1. Introduction
Diarrheal diseases remain one of the leading causes of morbidity and mortality worldwide. Globally, an estimated 4.5 billion cases and 1.7 million deaths were attributable to diarrheal diseases in 2016 (Troeger et al., 2018), with low and middle income countries (LMIC) being particularly burdened (Walker et al., 2013; Wazny et al., 2013). Despite the persistence of diarrheal diseases globally, there has been progress. The number of deaths due to diarrhea decreased by an estimated 20.8% from 2005–2015 (Troeger et al., 2017), demonstrating that diarrheal disease is a tractable high-priority target to meet the third Sustainable Development Goal (SDG) of ensuring healthy lives and promoting well-being (WHO & Unicef, 2013).
Evidence-based interventions to prevent and treat diarrheal diseases exist (WHO, 2005), but inequities prevent universal access to these basic interventions. Prevention of diarrheal diseases requires basic proven hygiene and sanitation interventions at the population level, which is lacking in many LMICs(Leung et al., 2016). Treatment often requires only low-cost oral rehydration solution (ORS) without antibiotics, but information and supplies (including potable water) may not be available for vulnerable and marginalized populations (Ellis et al., 2007; Santosham et al., 1997). Addressing barriers to access for diarrheal disease patients is a key step to extending the gains achieved globally over the past two decades (Ellis et al., 2007).
The patient experience for diarrhea management begins at the household, when decisions are made to seek care. Colvin et al. describe this decision making process for common diseases, including diarrhea, in Sub-Saharan Africa as a non-linear and uncertain dynamic process (Colvin et al., 2013). This process involves trial and error to identify pathways to desired clinical outcomes that meet social norms and respect financial constraints. Similar insights have been documented in Southeast Asia, where diseases like cholera are endemic in Bangladesh and Eastern India and can seed global pandemics (Andrews et al., 2017). Care-seeking for diarrheal disease in Bangladesh is similarly dynamic, and has benefitted from decades of education, resulting in knowledge and practices related to at-home ORS. However, outbreaks caused by diseases like cholera further strain systems (Farmer et al., 2011) and likely challenge the dynamic process of seeking and receiving care (Colvin et al., 2013).
In cases of severe or sustained diarrhea, families may decide to seek treatment at a hospital. In Bangladesh, poorly funded government facilities are often the only access point for life-threatening situations for poor patients. Diarrheal disease is one of the most common reasons to seek hospital-level care in Bangladesh (Sultana et al., 2015), and the country holds one of the highest child mortality rates from diarrhea (International Vaccine Access Center (IVAC), 2018). Despite the commonness of diarrheal presentation, treatment for diarrhea is inconsistent and often fails to meet standard guidelines, particularly during large seasonal outbreaks of diarrheal disease pathogens like cholera (Andrews et al., 2017; Das et al., 2014).
The hospital wards where diarrhea patients are treated can be chaotic, with social and structural factors limiting effective care (Hadley et al., 2007; Hadley & Roques, 2007; Zaman, 2004). Patients often enter care with desired treatment pathways, which may be inconsistent with providers’ clinical judgment, and/or not aligned with WHO guidelines for diarrheal management (Howteerakul et al., 2003). The resolution of this conflict may result in unnecessary intravenous (IV) fluid use (Haque et al., 2017) and inappropriate antibiotic prescriptions that drive antimicrobial resistance and treatment failures (Bojalil & Calva, 1994; Charanasri et al., 1995; Howteerakul et al., 2003).
Diarrheal disease represents significant economic burden on households (Rheingans et al., 2012; Shillcutt et al., 2016) that is exacerbated by hospitalization. The average societal cost of each episode of diarrheal disease in Bangladesh is 67 USD; the outpatient cost is 24 USD, and the inpatient cost is more than 110 USD (Sarker et al., 2018). A single hospitalization for diarrhea may cost a family one month salary (Sarker et al., 2013). After discharge, post-discharge morbidity and mortality add additional hardships (Kotloff et al., 2013). These financial pressures influence decision making around seeking clinical care, expectations at hospitals, and behaviors following discharge.
In order to improve diarrheal management in hospitals, it is important to understand the journey of patients who are admitted to the hospital with diarrheal disease. Ethnographic approaches, including observation and informal interviews, offer a flexible methodological approach to identify locally grounded evidence that can influence public opinion and policy (Hansen et al., 2013). In global health, ethnography is important to identify agendas that are patient inspired and respectful of the social context (Pigg, 2013). Hospital-based ethnography explores the culture of the facility and garners an appreciation of how the clinical environment reflects and reinforces social and cultural processes outside the hospital (Van der Geest & Finkler, 2004). While ethnographic studies of hospital care are increasingly of interest (Street & Coleman, 2012), there remain limited studies that use this approach to characterize standards of care for diarrheal disease in resource-limited hospitals, especially in Bangladesh (Hadley et al., 2007; Hadley & Roques, 2007).
In this study, we conducted a rapid ethnographic study at 10 public hospitals in Bangladesh with the aim of characterizing hospital-based diarrheal disease management (norms, attitudes, practices, behaviors and logics) from multiple perspectives. The overall goal was to explore factors that influence the management of patients, and to identify opportunities to improve clinical care. The results of the study may guide policy makers, public health officials and clinicians to improve care while minimizing cost to an already burdened public health infrastructure.
2. Methods
2.1. Setting
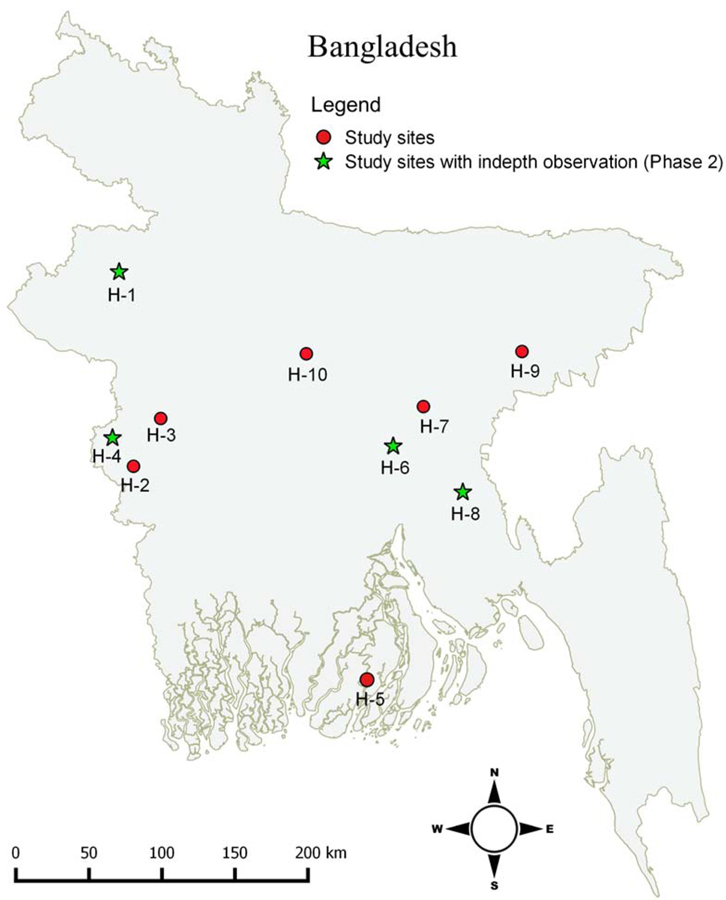
The study was conducted at ten district hospitals throughout Bangladesh (Figure 1, Table 1) that were participating in a clinical trial of a decision support tool for diarrhea management (A I Khan et al., 2020); data on doctor/patient interaction were collected before the clinical trial intervention was deployed. The facilities were selected from an original pool of 22 heterogenous government hospitals that participated in a national cholera surveillance study (M. T. Islam et al., 2019; A.I. Khan & Qadri, 2019). All of the hospitals provided general care with intermittent subspecialty care. Five hospitals had 250 approved beds and five had 100 approved beds.
Figure 1.
Distribution of district study hospitals in Bangladesh.
Table 1.
Characteristics of district study hospitals
| Hospital | H-1† | H-2 | H-3 | H-4† | H-5† | H-6† | H-7 |
|---|---|---|---|---|---|---|---|
| Founded | 1965 | 1970 | 1962 | 1997 | 1979 | 1999 | 2003 |
| Relative location | North | West | West | West | South-central | Central | Central |
| District population* | 2,385,900 | 1,120,098 | 1,946,838 | 655,392 | 15,57,137 | 29,48,217 | 22,24,944 |
| Approved beds, n | 100 | 100 | 250 | 250 | 250 | 100 | 100 |
| Total patients (2017)** | 461,580 | 324,000 | 470,876 | 223,347 | 212,576 | 270,553 | 253,291 |
| Diarrhea patient (2017) | |||||||
| Wards; n | 8 | 9 | 14 | 5 | 13 | 7 | 5 |
| Doctors; n | 29 | 16 | 47 | 13 | 22 | 34 | 37 |
| Nurses; n | 52 | 61 | 168 | 90 | 102 | 100 | 40 |
| Support staff; n | 57 | 24 | 53 | 30 | 35 | 34 | 40 |
Sites where the in-depth phase-2 research was conducted
Source: Bangladesh national census 2011 (Statistics., 2014)
Source: District hospital databases 2017. Courtesy of respective Ministry of Health and Family Planning, Government of Bangladesh.
2.2. Rapid ethnographic data collection
Rapid ethnographic research targets a specific problem and is a pragmatic strategy to collect context-specific data that is needed to inform policy and practice for urgent public health issues (Johnson & Vindrola-Padros, 2017; Pigg, 2013). The approach taken in this study included clinical observations and informal interviews with clinicians, staff nurses and patients (Pelto, 2016). Data collection was conducted by two anthropologists with Masters-level training in qualitative research methods; responsibilities were divided and each ethnographer worked independently. The study was conducted over 12 weeks between March-July 2018; this period in Bangladesh is typically associated with outbreaks of bacterial agents that cause diarrhea, most notably cholera (Haque et al., 2017; A.I. Khan & Qadri, 2019).
Data collection proceeded in two phases. Phase one was conducted at all ten hospitals over a four week period (3 days in each hospital) and included both clinical observations and informal interviews. Emergent findings were discussed in the team to identify common and divergent themes across sites. Based on this comparative analysis, we purposefully selected four disparate hospitals for further in-depth study. In phase two, the ethnographers spent ten additional days at the four selected hospitals, conducting clinical observations and informal interviews. For both phases, the ethnographers spent approximately 6 hours per day in emergency rooms and hospital wards that managed diarrheal disease patients; this equated to 420 total hours of observation and interviews (180 hours in phase 1; 240 hours in phase 2). To accommodate for variation in hospital activity level throughout the day, research was conducted during the peak activity period of 08:00 to 14:00, and the lower activity period of 16:00 to 22:00.
In order to understand patient flow, the ethnographers mapped the physical layout of the emergency departments and wards where diarrheal disease patients were treated. Observations focused on the clinical workflow, providers’ consultations and communication with patients and their families, and interactions among clinicians and other clinical staff. The ethnographers observed clinician-patient interactions during outpatient and inpatient consultations (n=76), and created profile notes on clinician-patient interactions, including body language, methods of history taking, physical examination, and use of equipment. Each day, the ethnographers transcribed detailed notes of their observations and experiences.
Informal interviews included discussions with clinicians and hospital staff, as well as patients and their families, about their experiences, perceptions and thoughts on diarrheal disease management in the hospital (n=138; Table 2). Notes taken during interviews were brief and expanded after each interview to maintain the informality of an ethnographic approach; efforts were made to triangulate data across participant types and between observations and self-reports. Unplanned natural interactions between the ethnographers and stakeholders provided additional insights; these were documented in notebooks discretely to avoid disrupting natural flows of conversation and events. Key statements were written in direct quotes. All field notes were digitized for analysis and archiving.
Table 2.
Distribution of unique individuals interviewed
| Hospitals | H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | H9 | H10 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Physicians | 2 | 3 | 3 | 5 | 3 | 6 | 3 | 4 | 5 | 3 | 37 |
| Nurses | 2 | 2 | 3 | 4 | 2 | 4 | 3 | 4 | 2 | 2 | 24 |
| Patients/ Family | 4 | 3 | 4 | 14 | 3 | 15 | 2 | 19 | 12 | 3 | 72 |
| Other hospital staff* | 2 | - | - | 3 | - | 2 | - | 3 | 3 | 1 | 12 |
| Total | 10 | 8 | 10 | 24 | 8 | 27 | 8 | 30 | 22 | 9 | 138 |
Other hospital staff includes administrative officers, medical assistants and sub-assistant community medical officers (SACMOs)
Our final sample was a result of the observations and interviews that the team was able to conduct in the data collection time periods that were determined a priori. Although the sample was not based on a goal of data saturation, analysis was conducted iteratively and the team felt at the end of the data collection that they had reached saturation of themes.
2.3. Analysis
Data analysis was informed by applied thematic analysis, an inductive analytic approach that is appropriate to the applied research context (Guest et al., 2014). Analysis was conducted iteratively throughout the collection period in order to be responsive to emergent findings. Field notes were expanded daily after direct observations and interviews, and subsequently translated from Bengali to English. The transcribed data were used to identify inductive themes across socio-cultural, clinical, institutional and financial interest categories with sub-stratification. For example, under ‘institutional’, the sub-categories were patient flow, lack of equipment, and human resource limitations. A codebook was created by the two field anthropologists that included categories (parent codes) and emergent themes under each category (child codes). Coded data were reviewed by two non-field supervising anthropologists to identify relationships between codes, and to “lump” codes for summarization (Leech & Onwuegbuzie, 2007). In order to describe the sample, clinical features and patient characteristics were enumerated and summarized.
2.4. Ethics
The study protocol was approved by the Ethics Review Committees of the icddr,b (PR-17036), IEDCR (IEDCR/IRB/2017/10), and the University of Florida (IRB201601762); this study was part of a larger clinical trial registered at clinicaltrials.gov (NCT031542290) (A I Khan et al., 2020). Central (IEDCR) and local Ministry of Health and Family Welfare (MOHFW) governmental permission was granted to conduct research within the hospitals. Written informed consent/assent was obtained from patient participants. Verbal permission was obtained from physicians at the start of each observed consultation. Verbal permission from non-patient participants was obtained before conducting interviews. Personally identifying information was removed from all ethnographic notes. At the hospital level, data were aggregated and de-identified.
3. Results
Clinical observations and informal interviews were conducted over a total of 420 hours. Data were collected from 138 individuals, including 73 hospital personnel (doctors, nurses and other staff) and 72 patients or family members of patients (Table 2). Characteristics of 76 evaluations by doctors of patients with diarrheal disease were observed (Table 3); among these 72 of the patients were interviewed individually. Clinical features and characteristics of the 76 patients from these interactions are presented in Table 3.
Table 3.
Characteristics of patients observed in patient/doctor interactions at admission
| Characteristic | n (%) |
|---|---|
| Patients | |
| Male | 47 (62) |
| Female | 29 (38) |
| Total | 76 |
| Age | |
| <5 years | 37 (49) |
| 6–17 Years | 05 (07) |
| 18–50 years | 29 (38) |
| >50 years | 05 (07) |
| Dehydration status* | |
| No | 28 (37) |
| Some | 43 (57) |
| Severe | 05 (07) |
| Physical examination features performed by doctor | |
| Skin pinch | 13 (17) |
| Checked for sunken eye | 14 (18) |
| Checked BP | 04 (05) |
| Checked temperature | 01 (01) |
| Measured weight | 00 (00) |
| Checked Tongue | 12 (16) |
| No physical examination | 43 (57) |
| Treatment ordered | |
| IV fluid | 68 (89) |
| ORS only | 08 (11) |
| Antibiotic | 60 (79) |
| Zinc | 26 (34) |
| IV fluid ordered by dehydration status | |
| No | 21 (75) |
| Some | 42 (98) |
| Severe | 05 (100) |
| Antibiotic ordered by dehydration status | |
| No severe | 21 (75) |
| Some severity | 35 (81) |
| Severe | 04 (80) |
| Use of Zinc by patient age | |
| <5 years | 16 (43) |
| 6–17 Years | 00 (0) |
| 18–50 years | 08 (28) |
| >50 years | 02 (40) |
| Antibiotic by patient age | |
| <5 years | 25 (68) |
| 6–17 Years | 04 (80) |
| 18–50 years | 26 (90) |
| >50 years | 05 (100) |
Evaluated by the doctor and recorded in the patient’s chart
Specific themes emerged that impacted the management of diarrheal diseases in the hospital setting. Providers had robust knowledge about diarrhea management, but did not routinely apply this knowledge in practice. This was due to providers’ desire to meet patients’ expectations for clinical treatments (specifically antibiotics and IV fluids), and a high patient load that made unsubstantiated decisions about clinical treatment more expedient. At the provider level, shortages in personnel, as well as conflicts of interest by physicians, limited best-practice treatment of diarrhea. At the institutional level, overcrowding of facilities and poor hygiene and sanitation prevented both prioritization and adequate treatment of diarrheal diseases. Below, we elaborate on these themes.
3.1. Best practices for diarrheal management
Physicians explained what they viewed as best practice for evaluating cases of diarrheal disease, which was grounded in history taking and physical examination. They noted that an initial intake should consider the patient’s age, how the illness began, stool type, duration and frequency of diarrhea, secondary symptoms (e.g. fever, vomiting, nausea, abdominal pain), nutritional intake, past medical history and medication use. Physicians explained that these features were integrated with the physical examination to assess the dehydration status and generate a treatment plan. They explained that their examination consisted of assessing general condition, sunken eyes, thirst, and skin turgor (skin pinch). Additional features mentioned were weight, body temperature, heart rate, blood pressure, and abdominal exam.
Physicians defined diarrhea as a change in normal bowel movement that increased the frequency of stools to greater than, or equal to, three times per day, and changed the stool consistency to ‘liquid’/’loose’. They typically noted three types of diarrhea: acute watery diarrhea (often referred to as ‘cholera’;‘daeria/patla paykhana’ in local terms), acute bloody diarrhea (referred to as ‘dysentery’ or‘amasha/rokto amasha’), and ‘chronic diarrhea’ that lasts for a month or more. The latter category was seen as having multiple etiologies. Physicians perceived that most diarrhea cases were viral (e.g. rotavirus) and a few were bacterial, such as Escherichia coli, Salmonella spp. and Shigella spp.. Parasites were rarely mentioned as a cause of diarrhea. When asked about diagnostics to identify the etiology of the diarrhea presentation, physicians explained that laboratory testing delayed treatment, was an unnecessary expense for patients, and was not necessary per guidelines for most clinical situations.
Diarrhea was viewed as a non-serious disease by both physicians and nurses. Physicians and nurses often had a casual attitude towards their clinical approach and duty, expressing that diarrheal disease was “not a big deal” to manage. They stressed that all physicians working in a public hospital are competent in the management of diarrheal diseases and all district level hospitals have the capacity to treat diarrhea. Physicians acknowledged that diarrhea can be a “serious illness” for a sub-set of patients with uncontrolled chronic diseases, young/old age, malnutrition, and poverty. Among young children, malnutrition that was co-morbid with diarrhea was considered a high-risk situation for mortality, and these cases were referred to hospitals with pediatric sub-specialists. Across all patients presenting with diarrheal disease, physicians saw the primary risks to be dehydration; therefore, rehydration was viewed as the initial and most important treatment.
Providers’ views about diarrheal management were grounded in the context of widespread diarrheal disease among communities with low social-economic status. Physicians attributed the high rates of diarrhea to conditions of poverty, including unsafe drinking water, poor sanitation facilities, and lack of adequate hygienic conditions. Severely dehydrated patients seeking care at the hospital were labeled as patients with a “poor person’s disease.” Physicians and hospital management stressed that interventions aimed at improving nutrition, hygiene and sanitation at the household level would help prevent diarrhea-related mortality, and were an urgent public health priority.
3.2. A knowledge-practice gap in management of diarrhea
A comparison of providers’ self-report and clinical observations revealed a significant ‘know-do’ gap. Despite providers’ robust explanations of the procedures for clinical management of patients with diarrheal disease, the clinical observations revealed a cursory and variable approach to clinical care for this population. Physicians took a limited medical history, and did not routinely address the elements of clinical history described above; the median time of interaction between clinicians and patients was 2 minutes (Table 3). Medical history questions included the duration of illness, number of bowel movements and stool characteristics. Queries to address co-morbidities were rarely observed. Dehydration status was determined visually without physical examinations in 57% of observations (43/76), and skin turgor was assessed in 17% of observations (13/76). Scales to record patients’ weight were absent at all ten emergency rooms; this prevented the application of weight-based fluid and medication dosing, or required providers to visually estimate patients’ weight. Diagnostic testing for disease etiology (e.g. stool cultures) was extremely rare across all sites.
Providers’ decision-making regarding patient disposition (i.e., discharge to home or admit to hospital) was dependent on the assessment of dehydration status. Providers explained that patients with ‘No’ or ‘Some’ dehydration were advised to rehydrate at home by ingesting extra fluids such as fruit juice, soup or ORS. Patients with ‘Severe’ dehydration were treated at the hospital with IV fluids. However, physicians explained that even patients with non-severe dehydration may require admission to the hospital to monitor for the development of severe dehydration. Observations revealed that almost all patients were given IV fluids (90%, n=68/76), even those who were identified as ‘No’ dehydration. Patients with severe dehydration represented a small percentage of cases (6.6%; 5/76).
Physicians stated that they were mindful of their use of antibiotics out of concern for drug resistance. However, observations revealed that 79% of patients (60/76) were prescribed an antibiotic; the most common antibiotic was ciprofloxacin (Table 3). Physicians also provided zinc to adults frequently, which should be reserved for children less than 5 years of age per WHO guidelines.
The data suggested that the desire to be a “good doctor” dominated inter-personal interactions between physicians and patients, and informed clinical decision making. This included the desire to be seen as thorough and authoritative in ways that would maintain or improve their professional reputations (Text Box 1). One senior physician reported that, “a junior doctor is more likely to prescribe antibiotics to patients with acute diarrhea compared to a senior doctor.” However, deviation from best practices was observed across physicians, regardless of experience. Junior physicians favored an approach that relied on examination, while senior physicians were more likely to rely on “experience” without physical examination. Two examples of common exchanges between providers and patients are presented in Text Box 1. Providers often explained that their cursory assessments and treatments were due to limited resources. In the following example, the physician did not ask the age of the child (a baby of 4 months) and did not perform any physical examination or weight measurement. When the doctor was asked about this issue, s/he explained:
I always follow the standard protocol, and everyone should have to, but sometimes due to lack of time and work load, I do not perform all the physical examination, but I can assume the patient’s condition by taking some of the medical history.
The clinical approach was also influenced by the social and economic presentation of the patient. Patients who were viewed as having higher social status by healthcare providers might receive privileged access to services, amenities and time. Doctors carefully examined these patients and at times provided a special bed/“cabin” for them. However, the majority of patients were poor and had decided to seek care at the district only after home treatments (e.g. ORS), efforts at local medication vendors had failed, and elements of desperation led patients to seek care at the hospital.
Text Box 1: Common interactions between physician and caregiver/patient.
Example 1:
Senior doctor: “What’s the problem?”
Caregiver: “Sir, passing watery stool since last night. Stool passed about 7–8 times, and also vomited two times.”
Senior doctor: “Ok, give these medications”
Actions: Written prescription is given, no weight is measured and no physical exam is performed.
[Author comment (EJN): Unable to form an assessment and treatment plan based on this interaction]
Example 2:
Junior doctor: What happened?
Patient: Sir, diarrhea since last day.
Junior doctor: How many times did you pass stools since last night?
Patient: sir, 7–8 times
Junior doctor: Was there any blood with stools?
Patient: No sir
Junior doctor: Do you have any other complications? Like diabetes, pressure?
Patient: Sir, I had stroke once
Junior doctor: When?
Patient: 5 years ago
Junior doctor: Are you currently taking any medicine?
Patient: Yes sir, I am taking medicine for pressure
Junior doctor: Are you feeling weak?
Patient: Yes sir
Junior doctor: Ok, no problem. You will be alright.
Action: Writes medication prescription, asks a medical assistant to check blood pressure; no weight was measured and no physical exam was done]
[Author comment (EJN): Unable to form an assessment and treatment plan based on this interaction]
Patient satisfaction with care was closely linked to receiving expected clinical treatments, most notably medication and IV fluids. If the patient did not receive medication, the patient perceived the physician as providing inadequate care and was left dissatisfied. Patients’ expectations and their subsequent satisfaction with care influenced providers’ clinical decision-making, as this provider explained:
If a patient recovers very quickly by taking medicine that has been given by me, then I will be considered a ‘good’ doctor and this patient will come to me again in the future. Therefore, generally physicians are more likely to provide medicine and even antibiotics to all patients at their very first visit.
Similar observations on satisfaction and quality of care related to IV fluids were found. Physicians explained that they often prescribed IV fluids even when not clinically indicated because patients “demanded” it. We observed only a few situations (4/76) in which patients specifically requested IV fluids. More often, we observed physicians deciding themselves to provide IV fluids for mild diarrhea, as opposed to less invasive ORS. It is possible that the decision to provide IV fluids was driven by patients’ unstated expectations. In interviews, patients and their caregivers expressed that IV fluids were more effective than ORS, and considered it “a medicine” that shortened the duration of illness. One patient caregiver explained:
“There are more vitamins and minerals in the IV saline compared to ORS, so patients can overcome their weakness better than ORS.”
Many patients expressed that the only significant role of a physician was to prescribe “medicine” (including both antibiotics and IV fluids). Patients perceived that medicines would effectively treat the disease, and doctors knew what medicine were required. Thus, patients came to a doctor primarily to get medicines. One physician explained that, “Patients want medicine from us, but sometimes they don’t understand that a patient does not always need medicine… patients also don’t pay attention to the type of medication, they just want one.” Many patients felt unsatisfied if the physician ended the consultation without prescribing medicine. One patient expressed that, “This is a hospital and we come to a hospital to recover from the disease. We do not expect that a patient will suffer here as they suffer at home.” A pediatric consultant explained:
We are not only using unnecessary antibiotics at this hospital, most of the doctors overall in Bangladesh are prescribing more antibiotics because of the fast recovery of patients and they want to be a good doctor to the public. The patient’s mentality also supports the usage of antibiotics as they do not want to stay more days in the hospital bed.
Most patients and their families believed that the government provided adequate medicine to public hospitals, however some patients expressed concern for misuse and misappropriation of medications. Patients were frustrated because prescriptions often had to be filled at private pharmacies. This inflamed sentiment of conflicts of interest and accusations of misconduct. To avoid this situation, physicians sometimes avoided prescribing medications that were in short supply in the hospital pharmacy.
3.3. Human resource challenges in diarrhea management
Hospital managers, administrative staff and senior doctors expressed that the quality of medical care was severely impacted by the general lack of hospital personnel at all levels. Pressures to triage and manage large caseloads limited time for consultations and diagnosis; these pressures increased during outbreaks because diarrheal wards generally lacked surge capacity. Physicians reported that the high patient volume led them to prescribe “common regimens” to diarrheal patients without assessment of the individual patient’s presentation. A Residential Medical Officer (RMO) explained:
In a single shift I see around 200–250 patients. Can you imagine how difficult it is for me to handle all these patients? Because of this, sometimes I can’t check or examine dehydration conditions properly. I just ask a few questions such as the number of purges, vomiting or not, abdominal pain, etc, and based on this I provide treatment.
At another hospital, we observed large numbers of patients with diarrheal disease that overwhelmed the hospital capacity. Staff tried to rehydrate patients quickly and release them within two hours before complete resuscitation, as this doctor explained:
We do not maintain standard guidelines for the use of IV fluid. Most of the time we provide cholera saline [an IV fluid] with a running dose [not calculated] though it is not recommended. Due to the huge load of diarrheal patients, we follow the strategy to manage only dehydration at the hospital, and then discharge patient by prescribing other medicine.
Physicians and nurses explained that recurrence of diarrhea after discharge was common, and patients needed counseling on treatment and prevention. However, due to large caseloads there was no time for patient counseling and education on ORS and supportive care. In light of this, providers often resulted to unnecessary use of IV fluids and antibiotics as a matter of efficiency.
A shortage of trained health care workers also impacted clinical management. Physicians were often absent in the hospital because they were attending in a private clinic to supplement their salary, or they were preparing for licensing/board examinations. The shortage of physicians resulted in task-shifting of diarrhea management to lower cadre providers such as medical assistants. In the emergency room, physicians often prioritized the care of non-diarrheal patients, while medical assistants were tasked with managing diarrheal patients, whose care was seen as less complex. In addition, the gender composition of physicians and medical assistants (typically male) restricted the full clinical assessment of female patients due to cultural gender barriers.
Across all sites, there was no dedicated physician for the diarrheal wards. Physicians from the medicine and pediatric departments were responsible for rotating on the diarrheal wards, in addition to their primary departments. This created logistical challenges, as there was no single physician ‘in-charge’ of the diarrheal wards. Nursing staff provided the majority of clinical care in the diarrheal wards, but felt restricted in their ability to be responsive to patients’ needs. In one hospital, the diarrheal ward was located far from the medicine ward, and staff nurses reported that physicians were reluctant to visit the diarrheal ward. Nurses expressed that they had “no power” to address problems or make independent decisions; doctors were seen as superior and their role was to do what the doctor recommended, even if the doctor was rarely present in the ward.
3.4. Conflicts of interest that influence diarrhea management
Financial incentives appeared to play an important role in influencing the culture of care and clinical decision-making. As mentioned, physicians split their duties between private practice and public service in government hospitals, often by mandate. Many physicians owned their private practices and spent considerable effort building their clientele. They were frustrated with disproportionally low public salaries. It was perceived that a ‘good physician’ would be in high demand and would naturally take advantage of higher salaries through private practice and consultations. One pediatrician explained:
Most of the doctors are working in private clinics and also have their own chamber [office], and you know people will go to you if you are able to make them believe/trust that you are a good doctor. Otherwise, you will lose money.
Patients and families reported that doctors sometimes asked them to seek care at their private practice if their case (diarrhea and non-diarrhea) required more time or if they were too busy. This exposed a risk of financial conflicts of interest, since physicians received consultation fees at the private clinics.
Additionally, non-physician hospital staff suggested that physicians may receive financial incentives from pharmaceutical companies and their representatives, which could influence prescribing practices. Pharmaceutical representatives were observed in each of the hospitals and at the doctors’ private practices (‘chambers’) on a daily basis. Due to the sensitivity of the topic, it was not possible to verify if there were transactional agreements between physicians and the pharmaceutical representatives. How this might have impacted antibiotic use and choice was unclear. However, there was anecdotal evidence that pharmaceutical companies influenced behavior. For instance, one nurse reported that physicians prescribed different brands of antibiotics on different days to satisfy all of the pharmaceutical representatives.
Professional status and social hierarchy were important factors that influenced treatment decisions. A competitive clinical environment that blended private and public domains was observed, with an overarching focus of physicians wanting to “satisfy” patients in order to build their reputation as good doctors and expand their patient load in their private practice. One physician explained this competition for patients:
Now it is a time of competition. There are so many physicians available. Patients will visit the ones that are considered to be good doctors and have a [good] reputation. If I cannot satisfy my patients they will go to another doctor on their next visit. So I will lose money.
There was recognition that reputation as a ‘good’ or ‘bad’ doctors traveled rapidly through social networks. The motivation to become professionally popular and to maintain a private practice was strong. This motivation led physicians to prioritize a sense of accountability to patients, primarily those of higher socio-economic status, above accountability to hospital administration or clinical guidelines.
3.5. Impact of overcrowding on diarrhea management
The diarrheal wards and emergency departments were high-stress, crowded and noisy environments with constant movement of medical staff, patients, caregivers and visitors (Figure 2 and 3). Staff and patients expressed negative opinions of the wards, namely “too much noise”, an “unhygienic” and “dirty” environment, lack of amenities, and various “bad smells.” The hospital, especially the diarrheal ward, was seen as “pathogenic”. One caregiver explained that, “a healthy man will become sick if he stays in the hospital for a few days.” Hospital staff linked inadequate inpatient capacity with lack of investment in infrastructure and flawed hospital design. In addition, external considerations such as population growth and increases in chronic diseases were believed to have resulted in an overwhelmed public health system.
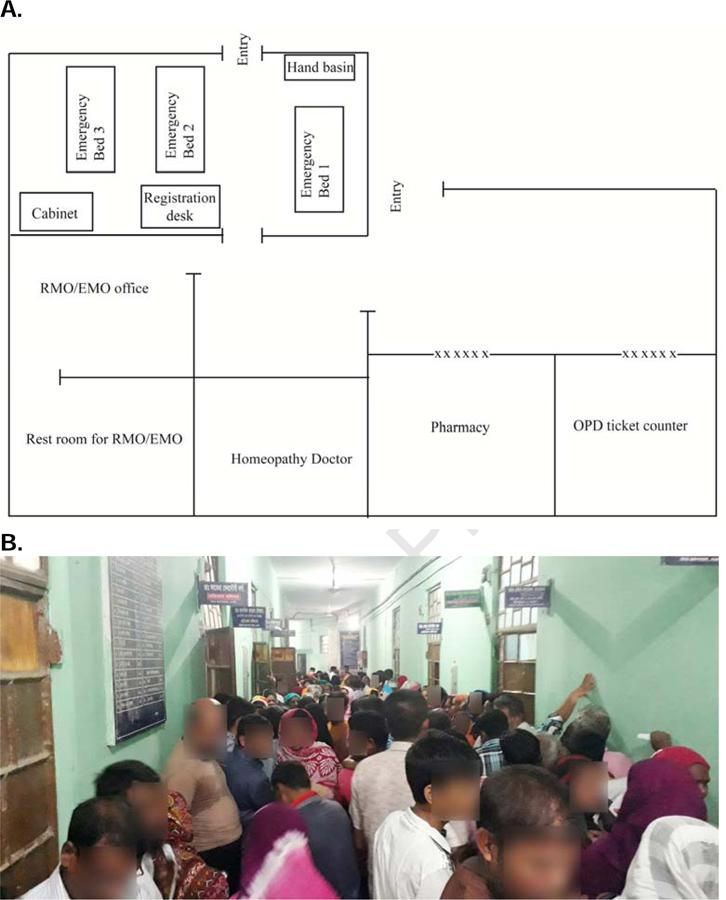
Figure 2.
A. Layout of a representative district hospital emergency department. B. Patients waiting in the hospital corridor for outpatient and emergency services.
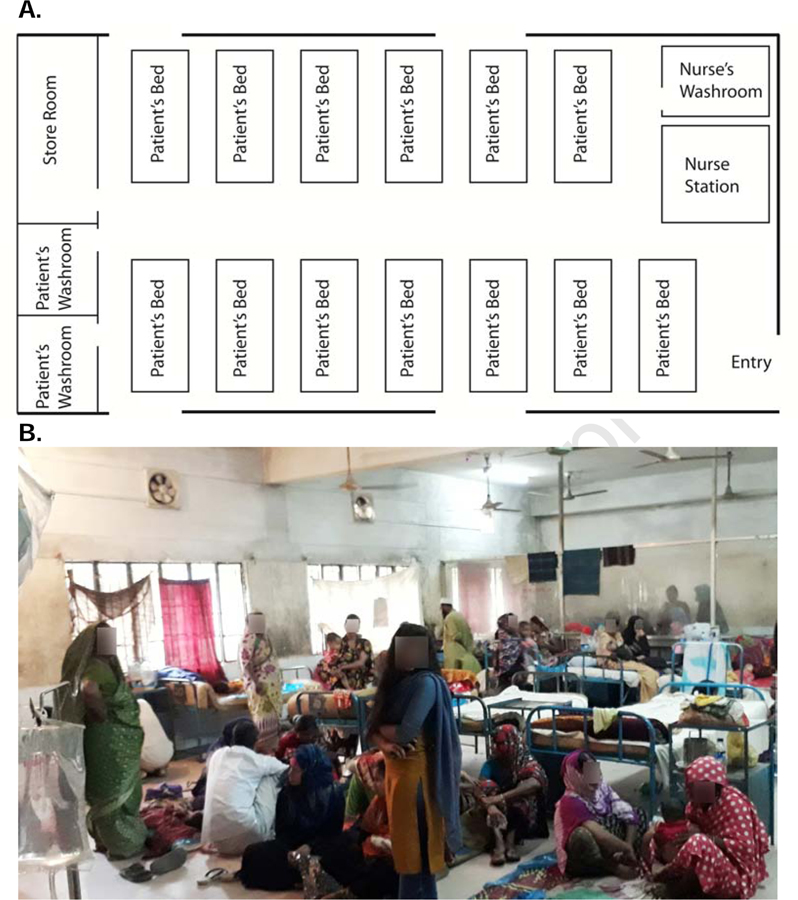
Figure 3.
A. Layout of a representative diarrheal treatment ward at a district hospital. B. An example of a ward with insufficient patient beds.
We found that the layouts of the emergency rooms (Figure 2A) and diarrheal wards (Figure 3A) were similar between hospitals. Buildings were constructed between 1962 and 2003 out of concrete and bricks. The majority of diarrheal patients first presented at emergency departments, which have a large primary room to receive patients, a separate room for Emergency and Residential Medical Officers (EMOs / RMOs) where most clinician-patient consultations were made, and a designated space for registration and ancillary tasks. Like the emergency department, diarrheal wards had an open floor plan with 6–23 beds (Figure 3A) and glass windows were covered with iron bars. In the eight hospitals with diarrheal wards, the diarrheal wards were located on the same floor as the emergency departments. The size of diarrheal wards ranged from 200–1000 square feet.
Patient experiences in the wards, and staff motivation, were influenced by a lack of resources, including beds, ventilation and privacy. All medical staff stressed that their ability to spend adequate time with individual patients was severely affected by overcrowding. In nearly all situations, more patients than beds were observed. Bed assignments were based on a first-come-first-service basis. Patients who did not get beds were assigned mattresses or blankets on the floor or adjacent verandas (Figure 3B). Patients sharing beds was observed and was more common for pediatric patients. “Cholera cots” (vinyl cots with a hole and plastic shoot to a bucket for stool) were not present in any of the facilities. The distance between bed placement was often within an arm’s distance. Seven out of eight diarrheal wards did not have separate wards for males and females; one hospital transferred patients to gender-specific medicine wards. The lack of gendered wards is problematic given the nature of diarrhea and the cultural norm to provide privacy for women in Bangladesh. It is customary for only women to attend at female wards. Patients commented that this was especially important when toilets were used. One woman explained:
I feel shy to use a toilet frequently in front of so many outsiders (men) because you know that our religion does not allow us to be seen openly. However, as I am sick, I have to stay here (in the ward with others), but I feel ashamed.
The wards were hot and humid. Although each ward had glass windows and ceiling fans, most of the windows could not be opened and staff complained regularly of the lack of air conditioning. The lack of basic amenities was perceived as stressful by nursing staff, who viewed it as a source of conflict, argumentation, and negativity.
The average number of patients visiting the emergency rooms per day varied from 300 to 500 patients. Some patients could not enter the emergency room due to overcrowding, and physicians often provided treatment without a physical examination. In the diarrheal wards, an average of 26 patients were seen per day. One hospital (with only 12 beds) averaged 70–100 patients per day in the diarrheal wards. Providers explained that diarrhea caseload varied with season and outbreak frequently occurred.
The crowded conditions created friction between families and hospital staff. Family members who were direct caregivers frequently refused to leave the ward despite nurses asking them to do so. Caregivers and non-caregiver visitors gathered in front of the nurse’s table with demands: asking for medicines, inquiring about a patient’s condition, looking for the physician, insisting on a bed for their patient, and requesting early release from the hospital. In response, staff nurses occasionally became angry, amplifying the collective stress level of the ward.
3.6. Access to hygiene and sanitation in the clinical environment
The lack of hygiene and sanitation infrastructure exacerbated the complications of hospital overcrowding. Hand-washing sinks were available in nine of ten emergency rooms. In the diarrheal wards, two of eight restrooms had sinks for patients. In both settings, soap was not available. Staff nurses explained that it was hard to maintain soap because of overcrowding and theft. Most patients and their families managed their own drinking water in the form of purchased bottled water, or water brought from home. Each hospital had a tube-well with a hand pump and some patients/their attendant secured water from these wells for drinking. Staff nurses recommended using bottled water for ORS use.
A similar situation was found with sanitation. There were 1–2 toilets attached to each diarrhea ward, with a separate toilet for nurse/staff and patients. Separate toilets for visitors or caregivers were not available and resulted in additional burden on patient toilets. These small bathrooms were used for urination, defecation, as well as bathing. Restroom sinks were also used for washing cloths and utensils. Most of the toilets were not clean, and floors were often wet and slippery. Under these conditions, caregivers had difficulty disposing human waste and managing used soiled clothes and linens. Toilets designated for nurses and doctors were comparatively clean and many, but not all, had soap and running water.
Hospitals had a general shortage of janitorial staff to manage sanitation on the wards. Human waste and medical waste was frequently visible in the diarrheal wards. Window grills, walls, bed covers, mattresses, bedrails and furniture were often visibly soiled. Large amounts of litter was present on the floors and under beds. Animals (e.g. cats) roamed freely in the wards. Cotton/synthetic single layer bedcovers were not changed during the observation period, for or between patients; families were not instructed to bring their own sheets.
Medical staff acknowledged that the conditions increased the risk of nosocomial infection. Staff felt that the lack of proper waste management increased the risk of hospital-acquired infections like “pneumonia”, “meningitis”, and “gastroenteritis”. To protect patients, physicians reported that they would prescribe antibiotics prophylactically to avert nosocomial infections. Nurses reported that the situation was made worse because patients were from the lower socio-economic strata with less education. The nurses perceived the patients had a higher tolerance for poor hygienic conditions due to their living conditions at home, and that they therefore did not appreciate the risks of transmission and contamination on the wards. The lack of drinking water on the wards, and physician attitudes about the hygienic practices of patients, influenced IV and antibiotic orders. For example, one physician told us: “If a patient prepares ORS with contaminated water then it would be risky for them.” This formed part of the explanation given by physicians for why IV fluids were prescribed for ‘No’ and ‘Some’ dehydration .
4. Discussion
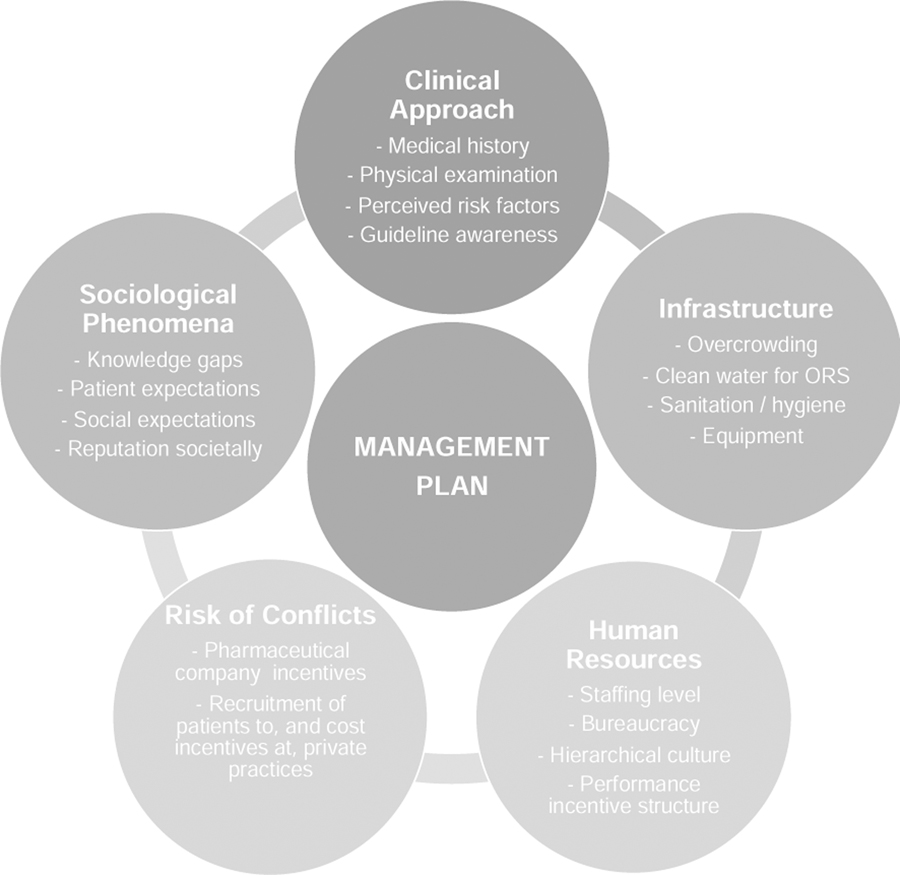
This ethnographic exploration found a significant gap between knowledge and practice in the management of diarrheal disease in Bangladeshi hospital. Following prior ethnographic studies on hospital settings and clinical encounters (d’Alessandro, 2015; Van der Geest & Finkler, 2004), we identified several domains that shape clinical treatment, and therefore health outcomes and quality of services (Figure 4). These domains (clinical approach, physical infrastructure, sociological phenomena, human resources and risk of conflicts of interest) are interconnected and form a complex ecosystem that influences management plans that align or deviate with WHO guidelines. The problems identified should be viewed as opportunities to identify solutions for improvement that are desired, feasible and sustainable in resource-limited hospitals like those in this study.
Figure 4.
Categories of thematic factors that influence the establishment of diarrheal disease management plans in resource-limited hospitals.
The WHO guidelines for the management of diarrheal diseases were developed to establish evidence-based standards of care that are still accommodating for the needs of specific locations. Our findings suggest that while guidelines are well intentioned, adherence may be nearly impossible to achieve because of non-clinical challenges within the ecosystem. This conflict between ‘what should be done’ and ‘what can be done’ culminated in a sense of accepted failure, neglect and even despair. The hospital itself at times was regarded “pathogenic.” This resulted in a sense of apathy associated with taking ‘shortcuts’ and devaluing efforts to improve the care for already marginalized patient populations. Consideration of increasing custodial staffing and deploying promising approaches to improve hospital hygiene in settings like those in this study need to be prioritized (George et al., 2016; George et al., 2019).
Navigating the conflicts in the gaps between ‘know’ and “do’ was a struggle that staff at multiple levels routinely face. The diarrheal wards were not positioned to effectively address patient and family needs. The overwhelming workload forced localized medical cultures where clinical assessments and treatments were brief, often without a routine clinical examination. This normalized guideline deviation and enabled the perception that diarrhea was ‘not a big deal’. However, from the perspective of the patients it was a ‘big deal’. Patients of low socio-economic strata are at increased risk of life-threatening dehydration from diarrhea disease (Andrews et al., 2017) and may not understand the importance of using ORS early and aggressively. Poverty compromises the social determinants of health, and in the study, promoted negative treatment pathways within public hospital systems.
Social and clinical expectations were an important factor of guideline deviation. Patients expected antibiotics and IV fluids regardless of the severity of dehydration. Doctors were aware of this expectation and wanted ‘to do something’, resulting in a complacency to order antibiotics and IV fluids even when it was not indicated. Doctors struggled to address patient expectations while responding to the emerging evidence-based adage, “Just don’t do something, stand there” (Petty, 1979). A clinical approach of ‘doing less’ is made even more difficult because conflicts of interest de-incentivize non-interventional approaches. In Bangladesh, previous investigation found financial incentives by pharmaceutical companies motivated physicians decisions to prescribe unnecessary and expensive antibiotics (M. S. Islam, 2006; Radyowijati & Haak, 2002; Saha & Promite, 2017). These events are also well-documented at a global level (Li et al. 2012).
The expectations of the medical teams were generally out of proportion with what was possible. Gaps in care were especially noticeable when doctors were not present for explained or unexplained reasons. Health assistants and physician assistants often covered for the physicians. However, this problem may present a work solution in that these medically trained assistants may offer a mechanism to decompress and reduce physician work-load. However, this might create friction between provider types that would need to be identified and addressed with multi-level training and hospital messaging before changes are made.
The study findings must be considered within the limitations of the study. The qualitative ethnographic methods rely on the skill of the ethnographer to ‘blend in’ to the environment. The more the ethnographer is viewed as part of the environment the more candid, honest, personal, and insightful the data becomes. Although Bangladeshi nationals conducted the research, the team was based in the capitol, educated, and carried paper instruments. Although great effort was made in reducing social distance, these differences may have nevertheless influenced the data collection. Second, the decision to conduct the study with phase 1 at all ten hospitals and phase 2 at a subset of four hospitals represents an effort to balance observing all sites while allowing for more granular observations at a subset of sites; this may have caused a reporting bias towards the phase 2 hospitals. Lastly, the methods were focused on problem finding and identifying themes with less emphasis on having participants self-identify solutions; solutions were noted when stated but were not the focus of this study.
Despite these limitations, this study has revealed important actionable insights (Text Box 2). These insights have originality because the ethnographers were granted rare complete multi-level access to conduct the research. This in itself represents an important institutional willingness to make positive change. We honor the responsibility of this access by providing recommendations that we hope catalyze positive change in challenging medical settings prone to outbreaks like those in this study. These recommendations are first steps to collectively promote improved guideline adherence and quality of care while being mindful of financial constraints of resource-limited medical systems.
Text Box 2: Recommendations to improve care and guideline adherence.
Clinical approach. Educate doctors, nurses, and medical staff on the management of ‘No’ dehydration and ‘Some’ dehydration with oral rehydration solution and the restriction of antibiotics to acute watery diarrhea with severe dehydration and patients with acute bloody diarrhea. The longevity of the education will benefit from paper and digital ‘job aids’, posters and placards.
Physical infrastructure. Collapsible vinyl ‘cholera cots’ that can be readily cleaned. They are designed to collect waste in a bucket below the cot for those patients unable to access the restroom. Ensure cleaning supplies. Provide a performance-based incentive structure. Investment in sufficient weight-scales, soap, sinks, and toilets. Increase space with surge capacity.
Sociologic phenomena. A behavior change intervention for providers and patients on setting expectations for sanitation, hygiene, ORS use, and the benefits and risks of antibiotics. Expectations must be set such that guidelines can be followed yet be accommodating for the realities of resource-limitations.
Human Resources. Reduce workload of the admitting physicians by empowering physician assistants to assess and initiate diarrheal treatment. Physicians would have oversight, yet have protected time to address patients with non-diarrheal disease conditions. Ward management mechanisms need to be strengthened with mechanisms for staff feedback. Hiring and adequately compensating custodial staff. Well-designed workshops on these elements would increase motivation and performance.
Risk of conflicts of interest. Developing an institutional policy on how best to engage with the pharmaceutical companies. Prescribing generic named drugs instead of brand named antibiotics may reduce cost and pharmaceutical influence. Creating policies on how to balance private and public practices.
There are opportunities to improve diarrheal disease management in seemingly change-resilient hospital settings.
Improving sanitation and hygiene may increase guideline adherence by reducing concern for hospital-acquired infections.
Human resource constraints, conflicts of interests and overcrowding expand the gap between knowledge and action.
ACKNOWLEDGEMENTS
We thank the hospital staff and patients interviewed in the study and the MOHFW central and district leadership for welcoming and permitting the conduct of this study. We are grateful to the administrative staff at each partner institution. This work was supported by the National Institutes of Health (USA) [DP5OD019893; R21TW010182] to EJN and internal support from the University of Florida, Stanford University and the icddr,b. The authors are grateful to the Governments of Bangladesh, Canada, Sweden and the United Kingdom for providing core/unrestricted support to the icddr,b. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Andrews JR, Leung DT, Ahmed S, Malek MA, Ahmed D, Begum YA, et al. (2017). Determinants of severe dehydration from diarrheal disease at hospital presentation: Evidence from 22 years of admissions in Bangladesh. PLoS Negl Trop Dis, 11, e0005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojalil R, & Calva JJ (1994). Antibiotic misuse in diarrhea. A household survey in a Mexican community. Journal of clinical epidemiology, 47, 147–156. [DOI] [PubMed] [Google Scholar]
- Charanasri U, Pornputtkul S, & Wongsaroj T (1995). Evaluating study of case management of diarrheal diseases in Thailand. Southeast Asian journal of tropical medicine and public health, 26, 453–456. [Google Scholar]
- Colvin CJ, Smith HJ, Swartz A, Ahs JW, de Heer J, Opiyo N, et al. (2013). Understanding careseeking for child illness in sub-Saharan Africa: a systematic review and conceptual framework based on qualitative research of household recognition and response to child diarrhoea, pneumonia and malaria. Soc Sci Med, 86, 66–78. [DOI] [PubMed] [Google Scholar]
- d’Alessandro E (2015). Human activities and microbial geographies. An anthropological approach to the risk of infections in West African hospitals. Social science & medicine, 136, 64–72. [DOI] [PubMed] [Google Scholar]
- Das S, Begum D, Ahmed S, Ferdous F, Farzana F, Chisti M, et al. (2014). Geographical diversity in seasonality of major diarrhoeal pathogens in Bangladesh observed between 2010 and 2012. Epidemiology & Infection, 142, 2530–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis AA, Winch P, Daou Z, Gilroy KE, & Swedberg E (2007). Home management of childhood diarrhoea in southern Mali--implications for the introduction of zinc treatment. Soc Sci Med, 64, 701–712. [DOI] [PubMed] [Google Scholar]
- Farmer P, Almazor CP, Bahnsen ET, Barry D, Bazile J, Bloom BR, et al. (2011). Meeting cholera’s challenge to Haiti and the world: a joint statement on cholera prevention and care. PLoS Negl Trop Dis, 5, e1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CM, Monira S, Sack DA, Rashid MU, Saif-Ur-Rahman KM, Mahmud T, et al. (2016). Randomized Controlled Trial of Hospital-Based Hygiene and Water Treatment Intervention (CHoBI7) to Reduce Cholera. Emerg Infect Dis, 22, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CM, Zohura F, Teman A, Thomas E, Hasan T, Rana S, et al. (2019). Formative research for the design of a scalable water, sanitation, and hygiene mobile health program: CHoBI7 mobile health program. BMC Public Health, 19, 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest G, MacQueen KM, & Namey EE (2014). Introduction to applied thematic analysis: Sage Publications, Inc. [Google Scholar]
- Hadley MB, Blum LS, Mujaddid S, Parveen S, Nuremowla S, Haque ME, et al. (2007). Why Bangladeshi nurses avoid ‘nursing’: social and structural factors on hospital wards in Bangladesh. Soc Sci Med, 64, 1166–1177. [DOI] [PubMed] [Google Scholar]
- Hadley MB, & Roques A (2007). Nursing in Bangladesh: rhetoric and reality. Soc Sci Med, 64, 1153–1165. [DOI] [PubMed] [Google Scholar]
- Hansen H, Holmes S, & Lindemann D (2013). Ethnography of health for social change: impact on public perception and policy. Soc Sci Med, 99, 116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque F, Ball RL, Khatun S, Ahmed M, Kache S, Chisti MJ, et al. (2017). Evaluation of a Smartphone Decision-Support Tool for Diarrheal Disease Management in a Resource-Limited Setting. PLoS Negl Trop Dis, 11, e0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howteerakul N, Higginbotham N, Freeman S, & Dibley MJ (2003). ORS is never enough: physician rationales for altering standard treatment guidelines when managing childhood diarrhoea in Thailand. Social science & medicine, 57, 1031–1044. [DOI] [PubMed] [Google Scholar]
- International Vaccine Access Center (IVAC), J.H.B.S.o.P.H. (2018). Pneumonia and Diarrhea Progress Report 2018.: Johns Hopkins Bloomberg School of Public Health. [Google Scholar]
- Islam MS (2006). A review on the policy and practices of therapeutic drug uses in Bangladesh. Calicut Med J, 4, e2. [Google Scholar]
- Islam MT, Khan AI, Sayeed MA, Amin J, Islam K, Alam N, et al. (2019). Field evaluation of a locally produced rapid diagnostic test for early detection of cholera in Bangladesh. PLoS Negl Trop Dis, 13, e0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GA, & Vindrola-Padros C (2017). Rapid qualitative research methods during complex health emergencies: A systematic review of the literature. Soc Sci Med, 189, 63–75. [DOI] [PubMed] [Google Scholar]
- Khan AI, Mack JA, Salimuzzaman M, Zion MI, Sujon H, Ball RL, et al. (2020). Electronic decision-support improves diarrhoeal disease guideline adherence (mHealth Diarrhoea Management, mHDM, Trial): a cluster randomized controlled trial. Lancet DH, 2, e250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AI, & Qadri F (2019). Epidemiology of cholera in Bangladesh: Findings from Nationwide Hospital-based Surveillance, 2014–2018. CID. [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. (2013). Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet, 382, 209–222. [DOI] [PubMed] [Google Scholar]
- Leech NL, & Onwuegbuzie AJ (2007). An array of qualitative data analysis tools: a call for data analysis triangulation. School psychology quarterly, 22, 557. [Google Scholar]
- Leung DT, Chisti MJ, & Pavia AT (2016). Prevention and Control of Childhood Pneumonia and Diarrhea. Pediatr Clin North Am, 63, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelto PJ (2016). Applied ethnography: Guidelines for field research: Routledge. [Google Scholar]
- Petty TL (1979). Don’t just do something—stand there! Archives of internal medicine, 139, 920–921. [PubMed] [Google Scholar]
- Pigg SL (2013). On sitting and doing: ethnography as action in global health. Soc Sci Med, 99, 127–134. [DOI] [PubMed] [Google Scholar]
- Radyowijati A, & Haak H (2002). Determinants of antimicrobial use in the developing world: Citeseer. [Google Scholar]
- Rheingans R, Kukla M, Faruque ASG, Sur D, Zaidi AK, Nasrin D, et al. (2012). Determinants of household costs associated with childhood diarrhea in 3 South Asian settings. Clinical infectious diseases, 55, S327–S335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, & Promite S (2017). Factors influencing clinician’s antibiotic prescribing behaviors (apb) in Bangladesh: an in-depth review using comb model. Open Access J Trans Med Res, 1, 00019. [Google Scholar]
- Santosham M, Keenan EM, Tulloch J, Broun D, & Glass R (1997). Oral rehydration therapy for diarrhea: an example of reverse transfer of technology. Pediatrics, 100, e10–e10. [DOI] [PubMed] [Google Scholar]
- Sarker AR, Islam Z, Khan IA, Saha A, Chowdhury F, Khan AI, et al. (2013). Cost of illness for cholera in a high risk urban area in Bangladesh: an analysis from household perspective. BMC infectious diseases, 13, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker AR, Sultana M, Mahumud RA, Ali N, Huda TM, Haider S, et al. (2018). Economic costs of hospitalized diarrheal disease in Bangladesh: a societal perspective. Global health research and policy, 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillcutt SD, LeFevre AE, Fischer Walker CL, Taneja S, Black RE, & Mazumder S (2016). Economic costs to caregivers of diarrhoea treatment among children below 5 in rural Gujarat India: findings from an external evaluation of the DAZT programme. Health policy and planning, 31, 1411–1422. [DOI] [PubMed] [Google Scholar]
- Street A, & Coleman S (2012). Introduction: real and imagined spaces. Space and Culture, 15, 4–17. [Google Scholar]
- Sultana M, Mahumud RA, & Sarker A (2015). Emerging patterns of mortality and morbidity in district level hospitals in Bangladesh. Ann Public Heal Res, 2, 2–4. [Google Scholar]
- Troeger C, Blacker BF, Khalil IA, Rao PC, Cao S, Zimsen SR, et al. (2018). Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Infectious Diseases, 18, 1211–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Reiner RC Jr, et al. (2017). Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Infectious Diseases, 17, 909–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Geest S, & Finkler K (2004). Hospital ethnography: introduction. Social science & medicine, 59, 1995–2001. [DOI] [PubMed] [Google Scholar]
- Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. (2013). Global burden of childhood pneumonia and diarrhoea. The Lancet, 381, 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wazny K, Zipursky A, Black R, Curtis V, Duggan C, Guerrant R, et al. (2013). Setting research priorities to reduce mortality and morbidity of childhood diarrhoeal disease in the next 15 years. PLoS medicine, 10, e1001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2005). The treatment of diarrhea: a manual for physicians and other senior health workers. 4th rev. Geneva, Switzerland: World Health Organization. [Google Scholar]
- WHO, & Unicef (2013). Ending preventable child deaths from pneumonia and Diarrhoea by 2025: the integrated global action plan for pneumonia and Diarrhoea (GAPPD). [DOI] [PubMed]
- Zaman S (2004). Poverty and violence, frustration and inventiveness: hospital ward life in Bangladesh. Soc Sci Med, 59, 2025–2036. [DOI] [PubMed] [Google Scholar]