Abstract
Introduction:
The pathophysiological relevance of the endocannabinoid system has been widely demonstrated in a variety of diseases including cancer, neurological disorders and metabolic issues. Therefore, targeting the receptors and the endogenous machinery involved in this system can provide a successful therapeutic outcome. Ligands targeting the canonical cannabinoid receptors, CB1 and CB2, along with inhibitors of the endocannabinoid enzymes have been thoroughly studied in diverse disease models. In fact, phytocannabinoids such as cannabidiol or Δ9-tetrahydrocannabinol are currently on the market for the management of neuropathic pain due to spasticity in multiple sclerosis, or seizures in children epilepsy amongst others.
Areas covered:
Challenges in the pharmacology of cannabinoids arise from its pharmacokinetics, off-target effects and psychoactive effects. In this context, the current review outlines the novel molecular approaches emerging in the field discussing their clinical potential.
Expert opinion:
Even if orthosteric CB1 and CB2 ligands are on the forefront in cannabinoid clinical research, emerging strategies such as allosteric or biased modulation of these receptors along with controlled off-targets effects may increase the therapeutic potential of cannabinoids.
Keywords: cannabinoid, allosteric, bias, peripheral, mitochondrial, multitarget, off-target
1. Introduction
Members of the endocannabinoid system (ECS), cannabinoid (CB) receptors and enzymes responsible for synthesis and degradation of their endogenous ligands, have been largely validated as a therapeutic target for numerous neurological, metabolic, immune or oncologic pathologies [1]. Thus far, two G-protein-coupled receptors (GPCRs), CB1 and CB2, have been classified as canonical CB receptors. Other receptors, including the orphan GPCRs GPR55, GPR18 and the GPR3–6-12 subset; ionotropic receptors, such as specific transient receptor potential (TRP) channels; or nuclear receptors, such as peroxisome proliferator-activated receptors (PPARs) have been proposed to be related to this system [2,3]. To make the picture more complex, CB receptors have been reported to form homo- and heterodimers triggering pharmacological responses in nonlinear ways [4-6]. Thus, all of the above mentioned elements along with endocannabinoids and related lipid mediators form the endocannabinoidome [7].
The recent approval of phytocannabinoids in an increasing number of countries is raising the necessity to accurately determine their appropriate use in the management of diverse diseases and/or symptoms. In addition to phytogenic compounds, their endogenous counterparts and a heterogeneous array of cannabimimetic synthetic molecules have been studied in the last years [8-10]. Unfortunately, the abuse potential of cannabinoids constitutes a limitation to the therapeutic value of these compounds. Indeed, over the past decade, numerous synthetic CB receptor agonists (SCRAs) have proliferated as new psychoactive substances (NPS) in drug markets constituting a serious public health threat [11-13]. Cannabinoids effects do not only depend on their pharmacological targets but also on drug preparation, concentration and chosen route of administration. Therefore, extensive controlled clinical trials are needed to shed light on this field.
Due to the complexity and promiscuity of cannabinoids actions, a deeper understanding of their molecular pharmacology can help fine-tuning potential CB treatments for a wide variety of disorders. In this context, the recently elucidated complexes of certain CB ligands with their targets [14-19] will certainly aid in the design of the next generation of CB-based drug. So far, efforts have been focused on the development of agonists and antagonists of CB1 and/or CB2 receptors, as well as drugs acting on endocannabinoid metabolism However, none of these synthetic cannabinoids have reached the market. Thus, phytocannabinoids such as cannabidiol (CBD, Figure 1) or Δ9-tetrahydrocannabinol (Δ9-THC, Figure 1) are clearly at the forefront of current clinical research for diverse pathologies such as cancer, Parkinsońs (PD) or Alzheimer’s Disease (AD). In fact, CBD has been recently approved in some countries for the treatment of specific types of childhood refractory epilepsies [20,21].
Figure 1.
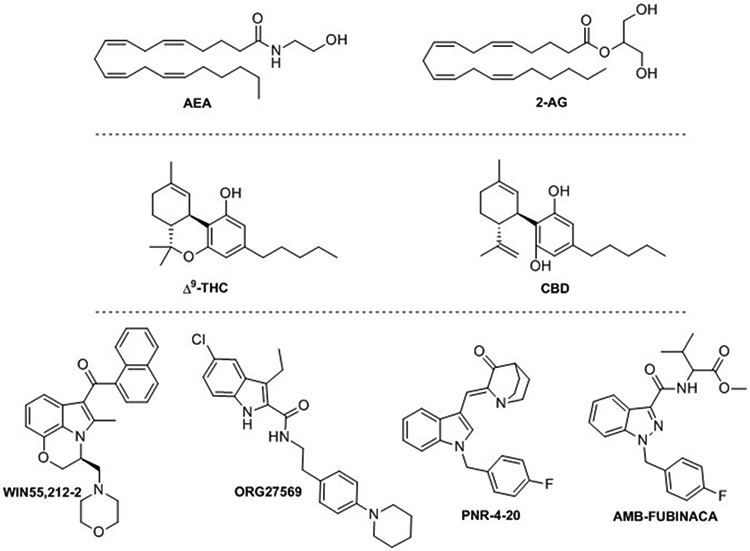
Structure of endocannabinoids AEA and 2-AG, phytocannabinoids Δ9-THC and CBD; and synthetic derivatives WIN55,212-2, ORG27569, PNR-4-20 and AMB-FUBINACA.
Since orthosteric cannabinoids and inhibitors of the endocannabinoid machinery have been extensively reviewed in the literature [22-24], herein, we will discuss novel strategies such as functionally selective, allosteric, peripheral or multitarget cannabinoids that are lately emerging to offer reduced adverse effects while maximizing the therapeutic value for specific diseases. Approaches to improve the pharmacokinetic profile of cannabinoids as well as to control their off-target effects are also being extensively studied in order to properly exploit the clinical potential of the ECS.
2. Targeting the ECS
2.1. Allosteric modulators at CB1 and CB2 receptors
Allosteric modulation has emerged as a viable drug discovery strategy for GPCRs. Is it the case for the CB receptors allosterism? Most reported cannabinoids activate or inhibit receptor signaling by binding at orthosteric sites [22]. In contrast, allosteric cannabinoids modulate receptor function by binding at spatially distinct binding sites. Positive allosteric modulators (PAMs) enhance the response of endogenous ligands or co-administered orthosteric cannabinoids while negative allosteric modulators (NAMs) decrease their response. The key characteristic of PAMs or NAMs is the ability to fine-tune physiological responses in presence of endogenous ligands. Another characteristic is an increase in receptor subtype specificity due to the fact that allosteric binding sites are not conserved through receptor subtypes. During the last decade, both CB1 PAMs and NAMS have been discovered providing new pharmacological tools. The structure and properties of these modulators (e.g. ORG27569, ORG27759, ORG29647, PSNCBAM-1, RTI-371, lipoxin A4, GAT211, ZCZ011, pregnenolone, CBD, Pepcans; Figures 1-2) have been extensively reviewed in recent literature [25-28]. Thus, only most recent advances are outlined here. More than discovering new CB1 allosteric scaffolds, recent studies have been focused on the identification of CB1 receptor allosteric sites, on the mechanism of action at cellular level and on the therapeutic usefulness.
Figure 2.
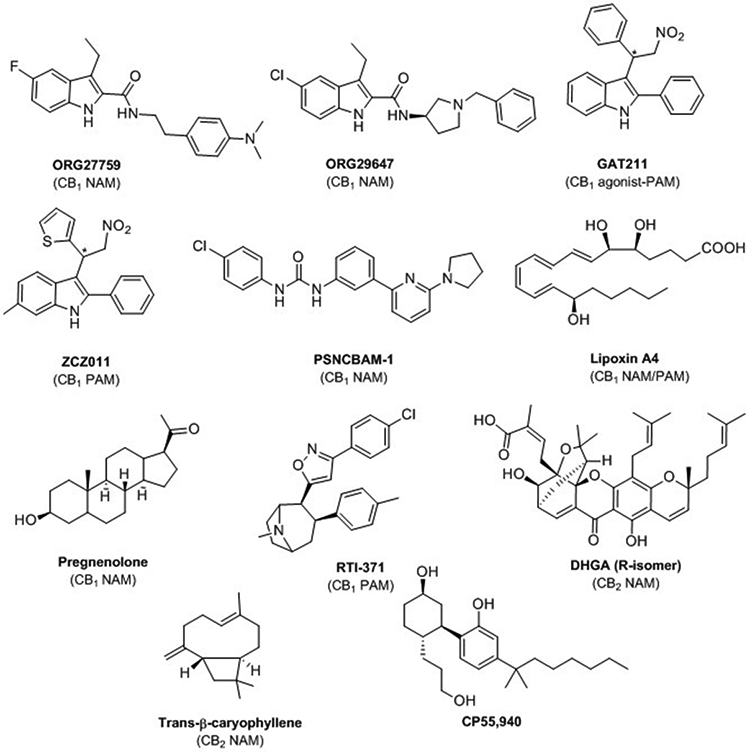
Structure of allosteric cannabinoids: ORG27759, ORG29647, GAT211, ZCZ011, PSNCBAM-1, lipoxin A4, pregnenolone, RTI-371, DHGA, trans-β-caryophyllene and structure of the agonist CB1/CB2 CP55,940.
Rational design around CB1 receptor allosteric sites has been studied by combining ligand docking, mutational analyses, and molecular dynamics (MD) simulations using first homology models of CB1, then using one of the five crystal structures of CB1 available (inactive state; partially agonist state; fully active Gi protein-bound state) [29-33]. The last research articles in this field point out the contribution of the phospholipid composition of the membrane in CB1 receptor activation due to PAM modulation by anionic phospholipids [34]. High-resolution structures of CB1 receptor bound to the CB1 agonist CP55,940 and to the CB1 NAM ORG27569 also highlight the role of the membrane in allosteric process [16]. CP55,940 (Figure 2) occupies the orthosteric pocket, while ORG27569 binds to an extrahelical site in the inner leaflet of the membrane [16]. Thus, the binding site of ORG27569 partially overlaps with the site of cholesterol binding. In reference to G-protein interaction, in presence of the agonist CP55,940, ORG27569 diminishes Gi coupling by conformational changes that pack transmembrane helix 6 (TM6) against TM3 and TM5. Deep neural networks, as well as conventional machine learning algorithms have been reported to help in identifying critical molecular properties, key substructures, and circular fingerprints for classifying CB orthosteric and allosteric ligands [35].
Enormous efforts need to be devoted to understand the complex mechanism of action of allosteric cannabinoids [27]. Assessing allosteric modulation requires combining binding assays, diverse functionality assays, kinetic studies, and finally in vivo efficacy control.
One of the most recent advances concerns the evidence of the effectiveness of PAMs and NAMs in vivo. Even though studies have been reported some years ago on the efficacy of the CB1 NAM PSNCBAM-1 in food intake and body weight in an acute rat feeding model, few in vivo evidences have been provided. For instance, GAT211 showed antinociceptive efficacy in models of neuropathic and inflammatory pain without eliciting psychotropic effects and physical dependence [36]. GAT211 also produced synergistic antinociceptive effects with the CB1/CB2 agonist WIN55,212–2 in these models. In a neuronal model involving the endocannabinoid 2-AG, GAT211 and ZCZ011 modulated the synaptic transmission in autaptic hippocampal neurons [37]. Drug-like properties of GAT211 have been very recently enhanced with structural modification following fluoro- and nitrogen-walk approaches leading to CB1 agonist-allosteric modulators with longer duration of action in inflammatory-pain model or with improved reduction of intraocular pressure in murine glaucoma models [38]. However, GAT211 is a racemic mixture meaning that in vivo assays will need to be revised with the resolved enantiomers, GAT228 (R) and GAT229 (S). Effectively, the enantiomers showed different pharmacological profiles. GAT228 (R) is a partial allosteric agonist and GAT229 is considered PAM. Moreover, they have been suggested to bind different allosteric binding sites [31].
Allosterism studies at CB2 receptor are much less developed than at CB1. However, allosteric modulation at CB2 may be useful in avoiding immunosuppression caused by direct chronic CB2 activation by orthosteric ligands. Allosteric profiles at CB2 of CBD, 1,1-dimethylheltyl-CBD, the peptide pepcan-12, an 2-oxopyridine-3-carboxamide, hydrogambogic acid (DHGA), and trans-β-caryophyllene (TBC) (Figure 2) have been already reported in a review published in 2018 [39]. Actually, none of them are CB2 specific; they target other biological targets with higher potency and specificity. There is clearly a lack of potent CB2 allosteric scaffolds and a lack of information on the allosteric binding site. Recently, potential CB2 allosteric binding sites have been proposed by the means of MD simulations [40]. CP55,940 was used as a CB2 agonist and TBC and DHGA as CB2 NAMs. The best-optimized CB2-CP55,940-TBC or DHGA complexes were studied by MD simulation resulting in a degree of flexibility of CP55,940 restricted in the presence of a NAM. The allosteric binding sites proposed could be the starting point for identifying new CB2 NAMs.
2.2. Cannabinoid biased signaling
Activation of CB receptors elicits a cascade of intracellular signals upon coupling to different effector proteins, including G proteins and β-arrestins [41]. CB1 receptors have shown G protein coupling promiscuity (Gαi, Gαs and Gαq), while CB2 primarily couple to Gαi-type G proteins [42,43]. Regarding β-arrestins, CB receptors can recruit the isoforms β-arrestin1 and β-arrestin2 upon activation [41]. The outcome of each downstream pathway evokes a unique pharmacological response, therefore, ligands capable to selectively induce receptor coupling to a specific transducer protein can offer optimized therapeutic effects. These are the so-called biased agonists or functionally selective ligands.
Most reported CB1 and CB2 ligands signal through G protein–dependent and independent pathways. β-arrestin recruitment can desensitize and internalize receptors, which may trigger tolerance reducing the pharmacological potential of cannabinoids for the management of chronic pathologies [41]. Consequently, identification of CB1 and/or CB2 biased ligands is currently emerging as a novel potential therapeutic approach.
In the search of bias agonists, not only novel synthetic ligands, but also well-known cannabinoids are being assessed using diverse functional endpoints. In fact, endogenous and plant-derived cannabinoids have been found to induce biased cellular responses [39,44]. For instance, the most abundant endocannabinoids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), have shown a CB1 and/or CB2 biased signaling profile in specific cellular models. Lapraire and colleagues demonstrated CB1 functional selectivity of these endocannabinoids in cell models of medium spiny projection neurons expressing wild-type (STHdhQ7/Q7) or mutant huntingtin protein (STHdhQ111/Q111) [45]. On the other hand, Soethoudt and coworkers observed that at CB2 2-AG signaling is biased towards the β-arrestin pathway, whereas unbiased results were obtained for AEA [46].
Moreover, phytocannabinoids such as Δ9-THC have also shown functionally selective responses at CB1 and CB2. While significant signaling bias toward β-arrestin1, and Gαq compared to Gαi/o was observed at CB1 [45], different studies reported Gαi/o protein preferential signaling at CB2 [46,47]. The non-psychoactive phytocannabinoid CBD has also been suggested to evoke bias responses despite a lack of orthosteric affinity to CB receptors. Thus, further studies are needed to confirm the intricate pharmacology of this ligand [4].
Recent studies have demonstrated that diverse synthetic CB ligands can exhibit biased signaling (previously reviewed [39,44,48]). The indole scaffold is a good example of these biased chemotypes. Derivatives such as the well-known CB1/CB2 potent aminoalkylindole WIN-55,212–2, the indole quinuclidinone PNR-4–20 or the indole-2-carboxamide ORG27569 (Figure 2) exert coupling preference towards specific transduction pathways [39,44]. It is worth mentioning that biased agonism has also been recently reported for some of the SCRAs that are emerging as NPS, the highly toxic indazole-3-carboxamide AMB-FUBINACA (Figure 1) among them. The pharmacology and toxicology of these recreational substances is being extensively studied due to their extended illegal consumption and health concerns [48].
Systematic functional profiling of reported cannabinoids as well as design of novel biased ligands is nowadays to be considered in order to find optimized therapeutics for specific pathologies. For instance, the development of CB1 G-protein biased ligands may offer reduced tolerance, opening new avenues for the treatment of chronic diseases.
2.3. Peripherally acting cannabinoids
Nowadays, among peripherally acting cannabinoids, CB1 antagonists are much more developed than CB1 agonist or CB2 ligands. The interest for peripherally restricted CB1 antagonists has been driven by the withdrawing of rimonabant from the European market in 2008 [49]. Despite its efficacy in reducing food intake and body weight in overweight or obese humans with beneficial effects on different metabolic and cardiovascular parameters, serious psychiatric problems overcame the benefits of rimonabant [49]. Although CB1 expression in peripheral organs is lower than within the central nervous system (CNS), selective inhibition of CB1 receptor activity at the periphery remains an interesting approach for metabolic syndromes, obesity, diabetes, lipogenesis, and liver diseases. Therefore, peripheral restricted antagonists have been explored as a novel strategy to avoid psychotropic effects. Reviews covering recent developments in CB1 antagonists/inverse agonists have been published recently [50-52]. Based on rimonabant structure, pyrazole derivatives such as AM6545, TXX522, TM-38837, DBPR211 (Figure 3) have been identified as therapeutic development candidate molecules in the control and amelioration of obesity in humans. Since polar surface areas can improve the likehood of producing compounds with limited brain penetration, functional groups such as carbamate, sulfonamide, sulfamide, amide, or piperidine have been incorporated into CB1 antagonist/inverse agonist structure. Other scaffolds, e.g. cannabinol, purine, or triazole, have also been explored as peripherally acting CB1 antagonists [51]. However, despite considerable attention to peripherally acting CB1 antagonists, none has reached the market yet.
Figure 3.
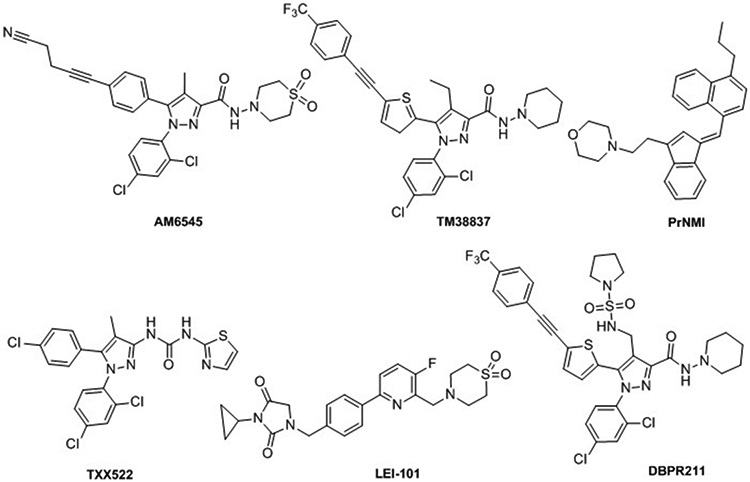
Structure of pheripheral cannabinoids: AM6545, TM38837, PrNMI, TXX522, LEI-101, DBPR211.
The widespread use of CB1 agonists is limited by CNS-mediated side effects. However, CB1 agonists suppress allodynia and hyperalgesia associated with chronic inflammatory and neuropathic pain states. Despite being known that part of these analgesic effects is peripherally CB1-mediated, very few restricted CB1 agonists have been developed. However, evidence supports the efficacy of this strategy. For instance, one of them, PrNMI (Figure 3) showed potent acute antinociceptive effect on spontaneous pain in the syngeneic murine model of cancer-induced bone pain [53]. PrNMI has been shown to suppress mechanical and cold allodynia in a chemotherapy-induced peripheral neuropathy model with minimal centrally-mediated side effects [53]. PrNMI was also efficient in alleviating the painful symptoms of neuropathy induced by unilateral sciatic nerve entrapment [54].
In contrast to CB1 receptors, CB2 receptors are mainly expressed in peripheral tissues and immune cells with limited expression in the CNS. Thus, CB2 acting compounds should have reduced psychoactive side effects. Developing CB2 peripherally restricted agonists likely won’t eliminate the possible adverse effect that could appear with chronic treatment with CB2 agonists, which is immune system suppression. But it will help avoiding CB1 side effects. In this sense, LEI-101 (Figure 3), which showed 100-fold selectivity in CB2 vs. CB1 in binding assays, did not produce CB1-mediated side effects up to 60 mg/kg in behavioural tests [55]. Thus, LEI-101 is only acting peripherally in ameliorating cisplatin-induced nephrotoxicity. Another CB2 peripheral agonist, olorinab, has recently reached phase II clinical trial for treatment of abdominal pain in patients with irritable bowel syndrome (IBS) [56].
2.4. Mitochondrial cannabinoid receptor
In the CNS, CB1 receptors are usually considered to be plasma membrane receptors with expression preferably at pre- and postsynaptic neurons and at astrocytes. Their internalization and their biosynthesis were believed to lead to non-functional intracellular receptors [57]. However, evidence points to the presence of intracellular CB1 receptors that response to cannabinoid activation. Rozenfeld and Devi [58] showed that CB1 receptors located in the late endosomal/lysosomal compartments could be activated by CB1 cannabinoids. Exploring their mechanism of action, they found that the heterotetrameric protein adaptor complex 3 (AP-3) involved in the sorting of lysosomal enzymes and in the generation of lysosome-related organelles governs the trafficking of intracellular CB1 receptors. Immunoelectron microscopy combined with immunoprecipitation and Western Blot demonstrated that CB1 receptors are also localized within mitochondria at brain and periphery (mtCB1 receptor) as compiled by Marsicano and Hebert-Chatelin and co-workers [59]. mtCB1 receptors mediate their effects through intra-mitochondrial Gi/o protein signaling, mitochondrial cyclic adenosine monophosphate (cAMP) synthesis, and decrease of intra-mitochondrial protein kinase A (PKA) activity [60]. Despite a low level of expression, activation of mtCB1 receptors alters mitochondrial metabolism, synaptic transmission and memory performance suggesting impact on brain physiology [61-63]. Extensive research in the field of mitochondrial dysfunction indicates that targeting mitochondria appears to be one of the most emerging pathological processes in senescence, apoptosis, inflammation and neurodegenerative diseases [64]. Considering the relevance of mitochondrial function, targeting mtCB1 receptors could be a promising strategy for these pathologies. Future studies should be aimed at elucidating the different actions of a particular CB1 ligand on the signaling pathways at cell surface, endosomes, and mitochondrial levels. Due to their lipophilicity, most cannabinoids penetrate the membrane cell bilayer by passive diffusion. Thus, activity differences should be observed between water-soluble and lipophilic ligands, between cell membrane impermeable and permeable cannabinoids. These physicochemical properties could be key points in this paradigm.
2.5. Multitarget agents
In the context of multifactorial diseases such as neurodegenerative disorders or cancer, targeting diverse proteins or mechanisms of action in a single chemical entity can offer optimized therapeutic outcomes. CB bivalent compounds or hybrid molecules bearing pharmacophoric features characteristic of different molecular targets (within the ECS or combined with other targets) have been reported [65,66]. These can be conjugated using a linker or integrated in the same structural framework.
Instances of those molecules are the cannabinoid-quinones reported in the last years [67-71]. Combining phytocannabinoid-like scaffolds with the cytotoxic moiety of quinones molecules such as HU331 [71] or chromenopyrazolediones 4 and 10 [69,70] (Figure 4) were developed as antitumor agents in diverse cancer models. Likewise, the quinol derivative of CBD VCE-004.8 (Figure 4) was synthesized and tested by Muñoz and coworkers [67,68]. This molecule exhibits therapeutic potential in multiple sclerosis or systemic scleroderma through activation of PPAR-γ and CB2 receptors, as well as the hypoxia inducible factor pathway. A lipidic formulation of this promising multitarget CBD derivative (EHP-101) has shown to be safe and well-tolerated in healthy volunteers (phase 1 clinical trials) and further development in patients with multiple sclerosis or scleroderma will be soon assessed [72]. The same molecular approach was used for the development of the cannabigerol-quinone VCE-003.2 (Figure 4) which has shown to improve clinical symptoms from Huntington disease and PD [73,74]. The neuroprotective effects of this molecule were proved to be mediated by activation of PPAR-γ.
Figure 4.
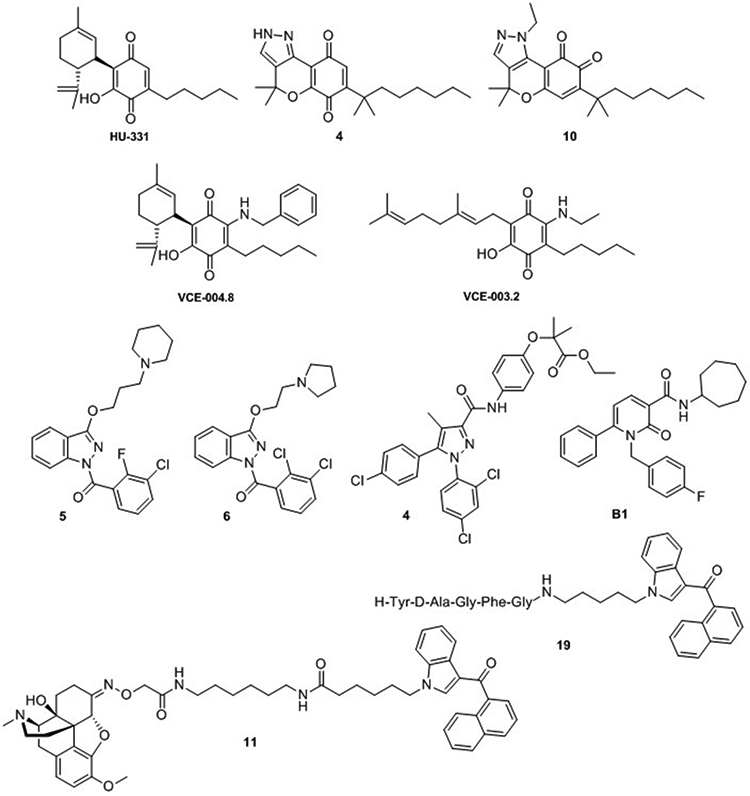
Structure of multitarget cannabinoids: CB-quinones HU-311 [71], VCE-004.8 and VCE-003.2 [67,68] and chromenopyrazolediones 4 and 10 [69,70]; indazolylketones 5 and 6 [75]; diarylpyrazole derivative 4 [76]; 1,2-dihydro-2-oxo-pyridine-3-carboxamide B1 [77] and CB-opioid bivalent ligands 11 and 19 [86]. Numbers that have been attributed to the structures refer to the corresponding number in original articles.
Moreover, multitarget indazolylketones have been proposed as potential therapeutic tools for the treatment of AD [75]. These compounds can activate CB2 receptors while inhibiting cholinesterase and/or β-secretase enzymes. In vitro activity in AD models indicates that indazole derivatives 5 and 6 (Figure 4) could be promising structures for further investigations.
Ligands targeting PPAR-α and CB1 receptors have been designed by fusing the pharmacophores of fibrates and the diarylpyrazole of the well-known CB1 inverse agonist rimonabant [76]. The dual profile of these compounds (chemotype exemplified by derivative 4, Figure 4) can be useful in the management of metabolic syndromes.
6-Aryl-1,2-dihydro-2-oxo-pyridine-3-carboxamides are also recent examples multitarget modulators of the ECS [77]. Derivative B1 (Figure 4) showed ability to modulate CB1, as partial agonist, CB2, as inverse agonist, and inhibit AEA uptake and fatty acid amide hydrolase (FAAH) [77].
Besides the aforementioned multitarget approaches, some ligands have been designed as probes for cannabinoid homo- or heterodimers. CB1 and CB2 receptors have been shown to homodimerize in specific tissues [78]. Similarly, heterodimers of these receptors with other GPCRs have been observed under specific physiopathological conditions. CB1 has been shown to form oligomers with opioid [79], serotonin [80], dopamine [81] and adenosine [6] receptors among others. On the other hand, CB2 heterodimers have been shown with GPR18 [82], with the serotonin receptor 5HT1A [83] or with the chemokine receptor CXCR4 [84].
From a drug targeting perspective, cannabinoid oligomerization offers novel pharmacological approaches with possible cross-talk effects or synergistic effects [85]. In this context, homo- and heterobivalent cannabinoids have been explored as multitarget ligands and potential tools for the study of their respective dimers (reviewed by Decker and colleagues [66]). Molecules of this class include the recently reported opioid-cannabinoids 11 and 19 (Figure 4) [86]. These compounds were designed following a multitargeting analgesic strategy using the naphthoylindole CB1/CB2 scaffold conjugated with the opiate analgesic oxycodone (11) or with an enkephalin related tetrapeptide (19).
Despite their promising multitarget pharmacological profile, available structural data challenges the fact that bivalent cannabinoids could simultaneously bind to both protomers within a dimer [87]. This fact, in addition to their poor pharmacokinetics emphasizes the importance of continuing efforts towards the design of efficient multivalent cannabinoids.
Hybrid orthosteric/ allosteric molecules (bitopic) are also emerging in the discovery of ligands for GPCRs, however, no cannabinoid of this type has yet emerged.
2.6. Non-CB1, non-CB2 targets
Besides their CB1/CB2 activity, numerous cannabinoids of endogenous, phytogenic and synthetic nature have shown to exert their effects through the modulation of non-CB1, non-CB2 targets. This includes orphan GPCRs, such as GPR55, GPR18, GPR3, GPR6, or GPR12; GPCRs from well-established families such as adenosine, opioid or serotonin receptors; TRP channels; nuclear receptors or ligand-gated ion channels [2,88].
Therefore, when testing cannabinoids, a complete pharmacological profiling in the following receptors should be considered regarding the physiopathological relevance of the targeted condition, tissue or organ.
2.6.1. Non-CB1, non-CB2 GPCRs
Although CB1 and CB2 are considered to be the canonical CB receptors, many cannabinoids have shown to interact with other orphan GPCRs.
The receptors GPR55 and GPR18 are Class A, rhodopsin-like GPCRs. Although they have few structural similarities with CB1 and CB2, GPR18 and GPR55 respond to endocannabinoids, phytocannabinoids and synthetic CB1/CB2 cannabinoids. This is also one of the main reasons they are considered putative CB receptors. Even though the endogenous N-arachidonoylglycine (NAGly, Figure 5), lysophosphatidylinositol (LPI, Figure 5) have been related to the activation of GPR18 and GPR55 respectively, they remain orphan receptors due to the lack of in vivo evidence of these endogenous ligands [2]. Regarding GPR3, GPR6, and GPR12, they all share more than 40% homology with CB1 and CB2, and over 60% among themselves [89,90]. Whereas cannabinoids such as CBD, HU-210 (Figure 5), CP55,940 (Figure 2) and WIN55,212–2 activate at least one of these three receptors, endocannabinoids failed to target these receptors [90]. As for GPR55 and GPR18, the International Union of Basic and Clinical Pharmacology (IUPHAR) still considers GPR3, GPR6, and GPR12 as orphan receptors even though diverse studies point out sphingolipids as endogenous ligands.
Figure 5.
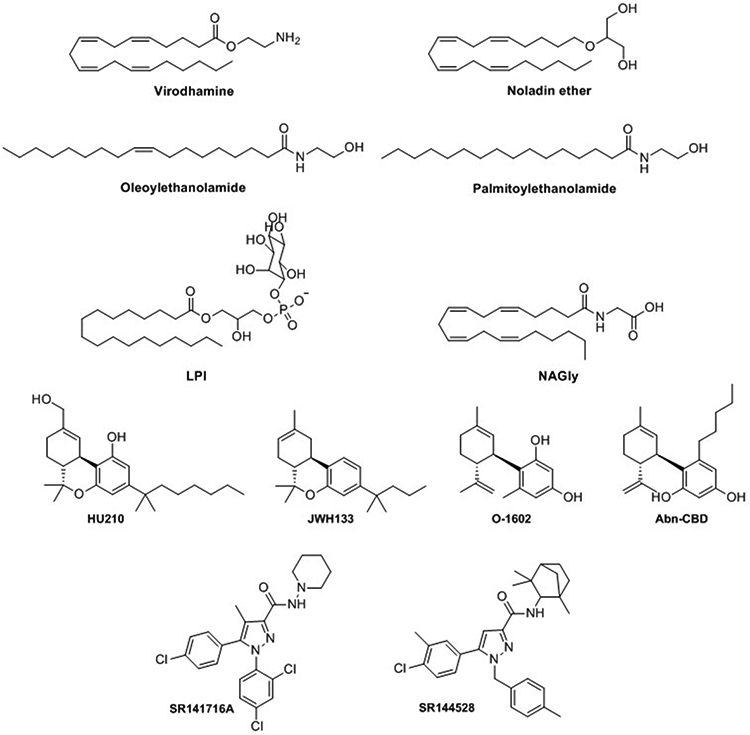
Structure of endocannabinoids virodhamine, noladin ether, oleylethanolamide and palmitoylethanolamide; putativee endogenous ligands for GPR55 and GPR18 LPI and NAGly; and synthetic cannabinoids HU210, JWH133, O-1602, Abn-CBD, SR141716A and SR144528.
The pharmacology at GPR55 and at GPR18 is quite intricate as illustrated in the following data. Several cannabinoids acting at GPR55 have shown agonistic properties in [35S]GTPγS binding assays [91]. That was the case of the endocannabinoids AEA, 2-AG, the endocannabonoid-like lipids noladin ether, palmitoylethanolamide, virodhamine, and oleoylethanolamine (Figure 5), the phytocannabinoid Δ9-THC or the synthetic cannabinoid HU210, among others. However, the conflicting data observed in the different bioassays readouts very often reflect biased signaling. The effect of CP-55,940, being a GPR55 agonist in [35S]GTPγS assays and an antagonist in β-arrestin recruitment, illustrates the complexity of the pharmacology at this receptor [91]. AEA and 2-AG were, for instance, ineffective in β-arrestin recruitment. Other cannabinoids including JWH-133 (Figure 5), tetrahydrocannabivarin, cannabidivarin and cannabigerovarin, inhibit the effect of the putative endogenous ligand LPI at GPR55. CBD, known to be a multitarget ligand, acts as GPR55 antagonist preventing [35S]GTPγS binding, whereas it is inactive in Ca2+ mobilization assays and β-arrestin recruitment experiments [91]. Several CB1 antagonists such as SR141716A (Figure 5) are GPR55 agonists but inhibit the effect of LPI inducing activation of ERK phosphorylation. Other cannabinoids such as WIN55,212–2 do not display any activity towards GPR55 through various functional assays [91].
Certains cannabinoids such as Δ9-THC, abnormal-CBD (Abn-CBD), Figure 5) and O-1602 (Figure 5), activate GPR18 activity whereas others such as AM251 and CBD act as antagonists at GPR18 [92]. Functional selectivity/biased agonism have been detected at GPR18. For example, the effect of Δ9-THC and CBD on GPR18 are mediated by β-arrestin at high concentration whereas others do not activate this signaling pathway [92].
GPR55 and GPR18 are emerging as interesting therapeutic targets of the ECS. GPR18 and GPR55 have a role in integrating, transmitting and/or alleviating pain whereas CB1 agonists induce inhibition of pain integration and CB2 agonists cause anti-inflammation via negative modulation of the immune system [93]. GPR55 is also emerging as therapeutic target for the non-dopaminergic symptomatic treatment of PD as shown by the effect of Abn-CBD in improving motor behaviour [94]. The role of GPR55 in energy balance and glucose metabolism has been thoroughly reviewed showing its potential in obesity and type 2 diabetes [95]. While the antitumor activity of certain cannabinoids is mostly mediated through activation of CB1 or CB2, other cannabinoids act partially through other targets such as GPR55 [96]. Interactions of GPR55 with other elements of the endocannabinoime have been shown to be possible therapeutic targets. For instance, crosstalk between CB2 and GPR55 has been identified as a determinant of cancer progression [97]. GPR18 has been proposed as a potential target for diverse pathologies different than pain, such as metabolic dysfunction [98], cardiovascular disease [99] or intraocular pressure [100].
Concerning the ECS-related orphan subset GPR3–6-12, a recent review summarizes their structure, pharmacology and biological relevance [90]. As the cannabinoid receptors, they are highly expressed in the CNS. Several studies support GPR3, GPR6, and GPR12 as potential targets for neurodegenerative disorders such as AD or PD [90]. Unfortunately, very few ligands of these receptors have been discovered so far. Consequently, there is a clear necessity for pharmacological tools to progress in the understanding of their relation with the ECS. In recent years, diverse cannabinoids have been reported to signal through these receptors. For instance, CBD was reported to be a moderate inverse agonist at GPR3, GPR6 and GPR12 [101]. Moreover, well-known synthetic cannabinoids such as WIN55212–2 or the arylpyrazoles SR141716A and SR144528 (Figure 5) have shown to exert biased β-arrestin2 inverse agonism at GPR6 [102]. Even though the endocannabinoids AEA and 2-AG do not display activity at GPR3, GPR6, or GPR12, several endocannabinoid-like N-acyl dopamines act as β-arrestin2 functionally selective inverse agonists for GPR6 [103].
Discovery of selective, potent ligands for these receptors and determination of their functions may provide interesting insight into physiological and pathological processes, as well as a possible contribution/relation with ECS.
2.6.2. Other cannabinoid related targets
Most cannabinoids present a complex pharmacology due to target promiscuity. Their lipophilic nature enhances their ability to reach a wide variety of biological tissues and therefore modulate receptors of different nature such as nuclear receptors, TRP or ligand-gated ion channels.
A wide variety of cannabinoids have been reported to modulate a specific subset of TRP channels. Six channels from three different TRP subfamilies [TRP vanilloid (TRPV), TRP ankyrin (TRPA), and TRP melastatin (TRPM)] have been reported to mediate CB activity: TRPV1, TRPV2, TRPV3, TRPV4, TRPA1, and TRPM8. Some cannabinoids have shown to interact with one or more of these channels showing a different functional profile [3]. For instance, phytocannabinoids such as Δ9-THC can activate TRPV2, TRPV3, TRPV4 and TRPA1, but antagonize TRPM8, whereas the endocannabinoid AEA is a potent TRPV1 agonist and TRPM8 antagonist. It is worth mentioning that the analgesic effects of certain cannabinoids are, at least partially, mediated via TRPV1 [3]. Due to the increasing research demonstrating CB interactions with these channels, they have been proposed as the “ionotropic CB receptors” [88].
Different phyto-, endo- and synthetic cannabinoids can also target the nuclear receptors PPARα and PPARγ [104]. In fact, certain therapeutic responses triggered by cannabinoids are, to some extent, mediated by these nuclear hormone receptors that control the transcription of target genes. Cannabinoid activation of PPARα and PPARγ is associated with some of the neuroprotective, anti-inflammatory and metabolic properties of these molecules (reviewed by ÓSullivan and coworkers [104]).
Other reported CB targets include ligand-gated ion channels [88] such as nicotinic acetylcholine (nACh) [105], sodium channels (Nav) [106], glycine (GlyR) [107] or GABAA receptors [108] and could be involved in CB-induced analgesia.
To sum up, the therapeutic potential of cannabinoids is tightly related with their activity at non-canonical receptors such as the ones detailed in this section. Therefore, to assess their pharmacological profile functional studies at these targets should be taken into account in the development of CB-based drugs.
2.7. Challenges in pharmacokinetics
Most research on the pharmacodynamic and pharmacokinetic effects of cannabinoids has been performed upon administration of inhaled cannabis and usually focuses on CBD and Δ9-THC. Nevertheless, studies realized with other phytogenic or synthetic cannabinoids exhibited similar kinetic profiles [109].
Cannabinoids bioavailability diverges depending on their formulation and route of administration [110]. Currently, cannabinoids for medical purposes include preparations designed for oral administration, oromucosal delivery, or transdermal application. Oral administration is particularly challenging due to the lipophilic nature of cannabinoids and therefore, increasing research efforts are focused on the search of suitable formulations. Attempts to improve oral bioavailability have been done through co-administration of cannabinoids with lipids [111]. Recent pharmacokinetic investigations have been directed to the development of nanoparticle-based formulations which have effectively shown to increase cannabinoids oral bioavailability [112,113]. Moreover, a nanomicellar formulation of WIN55,212–2 have not only exhibited better absorption but also milder psychoactive effects in vivo [114]. Bioisosteric approaches have also been reported to improve cannabinoid drug-like physicochemical properties while maintaining activity [115].
3. Experts Opinion
The road towards the therapeutic use of cannabinoids is raising high hopes especially since the recent legalization of medical marihuana in several countries. Phytogenic cannabinoids, CBD in particular, either alone or in combination with Δ9-THC, are being intensively studied as safe and efficacious drugs for the treatment of specific pathologies such as epilepsy, PD, AD, multiple sclerosis or cancer. However, regarding the synthetic cannabinoids, two events that occurred in 2016 have seeded bitter disappointments in the field. The illicit consumption of highly potent CB1 synthetic cannabinoids such as AMB-FUBINACA that caused deaths and serious adverse health events sounded the bell [11-13]. Even though the research community was aware of serious psychotropic effects that could be produced by such potent CB1 agonists, this episode contributed to intensifying the studies on pharmacological and toxicological aspects of cannabinoids as well as their metabolic and thermolytic degradants. It is worth mentioning that, in the same year, clinical trials with a FAAH inhibitor had to be interrupted [116]. The clinical development of the FAAH inhibitor BIA 10–2474 for the treatment of anxiety, chronic pain, multiple sclerosis, PD, cancer and hypertension had to be discontinued due to a fatal outcome in a phase II trial. One volunteer died and others were seriously affected neurologically. Even if the underlying mechanism of this toxic cerebral effect remains unknown, different studies point to critical off-targets in the brain [117]. In this context, cannabinoid discovery efforts have been focused on a deeper understanding of the ECS that could lead to new approaches to deliver potential drug candidates. Novel strategies including peripherally restriction, allosterism, and biased signaling are being currently explored to eliminate the central psychiatric adverse effects of CB1 receptor signaling pathway, while retaining its therapeutic benefits. Given the current lack of efficacy in vivo of CB2 agonists and their suspected immunosuppressive side-effects, there is also a need for exploring new therapeutic approaches. A summary of potential approaches to target the ECS are graphically described in Figure 6.
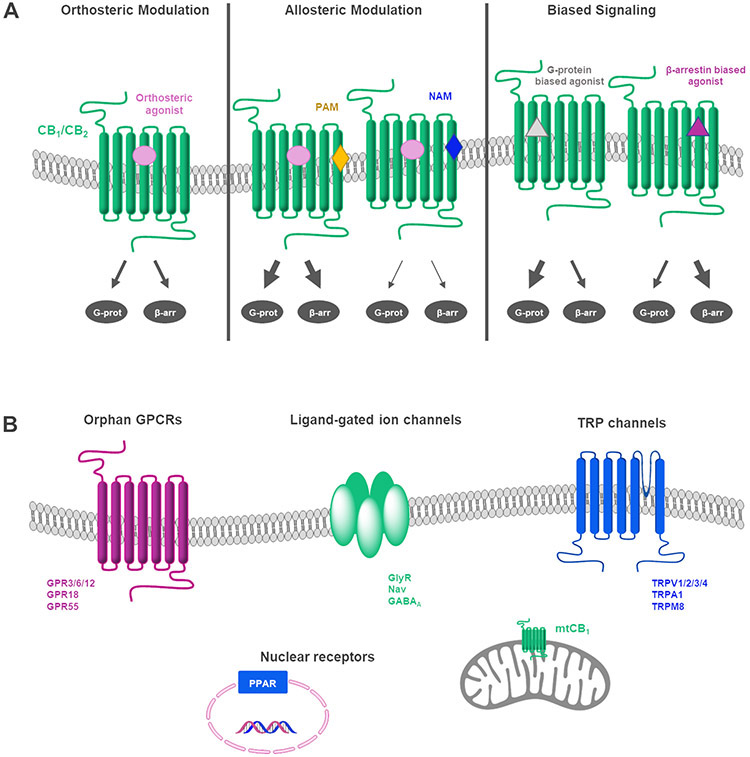
Figure 6.
Summary of potential approaches to target the ECS. A) Modulation at CB1 and CB2 receptors (G-prot refers to G protein signaling and β-arr to β-arrestin1 or 2 pathways). B) Other targets of cannabinoids.
Allosterism at GPCRs is currently proving to be a viable drug discovery strategy such as shown by the entrance in clinical phase I of HTL0014242, a selective NAM for the metabotropic glutamate (mGlu) receptor 5 subtype (mGlu5). Allosterism at CB receptors still remain a challenge. In one hand, extended in vivo assays need to validate CB allosterism as a therapeutic target. On the other hand, high-resolution structure of the CB1 receptor with a NAM CB combined with molecular modelling will certainly accelerate the discovery of allosteric cannabinoids. Targeting allosterism one should not underestimate the ability of allosteric compounds to engender signal pathway bias.
Alternatives such as biased cannabinoids are being studied in the search of selective pathway specificity of desired therapeutic outcomes. However, much research is needed to adequately pursue this goal. In fact, already known CB chemotypes should be re-evaluated using different cell types and functional endpoints to assess possible functional selectivity. Cannabinoids with particular pharmacological profiles such as CB1 G-protein bias could maximize the therapeutic benefit while reducing β-arrestin associated tolerance. These molecules could be useful for the management of chronic pathologies. In fact, even though it is generally considered that β-arrestins bind to activated GPCRs in absence of the G protein, GPCR–G protein–β-arrestin mega-complexes have only been reported recently [118]. Peripherally restricted cannabinoids.
Being one of the most abundant GPCRs in the CNS, CB1 receptor peripherally restricted ligands constitute a strategy to be explored to eliminate central psychiatric adverse effects but retain the therapeutic benefits. Much effort has been made on studying peripheral CB1 antagonists due to the considerable attention received by CB1 antagonist in regulating energy homeostasis and metabolism. Mitochondrial CB1 receptors
Mitochondrial dysfunction seems to be involved in senescence, apoptosis, inflammation, and neurodegenerative diseases. Thus, the activation of mtCB1 that directly alters mitochondrial energetic activity [61] might modulate high brain functions such as memory formation among others processes [62]. In this context, physicochemical properties of cannabinoids play a major role to allow or not allow penetration of the cell membrane to reach mtCB1.
Multitarget cannabinoids also offer unique potential therapeutic avenues in the treatment of multifactorial diseases. However, due to the challenges arising from their pharmacological evaluation and their poor pharmacokinetic properties, no lead compound has yet emerged with this profile in the CB field.
The therapeutic potential of GPR55, GPR18, GPR3, GPR6, and GPR12 remains to be fully appreciated and validated. Their complex pharmacology, receptor promiscuity, and lack of potent selective ligands delay the discovery of therapeutic agents. However, breakthroughs in combined pharmacology and molecular modelling will help the design selective ligands and will unravel the mechanisms of action of certain at molecular level. Other CB targets such as TRP channels and the nuclear receptors PPARα and PPARγ play a role in the ECS. Increasing evidence points out CB interactions with these nuclear receptors and these channels. All of these ECS related-receptors should be taken into account when developing cannabinoids or when designing/evaluating new cannabinoids.
As previously mentioned, oral administration due to intrinsic cannabinoid lipophilicity is still a challenge in drug development. Recent pharmacokinetic efforts to improve cannabinoid oral bioavailability include nanoparticle-based formulations or lipid co-administration. This approach may aid not only drug administration but also cannabinoid absorption at specific tissues for particular diseases or symptoms.
Lack of receptor selectivity and the intrinsic complexity of the ECS are the two main caveats currently faced by cannabinoids as medicines. Understanding the interactions of these compounds with their targeted proteins will guide the design of optimized molecules regarding the desired effect. Fortunately, an extraordinary number of high-resolution structures of cannabinoids with their targets have been reported in the last years [14-19]. CB1 has been solved in complex with agonists [15], antagonists [14] and allosteric modulators [16], in addition, very recent cryo-EM structures have elucidated CB1 and CB2-Gi signaling complexes [17,18]. This structural data will help in guiding the next stage of drug development in the CB field.
For this new decade, therapeutic exploitation of the ECS needs to be explored from a wider perspective. Activity at the aforementioned targets should be considered, and thus multitarget strategies can be promising for specific ECS–related disorders. Moreover, an array of functional assays needs to be accomplished for full elucidation of signalling pathways and therapeutic outcomes of a CB candidate for development. It is quite clear that nowadays, phytocannabinoids are at the forefront of clinical research. Therefore, drug design programs should focus on CB-based drugs with unique pharmacological profiles corresponding to particular pathophysiological conditions.
Article Highlights:
Novel strategies for targeting the endocannabinoid system include allosterism and bias signalling.
Multi-target approaches could be promising strategies for the treatment of endocannabinoid system-related disorders
Full characterization of signalling pathways needs to be accomplished for drug candidates targeting the ECS
High-resolution structures of cannabinoid receptors will help in guiding future drug design
The authors believe that phytocannabinoids are at the forefront of future clinical research
Acknowledgements:
The authors thank Dow H. Hurst for his English review.
Funding:
N Jagerovic is supported by the Ministry of Science, Innovation, and Universities, Spain (MCIU)/FEDER grant RTI2018–095544-B-I00, the Spanish National Research Council (CSIC) grant PIE-201580E033 and National Institutes of Health grant R01 DA0455698–01. Meanwhile, P Morales is supported by the CAM programme “Attraccion de Talentos” number 2018-T28MD-10819.
Abbreviation list
- Abn-CBD
abnormal cannabidiol
- AD
Alzheimer’s disease
- AEA
N-arachidonoylethanolamine o anandamide
- 2-AG
2-arachidonoylglycerol
- c-AMP
cyclic adenosine monophosphate
- CB
cannabinoid
- CBD
cannabidiol
- ECS
endocannabinoid system
- FAAH
fatty acid amide hydrolase
- GPCR
G-protein-coupled receptor
- LPI
lysophosphatidylinositol
- MD
molecular dynamics
- mtCB1
mitochondria CB1
- NAGly
N-arachidonoylglycine
- NAM
negative allosteric modulator
- PAM
positive allosteric modulator
- PD
Parkinson’s disease
- PPAR
peroxisome proliferator-activated receptor
- PKA
protein kinase A
- TM
transmembrane helix
- TRP
transient receptor potential
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- [1].Lu HC, Mackie K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016;516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Morales P, Reggio PH. An Update on Non-CB1 , Non-CB2 Cannabinoid Related G-Protein-Coupled Receptors. Cannabis Cannabinoid Res. 2017;2:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Muller C, Morales P, Reggio PH. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci 2019;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Navarro G, Reyes-Resina I, Rivas-Santisteban R, et al. Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem. Pharmacol. [Internet] 2018;In press. Available from: 10.1016/j.bcp.2018.08.046. [DOI] [PubMed] [Google Scholar]
- [5].Reyes-Resina I, Navarro G, Aguinaga D, et al. Molecular and functional interaction between GPR18 and cannabinoid CB2 G-protein-coupled receptors. Relevance in neurodegenerative diseases. Biochem. Pharmacol 2018;157:169–179. [DOI] [PubMed] [Google Scholar]
- [6].Moreno E, Chiarlone A, Medrano M, et al. Singular Location and Signaling Profile of Adenosine A2A-Cannabinoid CB1 Receptor Heteromers in the Dorsal Striatum. Neuropsychopharmacology. 2017;1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Veilleux A, Di Marzo V, Silvestri C. The Expanded Endocannabinoid System/Endocannabinoidome as a Potential Target for Treating Diabetes Mellitus. Curr. Diab. Rep 2019;19. [DOI] [PubMed] [Google Scholar]
- [8].Morales P, Hernandez-folgado L, Goya P, et al. Cannabinoid receptor 2 (CB2) agonists and antagonists : a patent update. Expert Opin. Ther. Pat 2016;3776. [DOI] [PubMed] [Google Scholar]
- [9].ElSohly MA, Gul W, Wanas AS, et al. Synthetic cannabinoids: Analysis and metabolites. Life Sci. 2014;97:78–90. [DOI] [PubMed] [Google Scholar]
- [10].Shevyrin V, Melkozerov V, Endres GW, et al. On a New Cannabinoid Classification System: A Sight on the Illegal Market of Novel Psychoactive Substances. Cannabis Cannabinoid Res. 2016;1:186–194. [Google Scholar]
- [11].Trecki J, Gerona R, Schwartz M. Synthetic Cannabinoid–Related Illnesses and Deaths. N. Engl. J. Med 2015;373:103–107. [DOI] [PubMed] [Google Scholar]
- [12].Scourfield A, Flick C, Ross J, et al. Synthetic cannabinoid availability on darknet drug markets—changes during 2016–2017. Toxicol. Commun 2019;3:7–15. [Google Scholar]
- [13].Banister SD, Connor M. The Chemistry and Pharmacology of Synthetic Cannabinoid Receptor Agonists as New Psychoactive Substances: Origins In: Maurer HH, Brandt SD, editors. New Psychoact. Subst Cham, Switzerland: Springer International Publishing; 2018. p. 165–190. [DOI] [PubMed] [Google Scholar]
- [14].Shao Z, Yin J, Chapman K, et al. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature. 2016;540:602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hua T, Vemuri K, Nikas SP, et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature. 2017;547:468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shao Z, Yan W, Chapman K, et al. Structure of an allosteric modulator bound to the CB1 cannabinoid receptor. Nat. Chem. Biol 2019;15:1199–1205. [DOI] [PubMed] [Google Scholar]
- [17].Hua T, Li X, Wu L, et al. Activation and Signaling Mechanism Revealed by Cannabinoid Receptor-Gi Complex Structures. Cell [Internet]. 2020; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32004463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xing C, Zhuang Y, Xing C, et al. Cryo-EM Structure of the Human Cannabinoid Receptor CB2-G i Signaling Complex. Cell [Internet]. 2020;180:1–10. Available from: 10.1016/j.cell.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li X, Hua T, Vemuri K, et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell. 2019;176:459–467.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Devinsky O, Nabbout R, Miller I, et al. Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia. 2019;60:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Billakota S, Devinsky O, Marsh E. Cannabinoid therapy in epilepsy. Curr. Opin. Neurol 2019;32:220–226. [DOI] [PubMed] [Google Scholar]
- [22].Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos. Trans. R. Soc. B Biol. Sci 2012;367:3353–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chicca A, Arena C, Manera C. Beyond the Direct Activation of Cannabinoid Receptors: New Strategies to Modulate the Endocannabinoid System in CNS-Related Diseases. Recent Pat. CNS Drug Discov 2016;10:122–141. [DOI] [PubMed] [Google Scholar]
- [24].Kohnz RA, Nomura DK. Chemical approaches to therapeutically target the metabolism and signaling of the endocannabinoid 2-AG and eicosanoids. Chem. Soc. Rev 2014;43:6859–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hryhorowicz S, Kaczmarek-ry M, Andrzejewska A, et al. Allosteric Modulation of Cannabinoid Receptor 1 — Current Challenges and Future Opportunities. 2019;1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khurana L, Mackie K, Piomelli D, et al. Modulation of CB1 cannabinoid receptor by allosteric ligands: Pharmacology and therapeutic opportunities. Neuropharmacology [Internet]. 2017;124:3–12. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0028390817302307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alaverdashvili M, Laprairie RB. The future of type 1 cannabinoid receptor allosteric ligands. Drug Metab. Rev. [Internet] 2018;0:1–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29355038%0Ahttps://www.tandfonline.com/doi/full/10.1080/03602532.2018.1428341. [DOI] [PubMed] [Google Scholar]
- [28].Morales P, Goya P, Jagerovic N, et al. Allosteric Modulators of the CB 1 Cannabinoid Receptor : A Structural Update Review. Cannabis Cannabinoid Res. 2016;1:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Saleh N, Hucke O, Kramer G, et al. Multiple Binding Sites Contribute to the Mechanism of Mixed Agonistic and Positive Allosteric Modulators of the Cannabinoid CB1 Receptor. Angew. Chemie - Int. Ed 2018;57:2580–2585. [DOI] [PubMed] [Google Scholar]
- [30].Tham M, Yilmaz O, Alaverdashvili M, et al. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. [Internet] 2019;176:1455–1469. Available from: http://doi.wiley.com/10.1111/bph.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hurst DP, Garai S, Kulkarni PM, et al. Identification of cb1 receptor allosteric sites using force-biased mmc simulated annealing and validation by structure-activity relationship studies. ACS Med. Chem. Lett 2019;10:1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sabatucci A, Tortolani D, Dainese E, et al. In silico mapping of allosteric ligand binding sites in type-1 cannabinoid receptor. Biotechnol. Appl. Biochem 2018;65:21–28. [DOI] [PubMed] [Google Scholar]
- [33].Chung H, Fierro A, Pessoa-Mahana CD. Cannabidiol binding and negative allosteric modulation at the cannabinoid type 1 receptor in the presence of delta-9-tetrahydrocannabinol: An In Silico study. PLoS One. 2019;14:e0220025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Díaz Ó, Dalton JAR, Giraldo J. Revealing the mechanism of agonist-mediated cannabinoid receptor 1 (CB1) activation and phospholipid-mediated allosteric modulation. J. Med. Chem. [Internet] 2019;1:acs.jmedchem.9b00612. Available from: http://pubs.acs.org/doi/10.1021/acs.jmedchem.9b00612. [DOI] [PubMed] [Google Scholar]
- [35].Bian Y, Jing Y, Wang L, et al. Prediction of Orthosteric and Allosteric Regulations on Cannabinoid Receptors Using Supervised Machine Learning Classifiers. Mol. Pharm 2019;16:2605–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Slivicki RA, Xu Z, Kulkarni PM, et al. Positive Allosteric Modulation of Cannabinoid Receptor Type 1 Suppresses Pathological Pain Without Producing Tolerance or Dependence. Biol. Psychiatry [Internet] 2017;011:1–12. Available from: 10.1016/j.biopsych.2017.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mitjavila J, Yin D, Kulkarni PM, et al. Enantiomer-specific positive allosteric modulation of CB1signaling in autaptic hippocampal neurons. Pharmacol. Res. [Internet] 2017;1–7 Available from: 10.1016/j.phrs.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Garai S, Kulkarni PM, Schaffer PC, et al. Application of Fluorine- and Nitrogen-Walk Approaches: De fi ning the Structural and Functional Diversity of 2 ‑ Phenylindole Class of Cannabinoid 1 Receptor Positive Allosteric Modulators. J Med Chem. 2020;63:542–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Morales P, Goya P, Jagerovic N. Emerging strategies targeting CB2 cannabinoid receptor: Biased agonism and allosterism. Biochem. Pharmacol 2018;157:8–17. [DOI] [PubMed] [Google Scholar]
- [40].Pandey P, Roy KK, Doerksen RJ. Negative Allosteric Modulators of Cannabinoid Receptor 2: Protein Modeling, Binding Site Identification and Molecular Dynamics Simulations in the Presence of an Orthosteric Agonist. J. Biomol. Struct. Dyn. [Internet] 2019;0:1–23. Available from: https://www.tandfonline.com/doi/full/10.1080/07391102.2019.1567384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ibsen MS, Connor M, Glass M. Cannabinoid CB1 and CB2 Receptor Signaling and Bias. Cannabis Cannabinoid Res. [Internet] 2017;2:48–60. Available from: http://online.liebertpub.com/doi/10.1089/can.2016.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Howlett A, Blume L, Dalton G. CB1 Cannabinoid Receptors and their Associated Proteins. Curr. Med. Chem 2010;17:1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol. Pharmacol. [Internet] 1999;56:1362–1369. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10570066. [DOI] [PubMed] [Google Scholar]
- [44].Al-zoubi R, Morales P, Reggio PH. Structural Insights into CB1 Receptor Biased Signaling. Int. J. Mol. Sci 2019;20:1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Laprairie RB, Bagher AM, Denovan-Wright EM. Cannabinoid receptor ligand bias: implications in the central nervous system. Curr. Opin. Pharmacol. [Internet] 2017;32:32–43. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1471489216301266. [DOI] [PubMed] [Google Scholar]
- [46].Soethoudt M, Grether U, Fingerle J, et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat. Commun 2017;8:13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dhopeshwarkar A, Mackie K. Functional Selectivity of CB2 Cannabinoid Receptor Ligands at a Canonical and Noncanonical Pathway. J. Pharmacol. Exp. Ther. [Internet] 2016. [cited 2016 Oct 21];358:342–351. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27194477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wouters E, Walraed J, Banister SD, et al. Insights into biased signaling at cannabinoid receptors: synthetic cannabinoid receptor agonists. Biochem. Pharmacol 2019;169:113623. [DOI] [PubMed] [Google Scholar]
- [49].Sam AH, Salem V, Ghatei MA. Rimonabant: From RIO to Ban. J. Obes. [Internet] 2011. [cited 2015 Nov 19];2011:432607 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3136184&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Amato G, Khan NS, Maitra R. A patent update on cannabinoid receptor 1 antagonists (2015–2018). Expert Opin. Ther. Pat. [Internet] 2019;29:261–269. Available from: 10.1080/13543776.2019.1597851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nguyen T, Thomas BF, Zhang Y. Overcoming the psychiatric side effects of the cannabinoid CB1 receptor antagonists: current approaches for therapeutics development. Curr. Top. Med. Chem 2020;19:1418–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tam J, Hinden L, Drori A, et al. The therapeutic potential of targeting the peripheral endocannabinoid/CB1 receptor system. Eur. J. Intern. Med. [Internet] 2018;49:23–29. Available from: 10.1016/j.ejim.2018.01.009. [DOI] [PubMed] [Google Scholar]
- [53].Zhang H, Lund DM, Ciccone HA, et al. Peripherally restricted cannabinoid 1 receptor agonist as a novel analgesic in cancer-induced bone pain. Pain. 2018;159:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am. J. Hosp. Palliat. Med 2010. [DOI] [PubMed] [Google Scholar]
- [55].Mukhopadhyay P, Baggelaar M, Erdelyi K, et al. The novel, orally available and peripherally restricted selective cannabinoid CB 2 receptor agonist LEI-101 prevents cisplatin-induced nephrotoxicity. Br. J. Pharmacol 2016;173:446–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].https://clinicaltrials.gov/ct2/show/NCT04043455?term=olorinab&draw=2&rank=1; consulting date January/31/2020.
- [57].Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev 2003;83:1017–1066. [DOI] [PubMed] [Google Scholar]
- [58].Rozenfeld R, Devi LA. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 2008;22:2311–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Melser S, Zottola ACP, Serrat R, et al. Functional Analysis of Mitochondrial CB1 Cannabinoid Receptors (mtCB1) in the Brain [Internet] 1st ed. Methods Enzymol. Elsevier Inc.; 2017. Available from: 10.1016/bs.mie.2017.06.023. [DOI] [PubMed] [Google Scholar]
- [60].Busquets-Garcia A, Bains J, Marsicano G. CB 1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacology [Internet]. 2018;43:4–20. Available from: 10.1038/npp.2017.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bénard G, Massa F, Puente N, et al. Mitochondrial CB 1 receptors regulate neuronal energy metabolism. Nat. Neurosci 2012;15:567. [DOI] [PubMed] [Google Scholar]
- [62].Hebert-Chatelain E, Desprez T, Serrat R, et al. A cannabinoid link between mitochondria and memory. Nature [Internet]. 2016;539:555–559. Available from: 10.1038/nature20127. [DOI] [PubMed] [Google Scholar]
- [63].Djeungoue-Petga MA, Hebert-Chatelain E. Linking Mitochondria and Synaptic Transmission: The CB1 Receptor. BioEssays. 2017;39:1–11. [DOI] [PubMed] [Google Scholar]
- [64].Wu Y, Chen M, Jiang J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion [Internet]. 2019;49:35–45. Available from: 10.1016/j.mito.2019.07.003. [DOI] [PubMed] [Google Scholar]
- [65].Oliveira Pedrosa M, Duarte da Cruz R, Oliveira Viana J, et al. Hybrid Compounds as Direct Multitarget Ligands: A Review. Curr. Top. Med. Chem 2017;17:1044–1079. [DOI] [PubMed] [Google Scholar]
- [66].Nimczick M, Decker M. New Approaches in the Design and Development of Cannabinoid Receptor Ligands: Multifunctional and Bivalent Compounds. ChemMedChem [Internet]. 2015;10:773–786. Available from: http://doi.wiley.com/10.1002/cmdc.201500041. [DOI] [PubMed] [Google Scholar]
- [67].García-Martín A, Garrido-Rodríguez M, Navarrete C, et al. Cannabinoid derivatives acting as dual PPARγ/CB2 agonists as therapeutic agents for systemic sclerosis. Biochem. Pharmacol 2019;163:321–334. [DOI] [PubMed] [Google Scholar]
- [68].Navarrete C, Carrillo-Salinas F, Palomares B, et al. Hypoxia mimetic activity of VCE-004.8, a cannabidiol quinone derivative: Implications for multiple sclerosis therapy. J. Neuroinflammation 2018;15:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Morales P, Blasco-Benito S, Andradas C, et al. A selective, non-toxic CB2 cannabinoid o-quinone with in vivo activity against triple negative breast cancer. J. Med. Chem 2015;58:2256–2264. [DOI] [PubMed] [Google Scholar]
- [70].Morales P, Vara D, Goméz-Cañas M, et al. Synthetic cannabinoid quinones: Preparation, in vitro antiproliferative effects and in vivo prostate antitumor activity. Eur. J. Med. Chem 2013;70:111–119. [DOI] [PubMed] [Google Scholar]
- [71].Kogan NM, Schlesinger M, Priel E, et al. HU-331, a novel cannabinoid-based anticancer topoisomerase II inhibitor. Mol. Cancer Ther. [Internet]. 2007. [cited 2012 Apr 12];6:173–183. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17237277. [DOI] [PubMed] [Google Scholar]
- [72].Evaluation of Safety, Tolerability and Preliminary Efficacy of EHP-101 in Diffuse Cutaneous Systemic Sclerosis - ClinicalTrials.gov [Internet]. [cited 2020 Jan 28]. Available from: https://clinicaltrials.gov/ct2/show/NCT04166552?term=EHP-101&draw=2&rank=1.
- [73].García C, Gómez-Cañas M, Burgaz S, et al. Benefits of VCE-003.2, a cannabigerol quinone derivative, against inflammation-driven neuronal deterioration in experimental Parkinson’s disease: Possible involvement of different binding sites at the PPARγ receptor. J. Neuroinflammation 2018;15:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Aguareles J, Paraíso-Luna J, Palomares B, et al. Oral administration of the cannabigerol derivative VCE-003.2 promotes subventricular zone neurogenesis and protects against mutant huntingtin-induced neurodegeneration. Transl. Neurodegener 2019;8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].González-Naranjo P, Pérez-Macias N, Pérez C, et al. Indazolylketones as new multitarget cannabinoid drugs. Eur. J. Med. Chem. [Internet] 2019;166:90–107. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0223523419300406. [DOI] [PubMed] [Google Scholar]
- [76].Pérez-Fernández R, Fresno N, MacÍas-González M, et al. Discovery of potent dual PPARα agonists/CB1 ligands. ACS Med. Chem. Lett. 2011;2:793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gado F, Arena C, Fauci C La, et al. Modification on the 1,2-dihydro-2-oxo-pyridine-3-carboxamide core to obtain multi-target modulators of endocannabinoid system. Bioorg. Chem 2020;94:103353. [DOI] [PubMed] [Google Scholar]
- [78].Mackie K Cannabinoid receptor homo- and heterodimerization. Life Sci. [Internet] 2005. [cited 2014 Sep 10];77:1667–1673. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15978631. [DOI] [PubMed] [Google Scholar]
- [79].Sierra S, Gupta A, Gomes I, et al. Targeting Cannabinoid 1 and Delta Opioid Receptor Heteromers Alleviates Chemotherapy-Induced Neuropathic Pain. ACS Pharmacol. Transl. Sci 2019;2:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Viñals X, Moreno E, Lanfumey L, et al. Cognitive Impairment Induced by Delta9-tetrahydrocannabinol Occurs through Heteromers between Cannabinoid CB1 and Serotonin 5-HT2A Receptors. PLOS Biol. 2015;13:e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Przybyla J a Watts VJ. Ligand-Induced Regulation and Localization of Cannabinoid CB1 and Dopamine D2L Receptor Heterodimers. Pharmacol. 2010;332:710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Reyes-resina I, Navarro G, Aguinaga D, et al. Molecular and functional interaction between GPR18 and cannabinoid CB2 G-protein-coupled receptors. Relevance in neurodegenerative diseases [Internet]. Biochem. Pharmacol 2018. Available from: 10.1016/j.bcp.2018.06.001. [DOI] [PubMed]
- [83].Franco R, Villa M, Morales P, et al. Increased expression of cannabinoid CB2 and serotonin 5-HT1A heteroreceptor complexes in a model of newborn hypoxic-ischemic brain damage. Neuropharmacology [Internet]. 2019;152:58–66. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0028390819300462. [DOI] [PubMed] [Google Scholar]
- [84].Coke CJ, Scarlett KA, Chetram MA, et al. Simultaneous activation of induced heterodimerization between CXCR4 chemokine receptor and cannabinoid receptor 2 (CB2) reveals a mechanism for regulation of tumor progression. J. Biol. Chem 2016;291:9991–10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Botta J, Appelhans J, Mccormick PJ. Continuing challenges in targeting oligomeric GPCR-based drugs [Internet] 1st ed. Oligomerization Heal. Dis. From Enzym. to G Protein-Coupled Recept. Elsevier Inc.; 2020. Available from: 10.1016/bs.pmbts.2019.11.009. [DOI] [PubMed] [Google Scholar]
- [86].Dvorácskó S, Keresztes A, Mollica A, et al. Preparation of bivalent agonists for targeting the mu opioid and cannabinoid receptors. Eur. J. Med. Chem 2019;178:571–588. [DOI] [PubMed] [Google Scholar]
- [87].Glass M, Govindpani K, Furkert DP, et al. One for the Price of Two…Are Bivalent Ligands Targeting Cannabinoid Receptor Dimers Capable of Simultaneously Binding to both Receptors? Trends Pharmacol. Sci. [Internet] 2016;xx:1–11. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0165614716000249. [DOI] [PubMed] [Google Scholar]
- [88].Pertwee RG, Howlett AC, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev 2010;62:588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Khan MZ, He L. Neuro-psychopharmacological perspective of Orphan receptors of Rhodopsin (class A) family of G protein-coupled receptors. Psychopharmacology (Berl). [Internet] 2017; Available from: http://link.springer.com/10.1007/s00213-017-4586-9. [DOI] [PubMed]
- [90].Morales P, Isawi I, Reggio PH. Towards a better understanding of the cannabinoid-related orphan receptors GPR3, GPR6, and GPR12. Drug Metab. Rev. [Internet] 2018;50:74–93. Available from: 10.1080/03602532.2018.1428616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Morales P, Jagerovic N. Advances towards the Discovery of GPR55 Ligands. Curr. Med. Chem 2016;23:2087–2100. [DOI] [PubMed] [Google Scholar]
- [92].Rajaraman G, Simcocks A, Hryciw DH, et al. G protein coupled receptor 18: A potential role for endocannabinoid signaling in metabolic dysfunction. Mol. Nutr. Food Res. [Internet] 2016;92–102. Available from: http://doi.wiley.com/10.1002/mnfr.201500449. [DOI] [PubMed] [Google Scholar]
- [93].Guerrero-Alba R, Barragán-Iglesias P, González-Hernández A, et al. Some Prospective Alternatives for Treating Pain: The Endocannabinoid System and Its Putative Receptors GPR18 and GPR55. Front. Pharmacol. [Internet] 2019;9 Available from: https://www.frontiersin.org/article/10.3389/fphar.2018.01496/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Celorrio M, Rojo-Bustamante E, Fernández-Suárez D, et al. GPR55: A therapeutic target for Parkinson’s disease? Neuropharmacology [Internet]. 2017; Available from: http://linkinghub.elsevier.com/retrieve/pii/S0028390817303842. [DOI] [PubMed] [Google Scholar]
- [95].Tudurí E, Imbernon M, Hernández-Bautista RJ, et al. GPR55: a new promising target for metabolism? J. Mol. Endocrinol. [Internet] 2017;58:R191–R202. Available from: http://jme.endocrinology-journals.org/lookup/doi/10.1530/JME-16-0253. [DOI] [PubMed] [Google Scholar]
- [96].Morales P, Jagerovic N. Antitumor Cannabinoid Chemotypes: Structural Insights. Front. Pharmacol. [Internet] 2019;10:Article 621. Available from: https://www.frontiersin.org/article/10.3389/fphar.2019.00621/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ramer R, Schwarz R, Hinz B. Modulation of the endocannabinoid system as a potential anticancer strategy. Front. Pharmacol 2019;10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rajaraman G, Simcocks A, Hryciw DH, et al. G protein coupled receptor 18: A potential role for endocannabinoid signaling in metabolic dysfunction. Mol. Nutr. Food Res 2016;60:92–102. [DOI] [PubMed] [Google Scholar]
- [99].Pirault J, Bäck M. Lipoxin and resolvin receptors transducing the resolution of inflammation in cardiovascular disease. Front. Pharmacol 2018;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Miller S, Leishman E, Oehler O, et al. Evidence for a GPR18 role in diurnal regulation of intraocular pressure. Investig. Ophthalmol. Vis. Sci 2016;57:6419–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Laun AS, Shrader SH, Brown KJ, et al. GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol. Acta Pharmacol. Sin. [Internet] 2018;1 Available from: http://www.nature.com/articles/s41401-018-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Laun AS, Shrader SH, Song ZH. Novel inverse agonists for the orphan G protein-coupled receptor 6. Heliyon [Internet]. 2018;4:e00933 Available from: 10.1016/j.heliyon.2018.e00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Shrader SH, Song Z. Discovery of endogenous inverse agonists for G protein-coupled receptor 6. Biochem. Biophys. Res. Commun. [Internet] 2019;10–14 Available from: 10.1016/j.bbrc.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Pistis M, O’Sullivan SE. The Role of Nuclear Hormone Receptors in Cannabinoid Function [Internet] 1st ed. Adv. Pharmacol Elsevier Inc.; 2017. Available from: 10.1016/bs.apha.2017.03.008. [DOI] [PubMed] [Google Scholar]
- [105].Mahgoub M, Keun-Hang SY, Sydorenko V, et al. Effects of cannabidiol on the function of α7-nicotinic acetylcholine receptors. Eur. J. Pharmacol. [Internet] 2013;720:310–319. Available from: 10.1016/j.ejphar.2013.10.011. [DOI] [PubMed] [Google Scholar]
- [106].Ghovanloo M-R, Shuart NG, Mezeyova J, et al. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J. Biol. Chem. [Internet] 2018;jbc.RA118.004929. Available from: http://www.jbc.org/content/early/2018/09/14/jbc.RA118.004929.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lu J, Fan S, Zou G, et al. Involvement of glycine receptor α1 subunits in cannabinoid-induced analgesia. Neuropharmacology [Internet]. 2018;133:224–232. Available from: 10.1016/j.neuropharm.2018.01.041. [DOI] [PubMed] [Google Scholar]
- [108].Bakas T, van Nieuwenhuijzen PS, Devenish SO, et al. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol. Res 2017;119:358–370. [DOI] [PubMed] [Google Scholar]
- [109].Grotenhermen F Clinical Pharmacokinetics of Cannabinoids. J. Cannabis Ther. [Internet] 2003;3:3–51. Available from: http://www.tandfonline.com/doi/abs/10.1300/J175v03n01_02. [Google Scholar]
- [110].Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol 2018;84:2477–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zgair A, Wong JCM, Lee JB, et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am. J. Transl. Res 2016;8:3448–3459. [PMC free article] [PubMed] [Google Scholar]
- [112].Bruni N, Pepa C Della, Oliaro-Bosso S, et al. Cannabinoid delivery systems for pain and inflammation treatment. Molecules. 2018;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Atsmon J, Cherniakov I, Izgelov D, et al. PTL401, a New Formulation Based on Pro-Nano Dispersion Technology, Improves Oral Cannabinoids Bioavailability in Healthy Volunteers. J. Pharm. Sci 2018;107:1423–1429. [DOI] [PubMed] [Google Scholar]
- [114].Greish K, Mathur A, Al Zahrani R, et al. Synthetic cannabinoids nano-micelles for the management of triple negative breast cancer. J. Control. Release 2018;291:184–195. [DOI] [PubMed] [Google Scholar]
- [115].Ji Y, Wang Z, Pei F, et al. Introducing nitrogen atoms to amidoalkylindoles: potent and selective cannabinoid type 2 receptor agonists with improved aqueous solubility. Medchemcomm [Internet]. 2019; Available from: 10.1039/C9MD00411D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kerbrat A, Ferré J-C, Fillatre P, et al. Acute Neurologic Disorder from an Inhibitor of Fatty Acid Amide Hydrolase. N. Engl. J. Med. [Internet] 2016. [cited 2020 Feb 10];375:1717–1725. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1604221. [DOI] [PubMed] [Google Scholar]
- [117].Van Esbroeck ACM, Janssen APA, Cognetta AB, et al. Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10–2474. Science (80-. ). 2017;356:1084–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Nguyen AH, Thomsen ARB, Cahill TJ, et al. Structure of an endosomal signaling GPCR–G protein–β-arrestin megacomplex. Nat. Struct. Mol. Biol 2019;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]