Abstract
Pulmonary exacerbations (PExs) are significant life events in people with cystic fibrosis (CF), associated with declining lung function, reduced quality of life, hospitalizations, and decreased survival. The adult CF population is increasing worldwide, with many patients surviving prolonged periods with severe multimorbid disease. In many countries, the number of adults with CF exceeds the number of children, and PExs are particularly burdensome for adults as they tend to require longer courses and more IV treatment than children. The approach to managing PExs is multifactorial and needs to evolve to reflect this changing adult population. This review discusses PEx definitions, precipitants, treatments, and the wider implications to health-care resources. It reviews current management strategies, their relevance in particular to adults with CF, and highlights some of the gaps in our knowledge. A number of studies are underway to try to answer some of the unmet needs, such as the optimal length of treatment and the use of nonantimicrobial agents alongside antibiotics. An overview of these issues is provided, concluding that with the changing landscape of adult CF care, the definitions and management of PExs may need to evolve to enable continued improvements in outcomes across the age spectrum of CF.
Key Words: cystic fibrosis, infection, review
Abbreviations: ACT, airway clearance technique; AST, antibiotic susceptibility testing; CF, cystic fibrosis; ECFS, European Cystic Fibrosis Society; HTS, hypertonic saline; NIV, noninvasive ventilation; PEx, pulmonary exacerbation; pwCF, people with cystic fibrosis
FOR EDITORIAL COMMENT, SEE PAGE 3
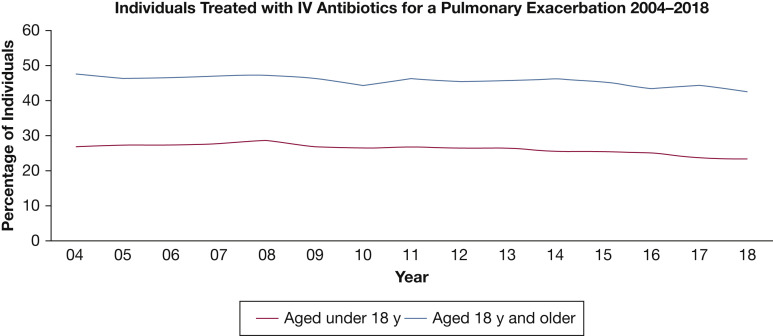
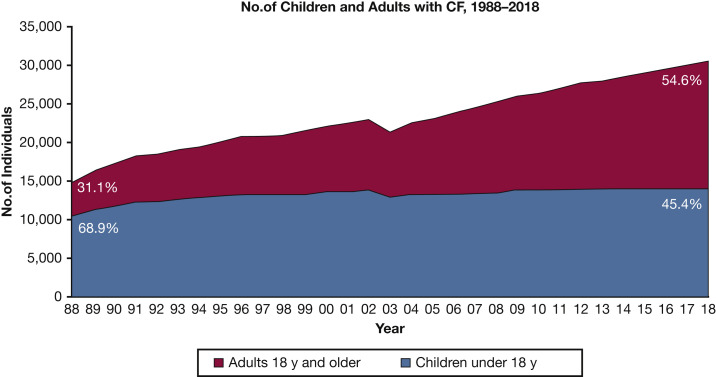
Pulmonary exacerbations (PExs) are recognized as important events in the lives of people with cystic fibrosis (pwCF), with prolonged and frequent exacerbations associated with declining lung function, reduced quality of life (QoL), and decreased survival.1 CF demography is changing: national registries containing data on > 90,000 pwCF2 reveal that in many countries, the adult CF population far outnumbers the pediatric CF population. Although PExs are significant events across all age groups, the prevalence is higher in adulthood, requiring more antibiotic treatments for longer periods compared with treatment of children with CF (Fig 1 ).1 In 2018, based on US CF registry data, approximately 43% of adults (aged ≥ 18 years) required IV antibiotics for a PEx compared with only 23% of children. In the United Kingdom, median (interquartile range) days of IV antibiotics per year for adults (aged ≤ 16 years) was 28 (14-48) compared with 16 (14-38) for children.3 Using western European data, Burgel et al4 predicted that the number of adults with CF would expand by up to 78% by 2025, while the number of pediatric CF cases would increase by just 20%. An increasing adult CF population (Fig 2 ), who have increasing multimorbidities,2 will require the approach to PEx management to evolve to recognize these differences. This review article focuses on PExs specifically in adults with CF to discuss these important emerging issues.
Figure 1.
IV antibiotic treatment for people with cystic fibrosis between 2004 and 2018, showing a higher prevalence in adults than children. (Reprinted with permission from the Cystic Fibrosis Foundation Patient Registry 2018 Annual Data Report.1)
Figure 2.
The increasing percentage of adults with CF. CF = cystic fibrosis. (Reprinted with permission from the Cystic Fibrosis Foundation Patient Registry 2018 Annual Data Report.1)
Definition of PExs
There remains no universally agreed definition of a PEx, making it difficult to standardize treatments. Historically, a PEx was defined as a deterioration in symptoms and biochemical markers, causing a physician to change treatments. However, by definition, this only accounts for exacerbations that cause management change, excluding those that resolve without antibiotics, and thus carries inherent problems due to variations in practice. Physician-led treatment remains the simplest definition of a PEx and has successfully been used in clinical trials,5 although this is unhelpful as a clinical tool to facilitate decision-making regarding antibiotic initiation. Models have been developed to try to standardize this, with the definition by Fuchs et al5 being perhaps the most widely recognized; they are not commonly used clinically (Table 1 ), however, and none of these definitions is exclusively for adult populations.6, 7, 8, 9
Table 1.
Summary of the Most Widely Recognized Definitions of a PEx
|
Definition |
Criteria to Define a PEx | Detail |
|---|---|---|
| EuroCareCF, 20116 | When additional antibiotics are needed due to a recent change in at least 2 items from a predefined list | Change in sputum volume or color; increased cough; increased fatigue, malaise, or lethargy; anorexia or weight loss; increased shortness of breath; decrease in pulmonary function by ≥ 10% compared with previous or radiographic changes consistent with a PEx |
| Rabin et al,7 2004 | Three or more signs/symptoms | In patients > 6 years old: relative decline in FEV1; increased cough frequency; new crackles; hemoptysis |
| Rosenfeld et al,8 2001 | Combined points system to diagnose a PEx and quantify its severity. Two models proposed, one using FEV1 | Model 1: decreased exercise tolerance; increased cough; increased sputum/cough clearance; increased sputum/cough congestion; school or work absenteeism; change in lung examination; decreased appetite Model 2: as per model 1; change in FEV1 |
| Ramsey et al,9 1999 | At least 2 signs/symptoms from a predefined list and 1 from a second list | List 1: Fever > 38°C; ≥ 50% increase in cough; 50% increase in sputum volume; loss of appetite; weight loss of ≥ 1 kg; absence from school or work for at least 3 of the preceding 7 days due to illness; symptoms of an upper respiratory tract infection List 2: decrease in FEV1 of at least 10%; increase in respiratory rate of at least 10 breaths/min; peripheral neutrophil count of > 15 |
| Fuchs et al,5 1994 | At least 4 signs/symptoms from a predefined list | Change in sputum; new or increased hemoptysis; increased cough; increased shortness of breath; malaise/fatigue/lethargy; temperature > 38°C; anorexia or weight loss; sinus pain or tenderness; change in sinus discharge; change in physical examination of the chest; decrease in pulmonary function by ≥ 10% compared with previous; radiographic changes consistent with a pulmonary exacerbation |
PEx = pulmonary exacerbation.
Antibiotic initiation is often based on a deterioration in FEV1. As a measurable and reproducible marker of lung health, possible for the majority of adults to complete, it remains a driver for guiding clinical decision-making. The Standardized Treatment of Pulmonary Exacerbations (STOP) study highlighted that some pwCF experience PExs without a change in FEV1. 10 In these cases, newer modalities such as the lung clearance index and/or MRI11 , 12 may be helpful, although they have yet to be established in this role, particularly for adults as most of the data are derived from children. In some countries, clinicians rely on C-reactive protein in clinical practice, although this and other biomarkers of inflammation have not yet been effectively incorporated as part of a PEx definition and its associated treatment.
Rather than the clinician-led diagnosis of PExs, patient-reported outcome measures are an attractive and promising approach.13 There have been several attempts to standardize these into scoring systems such as the Chronic Respiratory Infection Symptom Score.14 However, currently, they have largely been used to show the impact of PExs on patients and are mostly used in research studies.
The current suggested definitions5, 6, 7 , 9 aim to identify a PEx, but none categorizes severity or direct treatment. Importantly, they were all developed in an era predating CF transmembrane conductance regulator modulators, a new class of small molecule drug that treats the basic defect. These drugs have been shown to significantly reduce the rates of PEx15 , 16 with recent phase III trials of “triple therapy” modulators reducing PEx frequency by 63%.16
Etiology of PExs
The exact etiology of PExs and the underlying biological mechanisms driving disease are poorly understood. Pathogens infecting the pulmonary tract are believed to be the most common cause of PExs, but a variety of insults can change the homeostatic balance. PExs are most frequently caused by bacterial infections precipitating an amplified inflammatory response, leading to progressive and irreversible airway damage.17 Chronically infecting pathogens guide antibiotic treatment. Although Staphylococcus aureus and Haemophilus influenzae are most common in pediatrics, Pseudomonas aeruginosa dominates in subjects by 18 years of age. In 2019, 39.4% of UK adults with CF were chronically infected with P aeruginosa, while an additional 16.7% had intermittent P aeruginosa. 3 In the United States, approximately 70% of adults aged 30 years had at least one positive sputum culture of P aeruginosa in 2018, compared with only 20% of children aged 10 years.1 Other increasingly common bacteria in adults include Stenotrophomonas maltophilia and Achromobacter.
Nontuberculous mycobacteria are an increasing issue in CF, although their role in the setting of acute PExs is unclear.18 Allergic bronchopulmonary aspergillosis affects 8% to 9%3 , 19 of adults with CF and is associated with reduced lung function. Because allergic bronchopulmonary aspergillosis may cause some PExs, it is important to identify and treat this condition. The role of other Aspergillus-associated conditions (eg, Aspergillus bronchitis or sensitization) in PExs is unclear, as is the role of other fungal species, including Scedosporium, Candida families, and Exophiala species.18
Although respiratory viruses are not found more frequently in pwCF than in people with genetically normal lungs,20 they are believed to increase susceptibility to new bacterial infections or allow chronic bacteria to flare, causing a PEx.21 Data from a small sample of adults with CF highlighted that most PExs were caused by an existing strain of P aeruginosa, not a new bacterial growth.22 It remains unclear how much of an impact viruses have on deterioration in adults, whereas this is well established in pediatrics; studies in adults are varied, indicating minimal impact on lung function or rate of exacerbations.20 , 23 Influenza A and B, respiratory syncytial virus, rhinovirus, parainfluenza, cytomegalovirus, and adenovirus are found in CF, although influenza A is believed to be the most deleterious in adults.23 To date, the recent worldwide coronavirus disease 2019 pandemic seems to have had a lower impact on pwCF than predicted. A multinational report of 40 cases (median age, 33 years) concluded that this scenario may be due to effective shielding from exposure, but that the medium- and long-term effects on PwCF from this emerging pathogen are unknown.24
Not infrequently, the precise cause of the PEx is unknown, but patients respond to treatments regardless. Particularly relevant to adults is variable treatment adherence due to time constraints from work or family commitments. Reducing treatment burden and strategies to improve adherence were highlighted as research priorities in a survey of the CF community.25 Self-monitoring is an emerging field in CF: it may be effective to motivate patients to complete therapies and be vigilant for signs of exacerbations, while giving them greater responsibility away from a hospital-based environment. To date, research is scarce on the effectiveness of home monitoring; one large cohort study26 reported increased PEx identification but no difference in FEV1 decline over 52 weeks, which led to early trial termination.
Prevention and Management of PExs
The multifactorial presentation of PExs in adults with CF requires a multifaceted approach. Prevention of PExs in pwCF protects against lung injury and reduces rate of lung function decline. A number of pathways are targeted to achieve this goal, including optimizing nutrition and achieving diabetes control. Mucoactive agents are added early in infancy, and airway clearance techniques (ACTs) are used throughout the lives of pwCF. More specific interventions have been developed to eradicate bacteria and suppress chronic infections, including using oral and nebulized antibiotics.27 In severe presentations, adjunctive therapies such as noninvasive ventilation (NIV) and oxygen support may be required. Currently, there is no unified consensus for the best treatment or prevention of PEx, and there is a lack of robust evidence to guide clinical practice.28
Antibiotic Treatment
Antibiotics are key to PEx management and can be administered orally, by inhalation, or intravenously. Traditionally, particularly in P aeruginosa treatment,28 antibiotic combinations are used, aiming for synergistic antibacterial activity and reducing drug resistance. The STOP study reported that 54% of patients were prescribed two antibiotics, and 35% had three or more.10 This strategy is currently recommended by European Cystic Fibrosis Society (ECFS)27 and US28 guidelines, despite a lack of robust evidence.28 , 29 Although a consensus document on antibiotic treatment for CF30 identified aminoglycosides, polymyxins, β-lactams, cephalosporins, and carbapenems as potential antibiotics for use, a systematic review concluded that “no specific antibiotic combination can be considered superior to any other.”31 The CF community has highlighted the identification of the most effective/least toxic antibiotics as a research priority,25 while an international survey on antimicrobial stewardship perceptions found that health-care professionals wanted help with antibiotic choice, dose, and minimizing resistance.32
Most initial isolates of P aeruginosa are susceptible to commonly used antimicrobial therapies; however, resistance develops with repeated courses of antibiotics.33 Selection of the optimal antibiotic to use is highly debated, and treatments based on results of antibiotic susceptibility tests (ASTs) from traditional sputum cultures do not always predict an optimal clinical response.34 , 35 ASTs have limitations, especially in the context of phenotypic and genotypic diversifications of the CF lung microbiome and CF pathogens,34 although research has shown the airway microbiome to be relatively stable except for transient change with antibiotic treatment in PExs.36 The Cystic Fibrosis Microbiome-determined Antibiotic Therapy Trial in Exacerbations: Results Stratified (CFMATTERS) trial compared standard antibiotic therapy vs standard therapy plus an additional antibiotic selected from microbiome analysis of sputum; results showed no significant difference in clincal end points, and the active arm also required more IV days than standard therapy.37 In the context of AST, a Delphi consensus group recently recommended that decision-making be guided by clinical response to interventions instead of according to AST results.35
It has been suggested that 25% to 45% of adults with CF have chronic airway infection of multiresistant bacteria,38 which has been linked with faster disease progression.33 This creates an increasing challenge for adult CF teams as this prevalence could increase as median survival continues to improve. Given the complex issue of multiple bacterial growths, more research into optimal antibiotic combinations is required,16 as current treatments remain dependent on clinician experience and preferences. Increasing resistance necessitates alternative treatments, such as the cephalosporin/β-lactamase inhibitor combinations ceftazidime-avibactam and ceftolozane-tazobactam and the siderophore cephalosporin cefiderocol. It is believed that these new compounds are active against almost all current P aeruginosa isolates worldwide,39 thus offering possible emerging treatment options.
The decision to use oral, inhaled, or IV antibiotics as treatments is often based on exacerbation severity, with oral antibiotics often being used to treat patients with better lung function40 , 41 or milder exacerbations. Data for almost 45,000 PExs in the United States and Canada showed oral antibiotic use as the most prevalent PEx treatment, used in 73.2% of cases, IV antibiotics in 38.7%, and inhaled antibiotics in 23.9%.40 There was often an overlap of administration routes, with only 44% of PExs treated with oral antibiotics and 15% with inhaled antibiotics alone. Current evidence for the use of oral antibiotics yields conflicting results, one study suggesting oral antibiotics prevented IV use in 79% of mild PExs,42 while another retrospective study concluded that a significant proportion of patients did not recover baseline lung function when treated with oral agents, leading to decreased lung function long term.43 Although there is a relatively strong evidence base for inhaled antibiotics used as a chronic bacterial suppressive therapy, there is minimal evidence to support inhaled antibiotics being used alone to treat PExs.44 The US CF pulmonary guidelines28 and UK Cystic Fibrosis Trust Antibiotic Working Group guidelines30 acknowledge that inhaled antibiotics are often used alongside IV antibiotics when treating severe PExs, which might be more relevant to adults with CF; however, both concluded that there is not enough evidence to scientifically support this practice. The STOP study highlighted heterogeneity in the prescribing of adjunctive inhaled and/or oral antibiotics across US physicians.6 Using inhaled antibiotics alone as a first-line option to treat PExs may be beneficial as they do not have the same potential drug-related toxicity risks as IV formulations.44
Duration of Treatment
Traditionally, IV antibiotic courses last for 14 days27 , 45 but range from 10 to 21 days or extend further in severe infections. The STOP study reported a 15.0 day (SD, 6.0) mean IV treatment duration,6 although patients with FEV1 50% or below and those aged > 18 years were treated nearly 2 days longer. Retrospective data from the Epidemiological Study of CF have shown no relationship between IV treatment duration and recovery of FEV1.46
It may be argued that shorter IV courses have less risk of side effects and are better for the patient’s quality of life and adherence, while being cheaper for providers; however, courses that are too short may lead to only partial recovery and therefore necessitate further treatment.44 CFF registry data identified a significant increased risk of another PEx with treatment courses < 9 days or > 23 days.47 Interestingly, adult US CF physicians were uncomfortable enrolling patients into the STOP II study48 if it included IV courses < 10 days.41 With evidence lacking for the optimal duration of IV courses, results of the STOP II trial, concluding in early 2020, are eagerly awaited.
End points
When starting PEx treatment, the clinician must decide what defines treatment success; for example, reduction in biomarkers, improved FEV1, or an improvement in patient-reported outcome measures. The STOP study identified two common primary treatment objectives at the start of IV treatment for PExs: improvement of FEV1 and symptoms.41
ECFS best practice guidelines advocate assessing lung function at the beginning and end of PEx treatment,27 as many patients do not recover baseline lung function at the end of treatment or have another PEx within a short time,49 , 50 which is detrimental to their future health.49 Time to next exacerbation has been used in some trials as a marker of treatment success, but this is affected by many variables.
It is important to note from the STOP data that changes in objective clinical outcomes did not correlate well with the physician’s subjective judgment of treatment success51 and neither did it correlate with the patients’ symptom scores, particularly for adult patients.52 This highlights the necessity to incorporate both objective measures and patient-reported outcome measures when evaluating treatment effects.
Location of Treatment
Particularly relevant to adults with CF is the possibility of self-administering IV antibiotics at home, once trained to complete this safely and effectively. Although this option may be preferential for some, completing IV therapy alongside self-care can be time-consuming and effortful, with higher fatigue scores recorded in home IV candidates.53 Parkins et al50 reported that treatment for PExs was more likely to fail when adults had co-morbidities such as CF-related diabetes and CF liver disease, suggesting that for these complex cases inpatient surveillance may be beneficial. Importantly, ECFS guidelines recommend that if a patient with PEx requires hospitalization, this should not be delayed.27
Currently, studies show equal or inferior outcomes for home treatment compared with hospital treatment, but more robust evidence is required.53 Treatment location ranked as a priority research question for patients and clinicians in the STOP trial54 and remains in need of investigation.55 The STOP II trial includes patients with home and hospital treatment; thus, although location is not their primary outcome, insight into this question may be possible.
When deciding on PEx treatment location, clinicians should consider the individual’s circumstances53 and that medication is just one part of the care that a hospital admission includes. Multidisciplinary team interventions such as physiotherapy, dietetics, pharmacologic, or specialist nursing support are more readily available to inpatients, and may positively influence the outcome of treatment.56 Both the US and ECFS guidelines advocate home IV therapy only if all aspects of inpatient care can be replicated.27 , 28 With an increasingly multimorbid adult population, treating patients at home, particularly those with severe disease, may become even more challenging.
Mucoactive Drugs
Mucoactive medications include hyperosmolar drugs such as hypertonic saline (HTS) and mannitol, which work to increase the airway surface liquid, and dornase alfa, which breaks down DNA released by neutrophils in infected airways.57 Interestingly, trial data mostly support the use of inhaled mannitol in adults and not children with CF,58 perhaps relating to the better lung function seen in most children nowadays. Mannitol is not a therapeutic option for all pwCF because it is not commercially available worldwide. Although all of these agents have been shown to benefit stable pwCF,59, 60, 61 to date only HTS has been studied during an PEx,62 showing greater symptom resolution when added to usual care. A 2018 Cochrane review59 concluded that HTS “does appear to be an effective adjunct to physiotherapy during acute exacerbations.”
Airway Clearance Techniques
ACTs are a key part of daily CF management and are essential during PExs to clear infected sputum from the airways. With evidence growing that many adults replace ACT with exercise when well,63 it is imperative that all pwCF are competent to complete ACTs during episodes of PExs when sputum load may be increased and energy to exercise lowered. There is no consensus of which of the many ACTs is the best for pwCF,64 and there have been few studies investigating ACTs during PExs; however, it is recommended that usual ACT regimens are intensified,28 and ACTs may need alterations or additional support during PExs. As with research into long-term ACTs, acute ACT interventions require more robust studies using appropriate outcome measures for more evidence-based recommendations to be made.
NIV can be used to decrease the effort required by patients during ACT and can be helpful for individuals having difficulty expectorating sputum.64 NIV with ACT in adults has been shown to improve dyspnea,65 increase inspiratory muscle function, decrease fatigue,66 and improve FEV1 faster compared with usual ACT during PEx.
Other Treatments
There are significant variations in medication use to treat the inflammatory component of PEx such as systemic corticosteroids and oral antiinflammatories. Published evidence for steroid use is limited to a single clinical trial that investigated the effect of adding 5 days of prednisolone to standard therapy for acute PExs. Although their pilot data did not show any statistically significant differences, there was a trend toward improved pulmonary function in the steroid group that may guide future research work.67 Due to insufficient evidence, the 2009 US guidelines28 do not recommend the routine use of corticosteroids in PExs; however, they comment that a short course of steroids may offer benefit in this circumstance. In support of this, prospective observational data have shown that steroid use was significantly associated with a reduction in PEx symptom scores.68 The ongoing Prednisone in CF Pulmonary Exacerbations (PIPE) study aims to improve the current evidence base by investigating the effect of 7 days’ oral steroids on PEx outcomes in a randomized, double-blind, placebo-controlled trial.69
NIV and Oxygen Support
Because adults with CF have generally more severe disease than children, the use of oxygen and NIV is significantly higher (Fig 3 )70 and has been increasing steadily over the last decade.1 , 71 Currently, 8.9% of UK adults with CF use supplementary oxygen and 2.9% NIV, compared with 1.8% and 0.4% in the pediatric population, respectively.3 In the United States, the median FEV1 for children in 2018 was 94.3%, with only a very small minority in the severe lung disease category (< 40% predicted FEV1); the median value for adults was 69.4%, with about 20% of adults aged > 30 years in the severe category.1 There is no specific guidance on the use of emergency oxygen for CF, and thus much of the advice is extrapolated from generic respiratory guidelines for emergency oxygen use in adult patients.72 The presence of type II respiratory failure should be investigated during a severe PEx or in patients with advanced lung disease, especially those presenting with reduced resting oxygen saturations. A recent randomized controlled trial in adults with CF showed the effectiveness of long-term NIV over 1 year of treatment.73
Figure 3.
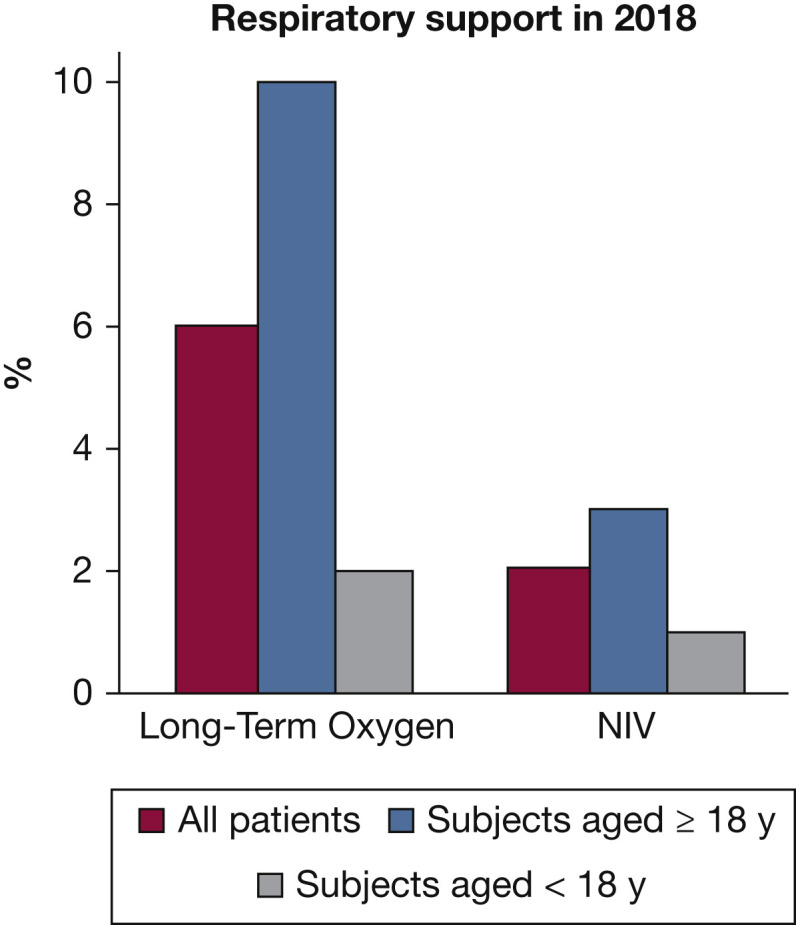
Oxygen and NIV usage in the UK population with cystic fibrosis, showing a higher prevalence in adults than children. NIV = noninvasive ventilation. (Reprinted with permission from the UK CF Trust Registry 2018.70)
Cost
Medical advancements in CF care have led to improvements in both lifestyle and survival for many. In 2019, 77% of the US adult CF population aged 18 to 29 years were working or in education.18 Working adults can be independent and not reliant on social care, while providing tax back into the economy; however, most governments continue to evaluate health economics on direct costs alone.74
As CF progresses, management becomes increasingly complex, and the costs mount. The Australian Registry analysis estimated annual costs of mild disease at US $10,151 compared with US $33,691 for severe CF.73 Patients require more medications, appointments, increasing and prolonged hospitalizations, home support, and often a lung transplant. The greatest cost in CF management is the hospital sector, ranging from 50% of cost in people with mild disease, to 77% of cost in people with severe disease. However, a PEx does not just have physical cost; psychological, social, and financial burdens must all be considered, and the longer a person with CF remains unwell, the more these costs rise. Further studies are needed to investigate the holistic costs of PExs and their overall economic impact.
Conclusions
The landscape in CF is changing with increasingly more adult patients. These patients have disease characteristics and treatment requirements that are diverging from the pediatric population, as they have more severe disease and multiple comorbidities. The introduction of CF transmembrane conductance regulator modulators may hail an era of PEx reduction, but there will remain an adult population for the foreseeable future with CF-related lung damage for whom PEx management is complicated and challenging.
PEx etiology is multifactorial and the management often complex, requiring a multifaceted approach. A better definition of PEx is still required and more effective management of the infective and noninfective components of the PEx is needed, supported by a stronger evidence base. Currently, there are ongoing studies aiming to answer some of the key questions in PEx management. Until there is more robust evidence for many aspects of management, clinicians need to supplement the evidence base by extrapolating data from stable state studies, clinical guidelines, and thorough assessment of the individual. It is not yet known if, in the future, adults with better lung function will experience PExs in the same way, but it is likely that definitions and management will need to evolve, as the issues we have highlighted will continue to change.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: N. J. S. has received consultancy fees from Vertex, Roche, Chiesi, and Pulmocide; and honoraria for speaking engagements from Vertex, Chiesi, Teva, and Zambon. None declared (G. E. S., K. D.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: The post of G. E. S. is supported by a Health Education England/National Institute for Health Research Clinical doctoral fellowship [Grant No. CDRF-2014-05-055].
References
- 1.Cystic Fibrosis Foundation (CFF). CFF 2018 Patient Registry Annual Data Report. https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2018-Patient-Registry-Annual-Data-Report.pdf. Accessed November 11, 2020.
- 2.Bell S.C., Mall M.A., Gutierrez H., et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2020;8(1):65–124. doi: 10.1016/S2213-2600(19)30337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Cystic Fibrosis Trust. UK Cystic Fibrosis Registry Annual Data Report 2019. https://www.cysticfibrosis.org.uk/the-work-we-do/uk-cf-registry/reporting-and-resources, 2020.
- 4.Burgel P.R., Bellis G., Olesen H.V., et al. Future trends in cystic fibrosis demography in 34 European countries. Eur Respir J. 2015;46(1):133. doi: 10.1183/09031936.00196314. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs H.J., Borowitz D.S., Christiansen D.H., et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331(10):637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 6.Bilton D, Canny G, Conway S, et al. Pulmonary exacerbation: towards a definition for use in clinical trials. Report from the EuroCareCF Working Group on outcome parameters in clinical trials. J Cyst Fibros. 2011;10(suppl 2):S79-S81. [DOI] [PubMed]
- 7.Rabin H.R., Butler S.M., Wohl M.E.B., et al. Pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2004;37(5):400–406. doi: 10.1002/ppul.20023. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld M., Emerson J., Williams-Warren J., et al. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr. 2001;139(3):359–365. doi: 10.1067/mpd.2001.117288. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey B.W., Pepe M.S., Quan J.M., et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 10.West N.E., Beckett V.V., Jain R., et al. Standardized Treatment of Pulmonary Exacerbations (STOP) study: physician treatment practices and outcomes for individuals with cystic fibrosis with pulmonary exacerbations. J Cyst Fibros. 2017;16(5):600–606. doi: 10.1016/j.jcf.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wielpütz MO, Mall MA. Imaging modalities in cystic fibrosis: emerging role of MRI. Curr Opin Pulm Med. 2015;21(6):609-616. [DOI] [PubMed]
- 12.Sonneveld N., Stanojevic S., Amin R., et al. Lung clearance index in cystic fibrosis subjects treated for pulmonary exacerbations. Eur Respir J. 2015;46(4):1055. doi: 10.1183/09031936.00211914. [DOI] [PubMed] [Google Scholar]
- 13.Goss C.H., Quittner A.L. Patient-reported outcomes in cystic fibrosis. Proc Am Thorac Soc. 2007;4(4):378–386. doi: 10.1513/pats.200703-039BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goss C.H., Edwards T.C., Ramsey B.W., Aitken M.L., Patrick D.L. Patient-reported respiratory symptoms in cystic fibrosis. J Cyst Fibros. 2009;8(4):245–252. doi: 10.1016/j.jcf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Ramsey B.W., Davies J., McElvaney N.G., et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middleton P.G., Mall M.A., Dřevínek P., et al. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferkol T., Rosenfeld M., Milla C.E. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006;148(2):259–264. doi: 10.1016/j.jpeds.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Chmiel J.F., Aksamit T.R., Chotirmall S.H., et al. Antibiotic management of lung infections in cystic fibrosis. II. Nontuberculous mycobacteria, anaerobic bacteria, and fungi. Ann Am Thorac Soc. 2014;11(8):1298–1306. doi: 10.1513/AnnalsATS.201405-203AS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cystic Fibrosis Foundation (CFF) Cystic Fibrosis Foundation Patient Registry 2019 Patient Registry Snapshot. https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2019-Cystic-Fibrosis-Foundation-Patient-Registry-Snapshot/2020
- 20.Ong E.L., Ellis M.E., Webb A.K., et al. Infective respiratory exacerbations in young adults with cystic fibrosis: role of viruses and atypical microorganisms. Thorax. 1989;44(9):739–742. doi: 10.1136/thx.44.9.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Ewijk B.E., van der Zalm M.M., Wolfs T.F.W., van der Ent C.K. Viral respiratory infections in cystic fibrosis. J Cyst Fibros. 2005;4:31–36. doi: 10.1016/j.jcf.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aaron S.D., Ramotar K., Ferris W., et al. Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2004;169(7):811. doi: 10.1164/rccm.200309-1306OC. [DOI] [PubMed] [Google Scholar]
- 23.Flight W.G., Bright-Thomas R.J., Tilston P., et al. Incidence and clinical impact of respiratory viruses in adults with cystic fibrosis. Thorax. 2014;69(3):247–253. doi: 10.1136/thoraxjnl-2013-204000. [DOI] [PubMed] [Google Scholar]
- 24.Cosgriff R., Ahern S., Bell S.C., et al. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J Cyst Fibros. 2020;19(3):355–358. doi: 10.1016/j.jcf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowbotham N.J., Smith S., Leighton P.A., et al. The top 10 research priorities in cystic fibrosis developed by a partnership between people with CF and healthcare providers. Thorax. 2018;73(4):388–390. doi: 10.1136/thoraxjnl-2017-210473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lechtzin N., Mayer-Hamblett N., West N.E., et al. Home monitoring of patients with cystic fibrosis to identify and treat acute pulmonary exacerbations. eICE Study Results. Am J Respir Crit Care Med. 2017;196(9):1144. doi: 10.1164/rccm.201610-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smyth A.R., Bell S.C., Bojcin S., et al. European Cystic Fibrosis Society Standards of Care: best practice guidelines. J Cyst Fibros. 2014;13:S23–S42. doi: 10.1016/j.jcf.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Flume P.A., Mogayzel P.J., Robinson K.A., et al. Cystic fibrosis pulmonary guidelines. Am J Respir Crit Care Med. 2009;180(9):802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 29.Elphick H.E., Scott A. Single versus combination intravenous anti-pseudomonal antibiotic therapy for people with cystic fibrosis. Cochrane Database Syst Rev. 2016;12(12):CD002007. doi: 10.1002/14651858.CD002007.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.UK Cystic Fibrosis Trust Antibiotic Working Group. Antibiotic treatment for cystic fibrosis - 3rd edition. https://www.cysticfibrosis.org.uk/∼/media/documents/the-work-we-do/care/consensus-docs-with-new-address/anitbiotic-treatment.ashx?la=en. Accessed November 11, 2020.
- 31.Hurley M.N., Prayle A.P., Flume P. Intravenous antibiotics for pulmonary exacerbations in people with cystic fibrosis. Cochrane Database Syst Rev. 2015;7:CD009730. doi: 10.1002/14651858.CD009730.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullington W., Smyth A., Elborn S., Drevinek P., Hempstead S., Muhlebach M. 1078. Expectations and attitudes toward antimicrobial stewardship among cystic fibrosis care providers. Open Forum Infect Dis. 2019;6(suppl 2):S382–S383. [Google Scholar]
- 33.Lechtzin N., John M., Irizarry R., Merlo C., Diette G.B., Boyle M.P. Outcomes of adults with cystic fibrosis infected with antibiotic-resistant Pseudomonas aeruginosa. Respiration. 2006;73(1):27–33. doi: 10.1159/000087686. [DOI] [PubMed] [Google Scholar]
- 34.Waters V.J., Kidd T.J., Canton R., et al. Reconciling antimicrobial susceptibility testing and clinical response in antimicrobial treatment of chronic cystic fibrosis lung infections. Clin Infect Dis. 2019;69(10):1812–1816. doi: 10.1093/cid/ciz364. [DOI] [PubMed] [Google Scholar]
- 35.Zemanick E., Burgel P.R., Taccetti G., et al. Antimicrobial Resistance International Working Group in Cystic Fibrosis. Antimicrobial resistance in cystic fibrosis: a Delphi approach to defining best practices. J Cyst Fibros. 2020;19(3):370–375. doi: 10.1016/j.jcf.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Einarsson G., Flanagan E., Lee A., Elborn J.S., Tunney M., Plant B.J. WS03.1 Longitudinal airway microbiota profiling in cystic fibrosis patients enrolled in the CFMATTERS clinical trial. J Cyst Fibros. 2017;16:S4. [Google Scholar]
- 37.Plant B. Final report summary—Cystic fibrosis microbiome-determined antibiotic therapy trial in exacerbations: results stratified. https://cordis.europa.eu/docs/results/603/603038/final1-cfmatters-final-report-draft-v3-mp-31082017.pdf
- 38.Aaron S.D., Vandemheen K.L., Ferris W., et al. Combination antibiotic susceptibility testing to treat exacerbations of cystic fibrosis associated with multiresistant bacteria: a randomised, double-blind, controlled clinical trial. Lancet. 2005;366(9484):463–471. doi: 10.1016/S0140-6736(05)67060-2. [DOI] [PubMed] [Google Scholar]
- 39.Tümmler B. Emerging therapies against infections with Pseudomonas aeruginosa. F1000 Research. 2019;8:F1000. doi: 10.12688/f1000research.19509.1. Faculty Rev-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagener J.S., Rasouliyan L., VanDevanter D.R., et al. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2013;48(7):666–673. doi: 10.1002/ppul.22652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders D.B., Solomon G.M., Beckett V.V., et al. Standardized Treatment of Pulmonary Exacerbations (STOP) study: observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros. 2017;16(5):592–599. doi: 10.1016/j.jcf.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Briggs E.C., Nguyen T., Wall M.A., MacDonald K.D. Oral antimicrobial use in outpatient cystic fibrosis pulmonary exacerbation management: a single-center experience. Clin Respir J. 2012;6(1):56–64. doi: 10.1111/j.1752-699X.2011.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanojevic S., McDonald A., Waters V., et al. Effect of pulmonary exacerbations treated with oral antibiotics on clinical outcomes in cystic fibrosis. Thorax. 2017;72(4):327. doi: 10.1136/thoraxjnl-2016-208450. [DOI] [PubMed] [Google Scholar]
- 44.Smith S., Rowbotham N.J., Charbek E. Inhaled antibiotics for pulmonary exacerbations in cystic fibrosis. Cochrane Database Syst Rev. 2018;10(10):CD008319. doi: 10.1002/14651858.CD008319.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abbott L., Plummer A., Hoo Z.H., Wildman M. Duration of intravenous antibiotic therapy in people with cystic fibrosis. Cochrane Database Syst Rev. 2019;9(9):CD006682. doi: 10.1002/14651858.CD006682.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schechter M.S., VanDevanter D.R., Pasta D.J., Short S.A., Morgan W.J., Konstan M.W. Treatment setting and outcomes of cystic fibrosis pulmonary exacerbations. Ann Am Thorac Soc. 2017;15(2):225–233. doi: 10.1513/AnnalsATS.201702-111OC. [DOI] [PubMed] [Google Scholar]
- 47.Vandevanter D.R., Flume P.A., Morris N., Konstan M.W. Probability of IV antibiotic retreatment within thirty days is associated with duration and location of IV antibiotic treatment for pulmonary exacerbation in cystic fibrosis. J Cyst Fibro. 2016;15(6):783–790. doi: 10.1016/j.jcf.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Institutes of Health Clinical Center. Standardized treatment of pulmonary exacerbations II (STOP2). NCT02781610. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2016. https://clinicaltrials.gov/ct2/show/NCT02781610. Updated March 17, 2020.
- 49.Sanders D.B., Zhao Q., Li Z., Farrell P.M. Poor recovery from cystic fibrosis pulmonary exacerbations is associated with poor long-term outcomes. Pediatric Pulmonol. 2017;52(10):1268–1275. doi: 10.1002/ppul.23765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parkins M.D., Rendall J.C., Elborn J.S. Incidence and risk factors for pulmonary exacerbation treatment failures in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. Chest. 2012;141(2):485–493. doi: 10.1378/chest.11-0917. [DOI] [PubMed] [Google Scholar]
- 51.Burgel P.R., Reid D.W., Aaron S.D. A first step to STOP cystic fibrosis exacerbations. J Cyst Fibro. 2017;16(5):529–531. doi: 10.1016/j.jcf.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Gold L.S., Patrick D.L., Hansen R.N., Goss C.H., Kessler L. Correspondence between lung function and symptom measures from the Cystic Fibrosis Respiratory Symptom Diary–Chronic Respiratory Infection Symptom Score (CFRSD-CRISS) J Cyst Fibro. 2019;18(6):886–893. doi: 10.1016/j.jcf.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sequeiros I., Jarad N. Home intravenous antibiotic treatment for acute pulmonary exacerbations in cystic fibrosis—is it good for the patient? Ann Thorac Med. 2009;4(3):111–114. doi: 10.4103/1817-1737.53346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heltshe S.L., West N.E., VanDevanter D.R., et al. Study design considerations for the Standardized Treatment of Pulmonary Exacerbations 2 (STOP2): a trial to compare intravenous antibiotic treatment durations in CF. Contemporary Clinical Trials. 2018;64:35–40. doi: 10.1016/j.cct.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West N.E., Flume P.A. Unmet needs in cystic fibrosis: the next steps in improving outcomes. Expert Rev Respir Med. 2018;12(7):585–593. doi: 10.1080/17476348.2018.1483723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skolnik K., Quon B.S. Recent advances in the understanding and management of cystic fibrosis pulmonary exacerbations. F1000Research. 2018;7:F1000. doi: 10.12688/f1000research.13926.1. Faculty Rev-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurt K., Bilton D. Inhaled interventions in cystic fibrosis: mucoactive and antibiotic therapies. Respiration. 2014;88(6):441–448. doi: 10.1159/000369533. [DOI] [PubMed] [Google Scholar]
- 58.Nevitt S.J., Thornton J., Murray C.S., Dwyer T. Inhaled mannitol for cystic fibrosis. Cochrane Database Syst Rev. 2018;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wark P., McDonald V.M. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev. 2018;9(9):CD001506. doi: 10.1002/14651858.CD001506. [DOI] [PubMed] [Google Scholar]
- 60.Yang C., Montgomery M. Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev. 2018;9(9):CD001127. doi: 10.1002/14651858.CD001127.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nevitt S.J., Thornton J., Murray C.S., Dwyer T. Inhaled mannitol for cystic fibrosis. Cochrane Database Syst Rev. 2018;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dentice R.L., Elkins M.R., Middleton P.G., et al. A randomised trial of hypertonic saline during hospitalisation for exacerbation of cystic fibrosis. Thorax. 2016;71(2):141. doi: 10.1136/thoraxjnl-2014-206716. [DOI] [PubMed] [Google Scholar]
- 63.Ward N., Stiller K., Holland A.E., et al. Exercise is commonly used as a substitute for traditional airway clearance techniques by adults with cystic fibrosis in Australia: a survey. J Physiother. 2019;65(1):43–50. doi: 10.1016/j.jphys.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Association of Chartered Physiotherapists in Cystic Fibrosis (ACPCF) Standards of care and good clinical practice for the physiotherapy management of cystic fibrosis. https://www.cysticfibrosis.org.uk/~/media/documents/life-with-cf/publications/consensus-on-physiotherapy-management--third-edition-2017.ashx 3rd edition; 2017.
- 65.Holland A.E., Denehy L., Ntoumenopoulos G., Naughton M.T., Wilson J.W. Non-invasive ventilation assists chest physiotherapy in adults with acute exacerbations of cystic fibrosis. Thorax. 2003;58(10):880. doi: 10.1136/thorax.58.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dwyer T.J., Robbins L., Kelly P., Piper A.J., Bell S.C., Bye P.T.P. Non-invasive ventilation used as an adjunct to airway clearance treatments improves lung function during an acute exacerbation of cystic fibrosis: a randomised trial. J Physiother. 2015;61(3):142–147. doi: 10.1016/j.jphys.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 67.Dovey M., Aitken M.L., Emerson J., McNamara S., Waltz D.A., Gibson R.L. Oral corticosteroid therapy in cystic fibrosis patients hospitalized for pulmonary exacerbation: a pilot study. Chest. 2007;132(4):1212–1218. doi: 10.1378/chest.07-0843. [DOI] [PubMed] [Google Scholar]
- 68.Heltshe S.L., Goss C.H., Thompson V., et al. Short-term and long-term response to pulmonary exacerbation treatment in cystic fibrosis. Thorax. 2016;71(3):223. doi: 10.1136/thoraxjnl-2014-206750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.National Institutes of Health Clinical Center. Prednisone in CF pulmonary exacerbations (PIPE). NCT03070522. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2017. https://clinicaltrials.gov/ct2/show/NCT03070522. Updated November 3, 2020.
- 70.UK Cystic Fibrosis Trust. UK Cystic Fibrosis Registry Annual Data Report 2018. https://www.cysticfibrosis.org.uk/∼/media/documents/the-work-we-do/uk-cf-registry/2018-registry-annual-data-report.ashx?la=en. Published 2019. Accessed November 11, 2020.
- 71.Archangelidi O., Carr S.B., Simmonds N.J., et al. Non-invasive ventilation and clinical outcomes in cystic fibrosis: findings from the UK CF registry. J Cyst Fibro. 2019;18(5):665–670. doi: 10.1016/j.jcf.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 72.O’driscoll B.R., Howard L.S., Davison A.G. BTS guideline for emergency oxygen use in adult patients. Thorax. 2008;63(suppl 6):vi1. doi: 10.1136/thx.2008.102947. [DOI] [PubMed] [Google Scholar]
- 73.Milross M.A., Piper A.J., Dwyer T.J., et al. Non-invasive ventilation versus oxygen therapy in cystic fibrosis: a 12-month randomized trial. Respirology. 2019;24(12):1191–1197. doi: 10.1111/resp.13604. [DOI] [PubMed] [Google Scholar]
- 74.van Gool K., Norman R., Delatycki M.B., Hall J., Massie J. Understanding the costs of care for cystic fibrosis: an analysis by age and health state. Value Health. 2013;16(2):345–355. doi: 10.1016/j.jval.2012.12.003. [DOI] [PubMed] [Google Scholar]