhu H. C., Wang L. M., Wang M., Song B., Tan S., Teng J. F., et al. (2012). MicroRNA-195 downregulates Alzheimer Disease amyloid-β production by targeting BACE1. Brain Research Bulletin, 88(6), 596–601. [DOI: 10.1016/j.brainresbull.2012.05.018] [PMID ] [DOI] [PubMed] [Google Scholar]


